Abstract
BACKGROUND AND PURPOSE
In addition to its analgesic functions, the peripheral opioid receptor system affects skin homeostasis by influencing cell differentiation, migration and adhesion; also, wound healing is altered in δ-opioid receptor knockout mice (DOPr–/–). Hence, we investigated δ-opioid receptor effects on the expression of several proteins of the desmosomal junction complex and on the migratory behaviour of keratinocytes.
EXPERIMENTAL APPROACH
Expression levels of desmosomal cadherins in wild-type and DOPr–/– mice, and the morphology of intercellular adhesion in human keratinocytes were analysed by immunofluorescence. To investigate the δ-opioid receptor activation pathway, protein expression was studied using Western blot and its effect on cellular migration determined by in vitro live cell migration recordings from human keratinocytes.
KEY RESULTS
Expression of the desmosomal cadherins, desmogleins 1 and 4, was up-regulated in skin from DOPr–/– mice, and down-regulated in δ-opioid receptor-overexpressing human keratinocytes. The localization of desmoplakin expression was rearranged from linear arrays emanating from cell borders to puncta in cell periphery, resulting in less stable intercellular adhesion. Migration and wound recovery were enhanced in human keratinocyte monolayers overexpressing δ-opioid receptors in vitro. These δ-opioid receptor effects were antagonized by specific PKCα/β inhibition indicating they were mediated through the PKC signalling pathway. Finally, cells overexpressing δ-opioid receptors developed characteristically long but undirected protrusions containing filamentous actin and δ-opioid receptors, indicating an enhanced migratory phenotype.
CONCLUSION AND IMPLICATIONS
Opioid receptors affect intercellular adhesion and wound healing mechanisms, underlining the importance of a cutaneous neuroendocrine system in wound healing and skin homeostasis.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Keywords: δ-opioid receptor, intercellular adhesion, migration, wound healing
Introduction
Epidermal integrity relies upon the adhesion of epithelial cell sheets, which is mediated by multi-protein complexes called desmosomes (Brooke et al., 2012). Desmosomes contain plakoglobins and plakophilins, armadillo proteins including β-catenin (β-cat), and the transmembrane cadherin proteins desmogleins (DSGs) as well as desmocollins (Dusek et al., 2007; Desai et al., 2009). While anchoring epithelial cells together during homeostasis, desmosomes must also adjust to the changes needed for wound healing. Controlled weakening of desmosome junctions is required to allow transition of wound-edge keratinocytes from a hyper-adhesive to a migratory state (Wallis et al., 2000), which is characterized by the formation of leading-edge actin filament protrusions (Kira et al., 2002; Kirfel et al., 2004; Beaudry et al., 2010). Careful modulation of desmosome junctions is thus critical for effective wound healing, and there is substantial evidence that PKC has a role in regulating this process (Osada et al., 1997; Denning et al., 1998; Mascia et al., 2012). Activation of PKCα results in the translocation of desmosomal β-cat from the cell membrane to the cytoplasm (Wallis et al., 2000; Garrod et al., 2005). Alterations in the phosphorylation of desmosomal proteins including desmoplakin (DSP), resulting in impaired assembly of keratin intermediate filaments into desmosomes, have been linked to PKC α (Green and Simpson, 2007). Accordingly, the PKC α/β inhibitor Gö6976 promotes cell clustering and restores desmosome formation (Koivunen et al., 2004).
Alongside the regulation of desmosomes by PKC, GPCRs have also been recognized for their role in cellular processes such as cell migration (Cotton and Claing, 2009). The δ-opioid receptor (see Alexander et al., 2013), belonging to the family of GPCRs, has been reported to affect keratinocyte migration during wound healing (Bigliardi-Qi et al., 2006). Mice lacking δ-opioid receptors exhibit delayed wound healing and hypertrophic wound margins (Bigliardi-Qi et al., 2006; Bigliardi et al., 2009). While the importance of δ-opioid receptors is increasingly recognized, the mechanisms underlying its effects on keratinocytes are largely undefined.
In the work described here, the effect of δ-opioid receptors on the expression pattern of several proteins of the desmosomal junction complex and on the migratory behaviour of keratinocytes was examined. The endogenous δ-opioid receptor ligand Met-enkephalin (Met-Enk) was used to activate δ-opioid receptors, and the downstream signalling that mediates δ-opioid receptor-controlled migration was elucidated. Following δ-opioid receptor activation, marked changes in cell morphology of keratinocytes were observed. The data revealed that δ-opioid receptors profoundly affect intercellular adhesion and migration of human keratinocytes in vitro, which accounts for the delayed wound healing in δ-opioid receptor (DOPr)–/– mice and enhanced wound recovery in human keratinocyte monolayers overexpressing δ-opioid receptors in vitro.
Methods
Animals
A total of four male δ-opioid receptor wild type (WT) and four male DOPr–/– mice were tested in the experimental set-up. The use and generation of mice lacking δ-opioid receptors was as previously described (Filliol et al., 2000; Bigliardi-Qi et al., 2006). Mice were fully backcrossed in a pure C56BL/6J genetic background. All animals used were originated from the same breeding series and were about 10 months old. Mice were housed 2–5 per cage in a temperature (21 ± 2°C) and humidity (55 ± 10%) controlled room with a 12 h light : 12 h dark cycle (lights on between 08:00 and 20:00 h). Food and water were available ad libitum. Mice were habituated to their new experimental environment in individual cages and handled for 10 days before starting the experiments. The mice were shaved over the rostral part of the back 8 days before the start of the experiment. Finally, the mice were killed and biopsies were taken and fixed in 4% formaldehyde. All experimental procedures and animal husbandry were conducted according to standard ethical guidelines (European Community Guidelines on the Care and Use of Laboratory Animals 86/609/EEC) and approved by the local ethical committee. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animal (Kilkenny et al., 2010; McGrath et al., 2010).
Antibodies
The following antibodies were used: rabbit polyclonal anti-phospho-PKC α/β II (Thr638/641) (Cell Signaling, Singapore) and monoclonal mouse anti-α-tubulin (Sigma Aldrich, Singapore) were for immunoblotting. Mouse anti-β-cat antibody AA 571-781 (BD Transduction Laboratories, Singapore) was used for both immunoblot and immunofluorescence staining. Rabbit monoclonal anti-DSG1 [(EPR6766(B)] and rabbit polyclonal DSG4 (both Abcam, Singapore) were used for immunofluorescence staining in mouse tissue sections. Mouse monoclonal anti-DSP was kindly provided by D. Garrod, University of Manchester, UK (Parrish et al., 1987). Rabbit anti-GFP (Abcam) and mouse anti-integrin β1 antibody clone N29 (Chemicon, Billerica, MA, USA) were used for immunofluorescence in cells. Secondary antibodies for immunoblot were anti-rabbit IRDye 700DX and anti-mouse IRDye 800 (Rockland, Gilbertsville, PA, USA). Secondary antibodies for immunofluorescence include: goat anti-rabbit and goat anti-mouse conjugated to fluorophores Alexa Fluor 488 and Alexa Fluor 594 (Molecular Probes, Life Technologies, Singapore). Hoechst dye (Bisbenzimide H33258) was used for nuclear staining (Sigma-Aldrich, Lausanne, Switzerland). Fluorophore-conjugated Alexa Fluor 594 phalloidin (Molecular Probes, Life Technologies) was used to label F-actin and fluorophore-conjugated Alexa Fluor 647 wheat-germ agglutinin (Molecular Probes, Life Technologies) was used to label the plasma membrane.
Chemical reagents
[Methionine]enkephalin (Met-Enk) was obtained from Sigma-Aldrich and dissolved in water to form a 1 mM stock solution. A concentration of 10 nM was used for drug treatment. Naltrindole (NTI) was obtained from Tocris Biosciences (Singapore) and dissolved in water at 25 mM.The final concentration used for drug treatment was10 μM. The PKC α/β inhibitor Gö6976 was obtained from Merck Chemicals (Singapore) in an anhydrous DMSO solution with a stock concentration of 0.5 mg·mL–1. The ERK inhibitor PD98059 was obtained from Promega (Singapore) and dissolved in DMSO to form a 20 mM stock solution. For the in vitro migration assay, both 50 ng·mL–1 Gö6976 and 20 μM PD98059 were added 15 min before drug treatment. For all other inhibition experiments, identical concentrations of Gö6976 and PD98059 were added 1 h before drug treatment. All of the control reactions were done with the same concentrations of DMSO as used in the drug treatments.
Cell culture
Human skin keratinocytes N/-TERT-1 were obtained and cultured as described by the Rheinwald Laboratory (Dickson et al., 2000; Rheinwald et al., 2002). Keratinocytes were used at 80–90% confluence for all experiments except for viral transduction. Human 293Ta cells were used during lentiviral production as packaging cell line and were cultured in Gibco DMEM high glucose, GlutaMAX™ media with 10% FBS (JR Scientific, Woodland, CA, USA), 1% MEM non-essential amino acids (NEAA) solution and sodium pyruvate (Gibco, Life Technologies, Singapore).
Plasmid purification, transfection and viral transduction
The open reading frame cDNA lentiviral vector for GFP-tagged δ-opioid receptors (pEZ-Lv122, GeneCopoeia, Rockville, MD, USA) was inoculated and purified using the standard protocol of the Qiagen Plasmid Maxi Kit (Singapore). 293Ta cells were transfected with 1.5 μg of purified δ-opioid receptor plasmid and 1 μg of human lentiviral packaging vectors (psPax, pMD2.G, a kind gift from C. Widmann) using Qiagen Effectene Transfection Reagent (Singapore), according to a standard protocol. Seventy-two hours post-transfection, supernatants containing viral recombinants were harvested, filtered and concentrated using Beckman Ultracentrifuge at 366.66 Hz for 2 h as described by Barde et al. (2008). For viral titration N/TERT-1, target cells were transduced with a serial dilution of concentrated virus. After 72 h of incubation, cells were fixed and analysed by flow cytometry. The titre was calculated based on dilutions yielding 1–20% of GFP positive cells. For experiments, cells were transduced with 5 μL of virus stock (multiplicity of infection of 10) for 48 h and δ-opioid receptor overexpression confirmed by quantitative real-time (RT) PCR.
RNA extraction and RT PCR
Total RNA was extracted using RNeasy Kit (Qiagen) according to manufacturer's protocol. Total RNA (400 ng) was reverse transcribed into cDNA in a 10 μL reaction using PrimeScript RT Reagent Kit (Takara, Japan). The resulting cDNA was diluted to obtain a concentration of 20 ng·μL cDNA.
Quantitative RT PCR
For the quantification of target genes, 100 ng of cDNA template per reaction was amplified in the presence of SYBR green master mix and specific QuantiTect primers for DSG1 and DSG4 (Qiagen). Amplification was measured by Stratagene Mx3005P. Target gene expression was normalized based on the values of the expression of RPL13A (forward primer 5′-CTC AAG GTC GTG CGT CTG AA −3′ and reverse primer 5′-TGG CTG TCA CTG CCT GGT ACT-3′). Quantification was performed using the comparative 2-ΔΔCT Method. The experiment was repeated five times with similar results.
Immunoblotting
Cells were seeded in six-well plates and subjected to treatments as indicated in the figures before lysis in RIPA-like lysis buffer, comprising 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, 1% IGEPAL CA-630, 0.1% SDS, 0.5% sodium deoxycholic acid at pH 7.4, supplemented with 1 mM DTT, 1 mM PMSF, 10 mM Na3VO4 and 1× protease inhibitor (Roche Reference No. 11697498001). Cell lysates were quantified by use of the Bradford assay and analysed for protein expression by applying 50 μg of protein extract from each lysate. Blots were blocked for 1 h with 5% BSA. Specific primary antibodies were diluted at 1:1000 in TBS/0.1% Tween 20/5% BSA. IRDyes 700DX and 800 were used as secondary antibodies. Protein expressions were visualized and measured by LI-COR Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Integrated intensity results from quantification were exported as Microsoft Excel template for calculations and relative PKC phosphorylation was calculated by normalization to the respective α-tubulin control.
Immunofluorescence staining
Mouse skin biopsies were fixed with 4% paraformaldehyde for 48 h, embedded in paraffin blocks and 7 μm sections obtained. Sections were deparaffinized in xylene, rehydrated in decreasing concentrations of ethanol, followed by heat-induced antigen retrieval at pH 6 for 20 min and subsequent cooling for 1 h. For immunocytochemistry, cells were seeded at low density on glass coverslips and incubated for 3–4 days. Upon harvest, cells were washed with PBS, fixed for 15 min in 4% paraformaldehyde, permeabilized with TBS/0.2% Triton-X and blocked for 1 h in TBS/10% goat serum (Dako, Singapore) at room temperature. For paraformaldehyde-fixed cells labelled for plasma membrane, Alexa Fluor 647-conjugated wheat germ agglutinin (WGA) was added at 1:200 for 10 min before permeabilization. Alexa Fluor 594-conjugated phalloidin was used at 1:100 dilution and incubated for 1 h at room temperature. All other specific primary antibodies were diluted at 1:100 in TBS/10% goat serum and incubated overnight at 4°C. All secondary antibodies were diluted in TBS/10% goat serum at 1:400 and incubated in the dark for 1 h at room temperature. Nuclei were counterstained using 1 μg·mL Hoechst dye in TBS for 5 min. Both skin sections and coverslips were mounted using Dako fluorescence mounting medium with samples imaged using a confocal microscope (Olympus Upright FV1000, Olympus, Tokyo, Japan).
Skin sections were captured using 20×/0.75 Plan Apo objectives, with optical sections of 1.1 μm thickness scanned through the z-plane. Images were taken from at least six random fields of each sample and quantified by ImageJ software (NIH, Bethesda, MD, USA). Labelled cells were imaged with both 60×/1.42 Plan Apo Oil and 100×/1.4 Plan Apo Oil objectives with optical sections of 0.45 μm scanned through the z-plane. For PKC inhibitor experiments, only XY-plane was taken. XZ-planes were obtained by using ortho-slicing function in IMARIS analysis software under IMB license (Bitplane, Zurich, Switzerland). Quantification of β1 integrin (ITGB1) was performed by measuring integrated intensity per field of view after average projection of z-stacks. β-cat staining was manually quantified by drawing a five-pixel thick line along the membranes measuring integrated intensity of sum-projected z-stacks. The obtained values from the ImageJ analysis were exported and statistically analysed using GraphPad Prism5 (GraphPad Software Inc., San Diego, CA, USA).
In vitro migration assay
In an attempt to create a clean wound gap between cells, Ibidi self-culture inserts (Ibidi, Martinsried, Germany) were used. About 20 000 cells were seeded on each side of the insert and incubated for 48 h. The cells were then placed at 37°C, 5% CO2, on a Nikon Eclipse TI microscope (Nikon, Tokyo, Japan). Images were acquired with a 10×/0.3 Plan Fluor phase contrast objective every 15 min for 9 h. The stage positions of each experiment condition were determined manually using MetaMorph and up to six different regions of interest were sequentially recorded during each experiment using an automated stage. Area of wound recovery at a fixed time point and area percentage of wound recovery over the total time course were analysed using ImageJ and exported as Microsoft Excel template for calculation. For normal drug treatments, cells were treated 5 min before imaging and 15 min prior for inhibitor experiments.
Data analysis
The results are expressed as mean ± SEM. Comparison between different treatment groups in the DSG1 qPCR was done using anova with Newman–Keuls post hoc test. Due to unequal variances between experimental groups, one representative experiment is shown. Migration assays and quantification of immunofluorescence staining were carried out using anova followed by Newman–Keuls post hoc test. Quantifications of phosphorylated PKCα were analysed using anova followed by Bonferroni post hoc test. A P-value ≤ 0.05 was considered statistically significant.
Results
Levels of DSGs are inversely correlated with δ-opioid receptors
Sections of non-wounded epidermis from DOPr–/– mice were analysed for DSG1 and DSG4 expression and localization. Image quantification of DSG staining in DOPr–/– mice showed significantly more DSG1 and DSG4 expression in the epidermis than in WT animals (Figure 1A,B). A complementary mRNA expression pattern was observed in δ-opioid receptor -GFP-overexpressing (δ-opioid receptor-OE) human N/TERT-1 keratinocytes after δ-opioid receptor activation by Met-Enk: DSG1 as well as DSG4 mRNA levels were repressed after Met-Enk addition as compared with GFP-expressing controls, but the DSG1 level was increased by addition of the specific δ-opioid receptor antagonist NTI (Figure 1C), whereas the DSG4 level was further repressed by NTI. Together, these results suggest that δ-opioid receptor activation may affect desmosomal integrity by alterating the levels of desmosomal proteins.
Figure 1.
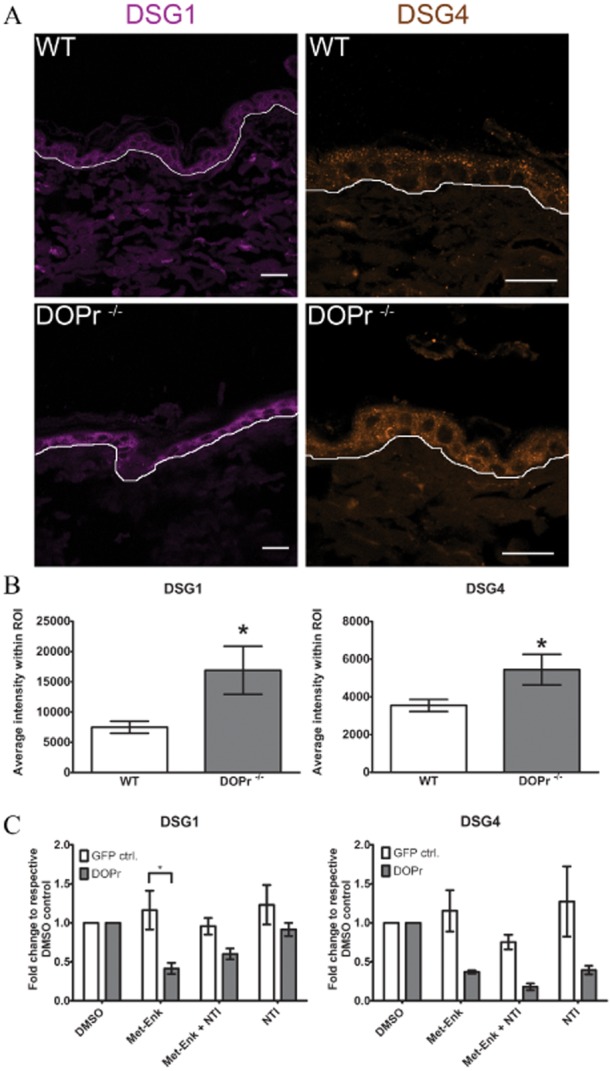
The δ-opioid receptor reduces the expression of desmogleins 1 and 4. (A) Representative images of DSG1 and DSG4 immnunofluorescence staining of non-wounded epidermis from WT and DOPr–/– mice. Scale bar 20 μm. (B) Quantification of DSG1 and DSG4 fluorescence intensity at the epidermis (region of interest). Results are represented as mean ± SEM of 4 mice per group and 5 images per individual mouse (n = 20). (c) Quantitative real-time PCR measured expression of DSG1 and DSG4 mRNA in DOPr-OE N/TERT-1 cells treated with Met-Enk for 12 h (+/− antagonist NTI 15 min prior to Met-Enk). Values are normalized to respective DMSO controls and represent the mean ± SEM of one representative experiment. One-way anova with Newman–Keuls post hoc test, *P ≤ 0.05.
To further characterize changes in desmosome morphology control, δ-opioid receptor-OE cells were treated with Met-Enk for 12 h before immunofluorescence staining of the desmosomal plaque protein DSP was carried out. DSP in Met-Enk-treated δ-opioid receptor-OE cells appeared punctate at the cell periphery where cell-cell contact is weakened. In contrast, control cells showed linear arrays of DSP emanating from cell-cell border (Figure 2), consistent with mature desmosome formation (Bass-Zubek et al., 2008). Additionally, β-cat staining was reduced at cell-cell borders of δ-opioid receptor-OE cells under all treatment conditions as compared with control cells (Supporting Information Fig. S1C,D). The quantity of β-cat protein was unchanged (Supporting Information Fig. S1A,B), indicating rather a redistribution of β-cat due to weakened cell-cell adhesion upon OE of δ-opioid receptors.
Figure 2.
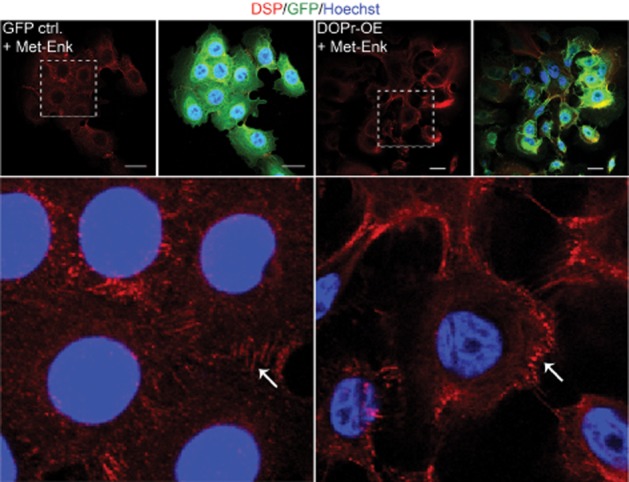
The δ-opioid receptor (DOPr) system alters expression patterns of intercellular desmosomal plaque proteins in N/TERT-1 keratinocytes. Representative confocal images of Met-Enk-treated GFP control and DOPr-OE N/TERT-1 cells labelled with antibodies recognizing fluorescent tag GFP (green), DSP (red), with Hoechst 33258 nuclear counterstain (blue). Scale bar 20 μm. Lower panel: magnified image of indicated regions showing difference in DSP localization. White arrows indicate linear arrays of DSP labelling on the left panel, compared with punctate distribution on the right.
The expression of β1 integrin is altered by activation of δ-opioid receptors
Cell motility is a product of changes in both the intracellular cytoskeleton and cell adhesion. Integrins mediate cell-substrate attachment and are associated with focal adhesion proteins, including the focal adhesion kinases. Mice lacking the ITGB1 subunit exhibit deficient epidermal integrity and skin blistering, suggesting a role for ITGB1 in wound healing. This led us to examine the effect of δ-opioid receptor -mediated signalling on ITGB1 in keratinocytes. Control and δ-opioid receptor-OE cells were treated for 1 h either with the endogenous δ-opioid receptor agonist Met-Enk or specific δ-opioid receptor antagonist NTI before analysis of ITGB1 expression. Activation of δ-opioid receptors by Met-Enk revealed an increased staining of ITGB1 mainly due to increased localization at the cell-matrix interface (Figure 3A, XZ-plane, second row). Quantification of the overall intensity of ITGB1 staining shows a significant increase in ITGB1 in δ-opioid receptor-OE cells treated with Met-Enk as compared with vehicle control cells and GFP control cells. NTI partially blocked this increased expression (Figure 3B). Furthermore, cell-cell adhesion was decreased in δ-opioid receptor-OE cells, resulting in reduced staining of ITGB1 at the cell membrane, which is visible in the XZ-plane images. NTI treatment reversed this δ-opioid receptor-mediated effect and increased cell-cell staining of ITGB1 (Figure 3A, XZ-plane, third row).
Figure 3.
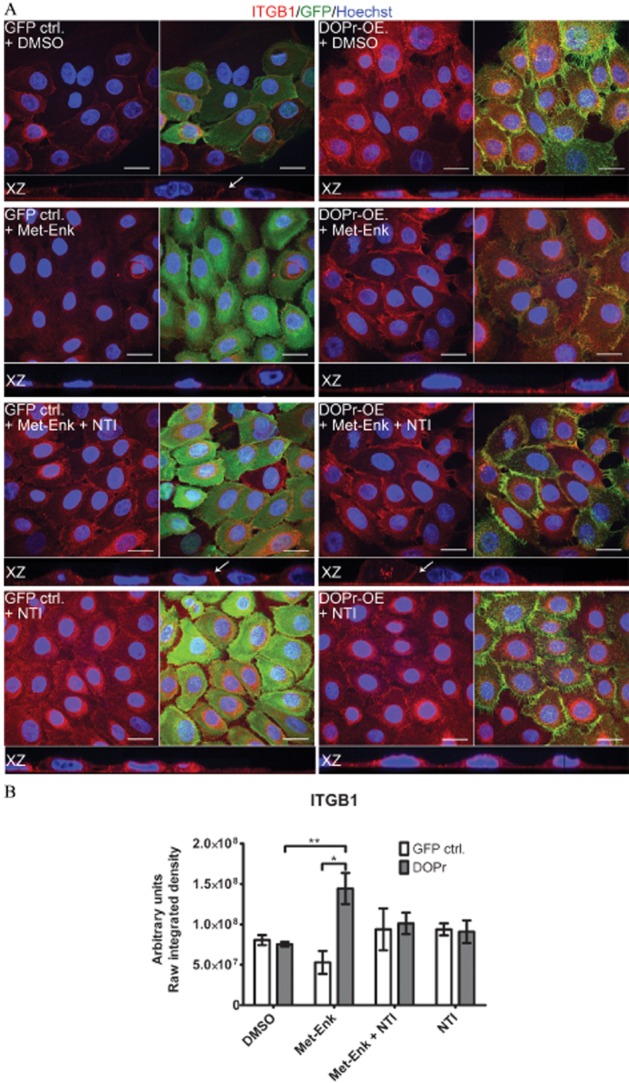
δ-opioid receptor (DOPr) activation causes changes in β1 integrin (ITGB1) expression and distribution that can be partially reversed by δ-opioid receptor antagonists. (A) Confocal images of Met-Enk-treated GFP control and DOPr-OE N/TERT-1 cells labelled with antibodies recognizing GFP fluorescence tag (green), and ITGB1 (red), with Hoechst 33258 nuclear counterstain (blue). Orthogonal slicing of XZ-plane images is shown. Scale bar 20 μm. (B) Quantification of ITGB1 integrated intensity per image from average z-projected images. The results are expressed as means ± SEM of two independent experiments with two images taken per condition. Statistical comparison was done by one-way anova with Newman–Keuls post hoc test. *P ≤ 0.05 and **P ≤ 0.01.
Activation of the δ-opioid receptor increases the migration of keratinocytes and promotes in vitro wound healing through PKCα-dependent pathways
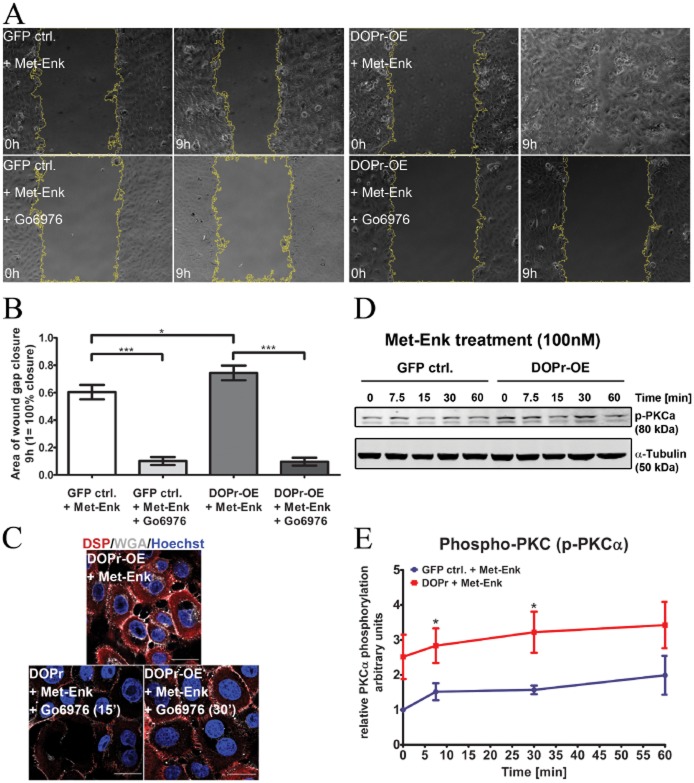
Loosening of intercellular adhesions and enhanced cell-matrix adhesion are required for cell motility (Pilcher et al., 1998; Gill and Parks, 2008; Wen et al., 2010). We performed in vitro scratch assays to examine whether loss of cell-cell contact through δ-opioid receptor activation enhanced keratinocyte migration. Control and δ-opioid receptor-OE cells were seeded into culture dishes with an inserted separation and grown to confluence. Removal of the inserted separation before imaging introduced a wound gap in the cell layer, and the area of the wound gap was recorded at 15 min intervals for 9 h. The results showed that, regardless of Met-Enk treatment, δ-opioid receptor-OE keratinocytes had significantly smaller areas of wound gap remaining (Figure 4A,B), indicating an innately faster cell migration phenotype. An obvious difference in cell migration over time can be seen between control and δ-opioid receptor-OE cells (Figure 4C), significantly as of 3 h migration. This demonstration of active migratory behaviour in δ-opioid receptor-OE keratinocytes confirms and provides functional evidence for δ-opioid receptor-mediated cell migration.
Figure 4.
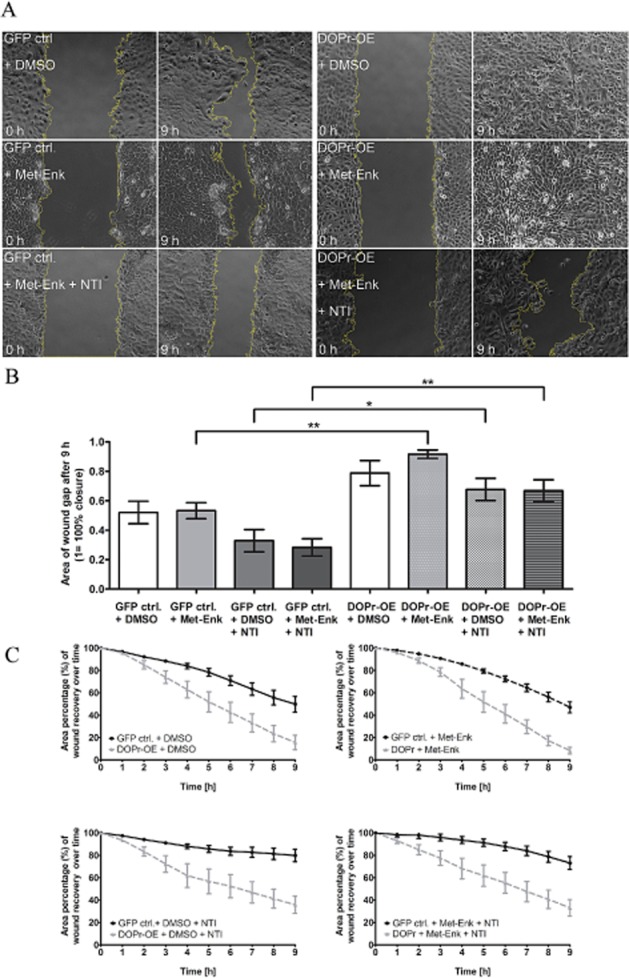
Activation of δ-opioid receptors (DOPr) by Met-Enk enhances keratinocyte migration. (A) Time-lapse microscopy over 9 h of in vitro wound healing using GFP control and DOPr-OE N/TERT-1 cells. Panels show representative images of the keratinocyte monolayer at the gap, at 0 and 9 h post-wounding. Graphs depict quantified average area (B) of gap closure at 9 h (1 = 100% closure) and percentage (C) of gap closure (gap at time 0 = 100%) over time. Bar chart shows mean ± SEM (n = 11 fields of view from three independent experiments) for all groups (one-way anova, with Newman–Keuls multiple comparison test, *P ≤ 0.05 and **P ≤ 0.01).
The involvement of classical PKC and ERK/MAPK pathways in cell migration is well established (Koivunen et al., 2004; Matsubayashi et al., 2004; Charbaji et al., 2012); however, initiation of PKC signalling by δ-opioid receptor activation influencing migration has not been extensively studied. Several isoenzymes of PKC with varying functions exist (Parker and Murray-Rust, 2004). Many studies have highlighted the involvement of PKCα/β in desmosomal adhesion (Wallis et al., 2000). As such, PKCα/β emerged as a good candidate to study the effect of cell migration attributed to a loss in intercellular adhesion. To investigate whether PKCα/β plays a role in δ-opioid receptor-mediated migration, we used the specific PKC inhibitor, Gö6976. Cells were pretreated with Gö6976 for 15 min before addition of the endogenous δ-opioid receptor ligand Met-Enk and monitoring of wound closure during 9 h of scratch-wound healing assay. The δ-opioid receptor-OE cells migrated faster than controls, (Figure 5A, upper panel; Figure 5B). Exposure to Gö6976 significantly impeded migration for both Met-Enk-treated control and δ-opioid receptor-OE cells (Figure 5A, lower panel; Figure 5B). Similar results were observed with the ERK inhibitor PD98059 (Supporting Information Fig. S2A,B).
Figure 5.
δ-opioid receptor (DOPr)-stimulated migration and accelerated in vitro wound healing require PKC α activation. (A) Time-lapse microscopy of in vitro wound healing using GFP control and DOPr-OE N/TERT-1 cells treated with Met-Enk and PKC α/β inhibitor Gö6976 (+/−). Representative images of the gap in the keratinocyte monolayer at the start of migration (0 h) and after 9 h. (B) Quantified average mean ± SEM of area wound closure (1 = 100%) for all groups from three independent experiments (one-way anova, with Newman–Keuls multiple comparison test, **P ≤ 0.05 and **P ≤ 0.01). (C) Immunolabelling of DSP (red) and plasma membrane marker WGA67 (grey) with Hoechst 33258 nuclear staining (blue) showed differences in cell clustering. Scale bar 20 μm. (D) Representative immunoblot and (E) graph showing increased phosphorylated PKCα/β expression in DOPr-OE N/TERT-1 cells (+Met-Enk) compared with GFP control cells. PKC phosphorylation was normalized to the respective tubulin expression. Graph shows mean ± SEM (n = 6 independent experiments) (two-way anova with Bonferroni post hoc test, *P ≤ 0.05).
To affirm our observations that the difference in migration was due to changes in intercellular adhesion among cells upon PKCα/β activation in δ-opioid receptor-OE keratinocytes, we used Alexa Fluor 647-conjugated WGA647 to visualize the plasma membrane. As previously observed (Figures 2 and 3), δ-opioid receptor-OE cells exposed to Met-Enk displayed loosely organized cell-cell connection morphology with cytoplasmic DSP staining (Figure 5C). However, 15 min pretreatment of δ-opioid receptor-OE cells with Gö6976 before Met-Enk treatment resulted in compact cell colonies with stronger cell-cell adhesion with DSP labelling showing tiny adherent junctions at neighbouring cell borders. A longer pretreatment of Gö6976 for 30 min resulted in the formation of robust interconnections between cells (Figure 5C, lower panel), resembling those of mature desmosome formation. The cell-cell connections seemed to be stabilized by PKCα/β inhibition, and it is possible that this maintenance of strong cell-cell connections prolongs closure of the wound gap in vitro. This implies that activation of PKCα/β is a possible downstream signalling target of δ-opioid receptor activation. Immunoblotting analysis for PKCα/β phosphorylation over a time course of Met-Enk incubation showed significantly increased phosphorylation of PKCα/β in δ-opioid receptor-OE cells (Figure 5D,E). At both 5 and 30 min, more phospho-PKCα is present in δ-opioid receptor-OE cells activated with Met-Enk as compared with control cells. This suggests that δ-opioid receptor-OE keratinocytes have an innately higher level of phosphorylated PKCα/β, correlating with their enhanced migration even when treated with vehicle DMSO only (Figure 4A,B).
Overexpression of δ-opioid receptors in keratinocytes enhances formation of membrane protrusions
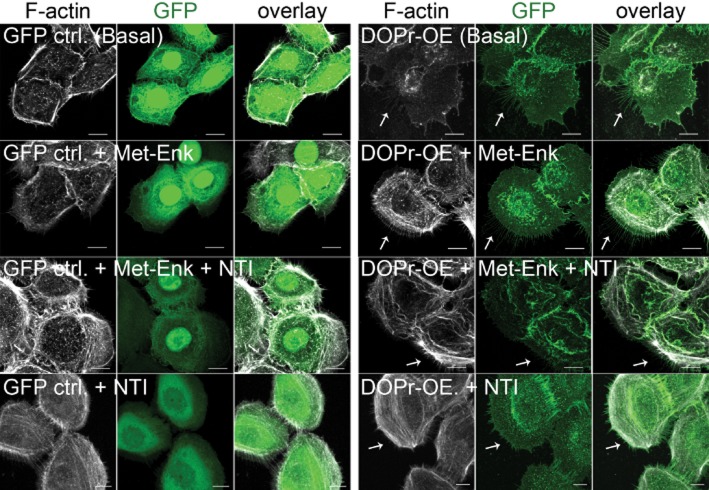
The migratory phenotype of wound-edge keratinocytes is characterized by the formation of leading-edge actin filament protrusions (Abercrombie et al., 1970; Imamura et al., 1999; Deramaudt et al., 2011), and so we determined whether δ-opioid receptors also affect this process. Close examination highlighted the presence of fine protrusions around the free edge of δ-opioid receptor-OE keratinocytes, resembling filopodia or retraction fibres. Visualization of filamentous actin through live cell imaging (Supporting Information Fig. S3A) and the immunofluorescence labelling with Alexa Fluor 594-conjugated phalloidin revealed staining at the free edge of δ-opioid receptor-OE cells and that these cells possessed longer filamentous actin compared with controls (Figure 6, first and second rows). Blocking δ-opioid receptor activation with the δ-opioid receptor antagonist NTI before the addition of Met-Enk had little or no effect on control cells (Figure 6, third row from top), but profoundly inhibited the formation of membrane protrusions in δ-opioid receptor-OE cells (Figure 6, third and fourth rows). Interestingly, these fine protrusions were found to leave behind trailing traces (Supporting Information Fig. S3B) near the cell-matrix surface. Together, these data led us to hypothesize that δ-opioid receptor expression enhances migration, at least in part, through promoting the formation of fine actin-containing protrusions.
Figure 6.
Morphological changes in vitro in δ-opioid receptor (DOPr)-OE (overexpressing) N/TERT-1 keratinocytes. Confocal images of GFP control and DOPr-OE N/TERT-1 cells labelled with anti-GFP antibody (green) and Alexa 594-conjugated phalloidin (grey). GFP control N/-TERT-1 cells generally do not display fine protrusions while obvious fine protrusions containing F-actin at the rear of DOPr-OE N/TERT-1 cells were observed (white arrows). Fewer protrusions were observed when DOPr N/TERT-1 cells were treated with DOPr antagonist NTI. Scale bar 10 μm.
Discussion and conclusions
Opioid receptors in the nervous system are predominantly located at synapses (Olive et al., 1997; Kieffer and Evans, 2009), and we observed δ-opioid receptors in keratinocytes preferentially accumulating at cell junctions, which would similarly facilitate directed intercellular signal transmission. Much speculation has surrounded the functional role of opioidergic signalling in keratinocytes (Wolf et al., 2009; Kuchler et al., 2010); effects on migration and wound healing have been observed in vitro and in mice in vivo (Bigliardi et al., 2002; Bigliardi-Qi et al., 2006; Wolf et al., 2009; Dai et al., 2010), but the pathways leading to such effects have not been analysed. Here, we study δ-opioid receptor-mediated alterations in cell-cell adhesion as a first step in the wound healing process.
DSGs are the transmembrane-anchoring components of desmosomal cell-cell junctions, and we found that DSG1 and DSG4 proteins were significantly more abundant in non-wounded epidermis of DOPr–/– mice compared with WT. Conversely, activation of the δ-opioid receptor results in reduced DSG expression, predicting loosening of cell-cell adhesion and so facilitating the change from stationary to migratory keratinocyte. Although both DSG1 and DSG4 are expressed at suprabasal epidermis, DSG4 is also strongly expressed in hair follicles (Kljuic et al., 2003). NTI seems to work as a more conventional antagonist in Met-Enk-regulated DSG1 expression, but not in Met-Enk-regulated DSG4 expression, which indicates additional regulatory mechanisms. Analysis of desmosomal plaque proteins revealed a redistribution of β-cat and DSP localization in δ-opioid receptor-activated cells, indicating reorganization of the desmosomal cell-cell adhesion complex in order to promote cell detachment and migration. In addition, the integrin-mediated focal adhesion complex was similarly influenced by the δ-opioid receptor. The connection between integrins and actins drives cell migration (Vicente-Manzanares et al., 2009), and ITGB1 in particular is thought to be important for keratinocyte migration (Kirfel et al., 2003; 2004). ITGB1 in δ-opioid receptor-OE cells activated by Met-Enk was increased in areas of cell-matrix adhesion. This suggests a connection of cytoskeleton and extracellular matrix mechanics to adhesion-dependent motility with the consequence of enhanced motility in δ-opioid receptor-activated cells. While the role of ITGB1 is more focused on cell-matrix adhesion, little is known about its involvement in cell-cell adhesion. Weitzman et al. (1995) reported no direct evidence of cell-cell adhesion in cells expressing β1 subunits. However, Martinez-Rico et al. (2010) demonstrated more recently some crosstalk between integrins and cadherins. We observed clear cell-cell border localization of ITGB1 in control keratinocytes, which was only replicated in δ-opioid receptor-OE keratinocytes in the presence of the δ-opioid receptor inhibitor NTI. δ-opioid receptor activation has previously been shown to be capable of triggering integrin signalling (Pello et al., 2006; Berg et al., 2007; Eisinger and Ammer, 2008a; b) and could explain our observation of redistribution of ITGB1 to cell-matrix regions.
These profound changes in protein expression and redistribution of cell adhesion components following δ-opioid receptor activation predicted a strong migratory phenotype, and δ-opioid receptor signalling promoted keratinocyte migration and significantly accelerated wound healing in vitro (Figure 4). Due to the short observation time of keratinocyte migration for 9 h, the enhancing effect of proliferation on wound healing can be ignored. Additionally, no increase in proliferation of δ-opioid receptor-OE cells as compared with control cells is observed (unpublished data). In order to better understand the underlying mechanisms of δ-opioid receptor-mediated effects on keratinocytes, we examined the PKC signalling pathway. We focused on the PKCα isoenzyme because Ng et al. (1999) linked ITGB1 re-localization with that of activated PKCα; findings reminiscent of our observed re-localization due to δ-opioid receptor activation. This suggested the possibility of a common link with δ-opioid receptors. Indeed, δ-opioid receptor activation led to a higher level of phosphorylated PKCα, in parallel with cell detachment, while inhibition of PKCα activation markedly reduced cell detachment. Consequently, keratinocyte migration could successfully be manipulated with PKC inhibition, confirming that δ-opioid receptor-mediated migration is PKC-dependent.
The morphological changes in δ-opioid receptor-OE cells are indicative of a switch to a migratory keratinocyte phenotype. We observed fine protrusions through live cell imaging and in fixed confocal images. These protrusions were frequently seen near the cell matrix and sometimes left detaching trailing traces (Supporting Information Fig. S3B). Based on these collective observations, we propose that these protrusions are filopodia. Filopodia are dynamic structures of varying size, growth and retraction velocity (Husainy et al., 2010), whose main role is initiating migration. It has been shown that depletion of capping proteins resulted in a strongly increased formation of filopodia (Mejillano et al., 2004). δ-opioid receptor activation might be involved in the regulation of these proteins, therefore, influencing the cytoskeletal architecture. However, the phenotype variation makes definitive identification challenging, and further characterization of the observed fine protrusions in δ-opioid receptor-OE keratinocytes is required.
In summary, our results show that activation of δ-opioid receptors destabilizes intercellular adhesion and promotes the migratory keratinocyte phenotype, which is required for fast wound closure. These observations have clinical implications. The release of opioid peptides at wounding sites (Schafer, 2003; Cavallini and Casati, 2004) indicates a contributive role of the opioid receptor system to the earliest phases of wound healing in the seconds and minutes following injury. Our findings provide a mechanism by which peptide activation of the δ-opioid receptor induces mobilization of keratinocytes through promoting intercellular dissociation and altering the migration pattern of these skin cells. Understanding the role of opioids and specifically the role of the δ-opioid receptor system in wound healing will permit rational investigation of the optimal means by which to exploit the opioid receptor pathways for analgesia and improved wound healing in the clinic.
Acknowledgments
This work was supported by the funds from the Singapore Biomedical Research Council of the Singapore Agency for Science, Technology and Research (A*STAR). The skin samples from DOPr knockout mice are the mice used in previous publications (Bigliardi-Qi et al., 2006), and therefore we want to acknowledge the importance for the support and help of Professor Brigitte Kieffer and Professor Gavériaux-Ruff by allowing us access to these mice. In addition, we want to thank Dr Graham Wright, John Lim and the IMB Microscopy Unit.
Glossary
Abbreviations
- β-cat
β-catenin
- DSG
desmoglein
- DSP
desmoplakin
- ITGB1
β1 integrin
- Met-Enk
Met-enkephalin
- NTI
naltrindole
- OE
overexpression
- WT
wild type
Author contributions
PLB, CN, YT and MB conceived and designed the experiments. YT, CN and AP performed the experiments. CN, YT, AP, MB and PLB analysed the data. YT, CN, MB and PLB wrote the paper.
Conflict of interest
The authors have declared that no conflict of interest exists.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12687
Figure S1 β-catenin distribution is affected by δ-opioid receptor (DOPr) expression. (a) Representative images of β-cat staining in δ-opioid receptor and control cells subjected to respective treatments. Prominent localization of β-cat at the membrane of control cells is observed while DOPr-OE cells show reduced membrane localization. (b) Manual quantification of cell-cell border staining after sum projection of z-stack images reveals significantly decreased staining in all DOPr-OE cultures regardless of drug treatment. (c) Immunoblot and (d) graph of quantification reveal no changes in β-catenin expression regardless of treatment as well as in both GFP control and DOPr overexpressing N/TERT-1s. Values shown are normalized values to loading control α-tubulin and then to the respective vehicle (DMSO) only control. Values represent the mean ± SEM of four independent N/TERT cultures (n = 4).
Figure S2 δ-opioid receptor (DOPr)-stimulated migration and accelerated in vitro wound healing can be alternately influenced by ERK activation. (a) Time-lapse microscopy of in vitro wound healing using GFP control and DOPr-OE N/TERT-1 treated with Met-Enk and ERK 1/2 inhibitor PD98059 (+/−). Representative images of the gap in the keratinocyte monolayer at the start of migration (0 h) and after 6 h. (B) Quantified mean ± SEM of area wound closure (1 = 100%) for all groups.
Figure S3 δ-opioid receptor (DOPr)-overexpressing N/TERTs exhibit dendritic-like protrusions. (a) Fluorescence time-lapse image of DOPr N/TERTs at basal state was taken at every 5 min interval for 1 h and viewed using pseudo-colour scheme to facilitate visualizations of the protrusion. (B) Representative confocal image at 100× magnification of DOPr-OE N/TERT-1 at basal state shows long and fine protrusions at the cell periphery. DOPr-OE N/TERT-1 was stained with anti-GFP antibody.
References
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res. 1970;59:393–398. doi: 10.1016/0014-4827(70)90646-4. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-couple receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde I, Salmon P, Trono D. Production and titration of lentiviral vectors. Curr Protoc Neurosci. 2010 doi: 10.1002/0471142301.ns0421s53. Chapter 4:Unit 4.21. doi: 10.1002/0471142301.ns0421s53. [DOI] [PubMed] [Google Scholar]
- Bass-Zubek AE, Hobbs RP, Amargo EV, Garcia NJ, Hsieh SN, Chen X, et al. Plakophilin 2: a critical scaffold for PKC alpha that regulates intercellular junction assembly. J Cell Biol. 2008;181:605–613. doi: 10.1083/jcb.200712133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry VG, Ihrie RA, Jacobs SB, Nguyen B, Pathak N, Park E, et al. Loss of the desmosomal component perp impairs wound healing in vivo. Dermatol Res Pract. 2010 doi: 10.1155/2010/759731. 2010: 759731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Zardeneta G, Hargreaves KM, Clarke WP, Milam SB. Integrins regulate opioid receptor signaling in trigeminal ganglion neurons. Neuroscience. 2007;144:889–897. doi: 10.1016/j.neuroscience.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigliardi PL, Buchner S, Rufli T, Bigliardi-Qi M. Specific stimulation of migration of human keratinocytes by mu-opiate receptor agonists. J Recept Signal Transduct Res. 2002;22:191–199. doi: 10.1081/rrs-120014595. [DOI] [PubMed] [Google Scholar]
- Bigliardi PL, Tobin DJ, Gavériaux-Ruff C, Bigliardi-Qi M. Opioids and the skin – where do we stand? Exp Dermatol. 2009;18:424–430. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- Bigliardi-Qi M, Gavériaux-Ruff C, Zhou H, Hell C, Bady P, Rufli T, et al. Deletion of delta-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation. 2006;74:174–185. doi: 10.1111/j.1432-0436.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- Brooke MA, Nitoiu D, Kelsell DP. Cell-cell connectivity: desmosomes and disease. J Pathol. 2012;226:158–171. doi: 10.1002/path.3027. [DOI] [PubMed] [Google Scholar]
- Cavallini M, Casati A. A prospective, randomized, blind comparison between saline, calcium gluconate and diphoterine for washing skin acid injuries in rats: effects on substance P and beta-endorphin release. Eur J Anaesthesiol. 2004;21:389–392. doi: 10.1017/s0265021504005071. [DOI] [PubMed] [Google Scholar]
- Charbaji N, Schafer-Korting M, Kuchler S. Morphine stimulates cell migration of oral epithelial cells by delta-opioid receptor activation. PLoS ONE. 2012;7:e42616. doi: 10.1371/journal.pone.0042616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cell Signal. 2009;21:1045–1053. doi: 10.1016/j.cellsig.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Dai X, Song HJ, Cui SG, Wang T, Liu Q, Wang R. The stimulative effects of endogenous opioids on endothelial cell proliferation, migration and angiogenesis in vitro 4. Eur J Pharmacol. 2010;628:42–50. doi: 10.1016/j.ejphar.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Denning MF, Guy SG, Ellerbroek SM, Norvell SM, Kowalczyk AP, Green KJ. The expression of desmoglein isoforms in cultured human keratinocytes is regulated by calcium, serum, and protein kinase C. Exp Cell Res. 1998;239:50–59. doi: 10.1006/excr.1997.3890. [DOI] [PubMed] [Google Scholar]
- Deramaudt TB, Dujardin D, Hamadi A, Noulet F, Kolli K, De MJ, et al. FAK phosphorylation at Tyr-925 regulates cross-talk between focal adhesion turnover and cell protrusion. Mol Biol Cell. 2011;22:964–975. doi: 10.1091/mbc.E10-08-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BV, Harmon RM, Green KJ. Desmosomes at a glance. J Cell Sci. 2009;122:4401–4407. doi: 10.1242/jcs.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek RL, Godsel LM, Green KJ. Discriminating roles of desmosomal cadherins: beyond desmosomal adhesion. J Dermatol Sci. 2007;45:7–21. doi: 10.1016/j.jdermsci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Ammer H. Delta-opioid receptors activate ERK/MAP kinase via integrin-stimulated receptor tyrosine kinases. Cell Signal. 2008a;20:2324–2331. doi: 10.1016/j.cellsig.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Ammer H. Delta-Opioid receptors stimulate ERK1/2 activity in NG108-15 hybrid cells by integrin-mediated transactivation of TrkA receptors. FEBS Lett. 2008b;582:3325–3329. doi: 10.1016/j.febslet.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L. Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J Cell Sci. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Husainy AN, Morrow AA, Perkins TJ, Lee JM. Robust patterns in the stochastic organization of filopodia. BMC Cell Biol. 2010;11:86. doi: 10.1186/1471-2121-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL, Evans CJ. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology. 2009;56(Suppl. 1):205–212. doi: 10.1016/j.neuropharm.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira M, Sano S, Takagi S, Yoshikawa K, Takeda J, Itami S. STAT3 deficiency in keratinocytes leads to compromised cell migration through hyperphosphorylation of p130(cas) J Biol Chem. 2002;277:12931–12936. doi: 10.1074/jbc.M110795200. [DOI] [PubMed] [Google Scholar]
- Kirfel G, Rigort A, Borm B, Schulte C, Herzog V. Structural and compositional analysis of the keratinocyte migration track. Cell Motil Cytoskeleton. 2003;55:1–13. doi: 10.1002/cm.10106. [DOI] [PubMed] [Google Scholar]
- Kirfel G, Rigort A, Borm B, Herzog V. Cell migration: mechanisms of rear detachment and the formation of migration tracks. Eur J Cell Biol. 2004;83:717–724. doi: 10.1078/0171-9335-00421. [DOI] [PubMed] [Google Scholar]
- Kljuic A, Bazzi H, Sundberg JP, Martinez-Mir A, O'Shaughnessy R, Mahoney MG, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired Pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- Koivunen J, Aaltonen V, Koskela S, Lehenkari P, Laato M, Peltonen J. Protein kinase C alpha/beta inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res. 2004;64:5693–5701. doi: 10.1158/0008-5472.CAN-03-3511. [DOI] [PubMed] [Google Scholar]
- Kuchler S, Wolf NB, Heilmann S, Weindl G, Helfmann J, Yahya MM, et al. 3D-wound healing model: influence of morphine and solid lipid nanoparticles. J Biotechnol. 2010;148:24–30. doi: 10.1016/j.jbiotec.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Martinez-Rico C, Pincet F, Thiery JP, Dufour S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J Cell Sci. 2010;123:712–722. doi: 10.1242/jcs.047878. [DOI] [PubMed] [Google Scholar]
- Mascia F, Denning M, Kopan R, Yuspa SH. The black box illuminated: signals and signaling. J Invest Dermatol. 2012;132:811–819. doi: 10.1038/jid.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y, Ebisuya M, Honjoh S, Nishida E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr Biol. 2004;14:731–735. doi: 10.1016/j.cub.2004.03.060. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, et al. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT. Presynaptic versus postsynaptic localization of mu and delta opioid receptors in dorsal and ventral striatopallidal pathways. J Neurosci. 1997;17:7471–7479. doi: 10.1523/JNEUROSCI.17-19-07471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada K, Seishima M, Kitajima Y. Pemphigus IgG activates and translocates protein kinase C from the cytosol to the particulate/cytoskeleton fractions in human keratinocytes. J Invest Dermatol. 1997;108:482–487. doi: 10.1111/1523-1747.ep12289726. [DOI] [PubMed] [Google Scholar]
- Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- Parrish EP, Steart PV, Garrod DR, Weller RO. Antidesmosomal monoclonal antibody in the diagnosis of intracranial tumours. J Pathol. 1987;153:265–273. doi: 10.1002/path.1711530311. [DOI] [PubMed] [Google Scholar]
- Pello OM, Duthey B, Garcia-Bernal D, Rodriguez-Frade JM, Stein JV, Teixido J, et al. Opioids trigger alpha 5 beta 1 integrin-mediated monocyte adhesion. J Immunol. 2006;176:1675–1685. doi: 10.4049/jimmunol.176.3.1675. [DOI] [PubMed] [Google Scholar]
- Pilcher BK, Sudbeck BD, Dumin JA, Welgus HG, Parks WC. Collagenase-1 and collagen in epidermal repair. Arch Dermatol Res. 1998;290(Suppl):S37–S46. doi: 10.1007/pl00007452. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, et al. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M. Cytokines and peripheral analgesia. Adv Exp Med Biol. 2003;521:40–50. [PubMed] [Google Scholar]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration–the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol Biol Cell. 2000;11:1077–1092. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman JB, Chen A, Hemler ME. Investigation of the role of beta 1 integrins in cell-cell adhesion. J Cell Sci. 1995;108((Pt 11)):3635–3644. doi: 10.1242/jcs.108.11.3635. [DOI] [PubMed] [Google Scholar]
- Wen TT, Zhang Z, Yu Y, Qu H, Koch M, Aumailley M. Integrin alpha3 subunit regulates events linked to epithelial repair, including keratinocyte migration and protein expression. Wound Repair Regen. 2010;18:325–334. doi: 10.1111/j.1524-475X.2010.00590.x. [DOI] [PubMed] [Google Scholar]
- Wolf NB, Kuchler S, Radowski MR, Blaschke T, Kramer KD, Weindl G, et al. Influences of opioids and nanoparticles on in vitro wound healing models. Eur J Pharm Biopharm. 2009;73:34–42. doi: 10.1016/j.ejpb.2009.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 β-catenin distribution is affected by δ-opioid receptor (DOPr) expression. (a) Representative images of β-cat staining in δ-opioid receptor and control cells subjected to respective treatments. Prominent localization of β-cat at the membrane of control cells is observed while DOPr-OE cells show reduced membrane localization. (b) Manual quantification of cell-cell border staining after sum projection of z-stack images reveals significantly decreased staining in all DOPr-OE cultures regardless of drug treatment. (c) Immunoblot and (d) graph of quantification reveal no changes in β-catenin expression regardless of treatment as well as in both GFP control and DOPr overexpressing N/TERT-1s. Values shown are normalized values to loading control α-tubulin and then to the respective vehicle (DMSO) only control. Values represent the mean ± SEM of four independent N/TERT cultures (n = 4).
Figure S2 δ-opioid receptor (DOPr)-stimulated migration and accelerated in vitro wound healing can be alternately influenced by ERK activation. (a) Time-lapse microscopy of in vitro wound healing using GFP control and DOPr-OE N/TERT-1 treated with Met-Enk and ERK 1/2 inhibitor PD98059 (+/−). Representative images of the gap in the keratinocyte monolayer at the start of migration (0 h) and after 6 h. (B) Quantified mean ± SEM of area wound closure (1 = 100%) for all groups.
Figure S3 δ-opioid receptor (DOPr)-overexpressing N/TERTs exhibit dendritic-like protrusions. (a) Fluorescence time-lapse image of DOPr N/TERTs at basal state was taken at every 5 min interval for 1 h and viewed using pseudo-colour scheme to facilitate visualizations of the protrusion. (B) Representative confocal image at 100× magnification of DOPr-OE N/TERT-1 at basal state shows long and fine protrusions at the cell periphery. DOPr-OE N/TERT-1 was stained with anti-GFP antibody.