Abstract
BACKGROUND AND PURPOSE
A number of experimental procedures require single housing to assess individual behaviour and physiological responses to pharmacological treatments. The endogenous opioids are closely linked to social interaction, especially early in life, and disturbance in the social environment may affect opioid peptides and thereby confound experimental outcome. The aim of the present study was to examine time-dependent effects of single housing on opioid peptides in rats.
EXPERIMENTAL APPROACH
Early adolescent Sprague Dawley rats (post-natal day 22) were subjected to either prolonged (7 days) or short (30 min) single housing. Several brain regions were dissected and immunoreactive levels of Met-enkephalin-Arg6Phe7 (MEAP), dynorphin B and nociception/orphanin FQ, as well as serum corticosterone were measured using RIA.
KEY RESULTS
Prolonged single housing reduced immunoreactive MEAP in hypothalamus, cortical regions, amygdala, substantia nigra and periaqueductal grey. Short single housing resulted in an acute stress response as indicated by high levels of corticosterone, accompanied by elevated immunoreactive nociceptin/orphanin FQ in medial prefrontal cortex, nucleus accumbens and amygdala. Neither short nor prolonged single housing affected dynorphin B.
CONCLUSIONS AND IMPLICATIONS
Disruption in social environmental conditions of rats, through single housing during early adolescence, resulted in time-, area- and peptide-specific alterations in endogenous opioids in the brain. These results provide further evidence for an association between early life social environment and opioids. Furthermore, the results have implications for experimental design; in any pharmacological study involving opioid peptides, it is important to distinguish between effects induced by housing and treatment.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Keywords: enkephalin, dynorphin, nociceptin/orphanin FQ, early stress, rats, social environment
Introduction
Disturbance in the social context is widely used in animal experimental models to study the effects of environmental adversity in young animals in order to evaluate neurobiological mechanisms underlying psychiatric disorders (de Kloet et al., 2005; Crews, 2008; Miczek et al., 2008). In neonatal animals, different forms of social deprivation are commonly used, for example, maternal separation models (Pryce et al., 2005; Nylander and Roman, 2013) or social isolation (Neisewander et al., 2012). In adolescent animals, isolation and other social stressors, for example, unstable hierarchies and social defeat, are often used (Miczek et al., 2008). These studies have generated new knowledge within a number of research fields, including behaviour, neurobiology and pharmacology, of how exposure to social stressors affects young animals and about the consequences for development of specific phenotypes, for example, depression- and anxiety-like behaviour, susceptibility to drug addiction and also for responses to pharmacological treatments (Smith et al., 2003; Miczek et al., 2008; Daoura and Nylander, 2011).
However, besides studies in which the purpose is to induce social distress, there are many occasions when the experimental animal is subjected to shorter or longer restrictions in social interactions due to single housing. Knowledge of how disturbances in the normal environment can affect the animals is important in study design to avoid confounding effects, especially when using young animals during critical social and cognitive developmental time windows (Andersen, 2003; Romeo and McEwen, 2006). Single housing is sometimes inevitable when conducting an experiment. One common example is during individual assessment of consumption behaviour, for example, food or drug intake. If the housing condition per se affects the brain, it can affect the assessment of neurobiological outcome or the effects of pharmacological treatment. Another common experimental situation is that some animals are taken out or left alone in a cage for shorter periods during treatment and other experimental procedures or before being killed. Both corticosterone and peptide levels can be affected by such procedures (Rosen et al., 1992; Ferland and Schrader, 2011). Therefore, it is important to acquire knowledge about possible effects induced by single housing conditions on the target system of interest in an experiment.
Knowledge of the effects of housing conditions on endogenous opioids is of importance for several reasons. Stressful stimuli induce activation of opioid networks and opioids regulate stress responses at the behavioural, endocrinological and neuronal levels (McCubbin, 1993; Drolet et al., 2001; Przewlocki, 2002). Opioid peptides are involved in parental bonding and play a key role in early social interactions (Panksepp et al., 1980; Moles et al., 2004) and in juvenile play behaviour (Vanderschuren et al., 1995). Also, disturbance of interactions between dam and pups during the first weeks of life causes long-term changes in opioids, possibly due to altered development of the endogenous opioid system (Nylander and Roman, 2012). However, the knowledge of how social disturbances affect opioids post-weaning, in early adolescence, has not been systematically investigated. The research interest in adolescent individuals is high, and to avoid confounding effects due to single housing in these studies, we need more information as to its effects on opioid peptides.
The aim in this study was to examine the effect of short and longer single housing on three opioid peptides previously known to be affected by pre-weaning social disturbance (Ploj et al., 2001; 2002; Nylander and Roman, 2012). The study design included rats in three different groups that relate to common experimental situations: (i) single housing for 7 days (long single housing; LSH); for example, used in studies measuring individual food, fluid and drug intake; (ii) single housing for 30 min prior to decapitation (short single housing; SSH); used for experimental procedures outside the home cage (e.g. various behavioural tests or treatment paradigms) or during animal euthanasia; and (iii) group housing until decapitation; a common control group. The aim of this study was to identify, map and show patterns of brain areas sensitive to single housing conditions and to examine peptides with distinct actions in the brain. To this end, the neurobiological analysis included the opioid peptides Met-enkephalin-Arg6Phe7 (MEAP), dynorphin B (DYNB) and nociceptin/orphanin FQ (N/OFQ) in several brain regions and the pituitary gland. The chosen peptides are widely distributed in the brain; they have different selectivity towards the opioid receptors and distinct neurobiological functions. These peptides regulate stress response in the brain through effects on different receptor system; MEAP on δ and μ opioid receptors; DYNB on κ opioid receptors and N/OFQ on NOP receptors (Drolet et al., 2001; Przewlocki, 2002; Caló and Guerrini, 2013; receptor nomenclature follows Alexander et al., 2013).
The results revealed that SSH during early adolescence produces an acute stress response as indicated by elevated immunoreactive (IR) corticosterone accompanied by increased levels of central IR-N/OFQ levels. Rats subjected to LSH had normal IR-corticosterone levels but alterations in IR-MEAP levels. IR-DYN was not affected by any condition. The findings provide new knowledge about time-, region- and peptide-specific effects caused by single housing that are of importance to consider in studies on opioids in young rats.
Methods
Animals
All animal care and experimental procedures followed the guidelines of the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Communities Council Directive (86/609/EEC) and were approved by the Uppsala Animal Ethical Committee. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 65 animals were used in the experiments described here.
Fifteen time-mated Sprague Dawley (B&K Universal, Sollentuna, Sweden) dams arrived at the animal facility on gestational day 17. The dams were individually housed in standard Makrolon cages (59 × 30 × 20 cm) containing wood chip bedding and nesting material. The cages were changed once a week. The animals were housed in a temperature (22 ± 1°C) and humidity (50 ± 10%) controlled animal room on a 12 h light/dark cycle with lights on at 60:00 h. The dams were maintained on standard pellet food (R36 Labfor, Lactamin, Lantmännen, Kimstad, Sweden) and water ad libitum in a room used only for this experiment. On the day of birth, post-natal day 0 (PND 0), the pups were sexed by measurement of ano-genital distance. The litters were cross-fostered and arranged so that each litter contained six males and four females as litter size and sex composition may affect the time the mother spends with each pup. The dams were left alone with their litters until PND 22, with the exception of cage change once a week. The experimental time-course is shown in Figure 1.
Figure 1.
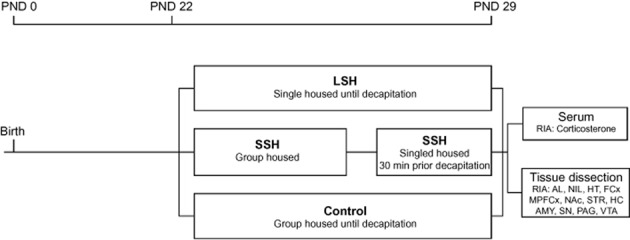
The experimental time-course. Abbreviations: AMY, amygdala; FCx, frontal cortex; HC, hippocampus; HT, hypothalamus; MPFCx, medial prefrontal cortex; NAc, nucleus accumbens; PAG, periaqueductal gray; PND, post-natal day; RIA, radioimmunoassay SN, substantia nigra; STR, dorsal striatum (caudate putamen); VTA, ventral tegmental area.
Housing conditions
At PND 22, the rats were weaned. Only males were used in the study and they were randomly assigned into three different experimental groups; (i) 10 rats were exposed to LSH; they were individually housed in Makrolon cages (42 × 26 × 18 cm) for 7 days; (ii) 10 rats were group housed (5 rats per cage) in Makrolon cages (59 × 38 × 20 cm), and prior to decapitation, each rat was exposed to 30 min of SSH in a separate cage; and (iii) 10 rats serving as controls were group housed (5 animals per cage) in Makrolon cages (59 × 38 × 20 cm) and were left undisturbed until decapitation. The rats were able to communicate with and smell other animals but they were deprived of tactile contact during the single housing. On PND 29, all animals were weighed and thereafter killed by guillotine decapitation without prior anaesthesia.
Blood sampling
Trunk blood was collected for corticosterone measurements according to the manufacturer's instruction. The blood samples were kept at room temperature for 20–30 min and centrifuged at 3000× g for 10 min at 4°C. The serum was stored at −20°C until further analysis.
Dissection
Immediately after decapitation, the brains were dissected using a pre-cooled rat brain matrix (ASI Instruments, Inc., Warren, MI, USA). The pituitary gland was divided into anterior and neurointermediate lobes, and the hypothalamus was removed from the brain. The brains were manually sliced with pre-cooled razor blades in coronal sections (1 mm slots) within 10–15 min. The frontal cortex, medial prefrontal cortex, nucleus accumbens, dorsal striatum (caudate putamen), hippocampus, amygdala, substantia nigra, ventral tegmental area (VTA) and periaqueductal gray (PAG) were dissected according to the rat brain atlas of Paxinos and Watson (1997) and immediately frozen on dry ice and stored at −80°C until further analysis.
Tissue homogenization and peptide extraction
The tissues were heated in 1 M acetic acid for 5 min at 95°C, cooled on ice and homogenized by sonication using a Branson Sonifier (Danbury, CT, USA) and then re-heated for 5 min at 95°C. The homogenates were cooled on ice and centrifuged for 10 min at 12 000× g in a Beckman GS-15R centrifuge (Fullerton, CA, USA). The supernatants were purified using a cation exchange chromatography procedure (Christensson-Nylander et al., 1985). The samples were dried in a vacuum centrifuge and stored at −20°C.
RIA
The peptides were analysed using specific RIAs according to methods previously described in detail for DYNB and MEAP (Nylander et al., 1995; 1997) and for N/OFQ (Ploj et al., 2000). Samples subjected to the MEAP assay were oxidized prior to the RIA procedure. An aliquot of either 25 μL of the samples or the standard peptide was incubated with 100 μL of antiserum or 125I-labelled peptide for 24 h at 4°C. Antiserum for the respective peptide was generated in rabbits and used in the following final dilutions: MEAP 90:3D II; 1:180 000; DYNB 113+; 1:562 500 and N/OFQ 96:2 + 1:112 500. Cross-reactivity for the MEAP antiserum with Met-enkephalin, Met-enkephalin-Arg6, Met-enkephalin-Arg6Gly7Leu8, Leu-enkephalin and dynorphin A (1–6) was less than 0.1%. The DYNB antiserum cross-reactivity with DYNB 29 was 1% and with big dynorphin (DYN 32) 100%. The N/OFQ showed 0.5% cross-reactivity with N/OFQ (1–13) and less than 0.1% cross-reactivity with nocistatin, dynorphin A and A(1–6), DYNB, Met- and Leu-enkephalin, MEAP and β-endorphin. Analysis of corticosterone was performed using the commercial ImmunChem™ Double Antibody Corticosterone 125I RIA kit for rats and mice (ICN Biomedicals Inc., Costa Mesa, CA, USA) using a highly specific corticosterone antiserum. The samples were analysed in accordance with the protocol in the RIA kit. All samples were assayed in duplicate and in the same assay in order to avoid inter-assay variations. The minimum level of detection was 25 ng·mL−1.
Data analysis
The body weight, IR-corticosterone levels and IR-peptide levels were analysed using a one-way anova followed by the Bonferroni post hoc test. Differences were considered statistically significant at P < 0.05. All statistical analyses were performed using Statistica 10 (Statsoft Inc., Tulsa, OK, USA).
In addition to conventional statistics, multivariate data analysis was used for investigation of the relationship between IR-peptide levels and IR-corticosterone levels. The multivariate method partial least square discriminant analysis (PLS-DA) enables visualization of how the individuals belonging to the different experimental groups relate to each other as well as how different analytes relate to each other. It can thus be used for pattern recognition analysis among individuals as well as analytes. The PLS-DA is a regression extension of principal component analysis for modelling the relationship between projections of dependent factors and independent responses involving a dummy variable for classification. The PLS-DA calculates the relationship between a Y-matrix (here experimental groups) and an X-matrix (here peptide levels and corticosterone levels). The weights for the X-variables (in the analysis denoted w) indicate the importance of these variables, whereas the weights for the Y-variables (in the analysis denoted c) indicate which Y-variables are modelled in the respective PLS model dimensions. The score plot is a map of the observations and shows the possible presence of outliers, groups, similarities and other patterns in the data. When the data are plotted in a w*c plot, a picture is obtained showing the relationships between X and Y (Eriksson et al., 2006). For the multivariate data analysis, SIMCA-P+ 12.0 (Umetrics AB, Umeå, Sweden) was used.
Results
Body weight
The mean body weight (g) ± SEM in the three experimental groups prior to the decapitation at PND 29 were 99.9 ± 2.5 (control), 99.0 ± 1.3 (SSH) and 94.2 ± 1.8 (LSH). No differences were seen between the groups [F(2,26) = 2.41; P = 0.11].
IR-corticosterone
The IR-corticosterone levels (ng·mL−1; mean ± SEM) in SSH (353.7 ± 45.3) rats were highly elevated compared to both LSH {135.8 ± 33.8, [F(2,27) = 13.9; P < 0.001]} and controls {85.1 ± 43.3, [F(2,27) = 13.9; P < 0.01]}. One outlier in the control group was observed and was identified as the last rat taken from the cage before decapitation. The statistically significant difference was the same with or without this sample. There was no difference between controls and LSH animals. Samples with IR-corticosterone levels below the detection limit were set to the detection level (25 ng·mL−1).
IR-N/OFQ levels
The IR-N/OFQ levels in the brain and the pituitary gland are shown in Table 1. SSH rats had significantly increased levels in the medial prefrontal cortex, nucleus accumbens and amygdala compared with controls (Figure 2A). The SSH rats had a significant increase in N/OFQ levels in the hypothalamus and medial prefrontal cortex compared with those in the LSH rats (Figure 2A).
Table 1.
Immunoreactive peptide content in dissected brain regions and in the pituitary gland
| MEAP (fmol·mg−1) | DYNB (fmol·mg−1) | N/OFQ (fmol·mg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LSH | SSH | Control | LSH | SSH | Control | LSH | SSH | |
| HT | 33.3 ± 2.2 (10) | 24.8 ± 1.4 (10) | 31.9 ± 1.5 (10) | 18.2 ± 1.0 (10) | 20.5 ± 1.2 (10) | 18.2 ± 0.7 (10) | 11.0 ± 0.4 (10) | 10.8 ± 0.6 (10) | 12.7 ± 0.5 (9) |
| FCx | 3.1 ± 0.4 (10) | 2.2 ± 0.1 (9) | 3.7 ± 0.2 (10) | 2.9 ± 0.2 (10) | 3.1 ± 0.2 (9) | 3.3 ± 0.2 (10) | 2.4 ± 0.05 (9) | 2.3 ± 0.09 (10) | 2.6 ± 0.1 (10) |
| MPFCx | 2.0 ± 0.2 (10) | 1.0 ± 0.2 (10) | 2.5 ± 0.2 (10) | n.d. | n.d. | n.d. | 2.6 ± 0.2 (10) | 2.4 ± 0.2 (10) | 3.2 ± 0.09 (10) |
| NAc | 29.4 ± 5.5 (10) | 25.1 ± 3.7 (10) | 21.5 ± 2.3 (9) | 38.0 ± 2.8 (10) | 41.0 ± 2.6 (10) | 41.9 ± 1.4 (9) | 2.7 ± 0.2 (10) | 3.2 ± 0.2 (10) | 3.5 ± 0.2 (10) |
| STR | 40.9 ± 3.2 (10) | 31.6 ± 1.3 (10) | 42.7 ± 4.3 (10) | 15.5 ± 0.6 (10) | 17.0 ± 0.8 (10) | 15.9 ± 0.9 (9) | 1.1 ± 0.04 (9) | 1.3 ± 0.04 (10) | 1.2 ± 0.08 (10) |
| HC | 6.2 ± 0.5 (9) | 5.0 ± 0.6 (10) | 7.8 ± 0.4 (9) | 18.5 ± 1.4 (10) | 19.0 ± 1.3 (10) | 21.1 ± 1.7 (10) | 3.0 ± 0.1 (10) | 2.8 ± 0.1 (10) | 3.0 ± 0.1 (10) |
| AMY | 11.3 ± 0.9 (10) | 7.1 ± 1.2 (10) | 12.6 ± 1.2 (10) | 6.8 ± 0.5 (9) | 8.0 ± 0.5 (9) | 7.5 ± 0.5 (9) | 3.5 ± 0.4 (10) | 4.2 ± 0.2 (9) | 4.9 ± 0.2 (10) |
| SN | 3.3 ± 0.4 (10) | 1.4 ± 0.4 (10) | 3.9 ± 0.4 (10) | 92.4 ± 3.9 (9) | 91.0 ± 4.3 (10) | 101.6 ± 6.1 (10) | 3.4 ± 0.3 (9) | 3.1 ± 0.3 (10) | 3.1 ± 0.3 (10) |
| VTA | 6.4 ± 0.8 (10) | 5.6 ± 0.9 (10) | 7.5 ± 0.6 (9) | 1.5 ± 0.2 (10) | 1.7 ± 0.2 (10) | 2.1 ± 0.4 (9) | 3.2 ± 0.4 (10) | 3.0 ± 0.6 (10) | 3.1 ± 0.6 (9) |
| PAG | 27.3 ± 2.1 (10) | 16.1 ± 1.2 (10) | 30.3 ± 1.5 (10) | 11.0 ± 0.6 (10) | 10.1 ± 0.7 (9) | 10.2 ± 0.4 (9) | 9.1 ± 0.5 (10) | 9.0 ± 0.3 (10) | 9.2 ± 0.4 (10) |
| AL | n.d. | n.d. | n.d. | 3.1 ± 0.4 (10) | 2.1 ± 0.3 (9) | 3.5 ± 0.6 (7) | n.d. | n.d. | n.d. |
| NIL | n.d. | n.d. | n.d. | 84.4 ± 5.8 (9) | 87.4 ± 8.3 (10) | 79.3 ± 3.2 (10) | n.d. | n.d. | n.d. |
Values are expressed as mean ± SEM (n). n.d., no data, values below detection limit in the RIA. Results from the statistical analyses are shown in Figure 2. Abbreviations: AMY, amygdala; AL, anterior pituitary lobe; FCx, frontal cortex; HC hippocampus; HT, hypothalamus; MPFCx, medial prefrontal cortex; NAc, nucleus accumbens; NIL, neurointermediate pituitary lobe; PAG, periaqueductal gray; SN, substantia nigra; SSH, short single housing; STR, dorsal striatum (caudate putamen); VTA, ventral tegmental area.
Figure 2.
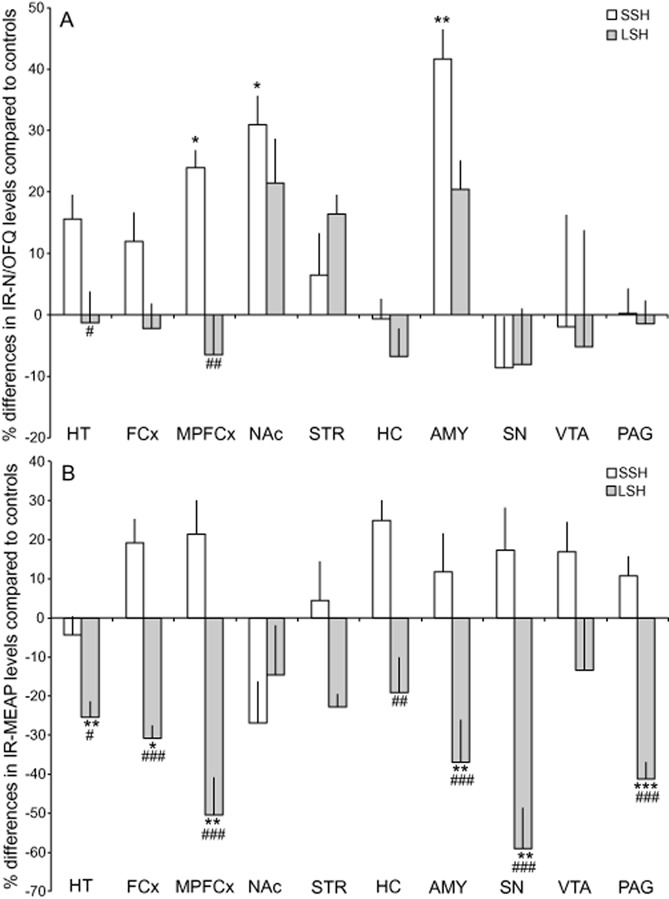
Long (LSH) and short single housing (SSH) and its effects on IR-N/OFQ (A) and IR-MEAP (B) levels in various brain areas. The values are presented as a percentage of the IR-levels in the control group. *P < 0.05; **P < 0.01; ***P < 0.001, compared with the controls; #P < 0.05: ##P < 0.01; ###p < 0.001, compared with SSH rats (one-way anova followed by the Bonferroni post hoc test). Abbreviations: AMY, amygdala; FCx, frontal cortex; HC, hippocampus; HT, hypothalamus; MPFCx, medial prefrontal cortex; NAc, nucleus accumbens; PAG, periaqueductal gray; SN, substantia nigra; STR, dorsal striatum (caudate putamen); VTA, ventral tegmental area.
IR-MEAP levels
The IR-MEAP levels in the brain and the pituitary gland are shown in Table 1. In the LSH group, IR-MEAP levels were significantly decreased in the hypothalamus, frontal cortex, medial prefrontal cortex, amygdala, substantia nigra and PAG compared with both controls and SSH rats (Figure 2B). A decreased level was also seen in the hippocampus when comparing the LSH and SSH groups (Figure 2B).
IR-DYNB levels
No significant differences between the groups were found in IR-DYNB levels. The IR-DYNB levels in the dissected brain regions are shown in Table 1.
Multivariate data analysis
Multivariate data analysis was used to investigate the relationship between IR-peptide levels and IR-corticosterone levels. Since the multivariate data analysis is used as a complement to conventional statistics in order to illustrate patterns among individuals and IR-levels of importance for creating such patterns, a PLS-DA was performed. The PLS-DA generated two significant components [R2X(cum) = 0.257; R2Y(cum) = 0.784; Q2(cum) = 0.578] (Figure 3). Figure 3A shows the individual score plot with a clear separation of control, LSH and SSH animals. Figure 3B illustrates how the IR-levels load in relation to the experimental groups. IR-levels loading close to a Y (experimental group) are of importance for this group. Notably, IR-corticosterone levels together with IR-N/OFQ levels in the amygdala, hypothalamus, frontal cortex and medial frontal cortex are important for the loading of the SSH group, which is in agreement with the conventional statistics.
Figure 3.
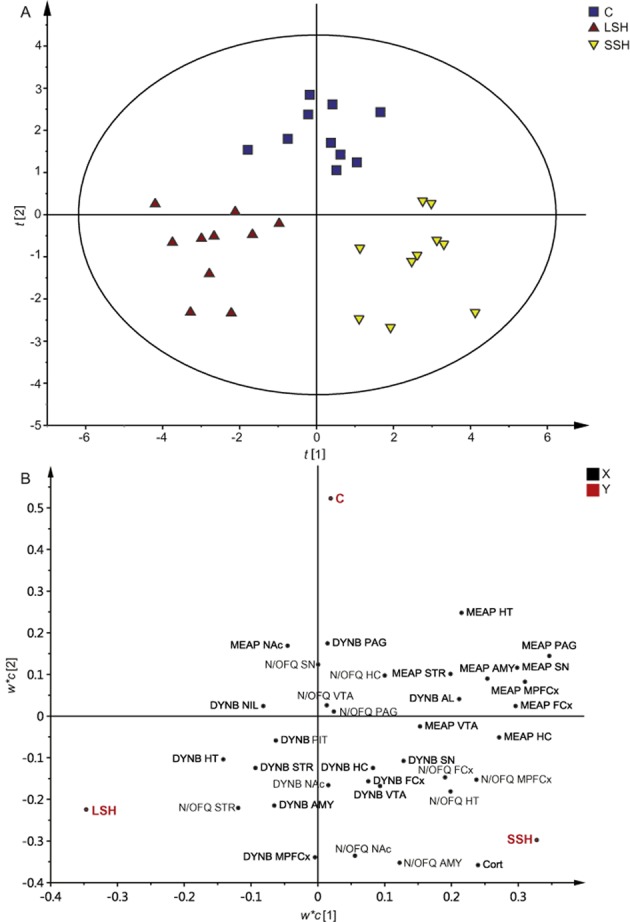
PLS-DA showing the individual animals (A) belonging to the C, LSH and SSH groups, respectively, and the IR-peptide levels and IR-corticosterone levels (B) important for the loading of the individuals. The PLS-DA generated two significant components [R2X(cum) = 0.257; R2Y(cum) = 0.784; Q2(cum) = 0.578]. Abbreviations: AL, anterior pituitary lobe; AMY, amygdala; C, control; Cort, corticosterone; FCx, frontal cortex; HC hippocampus; HT, hypothalamus; MPFCx, medial prefrontal cortex; NAc, nucleus accumbens; NIL, neurointermediate pituitary lobe; PAG, periaqueductal gray; PIT; pituitary gland (AL + NIL); SN, substantia nigra; STR, dorsal striatum (caudate putamen); VTA, ventral tegmental area.
Discussion
The main findings in the present study were that disturbance in the social environment through single housing in early adolescence results in time-, area- and peptide-specific changes in endogenous opioids. N/OFQ was affected after 30 min (SSH), whereas changes in MEAP were only found after 7 days (LSH) and DYNB was not affected at all.
The SSH rats were exposed to single housing 30 min before decapitation at PND 29. The serum IR-corticosterone levels were highly elevated, indicative of an acute hypothalamic–pituitary–adrenal (HPA) axis response. The IR-N/OFQ levels in the SSH group were also affected in several brain areas; higher levels were detected in the medial prefrontal cortex, nucleus accumbens and amygdala compared with control animals. Relative to the LSH animals, the SSH rats had higher levels in the hypothalamus; the same pattern was seen when comparing the SSH animals with controls but the difference was not significant (P = 0.07). These findings were further supported by the PLS-DA where IR-N/OFQ and IR-corticosterone loaded close to the SSH group. An association between elevated levels of central N/OFQ and increased corticosterone has previously been reported (Devine et al., 2001; Leggett et al., 2006). Intracerebroventricular (i.c.v) injections of N/OFQ cause elevated levels of serum corticosterone (Devine et al., 2001; Fernandez et al., 2004; Leggett et al., 2006) and increased anxiety-like behaviour (Fernandez et al., 2004). Stress-related behaviours observed after i.c.v injections of N/OFQ are attenuated with UFP-101, a selective NOP receptor antagonist (Fernandez et al., 2004; Leggett et al., 2006) and repeated injections with UFP-101 decrease corticosterone levels and reinstate sucrose consumption in rats exposed to the unpredictable chronic mild stress paradigm (Vitale et al., 2009). Increased levels of IR-N/OFQ in the SSH group in amygdala and a similar trend in hypothalamus are in line with the findings from Green and Devine (2009), showing that acute stress up-regulates NOP mRNA expression in the central and basomedial amygdala as well as in the paraventricular hypothalamus in adult rats (Green and Devine, 2009). In contrast to the present study, reduced basal N/OFQ levels were reported in forebrain regions after acute stress in adult animals (Devine et al., 2003). The discrepancy could be due to different dissection techniques, as several areas were included in the forebrain region, or to the different types of stress used, that is, restraint stress compared with social stress. Age-dependent responses to stress may also occur (Gomez et al., 2002; Romeo et al., 2006a; b), but to the best of our knowledge, age-related differences in stress-induced effects on N/OFQ have not been systematically examined. The negative feedback system in the HPA axis matures after PND 30 (Schapiro et al., 1971) and, before that, the corticosterone release is higher and more prolonged in young animals in comparison to adults (Goldman et al., 1973). Thus, it would be interesting to gather more knowledge about stress-induced effects on N/OFQ and, vice versa, the effects of N/OFQ on stress responses at different ages. The stress response seen after 30 min may be a result of disturbed social contact with cage mates but can also be a handling effect or induced by exposure to a novel environment. Nevertheless, the aim was to examine the effects of a short period of single housing and, as the results show that IR-N/OFQ levels were affected, caution should be taken whenever using single housing conditions.
Single housing for 7 days had no effects on IR-N/OFQ levels. These results are in line with a previous report where the basal levels were unaffected after another stress paradigm, that is, a chronic variable stress regime including several stressors such as foot shock, cold room and restraint (Devine et al., 2003). The animals may have adapted to the single housing conditions and the IR-N/OFQ levels may, like the IR-corticosterone levels, have returned to baseline levels. However, since only basal levels were measured and no challenge with acute stress was performed, we cannot exclude the possibility that the N/OFQ system was altered.
Seven days of single housing decreased the IR-MEAP levels in several brain regions, whereas the animals exposed to 30 min of single housing had similar IR-MEAP levels as the controls. Several of the affected regions (i.e. the hypothalamus, amygdala, hippocampus, frontal cortex and medial prefrontal cortex) are involved in the regulation of the HPA axis (Watts, 2000). Lower IR-MEAP levels may be an adaptation to the acute stress response with HPA activation and corticosterone release as seen in the SSH group. Enkephalin co-exists with corticotrophin-releasing factor in neurons projecting to the median eminence where opioids are implied in regulation of HPA axis activity (Przewlocki, 2002) and it is likely that MEAP has a role in adaptive regulation of the stress response.
Lower IR-MEAP levels could also be a result of disturbed development and maturation of enkephalin networks, either directly or indirectly through other opioid-regulating transmitters. Studies on pre-weanling rats have shown that MEAP is highly sensitive to daily deprivation of maternal contact as shown by lower basal levels in many brain regions (Nylander and Roman, 2012). Here, we show that single housing after weaning also results in reduced IR-MEAP levels. These results are in line with the notion that environmental conditions during adolescence are critical for normal brain development and that opioids are crucial for the development of social behaviour such as adolescent play (Panksepp et al., 1980; Spear, 2000; Trezza et al., 2010). The results also supports the brain opioid theory of social attachment that predicts that social isolation causes low levels of endogenous opioid peptides and that social contact releases endogenous opioids (see Machin and Dunbar, 2011).
Absence of social interactions can affect opioid-related systems in many ways, for example, isolated rats self-administer morphine to a higher extent (Marks-Kaufman and Lewis, 1984), respond differently to opioid treatment in condition place preference (Wongwitdecha and Marsden, 1996) and antinociception tests (Smith et al., 2003) and respond differently to naltrexone treatment during ethanol access (Daoura and Nylander, 2011). The results from the present study can shed some light over the effect that early life social interactions have on opioid-related neurobiological functions, drug response and behaviour later in life.
IR-DYNB was not affected in the present study, providing further evidence that disruption of social interactions early in life have little effect on its basal levels (Nylander and Roman, 2012). Dynorphin networks are more mature at birth, compared to those involving MEAP (McDowell and Kitchen, 1987), and may therefore be less sensitive to early life environmental influences. It is however important to note that only basal levels were measured in this study, so it is not known whether a stress challenge would unmask a possible underlying deranged dynorphin system.
In conclusion, disruption in social environmental conditions through single housing during early adolescence results in time-, area- and peptide-specific alterations in endogenous opioids. The results provide further evidence for a close association between early life environment and endogenous opioids. Furthermore, the results have implications for experimental design; in any pharmacological study involving opioid peptides, it is evident that care must be taken to distinguish between the effects induced by housing and treatment.
Acknowledgments
Funding from the Swedish Medical Research Council (K2012-61X-22090-01-3; I. N.), the European Foundation for Alcohol Research (EA 11 30; I. N. and E. R.) and the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly (I. N. and E. R.) supported this study.
Glossary
Abbreviations
- DYNB
dynorphin B
- HPA
hypothalamic–pituitary–adrenal
- IR
immunoreactive
- LSH
long single housing
- MEAP
Met-enkephalin-Arg6Phe7
- N/OFQ
nociceptin/orphanin FQ
- PAG
periaqueductal gray
- PLS-DA
partial least squares projection to latent structures discriminant analysis
- PND
post-natal day
- SSH
short single housing
- VTA
ventral tegmental area
Author contributions
I. N. supervised the project; I. N. and E. R. designed the experiment; E. R. performed the experiment; L. G. and E. R. performed the statistical analyses; L. G. prepared the manuscript. All authors interpreted the results and commented on the manuscript at all stages.
Conflict of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Caló GLDG, Guerrini R. Nociceptin/orphanin FQ. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. San Diego, CA: Academic Press; 2013. pp. 1577–1586. [Google Scholar]
- Christensson-Nylander I, Nyberg F, Ragnarsson U, Terenius L. A general procedure for analysis of proenkephalin B derived opioid peptides. Regul Peptides. 1985;11:65–76. doi: 10.1016/0167-0115(85)90032-1. [DOI] [PubMed] [Google Scholar]
- Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol. 2008;29:344–357. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoura L, Nylander I. The response to naltrexone in ethanol-drinking rats depends on early environmental experiences. Pharmacol Biochem Behav. 2011;99:626–633. doi: 10.1016/j.pbb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Devine DP, Watson SJ, Akil H. Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic-hypothalamic-pituitary-adrenal axis. Neuroscience. 2001;102:541–553. doi: 10.1016/s0306-4522(00)00517-0. [DOI] [PubMed] [Google Scholar]
- Devine DP, Hoversten MT, Ueda Y, Akil H. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J Neuroendocrinol. 2003;15:69–74. doi: 10.1046/j.1365-2826.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikström C, Wold S. Multi- and Megavariate Data analysis. Part I: Basic Principles and Applications. Umeå, Sweden: Umetrics AB; 2006. [Google Scholar]
- Ferland CL, Schrader LA. Cage mate separation in pair-housed male rats evokes an acute stress corticosterone response. Neurosci Lett. 2011;489:154–158. doi: 10.1016/j.neulet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143:2852–2862. doi: 10.1210/endo.143.8.8929. [DOI] [PubMed] [Google Scholar]
- Green MK, Devine DP. Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides. 2009;43:507–514. doi: 10.1016/j.npep.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Leggett JD, Harbuz MS, Jessop DS, Fulford AJ. The nociceptin receptor antagonist [Nphe1,Arg14,Lys15]nociceptin/orphanin FQ-NH2 blocks the stimulatory effects of nociceptin/orphanin FQ on the HPA axis in rats. Neuroscience. 2006;141:2051–2057. doi: 10.1016/j.neuroscience.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Machin AJ, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148:985–1025. [Google Scholar]
- Marks-Kaufman R, Lewis MJ. Early housing experience modifies morphine self-administration and physical dependence in adult rats. Addict Behav. 1984;9:235–243. doi: 10.1016/0306-4603(84)90015-7. [DOI] [PubMed] [Google Scholar]
- McCubbin JA. Stress and endogenous opioids: behavioral and circulatory interactions. Biol Psychol. 1993;35:91–122. doi: 10.1016/0301-0511(93)90008-v. [DOI] [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Development of opioid systems: peptides, receptors and pharmacology. Brain Res. 1987;434:397–421. doi: 10.1016/0165-0173(87)90006-3. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl) 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I, Roman E. Neuropeptides as mediators of the early-life impact on the brain; implications for alcohol use disorders. Front Molecular Neurosci. 2012;5:77. doi: 10.3389/fnmol.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I, Roman E. Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology (Berl) 2013;229:555–569. doi: 10.1007/s00213-013-3217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain Res. 1995;683:25–35. doi: 10.1016/0006-8993(95)00279-y. [DOI] [PubMed] [Google Scholar]
- Nylander I, Stenfors C, Tan-No K, Mathe AA, Terenius L. A comparison between microwave irradiation and decapitation: basal levels of dynorphin and enkephalin and the effect of chronic morphine treatment on dynorphin peptides. Neuropeptides. 1997;31:357–365. doi: 10.1016/s0143-4179(97)90072-x. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Ploj K, Roman E, Gustavsson L, Nylander I. Basal levels and alcohol-induced changes in nociceptin/orphanin FQ, dynorphin, and enkephalin levels in C57BL/6J mice. Brain Res Bull. 2000;53:219–226. doi: 10.1016/s0361-9230(00)00328-2. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Bergstrom L, Nylander I. Effects of neonatal handling on nociceptin/orphanin FQ and opioid peptide levels in female rats. Pharmacol Biochem Behav. 2001;69:173–179. doi: 10.1016/s0091-3057(01)00511-1. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Effects of maternal separation on brain nociceptin/orphanin FQ peptide levels in male Wistar rats. Pharmacol Biochem Behav. 2002;73:123–129. doi: 10.1016/s0091-3057(02)00778-5. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Przewlocki R. Stress, opioid peptides, and their receptors. In: Pfaff DW, editor. Hormones, Brain, and Behavior. San Diego, CA: Academic Press; 2002. pp. 691–733. [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann NY Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006a;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006b;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Rosen A, Brodin K, Eneroth P, Brodin E. Short-term restraint stress and s.c. saline injection alter the tissue levels of substance P and cholecystokinin in the peri-aqueductal grey and limbic regions of rat brain. Acta Physiol Scand. 1992;146:341–348. doi: 10.1111/j.1748-1716.1992.tb09428.x. [DOI] [PubMed] [Google Scholar]
- Schapiro S, Percin CJ, Kotichas FJ. Half-life of plasma corticosterone during development. Endocrinology. 1971;89:284–286. doi: 10.1210/endo-89-1-284. [DOI] [PubMed] [Google Scholar]
- Smith MA, Bryant PA, McClean JM. Social and environmental enrichment enhances sensitivity to the effects of kappa opioids: studies on antinociception, diuresis and conditioned place preference. Pharmacol Biochem Behav. 2003;76:93–101. doi: 10.1016/s0091-3057(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res. 1995;680:148–156. doi: 10.1016/0006-8993(95)00256-p. [DOI] [PubMed] [Google Scholar]
- Vitale G, Ruggieri V, Filaferro M, Frigeri C, Alboni S, Tascedda F, et al. Chronic treatment with the selective NOP receptor antagonist [Nphe1, Arg14, Lys15]N/OFQ-NH2 (UFP-101) reverses the behavioural and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacology (Berl) 2009;207:173–189. doi: 10.1007/s00213-009-1646-9. [DOI] [PubMed] [Google Scholar]
- Watts AG. Brain and brain regions. In: Fink G, editor. Encyclopedia of Stress. San Diego, CA: Academic Press; 2000. pp. 342–348. [Google Scholar]
- Wongwitdecha N, Marsden CA. Effect of social isolation on the reinforcing properties of morphine in the conditioned place preference test. Pharmacol Biochem Behav. 1996;53:531–534. doi: 10.1016/0091-3057(95)02046-2. [DOI] [PubMed] [Google Scholar]


