Abstract
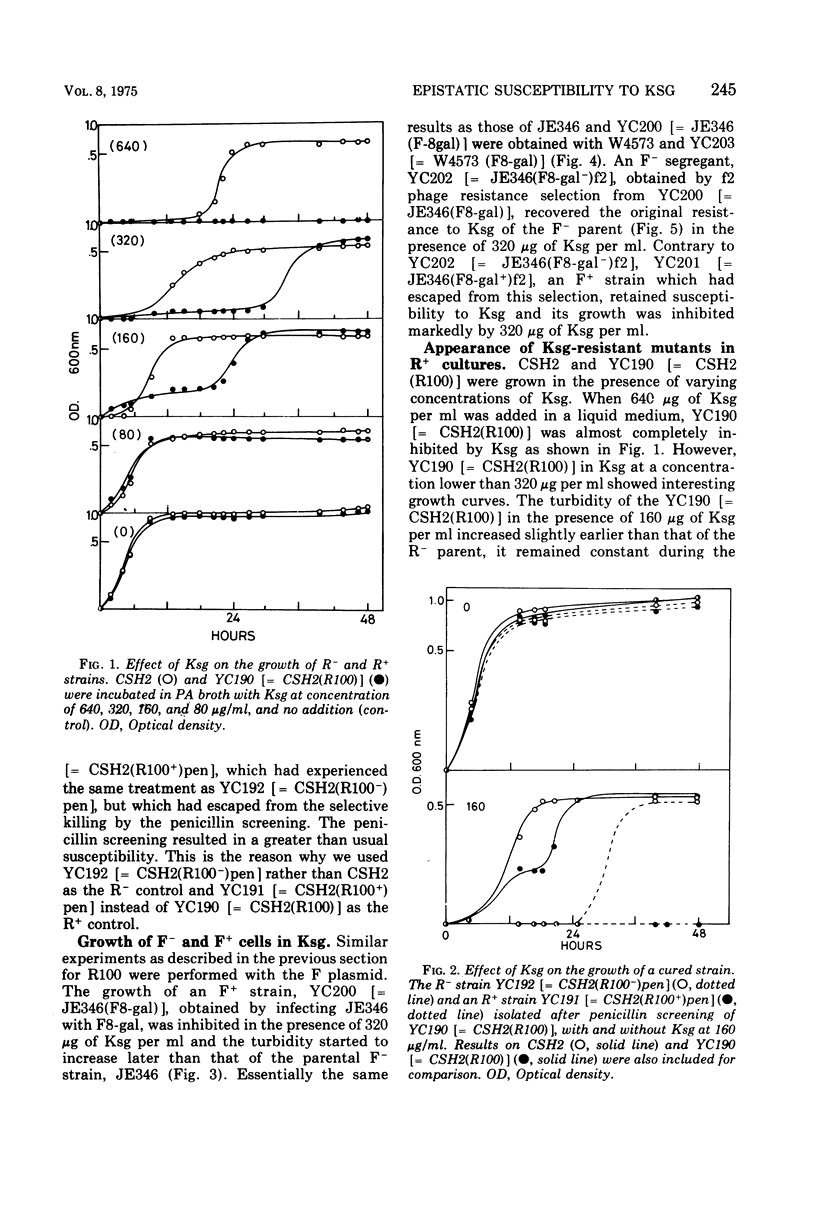
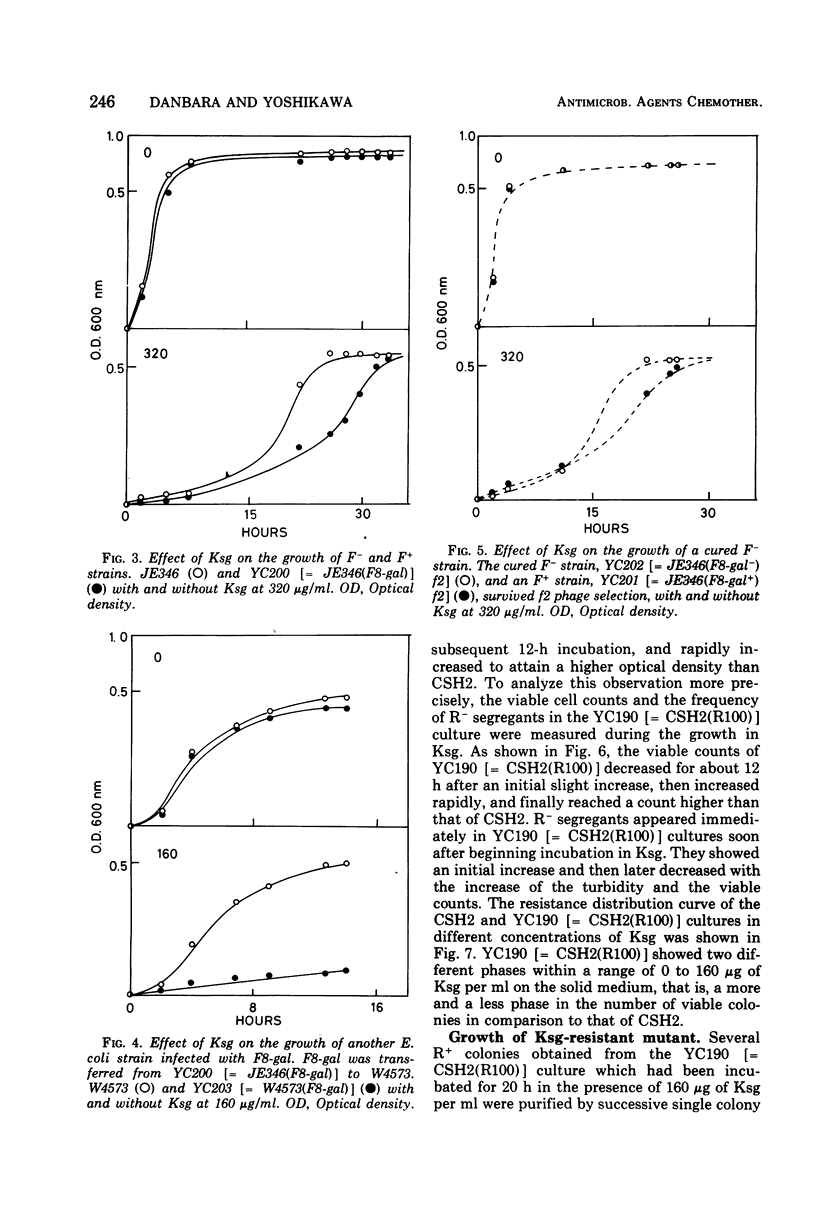
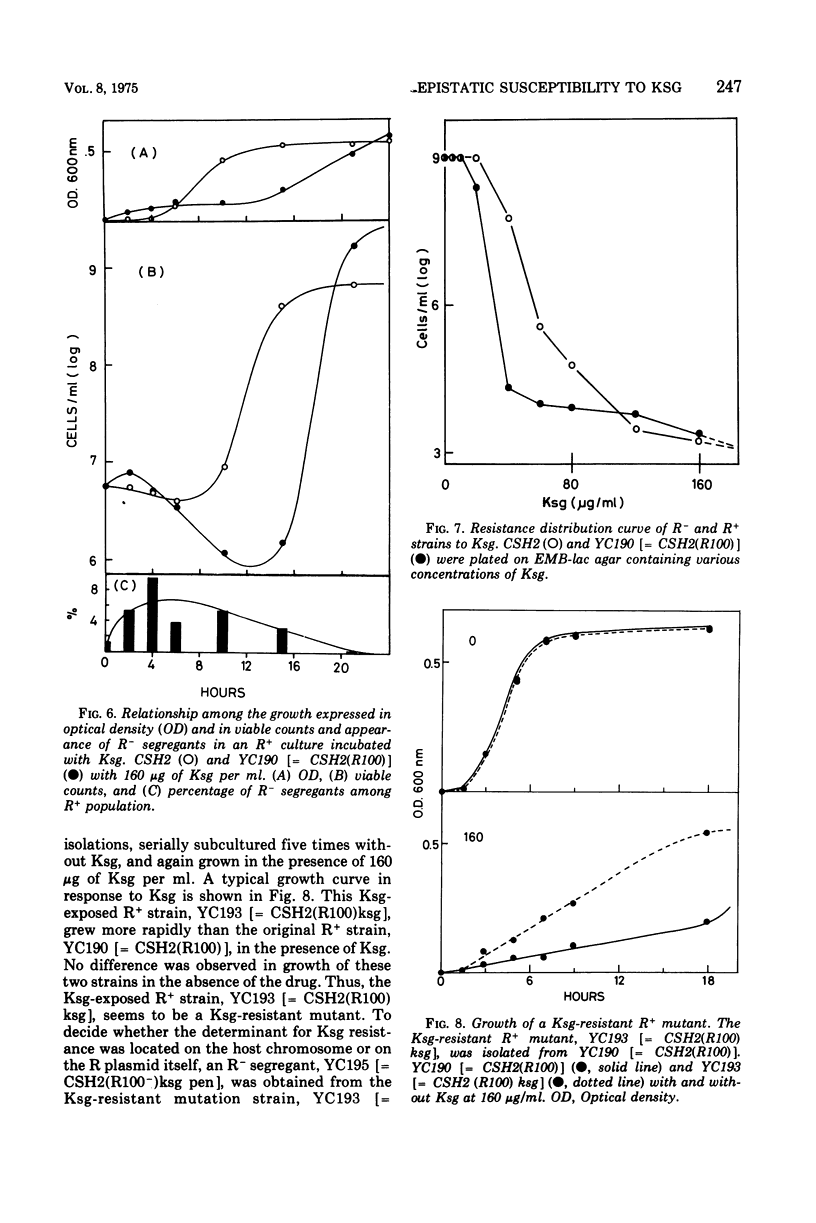
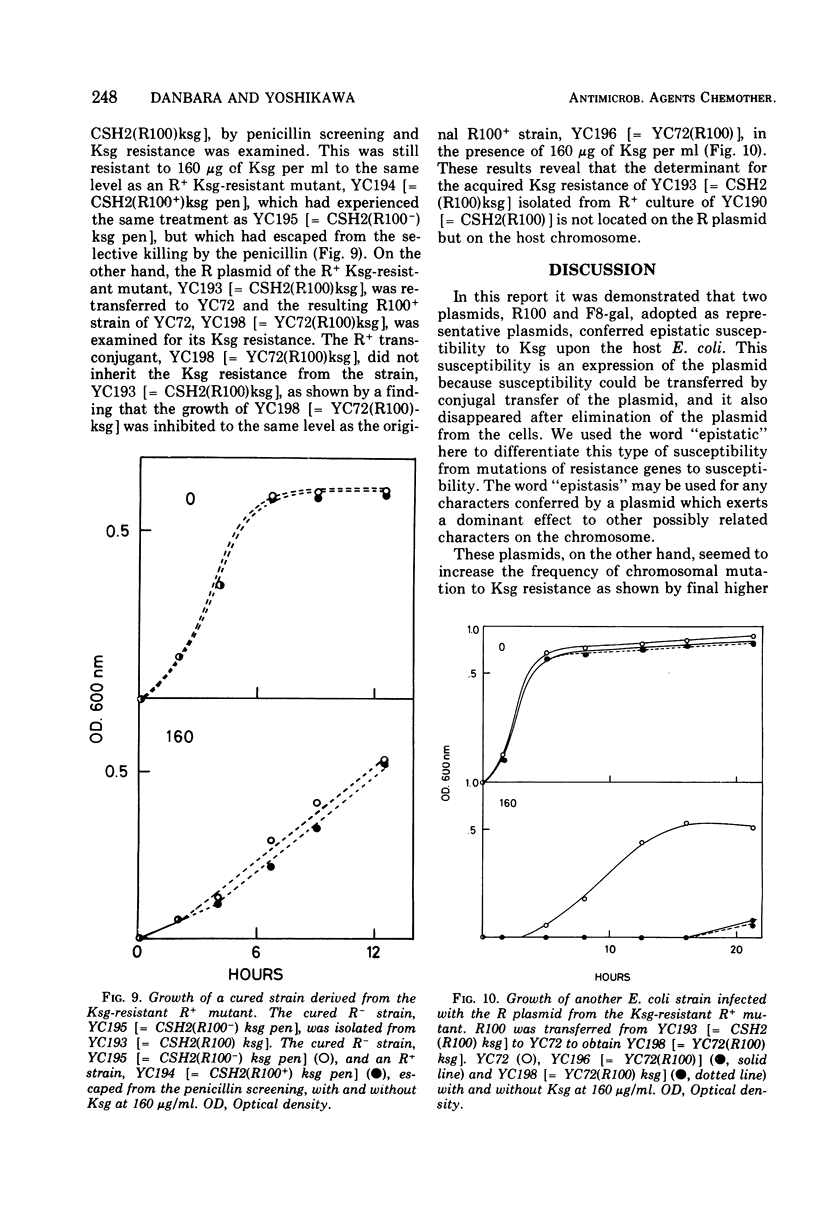
The effect of two representative plasmids, R100 and F8-gal, on the susceptibility of Escherichia coli to kasugamycin was studied. R+ and F+ cells were found to be more susceptible to this antibiotic than R− and F− cells, respectively. Retransfer and curing experiments of these plasmids show that this increased susceptibility of host cells to kasugamycin was conferred by either of the plasmids. At the early stage of growth of R100+ cells in the presence of kasugamycin, R− segregants overgrew the population and then they were replaced by kasugamycin-resistant mutants of the R+ cells which became the majority cell line of the population. The former phenomenon is assumed to be due to the increased susceptibility of R100+ cells to kasugamycin, and the latter is probably related to the finding that R100 enhances the spontaneous mutation of host cells to resistance to kasugamycin. The practical and experimental significance of these findings are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka N., Nakamura S., Inuzuka M., Tomoeda M. Specific action of sodium dodecyl sulfate on the sex factor of Escherichia coli K-12 Hfr strains. J Bacteriol. 1969 Nov;100(2):827–835. doi: 10.1128/jb.100.2.827-835.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Iyobe S., Hashimoto H., Umezawa H. [Preferential inhibition of the growth of Escherichia coli strains carrying episomes]. J Antibiot (Tokyo) 1970 Jul;23(7):319–323. doi: 10.7164/antibiotics.23.319. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Onishi Y. F factor promotes turnover of stable RNA in escherichia coli. Science. 1975 Jan 24;187(4173):257–258. doi: 10.1126/science.1089310. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Nishimura T., Yamaguchi H., Yamamoto C., Yoshida Y., Sashikata K., Umezawa H. Mechanism of action of kasugamycin. J Antibiot (Tokyo) 1965 Jul;18(4):139–144. [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMEZAWA H., OKAMI Y., HASHIMOTO T., SUHARA Y., HAMADA M., TAKEUCHI T. A NEW ANTIBIOTIC, KASUGSMYCIN. J Antibiot (Tokyo) 1965 Mar;18:101–103. [PubMed] [Google Scholar]
- Yoshikawa M. Drug sensitivity and mutability to drug resistance associated with the presence of an R factor. Genet Res. 1971 Feb;17(1):1–7. doi: 10.1017/s0016672300011988. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M. Selective enrichment of R- segregants as the main mechanism of "curing" of the R factor by acridine dyes. Genet Res. 1971 Feb;17(1):9–16. doi: 10.1017/s001667230001199x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Sevag M. G. Sensitivity of Escherichia coli to atabrine conferred by R factor and its potential clinical significance. J Bacteriol. 1967 Jan;93(1):245–253. doi: 10.1128/jb.93.1.245-253.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



