Abstract
Opioid receptors are highly homologous GPCRs that modulate brain function at all levels of neural integration, including autonomous, sensory, emotional and cognitive processing. Opioid receptors functionally interact in vivo, but the underlying mechanisms involving direct receptor–receptor interactions, affecting signalling pathways or engaging different neuronal circuits, remain unsolved. Heteromer formation through direct physical interaction between two opioid receptors or between an opioid receptor and a non-opioid one has been postulated and can be characterized by specific ligand binding, receptor signalling and trafficking properties. However, despite numerous studies in heterologous systems, evidence for physical proximity in vivo is only available for a limited number of opioid heteromers, and their physiopathological implication remains largely unknown mostly due to the lack of appropriate tools. Nonetheless, data collected so far using endogenous receptors point to a crucial role for opioid heteromers as a molecular entity that could underlie human pathologies such as alcoholism, acute or chronic pain as well as psychiatric disorders. Opioid heteromers therefore stand as new therapeutic targets for the drug discovery field.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Keywords: opioid receptors, GPCRs, heteromer, nociception, mood disorders, addiction, fluorescent knock-in mice
The opioid system
The opioid system is composed of three families of endogenous peptides, the enkephalins, dynorphins and β-endorphin, and three homologous GPCRs, the μ opioid receptor (MOP), δ opioid receptor (DOP) and κ opioid receptor (KOP) (Filizola and Devi, 2013; Cox et al., 2015; receptor nomenclature follows Alexander et al., 2013a). Opioid receptors and endogenous opioid peptides are expressed throughout the nervous system (Le Merrer et al., 2009). The opioid system plays a key role in reward and motivation, and regulates emotional responses and cognition. The system also modulates nociception, neuroendocrine physiology and autonomic functions (see Walwyn et al., 2010; Feng et al., 2012). The involvement of the three opioid receptors in pain control, drug abuse and mood disorders has been extensively studied and has been the focus of recent reviews (Bruchas et al., 2010; Gaveriaux-Ruff and Kieffer, 2011; Pradhan et al., 2011; Raehal et al., 2011; Lutz and Kieffer, 2012; 2013; Nadal et al., 2013; Charbogne et al., 2014).
Several decades of opioid pharmacology have uncovered the complexity of the opioid system physiology. In particular, the analysis of the effects of opioid drugs in vivo has revealed functional interactions across receptors, particularly documented for MOP and DOP receptors. However, whether in vivo receptor interactions occur at circuit, cellular or molecular level remains highly debated. In this context, formation of a new molecular entity with specific signalling and/or trafficking promoted by physical interaction between two receptors has been proposed. Such heteromers would constitute the molecular determinant of the integrated changes observed at system level. Numerous studies have reported heteromerization of all three opioid receptors between themselves or with non-opioid receptors in transfected cells, but data related to endogenous receptors are necessary to accurately describe the impact of in vivo heteromerization.
This review briefly covers the concept of receptor heteromers and the criteria required to validate their existence in vivo. Our current view of the structural determinants involved in opioid receptor association is presented. Techniques available to determine physical proximity between two endogenous receptors are then listed with emphasis on their limitations, and the functional outcome of heteromer formation between endogenous receptors with known in vivo physical interactions is reviewed. Finally, new tools for in vivo studies are presented and their expected effects on drug discovery discussed.
Opioid heteromers: the genesis
Receptor heteromers: a definition
GPCRs have long been considered to function as monomers, but this view has now been disputed for several years. The first breach arose from the observation that an obligatory association between two different monomers is required for GABAB receptor signalling, (Jones et al., 1998). The observation has now been extended to other class C GPCRs (Rondard et al., 2011). It then led to question whether such heterodimerization was restricted to class C GPCRs or could also be extended to class A GPCRs whose prototype is rhodopsin and to which opioid receptors belong. In particular, could direct physical interactions between two receptor types promote conformational changes and modulate ligand binding or receptor signalling properties? A large number of studies were initiated to address physical association between class A GPCRs and to investigate the functional impact in heterologous systems where the receptors are not naturally produced (Birdsall, 2010; Rozenfeld and Devi, 2011). This generated a long list of potential candidates to form pairs named heteromers. This term has been proposed in an attempt to provide a nomenclature that clarifies associations between receptors (Ferre et al., 2009). According to this classification, heteromeric receptors are defined as the minimal functional unit composed of two or more different subunits that are not functional on their own. Class C GABAB receptors fall into this category. On the other hand, heteromers refer to macromolecular complexes composed of at least two functional receptor units that show specific biochemical properties different from those of the individual components. This definition applies to a large number of the reported associations between class A receptors and will be adopted here.
How can opioid receptors form heteromers?
Recent crystallographic structures of the inactive forms of the four members of the opioid receptor family have brought to light interesting features (Granier et al., 2012; Manglik et al., 2012; Thompson et al., 2012; Wu et al., 2012). They revealed many common features in the four binding pockets with selective determinants essentially located on the extracellular surface (Filizola and Devi, 2013), therefore confirming the concept of message-address proposed some years ago (Décaillot et al., 2003; Rosenbaum et al., 2009). Interestingly, MOP receptors crystallized in a parallel dimeric form with a tight interface involving 28 amino acids of the transmembrane domains TM 5 and 6 (Manglik et al., 2012). It is thus tempting to conclude that MOP receptors exist as homodimers. However, the arrangement in dimers may also result from crystallization conditions and/or from the modifications introduced in the structure to make them amenable to crystal formation. Interestingly, an additional interface involving TM 1, TM 2 and helix H8 is present that can also be seen in the KOP receptor crystal (Wu et al., 2012 *).
Using a subtractive correlated mutation method, in silico modelling predicted that the most likely receptor interface in MOP/DOP receptor heteromers would involve MOP TM 1 and DOP TM 4, 5 and 6 (Filizola et al., 2002 *). In this approach, mutation analysis was correlated to the structural information contained in three-dimensional molecular models of the transmembrane regions using the rhodopsin crystal structure as a template (Filizola et al., 2002). Molecular dynamics on full-length, 3D models of MOP and DOP receptors also designated MOP TM 1 and 7, and DOP TM 4 and 5 as the most likely interface between the two receptors with an emphasis on MOP TM 1 and DOP TM 4 (Liu et al., 2009 *). TM 4 and 5 were also proposed as forming the interface in DOP receptor homodimers with TM 4/TM 4 associations being the most stable (Johnston et al., 2011 *).
Physical contact between MOP and DOP receptors through MOP TM 1 is also supported by experimental data. Expression of the MOP TM 1 fused to the transactivating transcriptional activator (TAT) sequence of the cell transduction domain of the human immunodeficiency virus (HIV) indeed interfered with endogenous MOP/DOP receptor co-immunoprecipitation (He et al., 2011 *).
On the other hand, experimental approaches pointed to an involvement of the receptor C-termini in heteromer formation. Decreased MOP/DOP receptor co-immunoprecipitation was observed in HEK 293 cells upon expression of the DOP C-terminus fused to the TAT peptide (Kabli et al., 2010 *). Similarly, reduced co-immunoprecipitation of MOP/DOP heteromers, but not DOP/DOP or MOP/MOP homomers, was observed upon truncation of the C-terminus of either receptors in transfected COS cells (Fan et al., 2005 *). Decreased co-immunoprecipitation was also observed when the sequence specific for the MOP1D splice variant (RNEEPSS) was fused to the TAT sequence and expressed in the mouse spinal cord suggesting a role for the C-termini in MOP1D/GRP heteromer formation (Liu et al., 2011b *). The distal part of the C-terminus, although involved in the formation of those heteromers, is not the only determinant as co-immunoprecipitation was only reduced and not abolished upon receptor truncation. MOP and NOP receptor co-immunoprecipitation was no longer detected in transfected HEK 293 cells upon truncation of the distal C-terminal part of either receptors suggesting that C-termini are also involved in their physical association (Wang et al., 2005 *). Finally, direct interaction between MOP and the NMDA NR1 subunit (see Alexander et al., 2013b for nomenclature) involved the C-termini of the two proteins, and the extent of association was modulated by PKC (Rodriguez-Munoz et al., 2012 *).
Interestingly, a peptide corresponding to the DOP receptor second intracellular loop fused to the TAT sequence reduced cell surface expression of this receptor in dorsal root ganglia (DRG) possibly through disruption of MOP/DOP heteromers as this peptide reduced MOP/DOP co-immunoprecipitation in transfected NG 108-15 cells (Xie et al., 2009 *).
Altogether, structural, biochemical and in silico data suggest that MOP TM 1 and DOP TM 4/TM 5 participate in the receptor interface in opioid heteromers and that additional interactions may occur between the C-termini.
Whether receptor pairs associate as early as the endoplasmic reticulum or whether opioid heteromer formation takes place at the plasma membrane remains unsolved. Some studies suggest that the receptors are exported together to the cell surface in heterologous system (Hasbi et al., 2007; Décaillot et al., 2008) or in vivo (Xie et al., 2009). Also, the increase in cell surface expression of DOP receptors observed upon chronic morphine treatment was dependent on MOP receptors (Cahill et al., 2001; Morinville et al., 2003 *). This observation is compatible with physical association taking place during export but can also reflect interactions forming at the cell surface. Interestingly, heteromerization between constitutively expressed MOP and DOP receptors expressed with an ecdysone-inducible system exclusively occurred at the surface of HEK 293 cells. In this system, cell surface expression of mutants unable to reach the cell surface on their own was not rescued by physical interaction with wild-type MOP and DOP receptor proteins (Law et al., 2005 *).
Minimal functional entity: monomer or dimer?
Despite growing evidence pointing to the existence of dimers, and possibly multimers between class A GPCRs, it is still strongly debated whether the minimal functional unit required to activate G proteins is a receptor monomer or dimer. It should be noted that the natural propensity of a given GPCR to form heteromers might be receptor-dependent. It is conceivable that some receptors do not require association with others to initiate intracellular cascades or only transiently interact to modulate downstream signalling as suggested for the dopamine D2 receptors (Fonseca and Lambert, 2009 *). Receptor-Gα fusion proteins that exhibit a fixed 1:1 receptor : G protein ratio were used in an attempt to better understand how opioid heteromers signal. Upon co-expression of receptor-Gα fusion proteins in transfected cells, physical association between DOP and MOP receptors (Snook et al., 2006), or between DOP receptors and the chemokine receptor CXCR2 (Parenty et al., 2008 *) was revealed by co-immunoprecipitation. Functional data suggested that activation of one receptor is sufficient to initiate G protein signalling though the underlying mechanism was dependent on the amount of available Gα subunits. G protein activation was attributed to allosteric modulation when Gα accessibility was not a limiting factor (Parenty et al., 2008; Snook et al., 2008 *). Alternatively, promiscuous contact between the functional receptor and the G protein was postulated when the two receptors were competing for a limited pool of Gα subunits (Molinari et al., 2003; Snook et al., 2006 *). In the latter case, ligand binding is affected, and the observed changes can be mistaken for ligand-binding cooperativity, leading to erroneous assumptions of the existence of functional heteromers (Chabre et al., 2009 *).
Opioid heteromers in heterologous systems
Devi's group was the first to report heteromer formation involving opioid receptors. Upon cell co-transfection with tagged KOP and DOP receptors, close physical vicinity was detected by co-immunoprecipitation and was accompanied by changes in ligand-binding and receptor-signalling properties (Jordan and Devi, 1999 *). Numerous studies in heterologous systems reported heteromerization of all three opioid receptors between themselves or with non-opioid partners. The alterations in ligand binding, receptor signalling or trafficking observed using epitope-tagged receptors in heterologous systems were attributed to specific properties elicited by physical association of the two transfected receptors (see Massotte, 2010; van Rijn et al., 2010b; Rozenfeld and Devi, 2011; Stockton and Devi, 2012). The results were not always congruent, and it was then argued that alterations in signalling and trafficking properties could be artificially created as a result of enforced interactions between receptors that were overexpressed in a non-native environment. In particular, the high level of expression that largely exceeds, by at least 10-fold, the endogenous level, may introduce bias in the interpretation of the results. Indeed, allosteric modulation of ligand binding was associated with MOP/DOP heteromerization (Gomes et al., 2011 *; Gomes et al., 2004). However, the amount of GTP available during the in vitro pharmacological experiments can affect the binding affinities of agonists to the monomers. Therefore, alterations in ligand-binding properties do not necessarily imply heteromer formation (Chabre et al., 2009 *).
With time, the physiological relevance of the reported heteromers became an increasing subject of concern. In a number of cases, there was no evidence supporting in vivo co-expression of the two partners within the same cell. In addition, it is widely acknowledged that the cellular content varies among cell lines and primary cultures (von Zastrow, 2010 *). Although fundamental processes are conserved across cell types, substantial differences may exist between transfected and naturally expressing cells. For example, the MOP receptor agonist morphine does not induce receptor internalization in several transfected cell lines (Borgland et al., 2003; Keith et al., 1996 *) or in neurons from the mouse locus coeruleus (Arttamangkul et al., 2008 *) but does induce sequestration upon MOP receptor transfection in rat striatal neurons (Haberstock-Debic et al., 2005 *). In the brain, MOP receptors can also respond differently to a stimulus when located in the soma or dendrites (Haberstock-Debic et al., 2003 *). In addition, the opioid pharmacology is complex, and in vitro ligand selectivities sometimes greatly differ from those demonstrable in vivo. For example, [D-Pen2,5]enkephalin, (DPDPE) is described in vitro as a DOP receptor selective agonist, but in vivo its analgesic effect is mainly mediated by MOP receptors (Scherrer et al., 2004 *). Also, the KOP receptor antagonist nor-binaltorphimine (norBNI) only exhibits moderate selectivity towards KOP receptors in vivo (Spanagel et al., 1994 *). All these observations underline the difficulty of extrapolating results obtained in transfected cells and the need to collect data using endogenous receptors.
Criteria to identify heteromers in native tissues
Limitations associated with heteromer identification in heterologous systems prompted efforts to delineate a framework that would encompass refined criteria. As a guideline, the International Union of Basic and Clinical Pharmacology therefore issued recommendations for the recognition and nomenclature of GPCR heteromers (Pin et al., 2007 *). According to these, receptor heteromers can only be accepted if their existence is unambiguously established in native tissues. Therefore, at least two of the following criteria should be met:
There should be evidence for physical association in native tissues or primary cells. This can be obtained by co-immunoprecipitation, co-localization with antibody at electron microscopic level or energy transfer technologies using labelled ligands or knock-in animals.
In native tissues, heteromerization must induce a specific functional property, either a new pharmacological profile or the activation of a specific transduction cascade, distinct from those induced by each receptor expressed alone.
The use of knock-out animals or RNAi technology must drastically alter the specific functional properties assigned to heteromers.
To date, in vivo physical association has only been validated for a limited number of heteromers identified in heterologous systems, often due to the lack of appropriate tools.
Tools and strategies to investigate physical proximity between endogenous receptors
In this section, technical approaches developed to provide evidence for physical association between endogenously expressed receptors are reviewed, and receptor pairs satisfying this criterion are listed (see Table 1).
Table 1.
Opioid heteromers. Receptor pairs with reported in vivo close physical proximity
| Receptor pair | In vivo physical proximity | In vivo functional impact | Reference* | |
|---|---|---|---|---|
| Tissue | Method | |||
| DOP/KOP | Rat trigeminal ganglia | coIP | Allosteric interactions Thermal allodynia | Berg et al., 2012 |
| Mouse | Bivalent ligand | Thermal analgesia KDAN-18, 6′-GNTI (i.t.) | Ansonoff et al., 2010; Bhushan et al., 2004; Waldhoer et al., 2005 | |
| DOP/MOP | Rat DRG | coIP | Morphine tolerance (TAT-DOP-2 L expression) | Xie et al., 2009 |
| Rat nucleus accumbens | ↓Anxiety, depression | Kabli et al., 2013 | ||
| Mouse spinal cord | coIP | Allosteric modulation of ligand binding Morphine thermal analgesia | Gomes et al., 2000; Gomes et al., 2004; Gomes et al., 2011 | |
| Mouse spinal cord | coIP | ↓Morphine thermal analgesia Morphine tolerance | He et al., 2011 | |
| Mouse hippocampus | coIP | Erbs et al., 2014 | ||
| Mouse brain | Selective antibody | CYM51010 analgesia | Gomes et al., 2013b; Gupta et al., 2010 | |
| Mouse | Bivalent ligand | Morphine tolerance, dependence | Daniels et al., 2005b | |
| Mouse | ↑MOP signalling | Walwyn et al., 2009 | ||
| DOP/NOP | Rat DRG | coIP | Evans et al., 2010 | |
| DOP/CB1 | Rat cortex | Selective antibody | DOP signalling (neuropathic pain) | Bushlin et al., 2012 |
| DOP/CXCR4 | Human monocytes | coIP | Silent | Pello et al., 2008 |
| Mouse brain Mouse glial culture | coIP | Silent | Burbassi et al., 2010 | |
| KOP/MOP | Proestrous female rat Sc | coIP | KOP mediated thermal analgesia | Chakrabarti et al., 2010; Liu et al., 2011a |
| KOP/NOP | Rat DRG | coIP | Evans et al., 2010 | |
| MOP/NOP | Rat DRG | coIP | Evans et al., 2010 | |
| MOP/CB1 | Rat caude putamen | Electron microscopy | Rodriguez et al., 2001 | |
| SKSNH | Neuritogenesis | Rios et al., 2006 | ||
| MOP1D/GRP | Rat Sc | coIP | Morphine itch specific | Liu et al., 2011b |
| MOP/NMDA NR1 | Mouse PAG | coIP | Morphine tolerance | Rodriguez-Munoz et al., 2012 |
6′-GNTI, 6′-guanidinonaltrindole; coIP, co-immunoprecipitation; DRG, dorsal root ganglia; GRP, gastrin-releasing peptide receptor; i.t., intrathecal; KDAN, KOP receptor DOP receptor agonist antagonist; PAG, periaqueducal grey matter; Sc, spinal cord; TAT, transactivating transcriptional activator.
These references have been amended after first publication to correct misaligned citations (2 September 2014).
mRNA
In the absence of selective antibodies, in situ hybridization (ISH) has often been used to map receptor expression in the brain. Although a good indication that the receptor may be produced in the cell, detection of mRNA transcript is no definite proof of its presence because mRNA may not be transcribed or protein synthesis may be restricted to specific conditions. Therefore, co-localization of two mRNAs represents a first hint but remains insufficient to establish in vivo receptor co-expression.
Approaches based on resonance energy transfer
FRET is based on the excitation of a donor fluorescent molecule that transfers its energy to a fluorescent acceptor that will re-emit with a lower energy. The efficiency of the transfer is proportional to the distance between the acceptor and the donor, and is also sensitive to their relative orientation (see Ishikawa-Ankerhold et al., 2012 *). Careful monitoring of several parameters such as the spectral overlap between the emission and the excitation spectra or the relative expression of the partners is required. Quantitative estimation of the monomer-dimer ratio by FRET thus remains difficult. In addition, the FRET signal that takes place between proteins located at the cell surface is difficult to isolate and can only be measured by sophisticated procedures such as time-resolved FRET (Maurel et al., 2008; Cottet et al., 2012 *).
Photobleaching FRET (pbFRET) is based on the decrease of donor fluorescence intensity due to irreversible photochemical destruction of the excited state of the fluorophore during prolonged exposure to excitation light (see Ishikawa-Ankerhold et al., 2012 *). The decrease in donor fluorescence intensity is monitored in the absence and in the presence of the acceptor. Any slowdown of the photobleaching process upon addition of the energy acceptor would reflect additional donor deactivation by FRET and would suggest that energy acceptor and donor are in close proximity, hence dimerization. pbFRET has been used to monitor the dynamics of dopamine D2 and somatostatin sst2 heterodimers in transfected cells and in primary cultures of striatal neurons (Baragli et al., 2007 *). Using pbFRET, the authors showed agonist-promoted heteromerization in transfected cells but constitutive heteromerization in cultured striatal neurons that was abolished in the presence of the D2 receptor antagonist eticlopride.
BRET is similar to FRET except that luciferase is used as the energy donor (Lohse et al., 2012 *; De et al., 2013). One advantage over FRET is the absence of potential direct excitation of the energy acceptor. This generates a high signal to background ratio and simplifies controls of background levels. However, BRET is clearly not very sensitive and high-resolution; single cell imaging and analysis are difficult. Despite these limitations, a proof of principle for the use of BRET-based microscopy to image protein interactions with subcellular resolution in a living cell has been obtained (Coulon et al., 2008 *).
So far, identification of opioid heteromers by FRET (Hojo* et al., 2008) or BRET (Ramsay et al., 2002; Rios et al., 2006; Juhasz* et al., 2008) has been performed in heterologous systems in which engineered receptors can be expressed. FRET-based techniques should gain more interest for in vivo functional studies especially when combined with engineered knock-in mice expressing fluorescent versions of the opioid receptors (De et al., 2013 *; Cottet et al., 2012; Aoki et al., 2013; Erbs et al., 2014).
Co-immunoprecipitation
To date, co-immunoprecipitation represents the most widely used approach to establish in vivo physical association. Immunoprecipitation is performed using an antibody selective for one receptor, and Western blot analysis is subsequently performed with an antibody selective for the other receptor. Detection of the second receptor by immunoblotting indicates that it is associated with the immunoprecipitated partner during the immunoprecipitation step, hence the co-immunoprecipitation denomination. Given the natural propensity of GPCRs to aggregate, proper controls need to be included to rule out the possibility of promiscuous receptor-receptor associations or the possibility of high-order aggregates that would artificially form during the solubilization step (Salim et al., 2002; De Harrison and van der Graaf, 2006 *). This is usually achieved by mixing membranes expressing one GPCR only before the immunoprecipitation step to ensure that detection of the two receptors by subsequent immunoblotting results from co-immunoprecipitation of physically close receptors and is not due to non-specific aggregation taking place during sample preparation. Such control membranes can be prepared from knock-out animals for one receptor or from tissues expressing only one of the two receptors as identified by ISH or by immunohistochemistry (IHC). Importantly, co-immunoprecipitation experiments depend on the selectivity of the antibodies. Therefore, the latter needs to first be validated using native tissues that do not express the target receptor. Another limitation is the requirement of a rather large quantity of tissue that may preclude the use of small brain areas. In addition, this approach does not provide cellular resolution but only indicates close physical proximity of two receptors in a given compartment. If a particular cellular population is of interest, target neurons must be isolated before receptor co-immunoprecipitation experiments. Finally, close proximity does not necessarily imply that changes in cellular responses result from heteromer formation rather than being merely due to downstream modulation of the signalling cascades initiated independently by each receptor.
Immunohistochemical and immunocytochemical co-localization by fluorescence or electron microscopy
Raising selective antibodies against GPCRs often proves to be very difficult. Most class A GPCRs exhibit low immunogenicity because the transmembrane domains constitute the greater part of the protein with usually short loops, and N- or C-termini. In addition, the N-terminus often bears N-glycosylation sites that mask potential epitopes. The selectivity of the antibodies therefore needs to be carefully checked using native tissues that do not express the target receptor. Co-localization using electron microscopy unambiguously establishes whether two receptors are close to each other within the same cell. However, the low expression levels together with a likely limited abundance of heteromers render this approach complicated. Importantly, neuronal co-localization identified by fluorescence microscopy does not necessarily translate into physical proximity. Indeed, two fluorescent signals can be identified at the cell surface with the two receptors present in separate domains of the plasma membrane (Kivell et al., 2004 *). Therefore, neuronal co-localization of the fluorescent signals on its own is not sufficient to assess heteromer formation that requires additional approaches such as co-immunoprecipitation.
Recently, heteromer-specific monoclonal antibodies were generated by subtractive immunization. In this strategy, mice are first made tolerant to unwanted epitopes, before being immunized with membranes from the same cell type that co-express the two receptors of interest. Clones specific for heteromers are identified from screens with membranes that do not express the receptors, express only one of them or co-express the two (Sleister and Rao, 2002 *; Gomes et al., 2013a). Antibodies generated according to this procedure were used to identify opioid heteromers and to probe their specific signalling (Gupta et al., 2010 *; Berg et al., 2012; Bushlin et al., 2012) (see below Functional outcome of heteromerization between endogenous receptors).
Bivalent ligands
Bivalent ligands are compounds composed of two ligands each selective for one receptor type and linked together by a spacer of defined length (Daniels et al., 2005b *; Schiller, 2010). Bivalent ligands called MDAN (MOP DOP receptor agonist antagonist) were generated by linking together the MOP agonist oxymorphone and DOP antagonist naltrindole with spacers of various lengths (Daniels et al., 2005b). Efficient binding only occurred for ligand moieties about 22 Å apart, corresponding to a 19-atom spacer, which is consistent with the distance between the binding pockets of two GPCRs making contact (Daniels et al., 2005b). Similarly, a series of bivalent ligands were generated to target KOP and DOP receptors. Efficient binding was observed for KDN21 with a 21-atom spacer between the κ receptor antagonist 5′-guanidinonaltrindole (GNTI) and the δ receptor antagonist naltrindole (Bhushan et al., 2004 *) and for KDAN-18 (KOP DOP receptor agonist antagonist) with an 18-atom spacer between the KOP agonist ICI-199, 441 and DOP antagonist naltrindole (Ansonoff et al., 2010 *). Therefore, bivalent ligands are able to bridge two adjacent receptors and can reveal their physical proximity and thus heteromer formation. The main limitation of this approach is the selectivity of each moiety. Naltrindole, for example, only shows moderate selectivity for DOP receptors with a 10- to 100-fold higher affinity for DOP compared with MOP receptors, depending on species and assay conditions. Therefore, binding of MDAN-19 to two physically close MOP receptors exerting antagonistic effects cannot be ruled out (Harvey et al., 2012 *). Designing bivalent ligands able to bridge two moieties that would each exhibit high in vivo selectivity for one receptor type is therefore essential because of the structural similarities existing between the opioid receptor binding pockets (see below Opioid heteromers: the genesis) (Filizola et al., 2013 *).
Opioid heteromers with identified physical proximity between endogenous receptors
Clear indication of close physical proximity has only been reported for a limited number of endogenous receptor pairs (see Table 1). However, more heteromers will undoubtedly be discovered in a near future with the arrival of new tools to efficiently tackle in vivo proximity. It is however essential to bear in mind that opioid heteromers have so far been identified in discrete regions of the nervous system, mostly the spinal cord and DRGs (Gomes et al., 2004; van Rijn and Whistler, 2009; Xie et al., 2009; Gupta et al., 2010; He et al., 2011 *), and in one case appeared hormone-dependent (Liu et al., 2011a *). Although current data unambiguously establish their existence in vivo, heteromer formation has to be validated for each region of interest.
To date, co-immunoprecipitation experiments revealed close proximity between all members of the opioid family (MOP-DOP, MOP-KOP, KOP-DOP) and the closely related opioid receptor-like NOP (NOP-MOP/DOP/KOP) (see Table 1). Co-immunoprecipitation experiments also uncovered physical proximity between DOP receptors and the chemokine CXCR4 receptor (Burbassi et al., 2010 *). In the case of cannabinoid CB1 receptors, physical proximity with MOP receptors was visualized by electron microscopy (Rodriguez et al., 2001 *) and close proximity with DOP receptors inferred from heteromer-specific antibodies (Bushlin et al., 2012 *). Finally co-immunoprecipitation of the MOP1D splice variant with the gastrin-releasing peptide receptor (GRP) was observed in the mouse spinal cord (Liu et al., 2011b *). Co-immunoprecipitation has also been observed in the synaptosomal fraction from different brain areas between MOP receptors and the NR1 subunit of the ionotropic receptor NMDA (Rodriguez-Munoz et al., 2012 *).
Receptor pairs with identified neuronal co-localization
In vivo co-localization in the same neuron has been reported for a few receptor pairs though physical proximity remains to be established, and therefore, heteromer formation remains hypothetical (see Table 2). MOP and 5HT2A receptors were co-localized in several rat brain areas at the mRNA level (Lopez-Gimenez et al., 2008 *). MOP and dopamine D1 receptors were co-localized in the rat cortex and striatum (Juhasz et al., 2008 *). DOP receptors were co-localized with α2A adrenoceptors in substance P neurons from the rat spinal cord by IHC (Riedl et al., 2009 *) and to a small extent with α2C adrenoceptors (Riedl et al., 2009). Limited co-localization in the rat spinal cord was also observed by IHC between MOP and α2A or α2C adrenoceptors (Riedl et al., 2009). Electrophysiological recordings indicated additional neuronal co-localization between MOP receptors and α2A adrenoceptors in the locus coeruleus (Illes and Norenberg, 1990 *). MOP receptors were also co-localized by IHC with the chemokine receptors CXCR4 or CX3CR1 in the hippocampus, cingulate cortex, periaqueductal grey, nucleus accumbens, ventral tegmental area and globus pallidus (Heinisch et al., 2011 *). In addition, co-expression in periaqueductal grey neurons was confirmed by electrophysiological recording (Heinisch et al., 2011). For these receptor pairs, indication of physical proximity is still lacking. Therefore, functional outcome cannot yet be attributed to heteromer formation as it may rather reflect interactions at the level of the signalling cascades as for MOP receptors and CXCR4 (Patel et al., 2006; Sengupta et al., 2009 *), or MOP and α2A adrenoceptors in the locus coeruleus (Stone and Wilcox, 2004 *).
Table 2.
Receptor pairs with reported neuronal co-localization
| Receptor pair | Tissue | Method | Reference* |
|---|---|---|---|
| DOP/α2A adrenergic | Rat spinal cord | IHC | Riedl et al., 2009 |
| DOP/α2C adrenergic | Rat spinal cord | IHC | Riedl et al., 2009 |
| MOP/α2A adrenergic | Rat spinal cord Rat locus coeruleus | IHC electrophysiology | Riedl et al., 2009; Illes and Norenberg, 1990 |
| MOP/α2C adrenergic | Rat spinal cord | IHC | Riedl et al., 2009 |
| MOP/dopamine D1 | Rat striatum,cortex | IHC | Juhasz et al., 2008 |
| MOP/serotonin 5HT2A | Rat brain | ISH | Lopez-Gimenez et al., 2008 |
| MOP/CXCR4 | Rat brain | IHC | Heinisch et al., 2011 |
| MOP/CX3CR1 | Rat brain | IHC | Heinisch et al., 2011 |
DOP, delta opioid receptor; IHC, immunohistochemistry; MOP, mu opioid receptor.
These references have been amended after first publication to correct misaligned citations (2 September 2014).
Functional outcome of heteromerization between endogenous receptors
In vivo physical proximity is a prerequisite to postulate the existence of heteromers. Although necessary, this requirement is however not sufficient. Indeed, evidence has to be brought that functional interactions directly result from physical association and are not merely produced by crosstalk between downstream signalling. This section reviews our current knowledge of the functional changes observed upon opioid heteromer formation between endogenous receptors.
MOP/DOP heteromers
MOP and DOP receptors are crucial modulators of both nociception and opiate analgesia (see Raehal et al., 2011 *; Gaveriaux-Ruff and Kieffer, 2011; Pradhan et al., 2011). In particular, the contribution of DOP receptors has been the subject of much interest because of its potential therapeutic impact (Bailey and Connor, 2005; Cahill et al., 2007; Zhang et al., 2006 *) because of the proposal that DOP receptors play a key role in opiate analgesia and morphine tolerance, possibly through formation of MOP/DOP heteromers.
In transfected cells, MOP/DOP receptor specific antibodies blocked ligand binding (Gupta et al., 2010 *) and β-arrestin recruitment (Gomes et al., 2013b*). In addition, they affected G protein-dependent signalling in membranes from both transfected cells and mouse brain by significantly blocking the potentiation of MOP receptor agonist-mediated cAMP by DOP receptor antagonists (Gupta et al., 2010 *). Changes in signalling properties upon MOP/DOP receptor co-expression were attributed to a switch from Pertussis-sensitive to Pertussis-insensitive G protein coupling (George et al., 2000; Fan et al., 2005; Hasbi et al., 2007 *) or to coupling to a new pathway (Rozenfeld and Devi, 2007 *), but this remains to be confirmed in vivo.
Treatment with a low dose of naltriben pharmacologically stabilized MOP/DOP receptors at the cell membrane in transfected HEK 293 cells by blocking methadone-promoted MOP/DOP receptor internalization without affecting MOP/DOP signalling (Milan-Lobo and Whistler, 2011 *). In vivo, chronic administration of the MOP receptor selective agonist methadone with a low dose of the DOP receptor selective antagonist naltriben resulted in reduced thermal analgesia that could be restored by promoting internalization with methadone or by blocking signalling with the antagonist naltrindole (Milan-Lobo et al., 2013 *).
In SK-NS-H cells endogenously expressing the two receptors, enhanced MOP receptor agonist binding and signalling were observed in the presence of agonists, antagonists or inverse agonists for DOP receptors (Gomes et al., 2000; 2011; Gomes et al., 2004 *). This suggests that occupancy of DOP receptors by any type of ligand acts as an allosteric modulator and triggers conformational changes in MOP receptors that potentiate ligand binding and receptor function.
Surface expression of MOP receptors was reduced in DRGs from DOP receptor knock-out mice, whereas the MOP receptor agonists, DAMGO and morphine, induced less inhibition of voltage-dependent calcium channels (Walwyn et al., 2009 *). Also, chronic morphine administration increased DOP receptor surface expression in neurons and was MOP receptor-dependent (Cahill et al., 2007 *). In addition, reduced tolerance was observed on pharmacological blockade of DOP receptors (Abdelhamid et al., 1991; Zhu et al., 1999 *) or in DOP receptor knock-out animals (Zhu et al., 1999; Nitsche et al., 2002 *). Finally, i.c.v. injection of the bivalent ligand MDAN-19 reduced dependence and tolerance in mice, however, as mentioned earlier (see Tools and strategies to investigate physical proximity between endogenous receptors); selectivity towards MOP/DOP heteromers remains questionable (Harvey et al., 2012 *).
Based on their pharmacological profile, MOP/DOP heteromers were postulated as the molecular entity corresponding to the δ 1 subtype (van Rijn and Whistler, 2009 *). TAN-67, identified as a MOP/DOP receptor selective agonist using co-transfected HEK 293 cells, decreased ethanol but not sucrose consumption in wild type but not DOP receptor knock-out mice (van Rijn and Whistler, 2009). This suggests that MOP/DOP receptor selective targeting may represent a more efficacious treatment of alcohol dependence. Recently, CYM51010 was reported as a biased MOP/DOP receptor agonist whose in vivo activity was blocked by MOP/DOP heteromer selective antibodies (Gomes et al., 2013b*). Systemic administration of CYM51010 induced thermal acute analgesia similar to morphine, whereas chronic administration of CYM51010 produced lesser antinociceptive tolerance compared with morphine. CYM51010 thus appears as the precursor of new class of analgesics drugs.
In the nervous system, MOP/DOP receptors are mainly co-localized in the nociceptive pathway [virtual brain atlas at https://mordor.ics-mci.fr/ (Erbs et al., 2014 *)] where MOP/DOP receptor physical proximity was established in several areas (see Table 1 and references therein). Accordingly, interfering with MOP/DOP receptor physical interaction in vivo modified the functional outcome. Expression of the MOP TM1 fused to the TAT sequence not only blocked endogenous MOP/DOP co-immunoprecipitation but also MOP/DOP degradation in the lysosomal compartment (He et al., 2011 *). Also, expression of the fusion construct in the spinal cord increased morphine thermal analgesia and decreased morphine tolerance (He et al., 2011). Similarly, expression of the DOP receptor C-terminus fused to the TAT sequence decreased co-immunoprecipitation and reduced anxiolytic and antidepressive effects induced by UFP 512, described as selective for MOP/DOP heteromers, when expressed in the rat nucleus accumbens (Kabli et al., 2010; Kabli et al., 2013 *). Finally, a peptide corresponding to the DOP second intracellular loop fused to the TAT sequence reduced morphine tolerance in rat and reduced cell surface expression of DOP receptors in DRGs (Xie et al., 2009 *).
DOP/KOP heteromers
Co-immunoprecipitation experiments performed on rat trigeminal ganglia cultures identified close proximity between DOP and KOP receptors in peripheral sensory neurons (Berg et al., 2012 *). On the functional point of view, the putative DOP/KOP heteromer-selective agonist 6′-GNTI, which was originally described as a KOP receptor agonist (Waldhoer et al., 2005) inhibited PGE2-stimulated cAMP accumulation in vitro and elicited a strong antinociceptive response in vivo. Either a DOP or a KOP receptor antagonist (Berg et al., 2012) blocked both of them. 6′-GNTI also exhibited high potency and efficacy in activating DOP/KOP heteromers in transfected cells and mediated thermal analgesia when injected at the spinal level but not upon i.c.v injection (Waldhoer et al., 2005 *). Similarly, the bivalent ligand KDN-21 composed of the KOP receptor antagonist 5′-GNTI and DOP receptor antagonist naltrindole presented a different selectivity profile upon intrathecal (i.t.) or i.c.v. administration (Bhushan et al., 2004 *). This suggests that DOP/KOP heteromers may only be present in the spinal cord with no functional analgesic DOP/KOP heteromers in the brain (Waldhoer et al., 2005 *). Alternatively, this could indicate that DOP and KOP receptors associate with different partners at the spinal and supraspinal levels therefore yielding distinct pharmacological and signalling profiles (Bhushan et al., 2004 *). Indeed, based on the binding and signalling properties of KDN-21, the pharmacologically defined δ 1 subtype was attributed to DOP/KOP heteromers (Bhushan et al., 2004; Xie et al., 2005 *). However, this subtype was also attributed to MOP/DOP heteromer formation using the selective agonist TAN-67 (see above) (van Rijn and Whistler, 2009 *). Once more, this illustrates the difficulty of interpreting in vivo pharmacological data from bivalent ligands. More recently, 6′-GNTI and KDAN-18 composed of the KOP agonist ICI-199, 441 and the DOP antagonist naltrindole linked by an 18-atom long spacer were tested on knock-out animals. Upon i.t. injection in DOP or KOP receptor knock-out mice, the two compounds showed reduced potency and efficacy in thermal analgesia, suggesting selective DOP/KOP receptor bridging (Daniels et al., 2005a; Ansonoff et al., 2010 *).
Using a subtractive strategy, Devi's group also generated monoclonal antibodies directed against DOP/KOP heteromers (Berg et al., 2012 *). In vivo, the anti-allodynic effect of the DOP receptor agonist DPDPE was increased by local injection of DOP/KOP heteromer selective antibodies in the plantar test suggesting that within DOP/KOP heteromers, KOP receptor antagonists can act as allosteric modulators of responses to DOP receptor agonists (Berg et al., 2012).
MOP/KOP heteromers
The spinal cord is a region of the CNS in which components of opioid analgesic pathways manifest sexual dimorphism in rodents. In males, spinal morphine antinociception results from the exclusive activation of spinal MOP receptors, whereas in females, spinal morphine antinociception requires the concomitant activation of spinal MOP and KOP receptors (Liu et al., 2007; Liu et al., 2011a *). Spinal KOP receptor density is indeed significantly greater in proestrous female rats (Liu et al., 2007 *). Accordingly, co-immunoprecipitation of MOP and KOP receptors was by far more abundant in the spinal cord of proestrous female rats compared with male or diestrous female rats (Chakrabarti et al., 2010 *). In addition, endogenous spinal dynorphin 1–17 appeared to bind spinal MOP/KOP heteromers because morphine analgesia was reduced by the KOP receptor antagonist norBNI and the anti dynorphin A antibody in proestrous but not diestrous female rats (Chakrabarti et al., 2010). The authors therefore postulated that MOP/KOP heteromers could be the molecular transducer for the female-specific dynorphin/KOP receptor component of spinal morphine antinociception. Estrogen would control the amount of MOP/KOP heteromers that would be predominant in female spinal analgesia and could represent a new target for pain management in females (Liu et al., 2011a *).
The monovalent ligand N-naphthoyl-β-naltrexamine (NNTA) selectively activated MOP/KOP heteromers in transfected HEK 293 cells (Yekkirala et al., 2011 *). In vivo, NNTA i.t. injection induced thermal analgesia in the mouse tail-flick test that was abolished in MOP receptor knock-out mice and was decreased by the KOP receptor antagonist norBNI (Yekkirala et al., 2011). In addition, NNTA (i.t.) did not induce tolerance or physical dependence although it presented aversive effects. NNTA i.c.v. injection only produced weak analgesia suggesting low functional MOP/KOP receptor expression in the brain and pointing to the potential interest in more detailed functional studies of MOP/KOP receptors in vivo.
OP/NOP heteromers
The opioid receptor-like NOP co-immunoprecipitated with MOP, DOP and KOP receptors in rat DRGs suggesting heteromer formation with each of them (Evans et al., 2010 *).
NOP physically interacts with N-type calcium channels in the brain and DRGs, as shown by co-immunoprecipitation (Beedle et al., 2004 *). In DRGs, this interaction mediates a tonic inhibition of calcium entry by an agonist-independent inhibition of N-type channels due to constitutive receptor activity (Altier et al., 2006 *). In addition, NOP receptors mediate N-type channel trafficking to and from the plasma membrane (Altier et al., 2006). NOP and MOP receptors, on the other hand, form heteromers in DRGs (Evans et al., 2010 *). In addition, co-immunoprecipitation with DOP or KOP receptors points to a potential contribution of NOP receptors in the regulation of the nociceptive information that deserves proper investigation.
Interestingly, co-expression of opioid receptors with NOP in TsA 2 cells affected nociceptin-induced inhibition of N type channels (Evans et al., 2010). Also, internalization of both NOP receptors and N type channels was promoted in these cells by the MOP receptor agonist DAMGO suggesting the existence of larger macromolecular complexes involving multiple receptor types and voltage-gated calcium channels (Evans et al., 2010). If such signalling complexes exist in vivo, they add further complexity to the regulation of pain mechanisms.
Opioid/CB1 receptor heteromers
Close vicinity between MOP receptors and cannabinoid CB1 receptors was established in rat striatal membranes using electron microscopy (Rodriguez et al., 2001 *). Negative allosteric modulation of both opioid and cannabinoid signalling was observed in SK-SN-H cells that endogenously express the two receptors and a decrease in cannabinoid signalling by the MOP receptor selective agonist DAMGO was observed in striatal membranes (Rios et al., 2006 *). Hence, heteromer formation may account for some of the functional interactions that underlie analgesia or addiction as both receptors are targets for drugs of abuse. Recently, DOP/CB1 heteromers were identified in membrane domains of mouse cortical primary neurons using heteromer-specific antibodies (Rozenfeld et al., 2012 *). In a model of neuropathic pain in rats, enhanced expression of DOP and CB1 receptors was observed in cortical membranes 2 weeks after L5 nerve lesioning. Non-signalling doses of CB1 receptor ligands allosterically enhanced DOP receptor activity, and this was blocked by the heteromer-specific antibody, suggesting that DOP/CB1 heteromers represent a suitable target for blockade of negative mood states associated with neuropathic pain (Bushlin et al., 2012 *).
DOP/CXCR4 heteromers
Physical proximity between DOP and the chemokine CXCR4 receptor was detected in human monocytes (Pello et al., 2008 *) as well as in brain tissue and glial culture from MOP receptor knock-out mice (Burbassi et al., 2010 *). DOP/CXCR4 heteromers appeared to be silent in immune cells (Pello et al., 2008 *) and brain glia (Burbassi et al., 2010 *). Although the physiological significance remains unclear, this observation deserves further investigation as it may open new therapeutic perspectives, in particular in HIV pathology.
MOP1D/GRP heteromers
A pivotal role in opioid-induced itch was attributed to heteromer formation between the MOP1D splice variant and the GRP receptor, supported by receptor co-immunoprecipitation, in the mouse spinal cord (Liu et al., 2011b *). Itch would result from the unidirectional transactivation of GRP receptors by the MOP agonist morphine, selectively activating MOP1D receptors at the level of the heteromer (Liu et al., 2011b). In vitro experiments in transfected HEK 293 cells suggested that morphine activated GRP receptor downstream calcium signalling as the effect of morphine was abolished by blocking PLCβ or by antagonizing IP3 receptors (Liu et al., 2011b). Injection (i.t.) of the MOP1D-specific sequence as a TAT fusion protein blocked morphine-induced scratching but did not affect morphine-induced thermal antinociception or GRP-induced scratching (Liu et al., 2011b). This study points to interesting directions for the design of novel agents with fewer side effects compared with morphine. It should however be noted that the expression of the MOP1D splice variant remains controversial as it could not be detected in rats (Oldfield et al., 2008 *), suggesting possible species specificity.
MOP/non-GPCR heteromers
Co-immunoprecipitation of MOP and the subunit NR1 of the ligand-gated channel NMDA was observed in the synaptosomal fraction prepared from different brain areas, supporting close physical proximity (Rodriguez-Munoz et al., 2012 *). Interestingly, morphine disrupted this complex by PKC-mediated phosphorylation of the NR1 C terminus. Morphine also potentiated the NMDA-CaMKII pathway implicated in morphine tolerance. This finding opens very exciting perspectives regarding the involvement of functional complexes formed between opioid receptors, and more generally GPCRs, and ligand-gated channels in the regulation of neuronal activity. It also suggests the possibility of selectively targeting these receptors with bivalent ligands as therapeutic drugs.
Effects on drug discovery
Few opioid heteromers have been validated in vivo. Nevertheless, their implication in the development of a number of pathological states affecting the nervous system cannot be ignored, in particular for pain management. Opioid heteromers represent excellent molecular targets because of their relative abundance in the nociceptive pathways. Indeed, MOP/DOP, MOP/KOP, DOP/KOP, MOP/CB1, MOP1D/GRP and (M-D-K)OP/NOP heteromers have been associated with antinociceptive responses. So far, in vivo data support specific ligand binding and signalling distinct from currently used opiate drugs such as morphine with less tolerance upon chronic use. Respiratory depression represents another major drawback associated with administration of high doses of morphine. Interestingly, MOP/DOP heteromers are not detected in the pre-Bötzinger complex that controls respiratory depression (Erbs et al., 2014 *). Selective ligands for MOP/DOP heteromers with no or low affinity for MOP receptors would therefore represent excellent candidates as the next generation of analgesic drugs. Similarly, selective targeting of MOP/DOP heteromers could provide new treatments for alcoholism (van Rijn et al., 2010a*), and selective targeting of DOP/CB1 heteromers could reduce anxiety and depressive states associated with chronic neuropathic pain (Bushlin et al., 2012 *).
One should however bear in mind that the observed behavioural modifications could reflect a change in the homeostasis of the system or could result from modifications in downstream signalling of the receptors without necessarily requiring their physical association or even their co-expression within the same cell. Although it is often difficult to unambiguously name heteromers as the molecular entity underlying a disease, identification of neuronal co-localization and establishment of physical proximity for an increasing number of receptor pairs will undoubtedly provide new potential targets for the pharmacological treatment of neurological disorders.
Future directions
Naturally expressing cell lines to identify functional heteromer-specific properties
As mentioned earlier, heteromer-specific properties identified in transfected cells often show limited predictive value when challenged in vivo. This is especially true when taking into account the complexity of the opioid receptor pharmacology that represents a significant limitation for the validation of new therapeutic drugs.
Whatever their rationale, new strategies exploring molecular and cellular aspects associated with heteromer formation should be tested only on cells endogenously expressing the receptors, either primary cultures derived from the tissue of interest or cell lines that closely resemble endogenous ones, such as immortalized primary cells or neuroblastoma. Embryonic and induced pluripotent stem cells on the other hand may offer a new development because of their capacity to differentiate into all types of somatic cells including neurons (Bibel et al., 2004; Plachta et al., 2004 *). In-depth phenotyping indicated the ability of stem cells to give rise to specialized populations corresponding to region and/or neurotransmitter-specific neuronal and glial types (Liu and Zhang, 2011 *; Peljto and Wichterle, 2011). This approach could be adapted to drug screening systems because it is reasonable to speculate that drugs identified in one of these cellular contexts will show the same pharmacological profile when administered in the intact organism. Another advantage of embryonic stem cells is the possibility to isolate them from genetically modified animals. Knock-out mice for one of the partner receptors, knock-in mice endogenously expressing GPCRs fused to fluorescent proteins and/or combinations of them represent powerful tools to assess in vivo the functional role of opioid heteromers.
Double fluorescent knock in mice
As previously emphasized, neuronal co-localization is mandatory before any attempt to associate changes in ligand binding or in receptor signalling and trafficking with opioid heteromer formation. Still, this prerequisite is too often missing, mostly due to the lack of appropriate tools for in vivo investigation. In particular, antibodies against opioid receptors have very often proven to be non-selective, which hampered in vivo mapping. Recently, fluorescent knock-in mice expressing a functional DOP receptor in fusion with the eGFP (DOP-eGFP) were successfully generated and used to address receptor trafficking in vivo (Scherrer et al., 2006; Pradhan et al., 2010; Pradhan et al., 2009 *). These mice were crossed with animals expressing functional MOP receptors in fusion with the red fluorescent protein mcherry (MOP-mCherry) to give rise to double fluorescent knock-in mice. Fine mapping of MOP and DOP receptors in the mouse CNS identified brain areas in which neuronal co-localization was visible under basal conditions (see Figure 1) (Erbs et al., 2014 *). These regions were mainly located in the brainstem within neuronal circuits essential for survival (see Figure 2). A large portion was present in the nociceptive pathway, but co-localization was also visible in circuits related to food and water consumption or to sexual behaviour (Erbs et al., 2014). Interestingly, MOP and DOP receptors appear essentially expressed on distinct neurons in the forebrain, suggesting a limited role of MOP/DOP heteromers in high-order processing.
Figure 1.
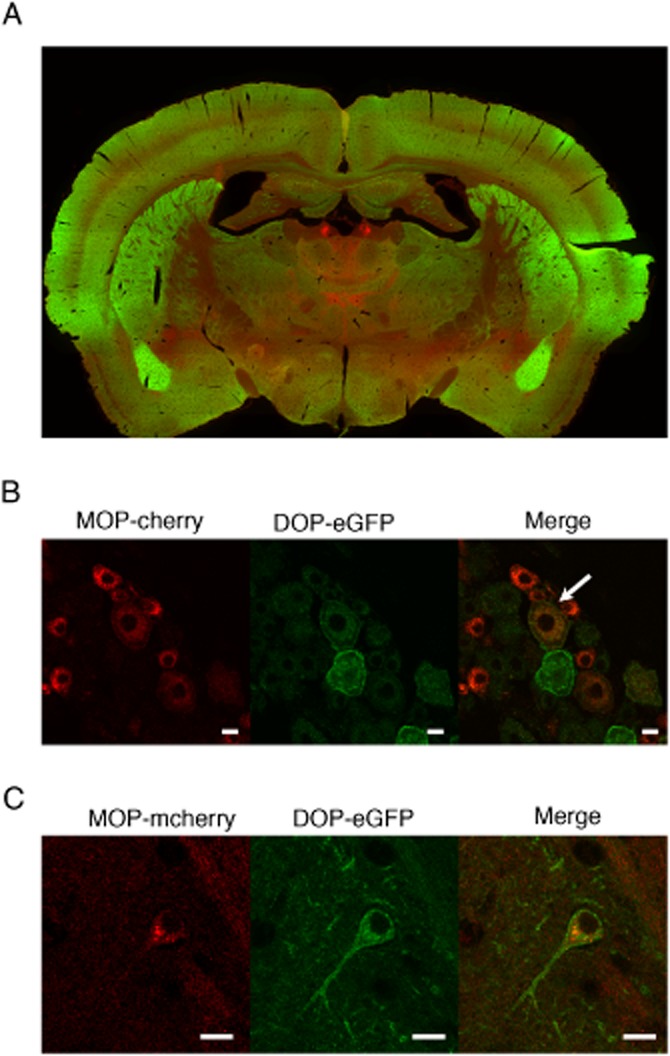
Double fluorescent knock in mice expressing DOP receptors fused to the GFP (green) and MOP receptors fused to the red fluorescent protein mcherry (red). (A) General view of a coronal brain section. (B) Neuronal co-localization of DOP and MOP receptors in dorsal root ganglia (white arrow). (C) Neuronal co-localization of DOP and MOP receptors in the hippocampus. Scale bars 10 μm.
Figure 2.
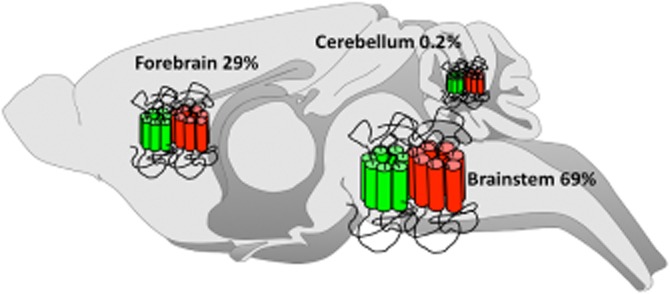
Relative distribution of neurons co-expressing DOP and MOP receptors in the mouse brain as determined using double fluorescent knock in mice expressing DOP receptors fused to the GFP and MOP receptors fused to the red fluorescent protein mcherry. Percentages of MOP/DOP receptor co-localization in the mouse brain are indicated.
Double knock-in mice therefore constitute an exceptional tool to address changes in MOP/DOP co-localization occurring at various stages of pathological conditions including chronic pain, psychiatric disorders or drug addiction. In addition, physical proximity can be addressed in regions of co-localization by co-immunoprecipitation with antibodies against the fluorescent proteins or by FRET providing solid ground for heteromer studies (see Tools and strategies to investigate physical proximity between endogenous receptors).
Finally, double knock-in mice enable direct visualization of heteromer trafficking in vivo in response to a pharmacological stimulation or a physiological challenge promoting endogenous opioid peptide release. Comparing receptor trafficking between neurons co-expressing the two receptors and neurons expressing one receptor type only will allow the separation of specific MOP/DOP behavioural outcomes, thereby providing a way to address the contribution of MOP/DOP heteromers to physiological processes and pathological states.
Conclusions
Despite a lot of effort and numerous studies performed on heterologous systems, our current knowledge of the mechanisms by which GPCRs physically assemble and function in vivo remains very limited. The extremely small number of heteromers formed between endogenous receptors that have been unambiguously identified is striking and suggests that the role of such associations is likely to be underrated. In particular, the consequences of opioid heteromer formation on cellular signalling are still poorly understood, as is our view of its contribution to physiopathological states. Involvement of opioid heteromer formation has been proposed in human pathologies such as alcoholism, acute or chronic pain, as well as psychiatric disorders but deserves further investigation. Altogether, our appraisal of opioid heteromers, as fully identified therapeutic targets, is still in its infancy. No doubt that the development of more sophisticated biophysical, biochemical and pharmacological tools together with the use of native cells and genetically modified animals will open unsuspected perspectives in the drug discovery field.
*Amended citations.
Acknowledgments
We would like to thank Dr K. Befort for critical reading of the manuscript and funding sources including the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université de Strasbourg, the Agence Nationale pour la Recherche (IMOP), and the National Institutes of Health (NIDA DA-05010).
Glossary
Abbreviations
- DOP
δ opioid receptor
- DPDPE
[D-Pen2,5]enkephalin, [D-Pen2,D-Pen5]enkephalin
- DRG
dorsal root ganglion
- GNTI
guanidinonaltrindole
- GRP
gastrin-releasing peptide
- HIV
human immunodeficiency virus
- IHC
immunohistochemistry
- ISH
in situ hybridization
- i.t
intrathecal
- KDOP
κ opioid receptor δ opioid receptor agonist antagonist
- KOP
κ opioid receptor
- MDAN
μ opioid receptor δ opioid receptor agonist antagonist
- MOP
μ opioid receptor
- NNTA
N-naphthoyl-β-naltrexamine
- norBNI
nor-binaltorphimine
- pbFRET
photobleaching FRET
- TAT
transactivating transcriptional activator
Conflict of interest
I have no conflict of interest to declare.
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- Ansonoff MA, Portoghese PS, Pintar JE. Consequences of opioid receptor mutation on actions of univalent and bivalent kappa and delta ligands. Psychopharmacology (Berl) 2010;210:161–168. doi: 10.1007/s00213-010-1826-7. [DOI] [PubMed] [Google Scholar]
- Aoki K, Kamioka Y, Matsuda M. Fluorescence resonance energy transfer imaging of cell signaling from in vitro to in vivo: basis of biosensor construction, live imaging, and image processing. Dev Growth Differ. 2013;55:515–522. doi: 10.1111/dgd.12039. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Baragli A, Alturaihi H, Watt HL, Abdallah A, Kumar U. Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2 Co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell Signal. 2007;19:2304–2316. doi: 10.1016/j.cellsig.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, et al. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci. 2004;7:118–125. doi: 10.1038/nn1180. [DOI] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, et al. Allosteric interactions between delta and kappa opioid receptors in peripheral sensory neurons. Mol Pharmacol. 2012;81:264–272. doi: 10.1124/mol.111.072702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci. 2004;7:1003–1009. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- Birdsall NJ. Class A GPCR heterodimers: evidence from binding studies. Trends Pharmacol Sci. 2010;31:499–508. doi: 10.1016/j.tips.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbassi S, Sengupta R, Meucci O. Alterations of CXCR4 function in mu-opioid receptor-deficient glia. Eur J Neurosci. 2010;32:1278–1288. doi: 10.1111/j.1460-9568.2010.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushlin I, Gupta A, Stockton SD, Jr, Miller LK, Devi LA. Dimerization with cannabinoid receptors allosterically modulates delta opioid receptor activity during neuropathic pain. PLoS ONE. 2012;7:e49789. doi: 10.1371/journal.pone.0049789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M, Deterre P, Antonny B. The apparent cooperativity of some GPCRs does not necessarily imply dimerization. Trends Pharmacol Sci. 2009;30:182–187. doi: 10.1016/j.tips.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Liu NJ, Gintzler AR. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc Natl Acad Sci U S A. 2010;107:20115–20119. doi: 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76(Pt B):204–217. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottet M, Faklaris O, Maurel D, Scholler P, Doumazane E, Trinquet E, et al. BRET and Time-resolved FRET strategy to study GPCR oligomerization: from cell lines toward native tissues. Front Endocrinol (Lausanne) 2012;3:92. doi: 10.3389/fendo.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon V, Audet M, Homburger V, Bockaert J, Fagni L, Bouvier M, et al. Subcellular imaging of dynamic protein interactions by bioluminescence resonance energy transfer. Biophys J. 2008;94:1001–1009. doi: 10.1529/biophysj.107.117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol. 2015;172:317–323. doi: 10.1111/bph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J Med Chem. 2005a;48:1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005b;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A, Jasani A, Arora R, Gambhir SS. Evolution of BRET biosensors from live cell to tissue-scale imaging. Front Endocrinol (Lausanne) 2013;4:131. doi: 10.3389/fendo.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décaillot FM, Befort K, Filliol D, Yue SY, Walker P, Kieffer BL. Opioid receptor random mutagenesis reveals how a G protein-coupled receptor turns on. Nat Struct Biol. 2003;10:629–636. doi: 10.1038/nsb950. [DOI] [PubMed] [Google Scholar]
- Décaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch J-L, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0717-9. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, You H, Hameed S, Altier C, Mezghrani A, Bourinet E, et al. Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem. 2010;285:1032–1040. doi: 10.1074/jbc.M109.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O'Dowd BF, George SR. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J Biol Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13:230–246. doi: 10.2174/138945012799201612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filizola M, Devi LA. Grand opening of structure-guided design for novel opioids. Trends Pharmacol Sci. 2013;34:6–12. doi: 10.1016/j.tips.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filizola M, Olmea O, Weinstein H. Prediction of heterodimerization interfaces of G-protein coupled receptors with a new subtractive correlated mutation method. Protein Eng. 2002;15:881–885. doi: 10.1093/protein/15.11.881. [DOI] [PubMed] [Google Scholar]
- Fonseca JM, Lambert NA. Instability of a class A GPCR oligomer interface. Mol Pharmacol. 2009;75:1296–1299. doi: 10.1124/mol.108.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol. 2011;22:405–414. doi: 10.1097/FBP.0b013e32834a1f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Ijzerman AP, Ye K, Maillet EL, Devi LA. G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Mol Pharmacol. 2011;79:1044–1052. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Devi LA. G-protein-coupled heteromers: regulation in disease. Methods Enzymol. 2013a;521:219–238. doi: 10.1016/B978-0-12-391862-8.00012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Gupta A, Saldanha SA, Negri A, Pinello CE, et al. Identification of a mu-delta opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci U S A. 2013b;110:12072–12077. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, et al. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, et al. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, van der Graaf PH. Current methods used to investigate G protein coupled receptor oligomerisation. J Pharmacol Toxicol Methods. 2006;54:26–35. doi: 10.1016/j.vascn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Harvey JH, Long DH, England PM, Whistler JL. Tuned-affinity bivalent ligands for the characterization of opioid receptor heteromers. ACS Med Chem Lett. 2012;3:640–644. doi: 10.1021/ml300083p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, et al. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Heinisch S, Palma J, Kirby LG. Interactions between chemokine and mu-opioid receptors: anatomical findings and electrophysiological studies in the rat periaqueductal grey. Brain Behav Immun. 2011;25:360–372. doi: 10.1016/j.bbi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, et al. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J Pharmacol Sci. 2008;108:308–319. doi: 10.1254/jphs.08244fp. [DOI] [PubMed] [Google Scholar]
- Illes P, Norenberg W. Blockade of alpha 2-adrenoceptors increases opioid mu-receptor-mediated inhibition of the firing rate of rat locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:490–496. doi: 10.1007/BF00169034. [DOI] [PubMed] [Google Scholar]
- Ishikawa-Ankerhold HC, Ankerhold R, Drummen GP. Advanced fluorescence microscopy techniques – FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 2012;17:4047–4132. doi: 10.3390/molecules17044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Aburi M, Provasi D, Bortolato A, Urizar E, Lambert NA, et al. Making structural sense of dimerization interfaces of delta opioid receptor homodimers. Biochemistry. 2011;50:1682–1690. doi: 10.1021/bi101474v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz JR, Hasbi A, Rashid AJ, So CH, George SR, O'Dowd BF. Mu-opioid receptor heterooligomer formation with the dopamine D1 receptor as directly visualized in living cells. Eur J Pharmacol. 2008;581:235–243. doi: 10.1016/j.ejphar.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, et al. Agonists at the delta-opioid receptor modify the binding of micro-receptor agonists to the micro-delta receptor hetero-oligomer. Br J Pharmacol. 2010;161:1122–1136. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Nguyen T, Balboni G, O'Dowd BF, George SR. Antidepressant-like and anxiolytic-like effects following activation of the mu-delta opioid receptor heteromer in the nucleus accumbens. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.115. doi: 10.1038/mp.2013.115. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, et al. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kivell BM, Day DJ, McDonald FJ, Miller JH. Mu and delta opioid receptor immunoreactivity and mu receptor regulation in brainstem cells cultured from late fetal and early postnatal rats. Brain Res Dev Brain Res. 2004;149:9–19. doi: 10.1016/j.devbrainres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, et al. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang SC. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci. 2011;68:3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, von Gizycki H, Gintzler AR. Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. J Pharmacol Exp Ther. 2007;322:654–660. doi: 10.1124/jpet.107.123620. [DOI] [PubMed] [Google Scholar]
- Liu NJ, Chakrabarti S, Schnell S, Wessendorf M, Gintzler AR. Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal kappa- and mu-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J Neurosci. 2011a;31:11836–11845. doi: 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kai M, Jin L, Wang R. Computational study of the heterodimerization between mu and delta receptors. J Comput Aided Mol Des. 2009;23:321–332. doi: 10.1007/s10822-009-9262-7. [DOI] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011b;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Nuber S, Hoffmann C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol Rev. 2012;64:299–336. doi: 10.1124/pr.110.004309. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Milligan G. Morphine desensitization, internalization, and down-regulation of the mu opioid receptor is facilitated by serotonin 5-hydroxytryptamine2A receptor coactivation. Mol Pharmacol. 2008;74:1278–1291. doi: 10.1124/mol.108.048272. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2012;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. 2013;23:473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massotte D. G protein-coupled receptor heterodimerization: a molecular determinant of neuronal activity. In: Nogueira APS, editor. Neuroscience Research Progress Nogueira. Vol. 2. Hauppage, NY: Novapublishers; 2010. [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan-Lobo L, Enquist J, van Rijn RM, Whistler JL. Anti-analgesic effect of the mu/delta opioid receptor heteromer revealed by ligand-biased antagonism. PLoS ONE. 2013;8:e58362. doi: 10.1371/journal.pone.0058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan-Lobo L, Whistler JL. Heteromerization of the mu- and delta-opioid receptors produces ligand-biased antagonism and alters mu-receptor trafficking. J Pharmacol Exp Ther. 2011;337:868–875. doi: 10.1124/jpet.111.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari P, Ambrosio C, Riitano D, Sbraccia M, Gro MC, Costa T. Promiscuous coupling at receptor-G alpha fusion proteins. The receptor of one covalent complex interacts with the alpha-subunit of another. J Biol Chem. 2003;278:15778–15788. doi: 10.1074/jbc.M300731200. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, et al. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal X, La Porta C, Andreea Bura S, Maldonado R. Involvement of the opioid and cannabinoid systems in pain control: new insights from knockout studies. Eur J Pharmacol. 2013;716:142–157. doi: 10.1016/j.ejphar.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield S, Braksator E, Rodriguez-Martin I, Bailey CP, Donaldson LF, Henderson G, et al. C-terminal splice variants of the mu-opioid receptor: existence, distribution and functional characteristics. J Neurochem. 2008;104:937–945. doi: 10.1111/j.1471-4159.2007.05057.x. [DOI] [PubMed] [Google Scholar]
- Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2-DOP receptor heterodimer. Biochem J. 2008;412:245–256. doi: 10.1042/BJ20071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto M, Wichterle H. Programming embryonic stem cells to neuronal subtypes. Curr Opin Neurobiol. 2011;21:43–51. doi: 10.1016/j.conb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pello OM, Martinez-Munoz L, Parrillas V, Serrano A, Rodriguez-Frade JM, Toro MJ, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–549. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- Plachta N, Bibel M, Tucker KL, Barde YA. Developmental potential of defined neural progenitors derived from mouse embryonic stem cells. Development. 2004;131:5449–5456. doi: 10.1242/dev.01420. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS ONE. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, et al. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63:1001–1019. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay D, Kellett E, McVey M, Rees S, Milligan G. Homo- and hetero-oligomeric interactions between G-protein-coupled receptors in living cells monitored by two variants of bioluminescence resonance energy transfer (BRET): hetero-oligomers between receptor subtypes form more efficiently than between less closely related sequences. Biochem J. 2002;365:429–440. doi: 10.1042/BJ20020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MS, Schnell SA, Overland AC, Chabot-Dore AJ, Taylor AM, Ribeiro-da-Silva A, et al. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol. 2009;513:385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–784. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of delta opioid receptor selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010a;335:133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010b;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology. 2012;37:338–349. doi: 10.1038/npp.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondard P, Goudet C, Kniazeff J, Pin JP, Prezeau L. The complexity of their activation mechanism opens new possibilities for the modulation of mGlu and GABAB class C G protein-coupled receptors. Neuropharmacology. 2011;60:82–92. doi: 10.1016/j.neuropharm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 2011;433:11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Bushlin I, Gomes I, Tzavaras N, Gupta A, Neves S, et al. Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS ONE. 2012;7:e29239. doi: 10.1371/journal.pone.0029239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim K, Fenton T, Bacha J, Urien-Rodriguez H, Bonnert T, Skynner HA, et al. Oligomerization of G-protein-coupled receptors shown by selective co-immunoprecipitation. J Biol Chem. 2002;277:15482–15485. doi: 10.1074/jbc.M201539200. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta R, Burbassi S, Shimizu S, Cappello S, Vallee RB, Rubin JB, et al. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 2009;29:2534–2544. doi: 10.1523/JNEUROSCI.5865-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleister HM, Rao AG. Subtractive immunization: a tool for the generation of discriminatory antibodies to proteins of similar sequence. J Immunol Methods. 2002;261:213–220. doi: 10.1016/s0022-1759(01)00567-1. [DOI] [PubMed] [Google Scholar]
- Snook LA, Milligan G, Kieffer BL, Massotte D. Mu-delta opioid receptor functional interaction: insight using receptor-G protein fusions. J Pharmacol Exp Ther. 2006;318:683–690. doi: 10.1124/jpet.106.101220. [DOI] [PubMed] [Google Scholar]
- Snook LA, Milligan G, Kieffer BL, Massotte D. Co-expression of mu and delta opioid receptors as receptor-G protein fusions enhances both mu and delta signalling via distinct mechanisms. J Neurochem. 2008;105:865–873. doi: 10.1111/j.1471-4159.2008.05215.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Shippenberg TS. Evidence that nor-binaltorphimine can function as an antagonist at multiple opioid receptor subtypes. Eur J Pharmacol. 1994;264:157–162. doi: 10.1016/0014-2999(94)00449-8. [DOI] [PubMed] [Google Scholar]
- Stockton SD., Jr Devi LA. Functional relevance of mu-delta opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:167–172. doi: 10.1016/j.drugalcdep.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Wilcox GL. Alpha-2-adrenergic and opioid receptor additivity in rat locus coeruleus neurons. Neurosci Lett. 2004;361:265–268. doi: 10.1016/j.neulet.2003.12.065. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature. 2012;485:395–399. doi: 10.1038/nature11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, et al. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci U S A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, John S, Maga M, Evans CJ, Hales TG. Delta receptors are required for full inhibitory coupling of mu-receptors to voltage-dependent Ca(2+) channels in dorsal root ganglion neurons. Mol Pharmacol. 2009;76:134–143. doi: 10.1124/mol.109.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn WM, Miotto KA, Evans CJ. Opioid pharmaceuticals and addiction: the issues, and research directions seeking solutions. Drug Alcohol Depend. 2010;108:156–165. doi: 10.1016/j.drugalcdep.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Hsu CY, Huang PC, Kuo YL, Li AH, Yeh TH, et al. Heterodimerization of opioid receptor-like 1 and mu-opioid receptors impairs the potency of micro receptor agonist. J Neurochem. 2005;92:1285–1294. doi: 10.1111/j.1471-4159.2004.02921.x. [DOI] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WY, He Y, Yang YR, Li YF, Kang K, Xing BM, et al. Disruption of Cdk5-associated phosphorylation of residue threonine-161 of the delta-opioid receptor: impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29:3551–3564. doi: 10.1523/JNEUROSCI.0415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. Interaction of bivalent ligand KDN21 with heterodimeric delta-kappa opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol. 2005;68:1079–1086. doi: 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, et al. N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. Proc Natl Acad Sci U S A. 2011;108:5098–5103. doi: 10.1073/pnas.1016277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M. Regulation of opioid receptors by endocytic membrane traffic: mechanisms and translational implications. Drug Alcohol Depend. 2010;108:166–171. doi: 10.1016/j.drugalcdep.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]


