Abstract
Contemporary theories emphasize the involvement of the endogenous opioid system in assigning hedonic values to rewards. Although earlier research supports this view, recent findings suggest that opioids play a larger and more complex role in reward processes than these theories suggest. For example, opioid activity in the basolateral amygdala is required for encoding incentive learning, a process by which the value of goal-directed actions is updated. Outside the amygdala, opioid receptors in the ventral striatum have been found to promote choice between different courses of action. Specifically, μ opioid receptors in the nucleus accumbens core and δ opioid receptors in the nucleus accumbens shell have been reported to mediate distinct aspects of incentive motivation; the core regulating the effect of experienced reward and the shell of predicted reward on choice. In both cases, the involvement of opioid receptors was restricted to the time of choice, although changes in their expression pattern could be observed prior to that point. This time-restricted involvement of opioid receptor-related processes is consistent with the view that opioids in the nucleus accumbens are central components of the limbic-motor interface, integrating reward-related information with instrumental learning to guide decision-making, particularly the selection and execution of goal-directed actions.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Table of Links
| TARGETS | LIGANDS |
|---|---|
| δ receptor | Naltrindole/Naloxone |
| κ receptor | Naloxone |
| μ receptor | Naloxone |
| NMDA receptor | Ifenprodil |
This Table lists the protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,c).
Many influential theories have attempted to describe the critical role played by the endogenous opioid system in reward processing (Koob and Le Moal, 1997; Peciña and Berridge, 2000; Barbano and Cador, 2007; Smith and Berridge, 2007; Berridge, 2009; Le Merrer et al., 2009; Nathan and Bullmore, 2009). The development of these theories has largely been motivated by seminal findings obtained in rodents showing that systemically administered opioid receptor agonists have reinforcing properties whereas antagonists of these same receptors induce aversive states (Grevert and Goldstein, 1977b; Mucha and Iversen, 1984; Mucha and Walker, 1987). The significance of these findings was further strengthened by the observation that antagonism of these receptors in humans produced dysphoria (Grevert and Goldstein, 1977a). Taken together, these studies and others led to the view that the opioid system acts as the ‘hedonic mediator’ of the CNS (Koob and Le Moal, 1997), computing and attributing emotional values to environmental stimuli. In fact, the idea that opioids maintain and adjust the ‘hedonic’ tone of the brain had long been postulated (Kosterlitz and Hughes, 1975) and remains today central to many contemporary theories on the role of these neurotransmitters (Peciña and Berridge, 2000; Barbano and Cador, 2007; Smith and Berridge, 2007). Nevertheless, recent studies on reward-related behaviour clearly suggest that the involvement of endogenous opioids cannot be limited to this ‘hedonic’ role alone (Wassum et al., 2009a; b,2011a,b; Laurent et al., 2012; Bertran-Gonzalez et al., 2013), a notion that is consistent with the widespread distribution of opioid receptors in the brain (Ding et al., 1996; Poulin et al., 2006; Le Merrer et al., 2009). In the present review, we will focus on some of these recent studies, emphasizing those investigating the role of opioids in decision-making and in choice between goal-directed actions (Laurent et al., 2012; Bertran-Gonzalez et al., 2013).
Goal-directed actions are those through which we and other animals exert control over the environment in the service of basic needs and desires. The ability to perform these actions is obviously critical for successful adaptation. But, it is no less critical to be able to select which actions are optimal given the current state of the environment. It is well established that this selection, or choice, can be influenced by two separate factors. The first is the reward value assigned to the consequences or outcome of an action (Adams and Dickinson, 1981; Rescorla, 1990; Dickinson, 1994; Dickinson and Balleine, 1994). Here, we will present evidence that opioids are necessary to learn about such reward values, and that these appear to be distinct from any involvement of opioids in the ‘hedonic’ processes previously described (Wassum et al., 2009b; 2011a,b). Furthermore, we will describe evidence that opioid activity is also required later when these values control choice between actions, that is, in value-based decision-making (Laurent et al., 2012) (see Figure 1).
Figure 1.
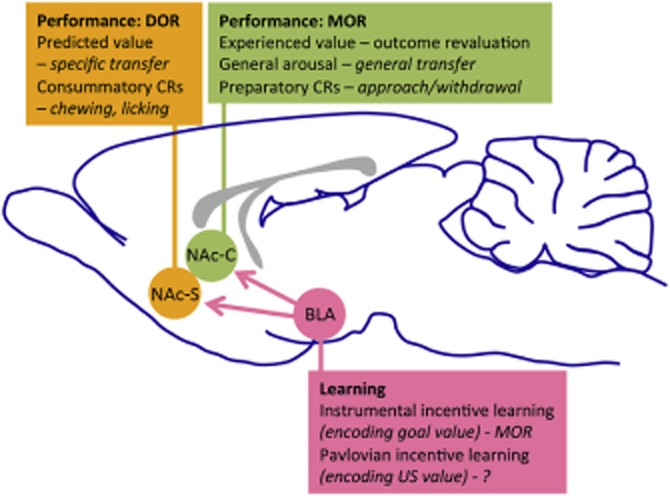
The role of opioid activity, particularly μ opioid receptors (MOR) and δ opioid receptors (DOR) processes, in incentive learning and in value-based and stimulus-based decision-making. These processes involve limbic inputs to the ventral striatum, particularly basolateral amygdala (BLA) inputs to the accumbens core (NAc-C) and shell (NAc-S), as part of the limbic–motor interface. US, unconditioned stimulus.
The second factor that influences choice is the presence of reward-related stimuli. For example, a Pavlovian stimulus associated with a particular outcome biases choice towards instrumental actions that deliver the same outcome (Colwill and Rescorla, 1988; Dickinson and Balleine, 1994; Holmes et al., 2010). This phenomenon, commonly referred to as specific Pavlovian-instrumental transfer (PIT – see Figure 2), provides an important example of the way predictive learning influences choice. Importantly, recent work has revealed that δ opioid receptors (δ receptors) play a critical role in this form of choice (Laurent et al., 2012; Bertran-Gonzalez et al., 2013), confirming that endogenous opioids play a complex role in reward learning and in the influence of that learning on performance.
Figure 2.
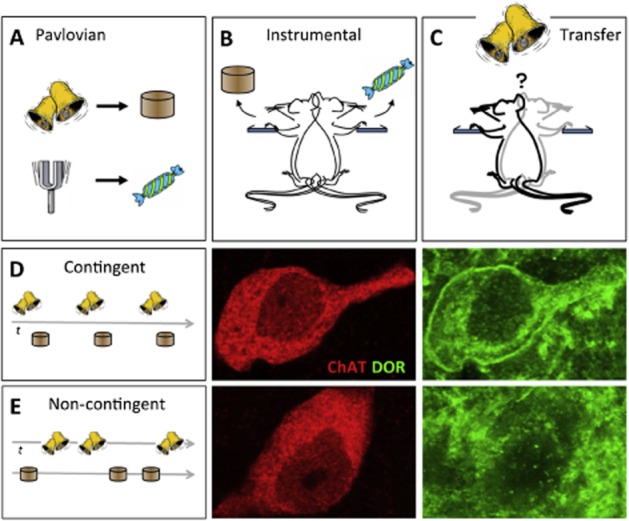
The role of a δ opioid receptor (DOR)-related process in specific PIT. (A–C) Design of specific PIT experiments. Rats or mice are given Pavlovian training (A) in which two stimuli (here a tone or bell) are paired with different foods (grain pellets or sugar). They are then given instrumental training (B) in which they learn to press different levers to earn the same outcomes (here left lever → pellet, right lever → sugar). The transfer test (C) is conducted in extinction (i.e. without food delivery) in which the stimuli are presented and the rats are allowed to choose between the two levers. (D, E) δ receptor activity, labelled for eGFP (green) in the somatic membrane of cholinergic interneurons, labelled for choline acetyl transferase (ChAT (red) in the accumbens shell reflects the strength of the contingency encoded between CS and US and the degree of PIT (D). Breaking the Pavlovian contingency by presenting the CS and US randomly on independent time (t) series and, therefore, in a ‘Non-contingent’ manner reduces δ receptor expression on the membrane of cholinergic interneurons compared to ‘Contingent’ Pavlovian training (E). CS, conditioned stimulus; US, unconditioned stimulus.
Taste reactivity ≠ reward value: the role of opioids in incentive learning and choice between actions
The performance of instrumental actions depends on the value assigned to the rewards they procure. It follows that these values depend, at least in part, on the emotional effects of those rewards. It is commonly agreed that these effects cannot be accurately determined through simple measures such as the amount or rate of reward consumption (Berridge and Robinson, 2003; Barbano and Cador, 2007), and there is now an extensive literature proposing that the ‘hedonic’ value of a reward can instead be assessed directly through the analysis of more complex behaviours including orofacial reactions in response to tastes and the microstructure of the licking responses as a result of liquid intake (Davis and Smith, 1992; Smith, 2001; Berridge, 2009; Johnson et al., 2010). A number of studies have therefore used these types of response to investigate the proposed role of opioids in reward. For example, it has been reported that an agonist of μ opioid receptors (μ receptors) increases the orofacial reactions elicited by sucrose when infused into either the nucleus accumbens shell (NAc-S) or the ventral pallidum (VP), and that disconnection of the NAc-S and VP removes this μ receptor-induced increase in palatability, as measured through these same orofacial reactions (Peciña et al., 2006; Smith and Berridge, 2007; Berridge et al., 2009). Such findings suggest that a circuit linking these structures is essential for establishing reward value and, consistent with this claim, mice with genetic deletion of μ receptors were found to display impairments in licking behaviour, suggesting an inability to properly process the palatability of liquid rewards (Ostlund et al., 2013). These studies using taste reactivity and licking behaviour have been interpreted by some authors to suggest that the NAc-S and VP are ‘hedonic hotspots’ where opioids modulate what they refer to as ‘liking responses’ (Berridge, 2009; Berridge et al., 2009). However, it does not necessarily follow that changes in licking reflect changes in liking or, for that matter, changes in reward value. In fact, changes in reward require an additional form of learning known as incentive learning and, although recent evidence suggests this learning is mediated by an opioid process, it appears to be independent of opioid-related changes in licking involving the NAc-S and the VP (Figure 1). As we shall see: changes in licking ≠ changes in liking.
Incentive learning
The hedonic impact of rewards can easily be manipulated through conditioned-taste aversion (CTA), a procedure during which a reward comes to be associated with illness by pairing its consumption with a systemic injection of the emetic agent LiCl (see Balleine, 2009, for review). Although this procedure clearly diminishes the hedonic value of the paired reward, surprisingly, it often has no immediate effect on instrumental performance. For example, in one study (Balleine and Dickinson, 1991), two groups of rats were trained to press a lever to obtain a liquid reward (i.e. sucrose solution). That reward was then devalued (i.e. its hedonic value was reduced) in one group by a single pairing of the sucrose with LiCl immediately after training. The other group received delayed LiCl treatment to prevent any associative relationship between the sucrose and the emesis. During a subsequent extinction test (i.e. in which no reward was given), no difference was found between the two groups of rats: both displayed similarly high levels of instrumental lever pressing. This lack of effect was not due to a failure to learn about the relationships between the reward and LiCl; rats treated immediately after training showed clear signs of aversion when given the sucrose subsequent to the pairing. These results suggest, therefore, that action values are preserved after devaluation until they are modified through consummatory experience (see also Rescorla, 1992; 1994, and subsequently, Balleine and Dickinson, 1992; Balleine et al., 2005). The authors tested this hypothesis in the same study by training additional groups of rats in the same manner except for the introduction of an additional stage. This stage occurred a day after the single pairing of sucrose and LiCl and consisted of giving rats the opportunity to freely consume the sucrose reward. Although innocuous in appearance, this additional stage had a dramatic effect: animals given re-exposure to the sucrose after receiving LiCl after training now exhibited a strong reduction in lever pressing, suggesting a significant decrease in the value assigned to the instrumental outcome. The effect of this additional ‘re-exposure’ stage has been observed in many situations other than CTA (Lopez et al., 1992; Dickinson and Balleine, 1994; Balleine and Dickinson, 1998) and appears to be required after motivational shifts before the reward value assigned of a specific outcome is changed (see Dickinson and Balleine, 1993; 1994; Balleine, 2011).
Importantly, substantial evidence suggests that incentive learning requires opioid activity in the basolateral complex of the amygdala (BLA). One study (Wassum et al., 2009b) employed shifts in motivational state to investigate the role of the endogenous opioid system in incentive learning. In that study, moderately sated rats (2 h food deprived) were initially trained to press one lever (the seeking lever) to access a second lever (the taking lever) that delivered a sucrose reward. The rats were then given a ‘re-exposure session’ during which they were allowed free access to the sucrose either when 2 h deprived, as in training, or when 23 h food deprived. Not surprisingly, the reward appeared to be more valuable for the latter group of rats than the former as revealed by higher rates of licking in the 23 h deprived rats. This experience allowed the rats to learn about the value of the sucrose in the 23 h deprived state, which was confirmed the following day in an extinction test: rats increased lever pressing when 23 h food deprived but only if previously allowed to consume the sucrose when 23 h food deprived; the increase in outcome value only emerged on test if direct experience of the effect of increased food deprivation on the reward value of sucrose was given prior to the test.
Critically, the increase in responding induced by re-exposure was blocked by an infusion of naloxone, a non-selective antagonist of opioid receptors, infused into the BLA prior to the ‘re-exposure session’, that is, at the time of incentive learning. Importantly, this infusion had no effect on the increase in licking induced by 23 h food deprivation in this session which, if such licking responses reflect reward value, must suggest that the increase in the value attributed to the reward was left unaffected. Furthermore, the opposite pattern of results was found when naloxone was infused into the NAc-S or the VP. In striking contrast to the effects of the BLA infusion, NAc-S and VP infusions blocked the increase in licking responses induced by the increase in food deprivation during the re-exposure session but had no effect on incentive learning. Despite naloxone blockade of the deprivation-induced increase in licking responses during re-exposure, lever press responding was elevated by the re-exposure in the subsequent extinction test. It should be noted that these results oppose previous interpretations of opioid control of orofacial reactions (Peciña et al., 2006; Smith and Berridge, 2007; Berridge et al., 2009), suggesting instead that these changes reflect changes in consummatory reactions not in liking; that is, μ receptors in the NAc-S and VP appear more likely to mediate conditioned consummatory reactions to taste-related stimuli than emotional responses associated with ‘hedonics’ or ‘liking’ (Balleine, 2011). Consistent with this suggestion, a subsequent series of experiments confirmed the importance of BLA opioids specifically in incentive learning, showing that the effects described previously were restricted to the re-exposure phase and did not affect the retrieval of incentive value during the lever press test. Further, using more selective antagonists, the effects of naloxone on incentive learning were found to be mediated by its effects on μ, rather than δ or κ receptors (Wassum et al., 2011a). Furthermore, whereas a μ receptor antagonist blocked increases in reward value, it was ineffective in blocking reductions in reward value induced by a reduction in food deprivation. Such reductions in value were, however, reversed by the μ receptor agonist DAMGO when it was infused into the BLA before the re-exposure session (Wassum et al., 2011a).
It is important to recognize that conclusions based on changes in licking and in the orofacial responses associated with taste reactivity have sometimes been found to differ. This was the case in the context of studies assessing the role of dopamine in reward and the effect particularly of dopamine antagonists, which appeared to reduce sucrose licking and so to induce anhedonia (Schneider et al., 1990) but were found to have no effect on taste reactivity (Treit and Berridge, 1990; Peciña et al., 1997). Although it may ultimately prove necessary to compare taste reactivity and incentive learning more directly, in the experiments described here, we found, as has been described for taste reactivity, that licking microstructure induced by sucrose is sensitive to opioid manipulations, as the change in licking microstructure associated with an increase in food deprivation was blocked by naloxone. Nevertheless, this modulation did not affect incentive learning. Taken together, therefore, the findings described here provide clear evidence that assigning incentive value to rewarding outcomes involves an evaluative learning process that does not rely on those mediating changes in orofacial reactions in the NAc-S or VP. Although the effects of changes in reward palatability on conditioned consummatory responses require μ receptor activity in the NAc-S and the VP, the desirability of rewards (i.e. their incentive value) involves activation of μ receptors in the BLA. Consistent with the latter involvement, incentive learning is impaired by lesion (Balleine et al., 2003), pharmacological inactivation (Shiflett and Balleine, 2010) or other pharmacological manipulations (Wang et al., 2005; Parkes and Balleine, 2013) of the BLA. In addition, these studies shed light on the neural circuitry that, with the BLA, promotes incentive learning and the influence that this learning exerts subsequently on the performance of actions. Indeed, as explained below, the performance of value-based actions also involves opioid activity (see Figure 1).
Value-based choice
The influence of outcome value on choice is often studied using sensory-specific satiety (Rolls et al., 1981; 1984) to devalue the outcome associated with an action. Typically, rats are trained to perform two actions, A1 and A2, which earn two distinct food outcomes, O1 and O2 (i.e. such that A1→O1 and A2→O2) after which one of these outcomes is devalued by allowing free access to that particular outcome for a relatively brief period of time (about 1 h). Immediately after this specific satiety treatment, the rats are given a choice test, during which they can perform either A1 or A2, but no outcome is delivered. The classic results show that rats avoid A1 and choose A2 when sated on O1, but avoid A2 and choose A1 if sated on O2 prior to the test. In other words, they bias their choice towards the action whose outcome has the relatively higher value. This procedure has been influential because it is particularly amenable to pharmacological manipulation. Furthermore, unlike CTA, the value of the outcomes is restored quite rapidly as satiety is diminished, allowing for additional and subsequent tests and within-subject analyses.
Given the role of the BLA in incentive learning (see above), it is not surprising that this region has been found to be involved in choice between actions after sensory-specific satiety (Corbit and Balleine, 2005; Ostlund and Balleine, 2008a; Shiflett and Balleine, 2010). However, this role is restricted to encoding changes in reward value (Parkes and Balleine, 2013). In this study, the authors employed the design described above and manipulated NMDA receptor activity using the NMDA antagonist ifenprodil during the sensory-specific satiety treatment (i.e. during outcome devaluation) or during the subsequent choice test. They found that the infusion of ifenprodil blocked the effect of outcome devaluation on subsequent choice but only when it was infused before the specific satiety treatment. It had no effect if infused before test. Interestingly, they also reported that ifenprodil infused in the insular cortex (IC), whether prior to specific satiety or the test, abolished the outcome devaluation effect in the choice test, suggesting a role for the IC in retrieving the change in outcome value. In fact, the authors provided strong evidence that the influence of the IC on retrieval involved interactions with the BLA. For example, the unilateral infusion of ifenprodil into the BLA before outcome devaluation combined with a unilateral infusion into the contralateral IC before test blocked outcome devaluation (i.e. BLA→IC). Importantly, this effect of ifenprodil was not observed if the order of infusions was reversed (IC→BLA). This study convincingly shows that the BLA encodes changes in outcome values whereas the IC retrieves those values to guide choice performance.
Another structure that is involved in this expression is the nucleus accumbens core (NAc-C – Figure 1), as local lesion or reversible inactivation just prior to the extinction test blocks the influence of outcome devaluation on choice (Corbit et al., 2001; Shiflett and Balleine, 2010; Corbit and Balleine, 2011). This time-restricted involvement of the NAc-C has led to the view that its role is essentially to guide and promote performance at the time of the choice test. Consistent with this view, the NAc-C receives strong projections from brain regions involved in incentive learning, such as the BLA and the IC (Wright and Groenewegen, 1996; Chikama et al., 1997; Sah et al., 2003; Naqvi and Bechara, 2009; Jones et al., 2010), indicating that it receives the information necessary to select and implement the performance of valued actions.
Of particular interest for the present review is the fact that changes in action selection based on changes in outcome value during a choice test involve opioid-related activity in the NAc-C. In a recent series of experiments (Laurent et al., 2012), we adopted a genetic and a pharmacological approach to tackle the role of opioids in the NAc-C during choice after outcome devaluation. We initially found that mice with a genetic deletion of μ receptors were unable to correctly alter their choice between actions following outcome devaluation; that is, they did not show a preference for the action that delivered the non-devalued outcome. The impairment was quite specific to choice performance after a change in the value of the outcome; the same animals showed no apparent deficits in tests of Pavlovian conditioning, instrumental conditioning or changes in choice induced by reward-related stimuli (see below). Interestingly, δ receptor knockout mice showed no deficit in the choice test and performed similarly to controls after outcome devaluation, suggesting a critical role for μ receptors during this test. Next, using rats, we assessed the effect of bilaterally infusing various opioid-related pharmacological agents in different sub-nuclei of the ventral striatum. Importantly, these infusions were made immediately prior to the choice test (we had previously excluded any role for the NAc-C in encoding incentive learning). Although intra-NAc-C naloxone was previously found not to affect changes in outcome value per se (Wassum et al., 2009b), blockade of μ receptors in the NAc-C did impair performance in the choice test conducted after outcome revaluation. This impairment was region- and receptor-specific. Thus, δ receptor blockade in the NAc-C or μ receptor blockade into the NAc-S did not affect performance in the choice test after outcome devaluation consistent with previous findings showing the critical role played by the NAc-C in the expression of changes in value in choice (Corbit et al., 2001; Shiflett and Balleine, 2010; Corbit and Balleine, 2011). They extend those previous findings by showing that this expression recruits local activity of μ receptors, indicating the critical role played by these receptors in learning about outcome values (i.e. incentive learning in the BLA) and using these values to choose which action to perform (i.e. value-based choice in the NAc-C).
Role of opioids in PIT
Choice is not only controlled by the incentive value assigned to the consequences of actions but is also influenced by predictive stimuli and the information they convey about forthcoming outcomes. The classic demonstration of the influence of predictive learning on choice involves a phenomenon referred to as specific PIT (Colwill and Rescorla, 1988; Dickinson and Balleine, 1994; Holmes et al., 2010). The protocol is similar to that of used to assess outcome devaluation (see Figure 2A–C); the subjects first learn the relationship between two instrumental actions (A1 and A2) and their respective outcomes (O1 and O2) before being presented with the opportunity to choose between the two actions in a test. However, during this choice, they are presented with two Pavlovian stimuli, S1 and S2, which have previously been trained to predict the two instrumental outcomes; that is, subjects learned that S1 predicted the delivery of O1 whereas S2 predicted that of O2. Upon testing, a stimulus typically elevates the action that in training delivered the outcome predicted by that stimulus, that is, S1 will elevate A1 whereas S2 will elevate A2 – see Figure 2. This effect has been observed using various types of rewards, such as food, money or drugs (cocaine or nicotine) in many species, including rats, mice, rabbits, horses, monkeys or humans (Henton and Brady, 1970; LoLordo, 1971; Lovibond, 1981; Corbit and Balleine, 2011; Nadler et al., 2011; Hogarth, 2012; Prévost et al., 2012; Bertran-Gonzalez et al., 2013; Lansade et al., 2013; Leblanc et al., 2013; Lewis et al., 2013; Lovibond and Colagiuri, 2013). The significance of PIT is that it provides a powerful insight into how animals extract predictive information from important events to guide their future actions. Such cognitive control of actions is essential for successful adaptation to a changing environment and deficits in this ability have been associated with many psychiatric disorders such as depression, schizophrenia, drug addiction or obesity (Hyman, 2005; Ostlund and Balleine, 2008b; Seymour and Dolan, 2008; Simpson et al., 2010; Petrovich, 2011). Despite the undoubted importance of this phenomenon, the underlying psychological and neural processes that mediate specific PIT remain largely unknown (Balleine and Ostlund, 2007; Holmes et al., 2010). One particular challenge at the neural level is to uncover the mechanisms by which two distinct forms of learning (Pavlovian and instrumental) established at different times can be integrated to promote choice between actions at a later time. Interestingly, one key to tackling this challenge appears to involve the recently established role of δ receptors in the ventral striatum (Laurent et al., 2012; 2014; Bertran-Gonzalez et al., 2013).
Role of δ receptors in the NAc-S
To date, a large number of brain regions have been shown to be necessary for specific PIT. They include the BLA, the mediodorsal thalamus, the orbitofrontal cortex, the dorsal striatum and the ventral tegmental area (Corbit and Balleine, 2005; Corbit and Janak, 2007; Corbit et al., 2007; Ostlund and Balleine, 2008a). However, the involvement of these regions is not so surprising as they are also involved in Pavlovian and/or instrumental learning, that is, the two forms of learning that promote specific PIT. Two notable exceptions, however, are the NAc-S and its main target region, the VP (Corbit et al., 2001; Corbit and Balleine, 2011; Leung and Balleine, 2013). Rats with NAc-S lesions exhibit no apparent deficits in their ability to learn (Corbit et al., 2001) and update (unpublished data from our laboratory) stimulus–outcome relationships during Pavlovian conditioning. They are also able to learn action–outcome associations during instrumental conditioning and to select a valued action over a devalued one during subsequent choice tests. However, such rats are unable to guide their choice between actions according to the predictive stimuli present (Corbit et al., 2001). The same results can be obtained when the NAc-S is pharmacologically inactivated or disconnected from the BLA prior to the PIT test (Shiflett and Balleine, 2010; Corbit and Balleine, 2011). Thus, a number of findings indicate that the NAc-S is essential for the expression of the effect of predictive learning on choice. In fact, there is now convincing evidence that this expression recruits δ receptor-related activity in the NAc-S.
As mentioned previously, mice carrying genetic deletion of δ receptors are able to display value-based choice (Laurent et al., 2012). However, these mice are impaired in PIT as, unlike their wild-type littermate controls, δ receptor knockout mice are unable to correctly select instrumental actions based on the predictive status of the stimuli present at the time of choice. The deficits of these mice are specific to the PIT test; they show no impairment in Pavlovian and instrumental training. Thus, δ receptor activity appears critical for the expression of stimulus-based choice. Given the role played by the NAc-S in that expression, we next assessed the role of δ receptor activity in the NAc-S at the time of choice in the PIT test. Rats were given Pavlovian and then instrumental training (cf. Figure 2), and then a PIT test immediately prior to which they were given either an infusion of vehicle or of the δ receptor antagonist naltrindole bilaterally into the NAc-S. As predicted, we found that naltrindole blocked the ability of rats to use predictive stimuli to bias choice in the PIT test. This effect was region and receptor specific: δ receptor blockade in the NAc-C or μ receptor blockade in the NAc-S had no effect on specific PIT (Laurent et al., 2012). Taken together, both our genetic and pharmacological approaches reveal a double dissociation at the level of the nucleus accumbens between the effect of outcome devaluation and of predictive stimuli on choice. Whereas the effect of outcome devaluation requires μ receptor activation in the NAc-C, PIT requires δ receptor activation in the NAc-S. However, although these results represent significant progress in our understanding of the neural mechanisms underlying specific PIT, they do not inform us as to how these two forms of learning, which are acquired at different points in time, are integrated to promote the effect of predictive learning on choice at a later time. Clearly, answering such a complex question requires a more precise level of analysis, involving, for example, the study of the changes occurring in the NAc-S at a cellular level.
δ receptor expression on cholinergic interneurons
Given the identified role of δ receptors in the expression of PIT, we have recently conducted a series of experiments using δ receptor-eGFP knock-in transgenic mice (δ receptor-eGFPki) to help identify the locus of these receptors mediating this effect. These mice express a fluorescent but functional form of δ receptors, a process achieved through fusion with the GFP protein, allowing the detection of δ receptor expression in the CNS (Scherrer et al., 2006). Initial experiments involving confocal imaging revealed substantial levels of δ receptor expression in the NAc-S (Bertran-Gonzalez et al., 2013). Most notably, these levels were significantly higher than those observed in the NAc-C, the adjacent region in which δ receptor blockade had no effect on PIT expression (Laurent et al., 2012). Further analysis showed that δ receptors were exclusively expressed post-synaptically. However, the main outcome of our confocal study was the striking contrast between the distribution of δ receptors on medium spiny neurons (MSNs) and that on cholinergic interneurons (CINs) in the accumbens shell (see Figure 2D). MSNs were a focus of attention because they constitute the vast majority of NAc-S cells and are the only output neurons of the structure (Bertran-Gonzalez et al., 2010; Gerfen and Surmeier, 2011). However, the pattern of δ receptor expression on those neurons was unclear compared to that on CINs. Indeed, we observed that δ receptors were almost exclusively localized in the perisomatic compartment of the membrane of CINs. This observation was particularly interesting given that CINs have been shown to be involved in predictive learning and theories have been developed concerning their role in promoting the influence of contextual cues on decision-making processes (Apicella, 2007; Brown et al., 2012; Stocco, 2012). Therefore, we conducted several experiments with the aim of establishing the role of δ receptors localized on CINs during specific PIT.
Food-deprived δ receptor-eGFPki mice were exposed to the specific PIT protocol, as described previously. They firstly learned that two stimuli (S1 and S2) predicted two distinct food outcomes (O1 and O2) and were then trained to perform two actions (A1 and A2) to earn O1 and O2. Mice given these two learning stages showed specific PIT during a subsequent choice extinction test (i.e. they showed S1: A1 > A2 and S2: A1 < A2). Importantly, these mice also displayed higher levels of δ receptor expression on the membrane of NAc-S CINs than control mice that had only been exposed to the conditioning chambers. More remarkably, however, the increase in δ receptor expression was not restricted to mice receiving a PIT test or showing stimulus-based choice. Indeed, δ receptor accumulation was present in trained mice in which either instrumental training or the PIT test had been omitted (Bertran-Gonzalez et al., 2013). Clearly, these findings suggest that Pavlovian training produced the change in δ receptor expression observed on NAc-S CINs. Consistent with this suggestion, we found a positive correlation between the levels of δ receptor expression and conditioned responding (CR; e.g. entry into the magazine where the food was delivered) across Pavlovian training; the higher the CR, the more δ receptors accumulated on NAc-S CINs (Bertran-Gonzalez et al., 2013 – see Figure 2D).
Although Pavlovian training appeared to trigger δ receptor accumulation, it remained unknown which aspect of this training was responsible for the accumulation. The only behavioural manipulation known to affect specific PIT involves degrading the specific stimulus–outcome (S–O) contingencies acquired across Pavlovian training (Delamater, 1995). We hypothesized, therefore, that it was establishing these contingencies that was inducing the observed changes in δ receptor expression. We tested this hypothesis by exposing food-deprived δ receptor-eGFPki mice to either contingent or non-contingent Pavlovian training (Bertran-Gonzalez et al., 2013). Contingent training was identical to that described above and involved mice learning the predictive relationships between the two stimuli, S1 and S2, and their two respective outcomes, O1 and O2. These relationships were degraded in non-contingent training; presentations of S1 and S2 and deliveries of O1 and O2 were uncorrelated preventing the mice from learning the predictive S–O relationships (see left panels of Figure 2D and E). As expected, non-contingently trained mice displayed lower levels of CR than contingently trained ones. More importantly, they also exhibited lower levels of δ receptor expression on NAc-S CINs compared to contingently trained mice (see Figure 2E). In fact, their levels were similar to those displayed by control mice that had simply been exposed to the conditioned chambers. In another experiment, we confirmed the detrimental effect of non-contingent training on the CR and showed that such training removed specific PIT such that the mice distributed their efforts evenly across the two instrumental actions. This contrasted with the behaviour of the contingent mice that biased their choice towards the action earning the same outcome as that predicted by the stimuli. The difference between contingent and non-contingent mice was also evident at the neural level; the former displayed higher levels of δ receptor accumulation on CINs than the latter. Critically, data from contingent mice revealed a positive correlation between δ receptor expression and specific PIT: the more δ receptor accumulation the larger the transfer effect. These effects were specific to CINs located in the NAc-S. No difference was found in the dorsal striatum or in the NAc-C (Bertran-Gonzalez et al., 2013).
In the course of these experiments we were also able to obtain evidence of functional and physiological changes associated with δ receptor accumulation (Bertran-Gonzalez et al., 2013). Indeed, this accumulation significantly altered the firing pattern of CINs by increasing the variance of action potential activity, an effect that was potentiated by a δ receptor agonist. This increase and its potentiation by the agonist were obtained both when we compared ‘high CR’ against ‘low CR’ mice, and when comparing ‘contingently trained’ against ‘non-contingently trained’ mice. The change in the physiological properties of the interneurons was of particular interest as it is widely agreed that CIN activity is a critical modulator of MSN function (Ding et al., 2010; Goldberg et al., 2012). This suggests that δ receptor-mediated modulation of CIN firing could provide a means quickly to alter NAc-S activity in order to express the effect of predictive learning on choice. Consistent with this suggestion and with previous findings (Laurent et al., 2012), δ receptor-eGFPki mice failed to show PIT when systemically treated prior to test with the δ receptor antagonist naltrindole. Importantly, the same treatment given across Pavlovian training has no apparent effect on the ability of δ receptor-eGFPki mice to learn specific S–O associations (unpublished data from our laboratory). For instance, we found that such mice selectively reduced Pavlovian CR to a stimulus that predicts a devalued outcome. This finding is entirely consistent with previous results showing normal Pavlovian performance in δ receptor knockout mice (Laurent et al., 2012) or NAc-S lesion rats (Corbit et al., 2001), and it confirms that the role of δ receptors is limited to the influence of predictive learning on choice between goal-directed actions and that they play no role in Pavlovian conditioning per se.
Taken together, these findings indicate that the acquisition of specific S-O contingencies across Pavlovian training triggers δ receptor accumulation on the membrane of NAc-S CINs. This accumulation is long lasting and survives at least for the time between Pavlovian training and the PIT test (i.e. 11 days). This accumulation substantially alters the firing pattern of CINs (Bertran-Gonzalez et al., 2013) and is not necessary for Pavlovian conditioning per se (Laurent et al., 2012) but is required for the influence of Pavlovian stimuli on choice between actions; that is, for PIT. Given its temporal and functional characteristics, this δ receptor-related plastic change constitutes a convincing neural substrate in the NAc-S by which two forms of learning established at different times are subsequently integrated to promote the expression of predictive learning on choice.
Summary and conclusions
The aim of the present review was to demonstrate the complex functions that the endogenous opioid system plays in decision-making. Opioids have traditionally been viewed as maintaining hedonic tone by, for example, assigning values to rewards (Koob and Le Moal, 1997). Although they do play a role in this process, we have seen here that they contribute more broadly. Opioid activity in the BLA is required for incentive learning, an essential process by which the values of reward are updated. However, we have also presented substantial evidence that opioid activity in the nucleus accumbens is essential for guiding choice between actions. Consistent with the view that this region acts as the limbic–motor interface (Mogenson et al., 1980; Alexander et al., 1986), we have described evidence showing that opioid activity in the accumbens is only essential at the time of choice. For example, δ-opioid receptor activation in accumbens shell is only necessary at the point of integration between Pavlovian and instrumental conditioning in specific PIT, even though the expression of these receptors was triggered much earlier at the time of predictive learning. In a similar manner, there is little evidence to suggest that μ receptor activity in the accumbens core plays any functional role in decision-making prior to the test assessing the effect of outcome devaluation on choice. Overall, there is clear evidence for the importance of opioids in decision-making particularly in the modulation of local circuits; for example, their control of cholinergic activity in the accumbens shell. Further experiments will be required to establish how these local changes modulate the output activity of the whole structure and, therefore, how this modulation promotes choice between goal-directed actions.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health, MH56646, and the National Health and Medical Research Council of Australia, #633267, and by an Australian Laureate Fellowship from the Australian Research Council, #FL0992409, to B. W. B.
Glossary
Abbreviations
- BLA
basolateral complex of the amygdala
- CINs
cholinergic interneurons
- CR
conditioned response
- IC
insular cortex
- MSNs
medium spiny neurons
- NAc-C
nucleus accumbens core
- NAc-S
nucleus accumbens shell
- PIT
Pavlovian-instrumental transfer
- S–O
stimulus–outcome
- δ receptor-eGFPki
δ receptor-eGFP knock-in
Author contributions
V. L., A. M. and B. B. all co-wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
References
- Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. Q J Exp Psychol Sect B. 1981;33:109–121. [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Balleine BW. Taste, disgust and value: taste aversion learning and outcome encoding in instrumental conditioning. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. New York, NY: Oxford University Press; 2009. pp. 262–280. [Google Scholar]
- Balleine B, Dickinson A. Instrumental performance following reinforcer devaluation depends upon incentive learning. Q J Exp Psychol Sect B. 1991;43:279–296. [Google Scholar]
- Balleine B, Dickinson A. Signalling and incentive processes in instrumental reinforcer devaluation. Q J Exp Psychol Sect B. 1992;45:285–301. [PubMed] [Google Scholar]
- Balleine BW. Sensation, incentive learning, and the motivational control of goal-directed action. In: Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton, FL: CRC Press; 2011. pp. 287–309. [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. The role of incentive learning in instrumental outcome revaluation by sensory-specific satiety. Anim Learn Behav. 1998;26:46–59. [Google Scholar]
- Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Ann N Y Acad Sci. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci Off J Soc Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Paredes-Olay C, Dickinson A. Effects of outcome devaluation on the performance of a heterogeneous instrumental Chain. Int J Comp Psychol. 2005;18:257–272. [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Hervé D, Girault J-A, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. 2010;4:1–9. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Laurent V, Chieng BC, Christie MJ, Balleine BW. Learning-related translocation of δ-opioid receptors on ventral striatal cholinergic interneurons mediates choice between goal-directed actions. J Neurosci Off J Soc Neurosci. 2013;33:16060–16071. doi: 10.1523/JNEUROSCI.1927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MTC, Tan KR, O'Connor EC, Nikonenko I, Muller D, Lüscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci Off J Soc Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Rescorla RA. Associations between the discriminative stimulus and the reinforcer in instrumental learning. J Exp Psychol Anim Behav Process. 1988;14:155–164. [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci Off J Soc Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci Off J Soc Neurosci. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Inactivation of the lateral but not medial dorsal striatum eliminates the excitatory impact of Pavlovian stimuli on instrumental responding. J Neurosci Off J Soc Neurosci. 2007;27:13977–13981. doi: 10.1523/JNEUROSCI.4097-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci Off J Soc Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH, Balleine BW. General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci. 2007;26:3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–228. [PubMed] [Google Scholar]
- Delamater AR. Outcome-selective effects of intertrial reinforcement in a Pavlovian appetitive conditioning paradigm with rats. Anim Learn Behav. 1995;23:31–39. [Google Scholar]
- Dickinson A. Instrumental conditioning. In: Mackintosh NJ, editor. Animal Learning and Cognition. San Diego, CA: Academic Press; 1994. pp. 45–79. [Google Scholar]
- Dickinson A, Balleine B. Actions and responses: the dual psychology of behaviour. In: Eilan N, McCarthy RA, Brewer B, editors. Spatial Representation: Problems in Philosophy and Psychology. Malden: Blackwell Publishing; 1993. pp. 277–293. [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012;208:223–241. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- Grevert P, Goldstein A. Effects of naloxone on experimentally induced ischemic pain and on mood in human subjects. Proc Natl Acad Sci U S A. 1977a;74:1291–1294. doi: 10.1073/pnas.74.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevert P, Goldstein A. Some effects of naloxone on behavior in the mouse. Psychopharmacology (Berl) 1977b;53:111–113. doi: 10.1007/BF00426478. [DOI] [PubMed] [Google Scholar]
- Henton WW, Brady JV. Operant acceleration during a pre-reward stimulus. J Exp Anal Behav. 1970;13:205–209. doi: 10.1901/jeab.1970.13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L. Goal-directed and transfer-cue-elicited drug-seeking are dissociated by pharmacotherapy: evidence for independent additive controllers. J Exp Psychol Anim Behav Process. 2012;38:266–278. doi: 10.1037/a0028914. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Marchand AR, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34:1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Sherwood A, Smith DR, Wosiski-Kuhn M, Gallagher M, Holland PC. An analysis of licking microstructure in three strains of mice. Appetite. 2010;54:320–330. doi: 10.1016/j.appet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Wheeler RA, Carelli RM. The basolateral amygdala differentially regulates conditioned neural responses within the nucleus accumbens core and shell. Neuroscience. 2010;169:1186–1198. doi: 10.1016/j.neuroscience.2010.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosterlitz HW, Hughes J. Some thoughts on the significance of enkephalin, the endogenous ligand. Life Sci. 1975;17:91–96. doi: 10.1016/0024-3205(75)90243-x. [DOI] [PubMed] [Google Scholar]
- Lansade L, Coutureau E, Marchand A, Baranger G, Valenchon M, Calandreau L. Dimensions of temperament modulate cue-controlled behavior: a study on Pavlovian to instrumental transfer in horses (Equus caballus. PLoS ONE. 2013;8:e64853. doi: 10.1371/journal.pone.0064853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Leung B, Maidment N, Balleine BW. μ- and δ-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice. J Neurosci Off J Soc Neurosci. 2012;32:1875–1883. doi: 10.1523/JNEUROSCI.4688-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Bertran-Gonzalez J, Chieng BC, Balleine BW. δ-opioid and dopaminergic processes in accumbens shell modulate the cholinergic control of predictive learning and choice. J Neurosci Off J Soc Neurosci. 2014;34:1358–1369. doi: 10.1523/JNEUROSCI.4592-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc KH, Maidment NT, Ostlund SB. Impact of repeated intravenous cocaine administration on incentive motivation depends on mode of drug delivery. Addict Biol. 2013 doi: 10.1111/adb.12063. doi: 10.1111/adb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. J Neurosci Off J Soc Neurosci. 2013;33:13848–13860. doi: 10.1523/JNEUROSCI.1697-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AH, Niznikiewicz MA, Delamater AR, Delgado MR. Avoidance-based human Pavlovian-to-instrumental transfer. Eur J Neurosci. 2013;38:3740–3748. doi: 10.1111/ejn.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoLordo VM. Facilitation of food-reinforced responding by a signal for response-independent food. J Exp Anal Behav. 1971;15:49–55. doi: 10.1901/jeab.1971.15-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Balleine B, Dickinson A. Incentive learning and the motivational control of instrumental performance by thirst. Anim Learn Behav. 1992;20:322–328. [Google Scholar]
- Lovibond PF. Appetitive Pavlovian-instrumental interactions: effects of inter-stimulus interval and baseline reinforcement conditions. Q J Exp Psychol Sect B. 1981;33:257–269. doi: 10.1080/14640748108400811. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Colagiuri B. Facilitation of voluntary goal-directed action by reward cues. Psychol Sci. 2013;24:2030–2037. doi: 10.1177/0956797613484043. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology (Berl) 1984;82:241–247. doi: 10.1007/BF00427782. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Walker MJ. Aversive property of opioid receptor blockade in drug-naive mice. Psychopharmacology (Berl) 1987;93:483–488. doi: 10.1007/BF00207239. [DOI] [PubMed] [Google Scholar]
- Nadler N, Delgado MR, Delamater AR. Pavlovian to instrumental transfer of control in a human learning task. Emot Wash DC. 2011;11:1112–1123. doi: 10.1037/a0022760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PJ, Bullmore ET. From taste hedonics to motivational drive: central μ-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP. 2009;12:995–1008. doi: 10.1017/S146114570900039X. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci Off J Soc Neurosci. 2008a;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models. 2008b;5:235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Kosheleff A, Maidment NT, Murphy NP. Decreased consumption of sweet fluids in mu opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology (Berl) 2013;229:105–113. doi: 10.1007/s00213-013-3077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Balleine BW. Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J Neurosci Off J Soc Neurosci. 2013;33:8753–8763. doi: 10.1523/JNEUROSCI.5071-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacol Biochem Behav. 1997;58:801–811. doi: 10.1016/s0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Forebrain circuits and control of feeding by learned cues. Neurobiol Learn Mem. 2011;95:152–158. doi: 10.1016/j.nlm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Poulin J-F, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496:859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- Prévost C, Liljeholm M, Tyszka JM, O'Doherty JP. Neural correlates of specific and general Pavlovian-to-Instrumental Transfer within human amygdalar subregions: a high-resolution fMRI study. J Neurosci Off J Soc Neurosci. 2012;32:8383–8390. doi: 10.1523/JNEUROSCI.6237-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Evidence for an association between the discriminative stimulus and the response-outcome association in instrumental learning. J Exp Psychol Anim Behav Process. 1990;16:326–334. [PubMed] [Google Scholar]
- Rescorla RA. Depression of an instrumental response by a single devaluation of its outcome. Q J Exp Psychol Sect B. 1992;44:123–136. doi: 10.1080/02724999208250606. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. A note on depression of instrumental responding after one trial of outcome devaluation. Q J Exp Psychol Sect B. 1994;47:27–37. [PubMed] [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiol Behav. 1981;27:137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Van Duijvenvoorde PM, Rolls ET. Pleasantness changes and food intake in a varied four-course meal. Appetite. 1984;5:337–348. doi: 10.1016/s0195-6663(84)80006-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LH, Davis JD, Watson CA, Smith GP. Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol. 1990;186:61–70. doi: 10.1016/0014-2999(90)94060-b. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur J Neurosci. 2010;32:1735–1743. doi: 10.1111/j.1460-9568.2010.07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP. John Davis and the meanings of licking. Appetite. 2001;36:84–92. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci Off J Soc Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco A. Acetylcholine-based entropy in response selection: a model of how striatal interneurons modulate exploration, exploitation, and response variability in decision-making. Front Neurosci. 2012;6:18. doi: 10.3389/fnins.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Berridge KC. A comparison of benzodiazepine, serotonin, and dopamine agents in the taste-reactivity paradigm. Pharmacol Biochem Behav. 1990;37:451–456. doi: 10.1016/0091-3057(90)90011-6. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Ostlund SB, Nader K, Balleine BW. Consolidation and reconsolidation of incentive learning in the amygdala. J Neurosci Off J Soc Neurosci. 2005;25:830–835. doi: 10.1523/JNEUROSCI.4716-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Maidment NT, Balleine BW. Disruption of endogenous opioid activity during instrumental learning enhances habit acquisition. Neuroscience. 2009a;163:770–780. doi: 10.1016/j.neuroscience.2009.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009b;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Balleine BW, Maidment NT. Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J Neurosci Off J Soc Neurosci. 2011a;31:1591–1599. doi: 10.1523/JNEUROSCI.3102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem Cold Spring Harb N. 2011b;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience. 1996;73:359–373. doi: 10.1016/0306-4522(95)00592-7. [DOI] [PubMed] [Google Scholar]


