Abstract
The folding and activation of furin occur through two pH- and compartment-specific autoproteolytic steps. In the endoplasmic reticulum (ER), profurin folds under the guidance of its prodomain and undergoes an autoproteolytic excision at the consensus furin site Arg-Thr-Lys-Arg107 ↓ generating an enzymatically masked furin-propeptide complex competent for transport to late secretory compartments. In the mildly acidic environment of the trans-Golgi network/endosomal system, the bound propeptide is cleaved at the internal site 69HRGVTKR75 ↓, unmasking active furin capable of cleaving substrates in trans. Here, by using cellular, biochemical, and modeling studies, we demonstrate that the conserved His69 is a pH sensor that regulates the compartment-specific cleavages of the propeptide. In the ER, unprotonated His69 stabilizes a solvent-accessible hydrophobic pocket necessary for autoproteolytic excision at Arg107. Profurin molecules unable to form the hydrophobic pocket, and hence, the furin-propeptide complex, are restricted to the ER by a PACS-2- and COPI-dependent mechanism. Once exposed to the acidic pH of the late secretory pathway, protonated His69 disrupts the hydrophobic pocket, resulting in exposure and cleavage of the internal cleavage site at Arg75 to unmask the enzyme. Together, our data explain the pH-regulated activation of furin and how this His-dependent regulatory mechanism is a model for other proteins.
The pH gradient formed by the various membranous compartments comprising the secretory and endocytic pathways has essential and manifold roles ranging from the regulation of protein traffic to the control of protein conformation and enzyme activities, including the processing of prohormones and proproteins by proprotein convertases (PCs).2 The PCs, a family of calcium-dependent serine endoproteases, cleave proproteins and prohormones at doublets or clusters of basic amino acids thus generating mature bioactive proteins as well as hormonal processing intermediates that require additional post-translational modifications to gain full bioactivity (1, 2). The proteolytic maturation of prohormones by the neuroendocrine-specific PC1/3 and PC2 requires the acidic pH of maturing secretory granules (3–5), whereas the proteolytic activation of proprotein substrates by the ubiquitously expressed PC furin in the trans-Golgi network (TGN)/endosomal system is compartment- and hence pH-specific (2, 6). This compartment-specific processing by furin is mediated in part by the pH-dependent changes in the conformation of furin substrates as well as the utilization of pH-sensitive furin cleavage sites. Furin efficiently cleaves a number of proprotein substrates at the consensus site -Arg-X-Lys/Arg-Arg- ↓ (2). Kinetic studies show that the P1 and P4 Arg residues are required for the efficient processing of furin substrates, whereas the P2 Arg has a modulatory role (7, 8). However, at acidic pH the absence of a P2 or P4 Arg can be compensated for by the presence of positively charged residues at P6 or possibly adjacent amino acids, demonstrating the relevance of pH for furin-dependent processing (8–12).
The pH-dependent processing of furin substrates by selective cleavage site utilization is exemplified by the autoproteolytic cleavages of its cognate prodomain. During transit from the pH-neutral ER to the acidic TGN/endosomal system, the ordered and compartment-specific furin prodomain processing guides the folding of the inactive proenzyme to the mature, active endoprotease. Similar to the evolutionarily related bacterial subtilisins, the 83-amino acid furin prodomain is necessary to correctly fold the catalytic domain in the ER (13, 14). The folded catalytic domain then rapidly (t1/2 <10 min) excises the prodomain at the canonical P1/P4 Arg furin cleavage site -Arg-Thr-Lys-Arg107 ↓, generating a transport-competent furin-propeptide complex. Inhibition of this excision step blocks transport of profurin molecules from the ER, suggesting that components of the cellular trafficking machinery detect formation of the furin-propeptide complex before directing their transit to late secretory pathway compartments (14). However, the machinery that localizes profurin to the ER is unknown. We identified PACS-1, which is a cytosolic sorting protein that connects the CK2-phosphorylated furin cytosolic domain to AP-1, thereby localizing the endopro-tease to the TGN (15, 16). Recently, we discovered PACS-2, which localizes membrane cargo to the ER by connecting them to COPI (17). But whether PACS-2 binds to furin or mediates ER trafficking of profurin is unknown.
Despite formation of the catalytic center, the furin-propeptide complex remains inactive toward substrates in trans because the bound propeptide is a potent furin inhibitor (K0.5 = 14 nm (18)). Release of the autoinhibitory propeptide from the enzyme requires trafficking of the furin-propeptide complex from the ER to the mildly acidic TGN/endosomal system (18). There, the noncovalently associated propeptide undergoes a second slow cleavage (t1/2 ~90 min) at the His-Arg-Gly-Val-Thr-Lys-Arg75 ↓ internal cleavage site, thus releasing the propeptide fragments and unmasking active furin capable of cleaving substrates in trans (14, 18). This second site cleavage is rate-limiting for activation of bacterial and human subtilisin-like proteases (14, 19). In vitro studies of furin show that the internal propeptide cleavage occurs optimally at pH 6.0, the pH of the TGN, demonstrating the differential pH requirements of the two prodomain cleavage sites (14, 18). Surprisingly, mutation of the P1/P6 Arg cleavage site to a canonical P1/P4 Arg furin site fails to yield mature, active furin but instead causes the accumulation of inactive profurin in the ER (14). Together, these studies suggest that the ordered, compartment-specific cleavages of the furin propeptide are necessary to guide the folding and activation of the endoprotease and that this activation process could be controlled by a pH sensor.
The requirement for exposure of the furin-propeptide complex to pH 6 to complete enzyme activation suggests a role for one or more histi-dine residues to serve as a pH sensor controlling furin activation. Protonation of the histidine imidazole ring, which has a pKa of 6.0, has profound effects on histidine chemistry under physiological conditions (20). Although no pH sensor has been described for processing enzymes, such a role for histidine is well established for generating allosteric changes, including control of O2/CO2 exchange by hemoglobin, gating of electrogenic molecules, and the pH-dependent conformational changes within class II major histocompatibility complex molecules that promote ligand exchange (21–24).
By using mutants that mimic the protonation state of histidine and through molecular modeling analysis we report the role of His69 in the folding and activation of furin. Our results demonstrate that the protonation state of His69, which is located in a solvent-accessible hydrophobic pocket, plays a critical role in regulating the secondary propeptide cleavage. Mutations that interfere with the His69 block propeptide excision, resulting in ER accumulation of profurin by a mechanism that requires the cytosolic sorting protein PACS-2 and COPI. Following propeptide excision, the furin-propeptide complex traffics to the mildly acidic TGN/endosomal system where protonation of His69 disrupts the solvent-accessible hydrophobic pocket to expose the P1/P6 Arg internal cleavage site His-Arg-Gly-Val-Thr-Lys-Arg75 ↓, leading to release of the inhibitory propeptide and furin activation.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Reagents were from Sigma except where stated. Antibodies against PACS-2 (25), FLAG (M1 and M2; Sigma), HA (HA.11; Covance), β-COP (Abcam), α-tubulin (Calbiochem), TGN46 (Serotec), PDI (R. Sitia), furin (PA-062, ABR), and Alexa (546 and 488)-conjugated secondary antibodies (Molecular Probes) and horseradish peroxidase-conjugated secondary antibodies (Southern Biotech) were described or provided as indicated.
Cell Culture, DNA Constructs, and Virus Construction
A7 and BSC-40 cells were cultured in minimal essential medium (MEM; Cellgro) containing 10% fetal bovine serum and 25 μg/ml gentamycin as described (14, 18). Vaccinia viruses (VV) expressing fur/f/ha, fur/f/haΔtc-k, and R75A:fur/f/ haΔtc-k were generated as described previously (14, 18). VV recombinants expressing H66L:fur/f/ha, H66L:fur/f/haΔtc-k, H69L:fur/f/ha, H69L:fur/f/ haΔtc-k, H69K:fur/f/ha, and H69K:fur/f/haΔtc-k were generated using standard PCR methods and subcloned into pZVneo fur/f/ha and fur/f/ haΔtc-k. Vaccinia virus recombinants were constructed using methods described previously (18).
Pulse-Chase Analysis
Pulse-chase analysis was performed as described (5). Briefly, BSC-40 cells grown in 35-mm dishes were infected with the indicated VV recombinants (m.o.i. = 5) for 3 h and labeled with [3H]arginine and [3H]leucine (100μCi each) in Arg/Leu-MEM (Invitrogen) for 30 min. The cells were then incubated at 37 °C with complete MEM containing an excess of cold arginine and leucine for 20 min and harvested at the indicated time points in mRIPA buffer (150 mm NaCl, 50 mm Tris, pH 8.0, 1% deoxycholate, 1% Nonidet P-40) containing 1 mm CaCl2. FLAG-tagged furin constructs were immunoprecipitated with mAb M1 (100 μg/ml) and captured with protein A-Sepharose, and the bound proteins were then separated by 15% Tris-glycine SDS-PAGE and detected by autoradiography as described (18).
Metabolic Labeling
A7 cells were infected with VV expressing the indicated furin constructs (m.o.i. = 3) for 12 h, washed, and incubated with phosphate-free MEM for 1 h after which 0.5 mCi/ml 32Pi (PerkinElmer Life Sciences) was added for 2 h. The labeled cells were washed with phosphate-buffered saline and lysed in mRIPA with 50 mm NaF, 0.1 μm orthovanadate, 10 μm pepstatin A, 10 μm leupeptin, 1 μm E64, 50 μg/ml aprotinin, 500 μm phenylmethylsulfonyl fluoride, and 1 mm CaCl2. FLAG-tagged furin proteins were immunoprecipitated with mAb M2, separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by autoradiography and Western blot.
Furin Activity Assays
To assay Δtc-k constructs, high speed cellular membrane preparations from BSC-40 cells infected with the indicated VV recombinants (m.o.i. = 2) for 14 h were prepared as described (18). The membrane samples were incubated in activation buffer (10 mm BisTris, 5 mm CaCl2, 1 mm 2-mercaptoethanol, 500 μm phenylmethylsulfonyl fluoride, 10 μm pepstatin A, 20 μm E64, 50 μg/ml aprotinin, pH 6.0 or 7.5 as indicated) at 30 °C either for 3 h (at pH 6.0 and 7.5) or at pH 7.5 for 1 h in the presence of trypsin (0.83 nm) followed by a 15-min incubation with soybean trypsin inhibitor (2.5 μm). Fluorometric assays of furin activity were performed with Abz-RVKRGLAY(NO2)D-OH furin substrate (Bachem) using a FluoroMax-2 spectrofluorometer equipped with a 96-well plate reader (18). To assay Fur/f/ha constructs, membrane preparations from BSC-40 cells expressing the indicated constructs were prepared, and furin activity was assayed in 100 mm HEPES, pH 7.5, 1 mm CaCl2 using the Abz-RVKRGLAY(NO2)D-OH peptide as described (14).
Fluorometric Assays of Internally Quenched Peptide Substrates
Each fluorogenic peptide substrate was prepared, characterized, and assayed as described (14). Briefly, each fluorogenic substrate was incubated with purified, soluble furin in 100 mm BisTris, 100 mm sodium acetate, pH 6.0 or 7.5, with 1 mm CaCl2. Incubations for the determination of kinetic constants were conducted with increasing concentrations of fluorogenic peptide substrate (corrected for peptide content) for up to 30 min. All assays were performed in duplicate, and the average values are reported (relative error, <11%). Fluorescence measurements were made with a FluoroMax-2 spectrofluorometer equipped with a 96-well plate reader (Instrument SA) using an excitation wavelength set at 320 nm and an emission wavelength set at 425 nm. The Km and Vmax values were determined using a computer-assisted algorithm (Enzfitter). Control studies showed no substrate inhibition for any of the fluorogenic peptides at concentrations 10-fold greater than their respective Km values.
Immunoblotting
BSC-40 cells were infected with VV expressing the indicated furin constructs (m.o.i. = 2) for 14 h and harvested in mRIPA. The samples were separated by 8% Tris-glycine SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blot. To detect the propep-tide, crude membrane extracts were incubated in activation buffer at pH 6.0 or 7.5 for 3 h at 30 °C, separated by 15% Tris-Tricine SDS-PAGE, transferred to nitrocellulose for 30 min at 36 V, and analyzed by Western blot with mAb HA.11 as described (14).
Immunofluorescence
BSC-40 cells were grown to 50–80% confluency on glass coverslips. After infection with the indicated VV recombinants (m.o.i. = 5, 5 h), cells were fixed with 4% paraformaldehyde, and immunofluorescence analysis was performed as described (26). For the siRNA experiments, A7 cells were transfected with control, PACS-2 (24), or β-COP (CAACUCCAGAUGGGAGACU) siRNA as described (25). After 48 h, the cells were infected with recombinant VV for an additional 4 h. The cells were fixed, permeabilized, and incubated with the indicated primary antisera followed by incubation with species- and subtype-specific fluorescently labeled secondary antisera. Images were then captured using a Zeiss Axioplan 2 microscope with a ×63 immersion objective and processed with the NIH image 1.62 program.
Sequence Analysis and Homology Modeling
Multiple sequence alignments of the mammalian PC sequences obtained from the Expasy server were aligned with ClustalW using default parameters. The alignments were then optimized manually using GeneDoc, and the model of the furin propeptide was built using the alignment interface of SwissModel, which predicts structures reliably with a root mean square deviation <2 Å for sequences with 50–60% identity (27) and with the solution structure of the PC1 propeptide (Protein Data Bank code 1KN6) as a template. The modeled structure was minimized in the SwissPDB Viewer package using 10,000 steps of steepest descent with the Gromos96 43B1 parameter set in vacuo and with a dielectric constant of 1. The model was further validated and manually checked for bad conformations and clashes by Whatcheck and ANOLEA software packages, and minimization procedures were repeated as necessary. The minimized model has an a root mean square deviation of 1 Å from that of the propeptide of PC1 (Protein Data Bank code 1KN6).
The furin propeptide was docked onto the crystal structure of the mouse furin catalytic domain ((9) Protein Data Bank code 1P8J) using the homologous bacterial protease-propeptide (Protein Data Bank code 1SCJ) complex (28) as template. The x-ray structure of the bacterial homologue depicts a side-on interaction between the propeptide and protease domains that appears to be conserved throughout the subtilase family. Using this as a template, the propep-tide was docked onto the x-ray structure of furin. Side chain clashes and deformations were then manually corrected using more favorable rotamers using InsightII. The docked structure was subsequently energy-minimized with CHARMm22 as the force field, initially using 10,000 steps of steepest descent followed by 10,000 steps of the Newton Rahpson's algorithm in the CHARMm module of InsightII.
RESULTS
Analysis of the furin propeptide revealed two histidine residues adjacent to the internal cleavage site and which are either strictly (His69) or partially (His66) conserved within all PC family members (Fig. 1A). To ascertain whether one or both of these histidine residues may be spatially arranged to affect the pH-dependent activation of furin, we undertook a structural analysis of the propeptide-furin complex by using homology modeling (27). The propeptide of furin was modeled using the solution structure of mouse PC1 propeptide (29) and docked onto the catalytic domain of furin by using information from the structures of the propeptide-subtilisin E complex and prokumamolysin, a serine carboxyl protease containing a subtilisin-like fold (Fig. 1, B and C) (28, 30). The correctness and accuracy of structures predicted using homology modeling were determined by the quality of the sequence alignment and the identity of the template, which, as evident from Fig. 1A, is high. The models were then refined and validated using the approaches described earlier (31). The internal cleavage site maps onto a loop between strands β3 and α3 and is part of a distinct hydrophobic pocket on the surface of the propeptide abutting the catalytic domain (Fig. 1, C and D). Although the cleavage site lies in a surface loop, close examination revealed that the conserved P7 His69 is buried at the center of a well formed hydro-phobic pocket. This pocket is lined by nonpolar residues, Gly53, Leu55, and aromatic residues, Phe54, Phe67, and Trp68 (Fig. 1D). By contrast, the P9 His66 mapped to the interface between the propeptide and the mature enzyme. In the homologous bacterial subtilisins, the interface between the propeptide and mature domains is dynamic with a relatively high average B factor (28), and increasing these dynamics can decrease the rates of autoprocessing (32). Furthermore, studies have established that modulating the propeptide binding to this interface by varying solvent conditions prolongs its release and hence the activation of the mature protease suggesting that this interface would be solvent-accessible (19). Therefore, our modeling analysis predicted that protonation of the His69 located in a hydrophobic pocket could have dramatic effects on the structure of the propeptide unlike protonation of the solvent-accessible His66.
FIGURE 1. Homology modeling of the furin propeptide.
A, multiple sequence alignment between propeptides of the PC family. Predicted secondary structure of the furin propeptide and sequence conservation (black highlight, 100% similarity; dark gray highlight, >80% similarity, light gray highlight, >50% similarity) between PCs is depicted. Propep-tide residues are numbered in reference to the furin propeptide, which begins at Gln25 (18). B, ribbon representation of the furin propeptide structure obtained by homology modeling. His66 and His69 (blue) and Arg75 (red) are highlighted. C, surface representation of the propeptide-furin complex. The modeled propeptide (yellow) is docked onto the active site of furin (green). The internal propeptide cleavage site (red) and His69 (blue) are highlighted. D, surface representation of the secondary cleavage site illustrates His69 (blue) buried in the solvent-accessible pocket formed by Gly53, Phe54, Leu55, Phe67, and Trp68 hydrophobic residues (yellow).
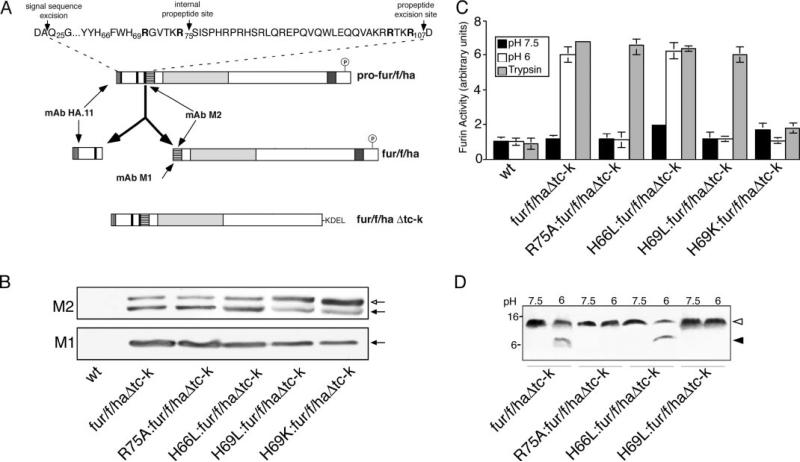
To test the importance of the two histidine residues for the pH-dependent profurin activation step in vitro, we measured the ability of profurin mutants containing a H66L or H69L substitution to undergo activation. We also tested a profurin variant H69K that mimics a constitutively protonated histidine residue, which the modeling analysis predicted would block formation of the propeptide hydrophobic pocket (Fig. 1D). As a control, we expressed a profurin construct that contains an R75A substitution at the P1 position of the internal cleavage site, which blocks profurin activation but not propeptide excision or ER export of the furin-propeptide complex (14). We assayed each variant using ER-localized profurin constructs in which the transmembrane and cytosolic domains were replaced by the luminal -Lys-Asp-Glu-Leu (KDEL) ER localization motif (Fig. 2A, referred to as Δtc-k constructs). The KDEL motif restricts the furin reporters to the neutral pH environment of the ER where they are able to undergo the first propeptide excision at Arg107 to form a furin-propeptide complex but are blocked from undergoing the second internal propeptide cleavage at Arg75 (14). To monitor propeptide excision at Arg107, we inserted a FLAG epitope between the C terminus of the propeptide and the N terminus of the catalytic domain. The FLAG epitope is recognized by the anti-FLAG mAb M2, which detects profurin and mature furin. Alternatively, mAb M1 requires exposure of the FLAG tag at the N terminus and therefore only recognizes mature furin following autoproteolytic excision of the propeptide at Arg107 (Fig. 2A). The constructs also contain an HA epitope tag in the propeptide to monitor the fate of this domain. Insertion of these epitope tags has no effect on the activation, activity, or the trafficking of furin (18, 26).
FIGURE 2. His69 controls the pH-sensitive furin propeptide cleavage and enzyme activation in vitro.
A, schematic representation of the furin constructs used in this study. Shown is full-length profurin containing the N-terminal prodomain with the excision and internal cleavage sites (black bars), the catalytic domain (light gray segment), the transmembrane domain (dark gray segment), and the cytosolic domain, which is phosphorylated by CK2 (circled P) to promote binding to PACS proteins (15, 17, 25). A FLAG epitope tag (horizontal bars) was inserted at the N terminus of mature furin such that the FLAG N terminus is exposed upon propeptide excision. mAb M2 recognizes either the blocked (profurin) or N-terminally exposed (mature furin) FLAG tag, whereas mAb M1 recognizes specifically the N-terminally exposed FLAG tag (mature furin). An HA epitope tag (vertical bars), which is recognized by mAb HA.11, was inserted at the N terminus of the prodomain. The sequence of the prodomain is shown at the top with the signal peptidase cleavage site as well as the propeptide excision and internal cleavage sites highlighted by vertical arrows. The P1/P4 Arg residues of the excision site and the P1/P6 Arg residues of the internal cleavage site are in boldface font. ER-localized, luminally restricted furin constructs (designated Δtc-k) were generated by replacing the transmembrane domain and cytosolic domains with the Lys-Asp-Glu-Leu (KDEL) ER localization signal. B, cell extracts from BSC-40 cells expressing a control viral vector (wt) or the indicated -Δtc-k ER-localized furin constructs were separated by SDS-PAGE and analyzed by Western blot using mAbs M1 or M2. Open arrow, profurin. Filled arrow, mature furin. C, membrane preparations from cells used in B were tested for furin activity using the Abz-Arg-Val-Lys-Arg-Gly-Leu-Ala-Tyr(NO2)-Asp-OH substrate, after incubation at pH 6.0 or 7.5 for 3 h, or at pH 7.5 for 1 h in the presence of trypsin followed by soybean trypsin inhibitor to block residual trypsin activity. Error bars represent the mean and S.E. of three independent experiments. D, membranes preparations from C were analyzed by 15% Tris-Tricine SDS-PAGE followed by Western blot using mAb HA.11. Mr values of the excised propeptide and the cleaved N-terminal propeptide fragment are indicated. Open arrowhead, intact propeptide. Filled arrowhead, cleaved propeptide.
Western blot analysis of extracts from cells expressing each reporter construct revealed a prominent doublet of profurin (mAb M2-positive) and mature furin (mAbs M2- and M1-positive; Fig. 2B). Ratiometric comparison of the mAb M2 cross-reactive bands showed that the R75A or H66L substitutions had no measurable effect on propeptide excision relative to the control (65% mature furin each). However, the H69L and H69K substitutions reduced the efficiency of propeptide excision (30 and 20% of mature furin, respectively), suggesting that His69 may be essential for folding, stability, or activation of the mature enzyme. The presence of the mature furin in each sample enabled us to test the ability of each ER-localized reporter to be activated in vitro following preincubation at pH 7.5 or 6.0 (Fig. 2C). In accordance with our previous studies (14, 18), fur/f/haΔtc-k, which contains the native propeptide sequence (see Fig. 2A), showed no detectable furin activity above a control sample when assayed at pH 7.5, whereas preincubation at pH 6.0 triggered a robust increase in furin activity. This increased activity was coupled with the cleavage of the furin propeptide (Fig. 2D) and was blocked by the furin-specific inhibitor α1-PDX (Ki = 0.6 nm (33, 34) and data not shown), demonstrating the pH-dependent autoproteolytic cleavage of the furin propeptide to unmask the active furin endoprotease. In addition, pretreatment of fur/f/haΔtc-k at pH 7.5 with trypsin to digest the bound inhibitory propeptide also activated furin, whereas the R75A:fur/ f/haΔtc-k construct, which cannot undergo propeptide cleavage at the internal site (14), failed to be activated by preincubation at pH 6.0 but could be activated by trypsinolysis of the propeptide. This demonstrates that the R75A substitution had no effect on the folding of the catalytic domain but blocked sensitivity of the furin-propeptide complex to acid pH. Like the native propeptide sequence, we found that the H66L substitution had no effect on the pH-triggered activation and propeptide release or on the trypsin-mediated unmasking of furin, suggesting His66 does not play an essential role in the pH-induced profurin activation (Fig. 2, C and D). However, the H69L substitution at the P7 subsite completely blocked the ability of acid pH to trigger furin activation and propeptide cleavage but had no effect on the trypsin-mediated activation step, demonstrating that H69L:fur/f/haΔtc-k was correctly folded but unresponsive to an acidic environment (Fig. 2, C and D). By contrast, H69K:fur/f/haΔtc-k failed to be activated by acidic pH or trypsinolysis (Fig. 2C). Together with the extremely inefficient formation of processed furin in cells expressing H69K:fur/f/haΔtc-k (Fig. 2B), our results suggest that the constitutively protonated H69K substitution affects correct folding and stabilization of the endoprotease in the ER.
The analysis of ER-localized profurin reporter constructs suggested that His69 has an essential role in the pH-dependent activation of full-length furin in late secretory pathway compartments. To test this possibility, we expressed full-length epitope-tagged furin (Fig. 2A, fur/f/ha) or furin mutants containing the H69L (H69L:fur/f/ha) or H69K (H69K: fur/f/ha) substitutions in cells. Western blot and enzyme activity assays of the profurin constructs showed marked differences in the efficiency of propeptide excision and enzyme activation (Fig. 3A). Analysis of fur/f/ha revealed a prominent doublet of equal intensity composed of profurin (mAb M2-positive) and mature furin (mAb M2- and M1-positive; Fig. 3A), which correlated with a robust increase in furin enzyme activity (Fig. 3B). H69K:fur/f/ha exhibited dramatically reduced propeptide excision and, correspondingly, enzyme activity near background levels. The near absence of mAb M1-positive mature furin in cells expressing H69K:fur/f/ha did not appear to result from instability of the mature domain as pulse-chase analysis or treatment of cells with proteosome or lysosomal hydrolase inhibitors failed to result in the generation of increased amounts of mature furin (data not shown). Consistent with the corresponding Δtc-k construct (Fig. 2), H69L:fur/f/ha underwent propeptide excision at Arg107 ↓ but did not produce active enzyme (Fig. 3, A and B).
FIGURE 3. The protonation state of His69 controls propeptide excision and enzyme activation in vivo.
A, cell extracts from BSC-40 cells expressing a control viral vector (wt) or full-length epitope-tagged furin molecules containing the indicated mutations were separated by SDS-PAGE and analyzed by Western blot with mAbs M1 and M2. Profurin (open arrow) and mature furin (filled arrow) are indicated. B, crude membrane preparations of cells used in A were tested for furin activity at pH 7.5 using the Abz-Arg-Val-Lys-Arg-Gly-Leu-Ala-Tyr(NO2)-Asp-OH substrate peptide. Error bars represent the mean and S.E. of three independent experiments.
The block of propeptide excision in cells expressing H69K:fur/f/ha and the lack of active furin in cells expressing H69L:fur/f/ha, despite correct propeptide excision, raised the possibility that the His69 mutations altered trafficking of these furin variants. Immunofluorescence microscopy analysis demonstrated that both fur/f/ha and H69L:fur/f/ha were co-localized with the TGN marker TGN46 (Fig. 4A). By contrast, H69K:fur/f/ha remained at the ER, similar to profurin and other furin mutants unable to undergo propeptide excision (14, 18). Our finding that the steady-state localization of H69L:fur/f/ha was at the TGN (Fig. 4A), but that this construct was enzymatically inactive (Fig. 3B), suggested that the inhibitory propeptide remained associated with furin. To test this possibility, we incubated cells expressing fur/f/ha or H69L:fur/f/ha with [3H]Arg and [3H]Leu to label the propeptide and then harvested the cells at increasing chase times. Mature furin proteins were immunoprecipitated with mAb M1, and co-immunoprecipitated propeptide molecules were resolved by SDS-PAGE followed by fluorography (Fig. 4B). This analysis showed that the native propep-tide released slowly from furin (t1/2 = 105 min) in agreement with earlier studies (18). By contrast, the H69L mutant propeptide failed to dissociate from mature furin during the 4-h course of this experiment. The inability for the H69L mutant propeptide to dissociate from mature furin in BSC-40 cells correlated with the absence of furin activity in extracts from cells expressing H69L:fur/f/ha (Fig. 3). As a control, we investigated the ability of H69L:fur/f/ha to internalize from the cell surface by mAb M1 uptake. Cells were incubated with mAb M1 to allow furin-dependent internalization of the antibody and then processed for immunofluorescence microscopy (Fig. 4C). We found that fur/f/ha and H69L:fur/f/ha internalized mAb M1 to the paranuclear region demonstrating that the H69L mutation had no obvious effect on the highly regulated trafficking itinerary of mature furin within the TGN/endosomal system.
FIGURE 4. The protonation state of His69 affects the subcellular localization of furin.
A, immunofluorescence microscopy of BSC-40 cells expressing fur/f/ha, H69L:fur/f/ha, or H69K:fur/ f/ha. Cells were fixed and stained with anti-furin PA1-062 and anti-TGN46 and then visualized with species-specific fluorescently labeled secondary antibodies. B, BSC-40 cells expressing fur/f/ha or H69L:fur/f/ha were pulse-labeled for 30 min with 100 μCi each of [3H]Arg and [3H]Leu and then chased with excess unlabeled Arg and Leu at 37 °C for the indicated times. Cell extracts were prepared, and mature furin molecules were immunoprecipitated with mAb M1, separated by 15% Tris-Tricine SDS-PAGE, and co-precipitating propeptide molecules (open arrowheads) were detected by fluorography and quantified by densitometry. C, immunofluorescence microscopy of BSC-40 cells expressing fur/f/ha or H69L:fur/f/ha. Cells were incubated with mAb M1 in the culture medium (30 μg/ml) for 1 h, and internalized mAb M1 was detected with a fluorescently labeled secondary antibody.
Our determination that H69K:fur/f/ha, similar to profurin (13, 25, 33), fails to undergo efficient autoproteolytic propeptide excision (Fig. 3) and is restricted to the ER (Fig. 4) suggested that this furin construct would enable us to identify the sorting machinery that restricts profurin to the ER. One candidate profurin ER sorting protein is PACS-2, which combines with COPI to localize membrane proteins containing phosphorylated acidic cluster motifs, such as that present on the furin cytosolic domain, to the ER (17, 25). Therefore, we examined whether these molecules are required to localize H69K:fur/f/ha to the ER. First, we used metabolic labeling studies with 32Pi to show that, like furin, H69K: fur/f/ha is phosphorylated in vivo (Fig. 5, A and ref. 35). Second, protein-protein binding studies showed PACS-2 bound selectively to the phosphorylated furin cytosolic domain (Fig. 5B). Third, we depleted cells of PACS-2 and the β-COP subunit of COPI to determine whether these proteins are required for the ER localization of H69K:fur/f/ha. Treatment of A7 cells with PACS-2 or β-COP siRNA caused a marked reduction in their respective protein levels after 48 h without causing cell toxicity (Fig. 5C and data not shown). In addition, depletion of either PACS2 or COPI caused H69K:fur/f/ha to redistribute from the ER to the Golgi/TGN without affecting the subcellular localization of the luminal ER chaperone protein-disulfide isomerase (PDI) under the experimental conditions used (Fig. 5D). Our finding is consistent with other studies reporting that PDI is localized to the ER by a retention-based mechanism in addition to the canonical COPI-dependent retrieval-based mechanism (36). These results suggest that PACS-2 combines with COPI to mediate the ER localization of profurin and that propeptide excision releases the furin-propeptide complex from PACS-2, allowing the mature enzyme to traffic to the TGN/endosomal system. Moreover, these data support our model that protonation of His69 disrupts the hydrophobic pocket in the propeptide, possibly by preventing correct folding of the enzyme and excision of the propeptide at Arg107.
FIGURE 5. PACS-2 and COPI localize H69K:fur/f/ha to the ER.
A, A7 cells expressing fur/f/ha or H69K:fur/f/ha were metabolically labeled with 32Pi, lysed, immunoprecipitated (IP) with mAb M2, separated by SDS-PAGE, and analyzed by autoradiography (upper panel) and Western blot (WB) using anti-furin PA1-062 (lower panel). B, glutathione S-transferase or GST-FurinCD, which contains the 56-amino acid furin cytosolic domain, was preincubated or not with CK2 and then incubated with thioredoxin-tagged PACS-2 FBR, which encodes the cargo binding region of PACS-2 (17, 25), separated by SDS-PAGE, and analyzed by Western blot using anti-Trx mAb. C, A7 cells were treated with control (scr.) or siRNAs specific for PACS-2 or β-COP for 48 h, lysed, separated by SDS-PAGE, and analyzed by Western blot using anti-PACS-2 and anti-β-COP antibodies. The blots were also incubated with anti-tubulin mAb to control for protein loading. D, A7 cells were treated with the indicated siRNAs for 48 h and then infected with virus expressing H69K:fur/f/ha for an additional 4 h. The cells were then processed for immunofluorescence microscopy and stained with mAb HA.11 and anti-PDI followed by subtype-specific fluorescently labeled secondary antibodies.
Our results suggest that one role for protonation of His69 in the TGN/endosomal system is to disrupt the propeptide structure, thereby promoting cleavage of the internal propeptide cleavage site at Arg75. However, recent studies suggest that protonation of a His residue proximal to furin cleavage sites enhances proteolysis (10, 11). Therefore, to test whether protonation of His69 increases the efficiency of the internal propeptide cleavage, we conducted a kinetic analysis using internally quenched fluorogenic peptide substrates. The peptide substrates were designed with either the native propeptide excision site at Arg107 ↓ (PS-1; Table 1), the propeptide internal cleavage site at Arg75 ↓ containing His69 (PS-2), or the H69L (PS-2:H69L) or H69K (PS-2:H69K) substitutions. The PS-1 substrate exhibited a low Km value at both neutral and acidic pH (Km = 1.99 and 1.62 μm, respectively). However, the PS-2 substrate revealed a preference for cleavage at pH 6.0 with a 3.6-fold lower Km value and increased cleavage efficiency. Unlike PS-2, PS-2: H69L exhibited a markedly high Km value and a low cleavage efficiency irrespective of pH. By contrast, PS-2:H69K, which mimics a constitutively protonated P7 residue, had a low Km value at both pH 7.5 and pH 6.0. Together, our data suggest that His69 is a pH sensor that allows enzyme activation following transport of the furin-propeptide complex from the ER to the mildly acidic TGN/endosomal system and that protonated His69 has the following dual role, it disrupts the propeptide to expose the internal cleavage site and increases the efficiency of cleavage at Arg75 to yield the active enzyme.
TABLE 1.
Peptidyl substrates
| Peptide sequence | pH | Km | k cat | kcat/Km | kcat/Km(relative) | |
|---|---|---|---|---|---|---|
| μ m | s–1 | s–1m–1 | ||||
| PS-1a | Abz-Ala-Lys-Arg-Arg-Thr-Lys-Arg107 ↓ Asp-Val-Tyr(NO2)-Ala | 7.5 | 1.99 | 1.90 | 9.58 × 105 | 1.000 |
| 6.0 | 1.62 | 1.03 | 6.34 × 105 | 0.661 | ||
| PS-2a | Abz-His-Arg-Gly-Val-Thr-Lys-Arg75 ↓ Ser-Leu-Tyr(NO2)-Ala | 7.5 | 23.75 | 6.37 | 2.68 × 105 | 0.279 |
| 6.0 | 6.59 | 2.92 | 4.43 × 105 | 0.458 | ||
| PS-2 H69K | Abz-Lys-Arg-Gly-Val-Thr-Lys-Arg75 ↓ Ser-Leu-Tyr(NO2)-Ala | 7.5 | 8.01 | 2.05 | 2.56 × 105 | 0.267 |
| 6.0 | 11.95 | 1.61 | 1.35 × 105 | 0.141 | ||
| PS-2 H69L | Abz-Leu-Arg-Gly-Val-Thr-Lys-Arg75 ↓ Ser-Leu-Tyr(NO2)-Ala | 7.5 | 38.20 | 2.90 | 7.59 × 104 | 0.079 |
| 6.0 | 45.93 | 2.66 | 5.79 × 104 | 0.060 |
Data are from Ref. 14.
DISCUSSION
We report the identification of His69 in the furin prodomain as a pH sensor that guides the multistep- and compartment-specific autoproteolytic activation of furin. We used cellular and cell-free furin activation assays to show that a nonpolar H69L substitution had no effect on propeptide excision at Arg107 (Fig. 2), nor on the TGN localization or the endosomal trafficking of the furin-propeptide complex (Fig. 4). However, this substitution blocked the acid pH-dependent cleavage of the furin propeptide at the internal Arg75 site and the release of the propeptide from the furin-propeptide complex (Figs. 2 and 4). By contrast, an H69K substitution, which mimics constitutively protonated histidine, blocked propeptide excision at Arg107, trapping the profurin molecule in the ER by a mechanism requiring the sorting protein PACS-2 and COPI (Figs. 2, 4, and 5).
Our experimental studies and modeling analysis together demonstrate a possible mechanism for the His69 protonation-dependent furin activation. In the neutral pH environment of the ER, unprotonated His69 is buried in a solvent-accessible hydrophobic pocket, stabilized by nonpolar amino acids, including Gly53, Leu55, Phe54, Phe67, and Trp68. This hydrophobic pocket may guide the correct folding of the catalytic domain while protecting the internal propeptide cleavage site. Once folded, the catalytic domain autoproteolytically excises the propeptide at Arg107 to form a transport-competent furin-propeptide complex that transits to the mildly acidic TGN/endosomal system. Profurin molecules unable to efficiently fold and undergo autoproteolysis remain in the ER by a PACS-2- and COPI-dependent mechanism (Fig. 5). Upon exposure to the mildly acidic TGN/endosomal system, protonation of His69 disrupts hydrophobic interactions, thereby resulting in the partial unfolding of the hydrophobic pocket. This puckering exposes the internal cleavage site, triggering cleavage at Arg75 to release the propeptide and unmask the catalytic center for substrate cleavage in trans. Thus, the H69L mutation would stabilize the solvent-accessible hydrophobic pocket, permitting propeptide excision at Arg107 and export of the furin-propeptide complex to the TGN/endosomal system. However, this nonprotonatable mimic prevents the pH-dependent cleavage of the propeptide at the internal site (Arg75) and subsequent enzyme activation. Similarly, blocking internal propeptide cleavage by a P1 R75A mutation has no measurable effect on furin folding, propeptide excision, or the pH-dependent puckering of the solvent-accessible hydrophobic pocket but blocks internal propeptide cleavage and enzyme activation (Fig. 2) (14). Hence, trypsinization of the propeptide in vitro is sufficient to recover active enzyme. By contrast, the H69K mutation, which mimics constitutively protonated His, prevents stabilization of the hydro-phobic pocket, blocking correct folding of the propeptide and propep-tide excision at Arg107, leading to the accumulation of the inactive profurin in the ER (Fig. 4). Similarly, a V72R mutation, which converts the P1/P6 Arg internal propeptide cleavage site to a consensus P1/P4 Arg site, blocks profurin folding and ER export by also destabilizing the solvent-accessible hydrophobic pocket (14).
NMR solution structures of the furin and PC1/3 propeptides suggest the molecular basis for the pH-dependent activation of the PCs. At neutral pH, the PC1/3 propeptide consists of four β-sheets and two α-helices in a β-α-β-β-α-β arrangement, with the internal cleavage site located in an extended loop between the β3 and α2 segments (37). Tangrea et al. (37) proposed that His66, which is present in furin but not PC1, serves as the pH sensor of the furin promoting dissociation of the catalytic and prodomains, whereas His69, which is conserved in all PCs (Fig. 1A), would point away from the putative binding surface and therefore would not contribute to the pH-dependent activation of furin. In addition, a separate study found that the furin prodomain was in a molten globule state at neutral pH with a measurable core and at acidic conditions undergoes unfolding largely in its C-terminal half that includes the internal cleavage site (38). Our results suggest four key differences underlying the mechanism of profurin activation compared with these studies. First, we found that His69 but not His66 has an overriding role as the pH sensor controlling furin activation. Second, our modeling studies indicate that unprotonated His69 is buried in a solvent-accessible hydrophobic pocket that guides the folding of the catalytic domain. This role for unprotonated His69 in the ER explains why the H69K mutation blocks propeptide excision of profurin, leading to restriction of the misfolded proenzyme to the ER. Third, following transport of the furin-propeptide complex to the mildly acidic TGN/ endosomal system, protonation of His69 disrupts the hydrophobic interactions, thereby exposing the internal cleavage site to the catalytic center. Fourth, protonation of His69 has an additional role in increasing the efficiency of propeptide cleavage (Table 1).
The utility of a protonatable His residue to regulate compartment specificity of furin cleavage is not restricted to the furin propeptide but is also used to order the furin-dependent cleavages of pro-BMP-4 and pro-α4 integrin in a compartment-specific manner (10, 11), underscoring the broad utility of the His pKa at physiological pH values. In addition, in vitro activation of PC1, which contains the evolutionarily conserved His residue corresponding to His69 of furin, also requires an acidic pH for activation.3 However, a recent study demonstrates that an H69A mutation had no effect on the ability of overexpressed furin to cleave pro-von Willebrand factor in 293 cells (39). This discrepancy between the H69L and H69A substitutions may be attributed to a larger van der Waals volume of Leu, which may result in a more prominent effect on cleavage and activation because of stronger hydrophobic interactions, differences in the assays used to detect furin activity, or perhaps differences in the cell lines used (10, 11).
Our demonstration that localization of H69K:fur/f/ha to the ER requires PACS-2 and COPI (Fig. 5) identifies components of the cellular trafficking machinery that maintain profurin in the ER. We recently identified PACS-2 and reported that it localizes the ion channel polycystin-2 to the ER by linking the CK2-phosphorylated acidic cluster in the polycystin-2 cytosolic domain to COPI (17, 25). Here we report that PACS-2 also binds to the CK2-phosphoryated furin cytosolic domain, and, like furin and profurin, H69K:fur/f/ha is phosphorylated in vivo (Fig. 4) (35). Thus, dephosphorylation of the furin cytosolic domain by a furin phosphatase (40) may be key for allowing ER export of the furinpropeptide complex. Alternatively, ER export of the furin-propeptide complex may occur if the PACS-2-binding site is masked, perhaps by furin dimerization (41). Regardless, our studies identify new components of the cellular sorting machinery that cooperate with the His69-based pH sensor to promote the stepwise- and compartment-specific activation of furin, and which may also control the activation of other PCs and secretory pathway proteins.
Acknowledgments
We thank E. Anderson for efforts in the early stages of this project, J. Larson for helpful discussions, and R. Sitia for the PDI antiserum.
Footnotes
This work was supported by National Institutes of Health Grants DK37274 and AI49793 (to G. T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: PC, proprotein convertase; ER, endoplasmic reticulum; TGN, trans-Golgi network; HA, hemagglutinin; mAb, monoclonal antibody; MEM, minimal essential medium; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; VV, vaccinia virus; m.o.i., multiplicity of infection; siRNA, short interfering RNA; PDI, protein-disulfide isomerase; Abz, 2-aminobenzoic acid.
S. S. Molloy and G. Thomas, unpublished results.
REFERENCES
- 1.Steiner DF. Curr. Opin. Chem. Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 2.Thomas G. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Lindberg I. J. Biol. Chem. 1993;268:5615–5623. [PubMed] [Google Scholar]
- 4.Zhou A, Webb G, Zhu X, Steiner DF. J. Biol. Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes CJ, Thorne BA, Lincoln B, Nielsen E, Hutton JC, Thomas G. J. Biol. Chem. 1993;268:4267–4275. [PMC free article] [PubMed] [Google Scholar]
- 6.Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. J. Biol. Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- 7.Krysan DJ, Rockwell NC, Fuller RS. J. Biol. Chem. 1999;274:23229–23234. doi: 10.1074/jbc.274.33.23229. [DOI] [PubMed] [Google Scholar]
- 8.Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Chem. Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]
- 9.Henrich S, Cameron A, Bourenkov GP, Kiefersauer R, Huber R, Lindberg I, Bode W, Than ME. Nat. Struct. Biol. 2003;10:520–526. doi: 10.1038/nsb941. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron E, Basak A, Decroly E, Seidah NG. Biochem. J. 2003;373:475–484. doi: 10.1042/BJ20021630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degnin C, Jean F, Thomas G, Christian JL. Mol. Biol. Cell. 2004;15:5012–5020. doi: 10.1091/mbc.E04-08-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockwell NC, Thorner JW. Trends Biochem. Sci. 2004;29:80–87. doi: 10.1016/j.tibs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Zhou A, Paquet L, Mains RE. J. Biol. Chem. 1995;270:21509–21516. doi: 10.1074/jbc.270.37.21509. [DOI] [PubMed] [Google Scholar]
- 14.Anderson ED, Molloy SS, Jean F, Fei H, Shimamura S, Thomas G. J. Biol. Chem. 2002;277:12879–12890. doi: 10.1074/jbc.M108740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 16.Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA, Thomas G. EMBO J. 2001;20:2191–2201. doi: 10.1093/emboj/20.9.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. EMBO J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G. EMBO J. 1997;16:1508–1518. doi: 10.1093/emboj/16.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbian E, Yabuta Y, Shinde UP. J. Mol. Biol. 2005;347:367–383. doi: 10.1016/j.jmb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Zamyatnin AA. Annu. Rev. Biophys. Bioeng. 1984;13:145–165. doi: 10.1146/annurev.bb.13.060184.001045. [DOI] [PubMed] [Google Scholar]
- 21.Wiebe CA, Dibattista ER, Fliegel L. Biochem. J. 2001;357:1–10. doi: 10.1042/0264-6021:3570001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotzschke O, Lau JM, Hofstatter M, Falk K, Strominger JL. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16946–16950. doi: 10.1073/pnas.212643999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen FB. Acta Physiol. Scand. 2004;182:215–227. doi: 10.1111/j.1365-201X.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- 24.Baukrowitz T, Tucker SJ, Schulte U, Benndorf K, Ruppersberg JP, Fakler B. EMBO J. 1999;18:847–853. doi: 10.1093/emboj/18.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwede T, Kopp J, Guex N, Peitsch MC. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain SC, Shinde U, Li Y, Inouye M, Berman HM. J. Mol. Biol. 1998;284:137–144. doi: 10.1006/jmbi.1998.2161. [DOI] [PubMed] [Google Scholar]
- 29.Tangrea MA, Alexander P, Bryan PN, Eisenstein E, Toedt J, Orban J. Biochemistry. 2001;40:5488–5495. doi: 10.1021/bi0026472. [DOI] [PubMed] [Google Scholar]
- 30.Comellas-Bigler M, Maskos K, Huber R, Oyama H, Oda K, Bode W. Structure (Camb.) 2004;12:1313–1323. doi: 10.1016/j.str.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Subbian E, Yabuta Y, Shinde U. Biochemistry. 2004;43:14348–14360. doi: 10.1021/bi048397x. [DOI] [PubMed] [Google Scholar]
- 32.Inouye M, Fu X, Shinde U. Nat. Struct. Biol. 2001;8:321–325. doi: 10.1038/86194. [DOI] [PubMed] [Google Scholar]
- 33.Anderson ED, Thomas L, Hayflick JS, Thomas G. J. Biol. Chem. 1993;268:24887–24891. [PubMed] [Google Scholar]
- 34.Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones BG, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, Thomas G. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorner AJ, Wasley LC, Raney P, Haugejorden S, Green M, Kaufman RJ. J. Biol. Chem. 1990;265:22029–22034. [PubMed] [Google Scholar]
- 37.Tangrea MA, Bryan PN, Sari N, Orban J. J. Mol. Biol. 2002;320:801–812. doi: 10.1016/s0022-2836(02)00543-0. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharjya S, Xu P, Xiang H, Chretien M, Seidah NG, Ni F. Protein Sci. 2001;10:934–942. doi: 10.1110/ps.41301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bissonnette L, Charest G, Longpre JM, Lavigne P, Leduc R. Biochem. J. 2004;379:757–763. doi: 10.1042/BJ20031902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molloy SS, Thomas L, Kamibayashi C, Mumby MC, Thomas G. J. Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolins N, Bosshart H, Kuster H, Bonifacino JS. J. Cell Biol. 1997;139:1735–1745. doi: 10.1083/jcb.139.7.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]