Abstract
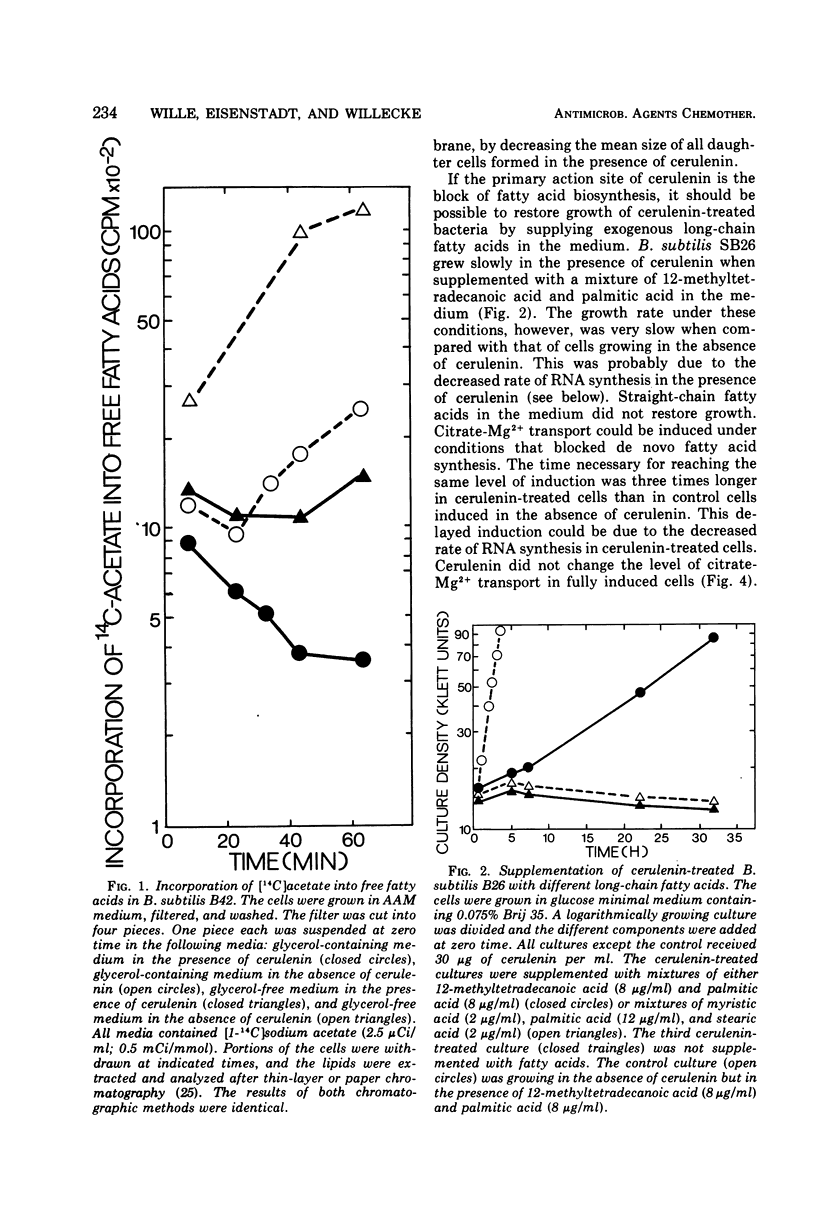
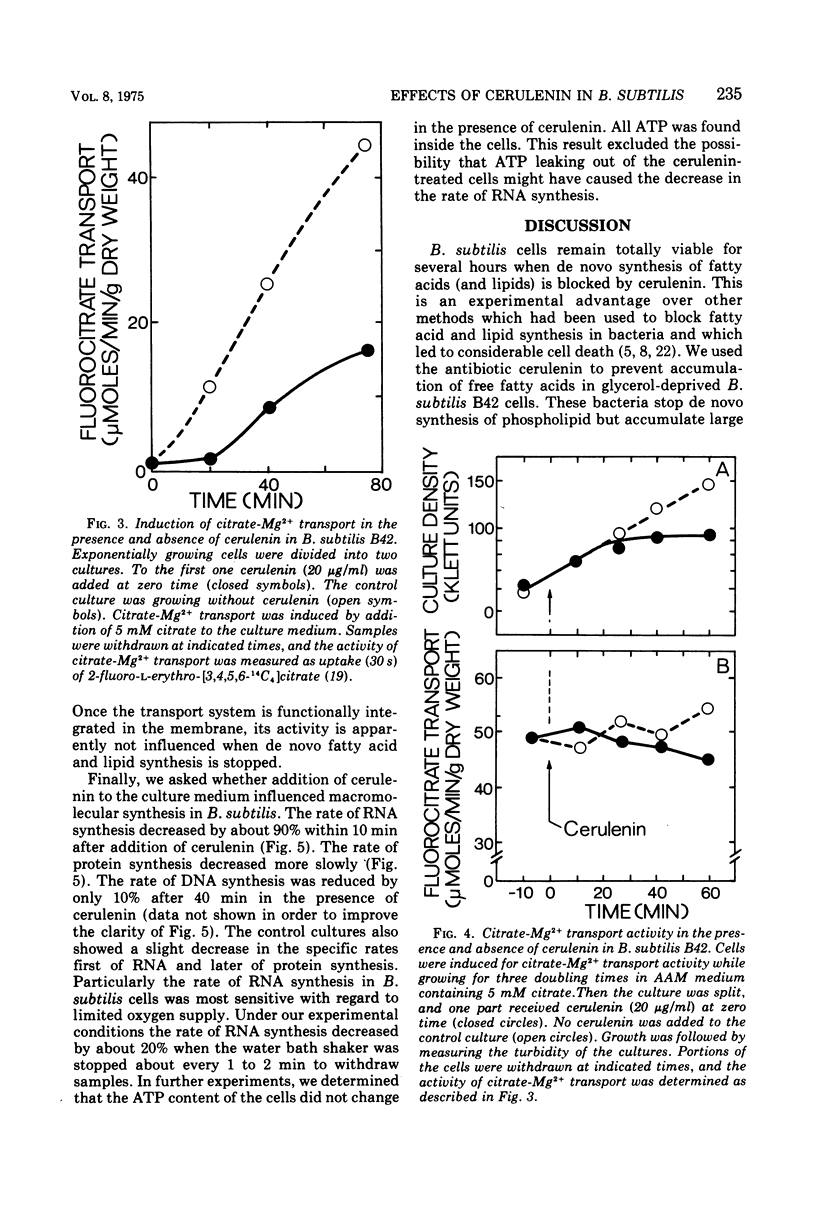
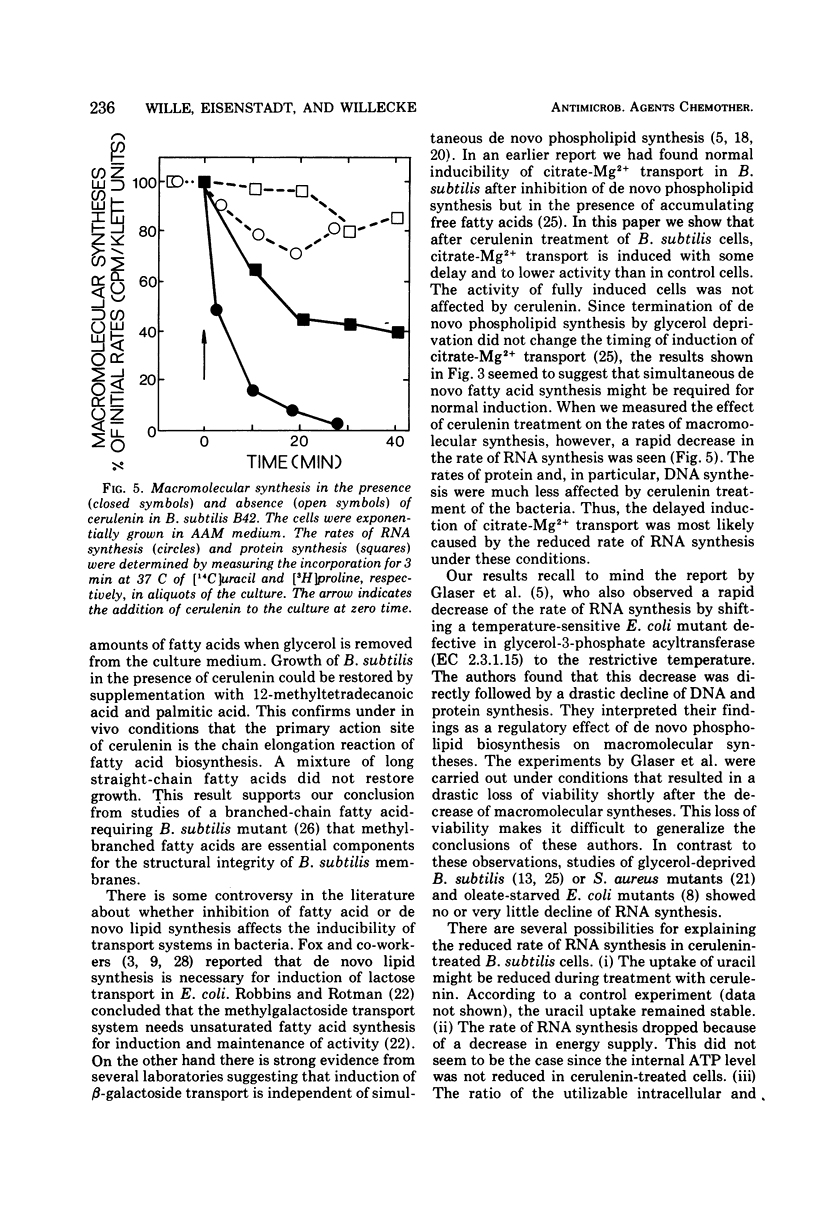
Inhibition of de novo fatty acid biosynthesis by the antibiotic cerulenin in Bacillus subtilis stopped de novo synthesis of neutral lipids and phospholipids. The bacteria ceased growing but remained completely viable. Addition of 12-methyltetradecanoic acid and palmitic acid to the culture medium of cerulenin-treated cells restored growth of the bacteria, albeit at a reduced rate. Although the de novo synthesis of all lipid components of the membrane was blocked, citrate-Mg2+ transport activity remained inducible, and induced cells did not lose this transport activity when treated with cerulenin. Shortly after the addition of cerulenin, the rate of ribonucleic acid synthesis dropped rapidly and was followed by a slower decrease in the rate of protein synthesis. The rate of deoxyribonucleic acid synthesis remained almost unaffected. The rapid decrease of ribonucleic acid synthesis in cerulenin-treated cells might be due to the inhibition of de novo fatty acid biosynthesis or it might be due to a secondary effect of cerulenin in B. subtilis cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arison B. H., Omura S. Revised structure of cerulenin. J Antibiot (Tokyo) 1974 Jan;27(1):28–30. doi: 10.7164/antibiotics.27.28. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 1967 Nov;2(4):448–453. doi: 10.1111/j.1432-1033.1967.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Walker J. R., Bloch K. Inhibition of lipid synthesis in Escherichia coli cells by the antibiotic cerulenin. Antimicrob Agents Chemother. 1973 May;3(5):549–554. doi: 10.1128/aac.3.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder M. E., Beacham I. R., Cronan J. E., Jr, Beacham K., Honegger J. L., Silbert D. F. Temperature-sensitive mutants of Escherichia coli requiring saturated and unsaturated fatty acids for growth: isolation and properties. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3105–3109. doi: 10.1073/pnas.69.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. C., Fox C. F. Induction of the lactose transport system in a lipid-synthesis-defective mutant of Escherichia coli. J Bacteriol. 1970 Aug;103(2):410–416. doi: 10.1128/jb.103.2.410-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L. R. The antibacterial activity of 3-decynoyl-n-acetylcysteamine. Inhibition in vivo of beta-hydroxydecanoyl thioester dehydrase. J Biol Chem. 1968 Jun 25;243(12):3223–3228. [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- Lillich T. T., White D. C. Phospholipid metabolism in the absence of net phospholipid synthesis in a glycerol-requiring mutant of Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):790–797. doi: 10.1128/jb.107.3.790-797.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Control of fatty acid synthesis in bacteria. J Bacteriol. 1972 Apr;110(1):96–102. doi: 10.1128/jb.110.1.96-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Hata T., Omura S. Inhibition of sterol and fatty acid biosyntheses by cerulenin in cell-free systems of yeast. J Antibiot (Tokyo) 1972 Jun;25(6):365–368. doi: 10.7164/antibiotics.25.365. [DOI] [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Omura S., Hata T. The action mechanism of cerulenin. I. Effect of cerulenin on sterol and fatty acid biosynthesis in yeast. J Biochem. 1972 May;71(5):783–796. doi: 10.1093/oxfordjournals.jbchem.a129827. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr Unsaturated fatty acid synthesis is not required for induction of lactose transport in Escherichia coli. J Biol Chem. 1974 Feb 10;249(3):724–731. [PubMed] [Google Scholar]
- Oehr P., Willecke K. Citrate-Mg2+ transport in Bacillus subtilis. Studies with 2-fluoro-L-erythro-citrate as a substrate. J Biol Chem. 1974 Apr 10;249(7):2037–2042. [PubMed] [Google Scholar]
- Overath P., Hill F. F., Lamnek-Hirsch I. Biogenesis of E. coli membrane: evidence for randomization of lipid phase. Nat New Biol. 1971 Dec 29;234(52):264–267. doi: 10.1038/newbio234264a0. [DOI] [PubMed] [Google Scholar]
- Ray P. H., White D. C. Effect of glycerol deprivation on the phospholipid metabolism of a glycerol auxotroph of Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):668–677. doi: 10.1128/jb.109.2.668-677.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Rotman B. Inhibition of methylgalactoside transport in Escherichia coli upon the cessation of unsaturated fatty acid biosynthesis. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2125–2129. doi: 10.1073/pnas.69.8.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum-Oliver D., Zamenhof S. Degree of participation of exogenous thymidine in the overall deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1972 May;110(2):585–591. doi: 10.1128/jb.110.2.585-591.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Willecke K., Mindich L. Induction of citrate transport in Bacillus subtilis during the absence of phospholipid synthesis. J Bacteriol. 1971 May;106(2):514–518. doi: 10.1128/jb.106.2.514-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Fatty acid-requiring mutant of bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J Biol Chem. 1971 Sep 10;246(17):5264–5272. [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Inducible transport of citrate in a Gram-positive bacterium, Bacillus subtilis. J Biol Chem. 1971 Feb 25;246(4):1032–1040. [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]



