Abstract
Environmental factors routinely influence an organism’s biology. The inheritance or transmission of such influences to descendant generations would be an efficient mode of information transfer across generations. The developmental stage at which a specific environment is encountered by the ancestral generation, and the number of generations over which information about that environment is registered, determines an inter- vs. trans-generational effect of ancestral influence. This commentary will outline the distinction between these influences. While seductive in principle, inter- and trans-generational inheritance in mammals is a hotly debated area of research inquiry. We present constructive criticism of such inheritance, and suggest potential experimental avenues for reconciliation. Finally, epigenetic mechanisms present an avenue for gene regulation that is dynamic. We briefly discuss how such malleability affords the potential for a reversal of any detrimental environmental influences that might have adversely impacted ancestral or descendant generations.
Keywords: epigenetics, inheritance, non-coding RNA, olfaction
Adapting to changing environments is often critical for survival. Events such as malnourishment, childhood maltreatment, terrorist attacks, and war violence have been shown to profoundly affect the exposed generation. Examples of the influence of ancestral environmental perturbations on descendant biology abound and are accumulating. For example, exposure to famine during the Dutch Hunger Winter of 1944 profoundly affected the F1 generation gestating in utero at the time that the F0 generation was subjected to famine conditions. Notably, that next, F1, generation went on to have a higher propensity for developing diabetes and obesity. What is striking is that this F1 generation, despite now living in non-impoverished conditions, gave birth to an F2 generation that also suffered from obesity and diabetes [1]. In another example, the more recent 9/11 terrorist attacks in New York saw the detection of lower cortisol levels in the 1-year old offspring that were gestating in utero at the time that their mothers witnessed the attacks [2]. Lower cortisol levels are predictive of PTSD-like symptoms, across generations, as has been shown in the case of adult offspring of Holocaust survivors [3]. We now possess the tools to begin to ask how such ancestral experiences could influence subsequent generations [4, 5].
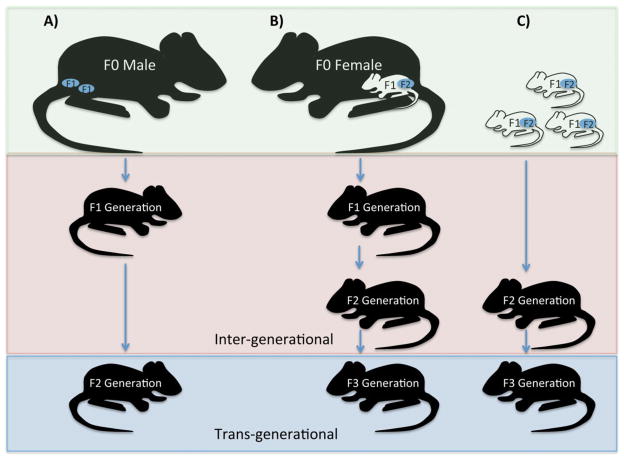
When discussing the influence of ancestral environments on descendant generations, distinctions between inter-and trans-generational influences must be made (Fig. 1). Inheritance implies information transfer via the germ-line (sperm and eggs); a phenomenon that cannot be disentangled if the germ cells of the descendant generations are themselves affected by the ancestral environment. The perturbation in the ancestral environment of pregnant females was also experienced by the descendant generation, in utero, and as such presents an example of inter-generational inheritance of ancestral exposures in the F1 and F2 generations. This is because of the in utero nature of the ancestral perturbation affecting not only the somatic and germ cells of the developing F1 fetus, but also the germ cells of the F2 generation. Effects must also be observed in the F3 generation to be considered trans-generational. In contrast, if the paternal environment were altered, any consequent effects on the F1 generation would be considered inter-generational, while persistence into the F2 generation would be considered trans-generational. The above discussed historical events and their effects are thus examples of inter-generational inheritance. For trans-generational inheritance to be supported, the F3 generation would have to be similarly affected.
Figure 1.
Inter- and trans-generational influences of ancestral environments in descendant populations are often investigated by manipulating the environment of A: the paternal ancestor, B: a pregnant maternal ancestor, or C: a peri-natal population. Exposing the paternal ancestor to an environmental perturbation affects not only the ancestor but also his sperm and as a consequence the F1 generation in an inter-generational manner. The persistence of any effects in the F2 generation would be considered true trans-generational inheritance. In the case of the maternal environment being altered during gestation, or early peri-natal environments like maternal care being manipulated, a trans-generational effect should survive into the F3 generation.
Should such intergenerational and trans-generational inheritance occur, what mechanisms might underlie these remarkable phenomena? With advances in modern molecular and genetic technology, epigenetic mechanisms have been heralded as being central to such inheritance and have come to represent the intersection of the genome with the environment [5, 6]. Complementing this thought is the notion that the DNA sequence is itself not affected by the environmental perturbation, but what is affected is whether that sequence is actively read or not. Broadly speaking, epigenetics (epi = over) allows for genetic loci to either be expressed or not by virtue of chemical modifications on histone proteins surrounding the DNA or on the DNA itself, as well as via non-coding RNA inhibiting the transcription and/or translation of a particular gene [7]. Definitive examples of such inheritance have been demonstrated for coat color and tail phenotype in mice [8–10].
Having talked about the observations of inter- and trans-generational effects of ancestral experiences, the distinction between those two effects and a broad scientific discipline that is thought to underlie such observations, how might we leverage animal studies to model and investigate such effects? To do so, animal researchers have typically subjected the ancestral population (F0) to a manipulation and then asked how the descendant generations are affected.
An eloquent example of the impact of maternal care quality on descendant biology has been highlighted by the work of Szyf, Meaney and colleagues [11, 12]. Taking advantage of naturally occurring variation in the maternal care exhibited by female rats post-parturition, these researchers have shown that higher levels of good maternal care at post-natal time-points causes the female rat pups to show the same high quality care toward their own offspring when they care for them. A cross-fostering strategy shows that this phenomenology is socially transmitted from generation to generation. By virtue of the generation in question needing to experience a certain quality of care, such an example speaks to the idea of social transmission of behavioral traits across generations. But note that it is distinctively different from inheritance. This distinction notwithstanding, follow-up studies using this model have provided insight into how alterations of DNA methylation around the glucocorticoid receptor gene in the hippocampus and the estrogen receptor in diverse brain regions profoundly affect behavior and physiology [12].
A model first generated by Michael Skinner’s group exemplifies the trans-generational influence of ancestral experience on descendant behavior. In this model, the exposure of pregnant female rats (F0 generation) to the fungicide, Vincozolin, has been shown to affect male fertility, and mate preference of subsequent generations [13, 14]. Among more recent works that document an inter- or trans-generational inheritance of ancestral experience, rodent F0 generations have been subjected to maltreatment during rearing, social defeat, and stress during adulthood [15–17]. All these studies have demonstrated that the ancestral environment affects anxiety- and depressive-like states in descendant generations. These examples utilize broad perturbations to the ancestral generation and query the inter-and trans-generational effects in the descendants.
Asking how manipulations of specific features of the ancestral environment affect the descendant generations is a useful way to focus effort on where and how in the (epi)genome might the effects of ancestral experience reside. An ecological example of this comes from the work in which the diet of a pregnant mouse female was supplemented with “cherry” or “mint” odors. This resulted in the descendant F1 generation showing a preference for those odors. Accompanying this behavioral preference was increased volumes in the olfactory bulbs of the glomeruli that process cherry (M71 expressing olfactory sensory neurons (OSNs) and glomeruli), and mint (M72 expressing cells and glomeruli) [18]. Manipulating the ancestral paternal environment with specific environmental cues prior to conception and examining the effect of such manipulation on how descendants might perceive those cues has received even less attention, but such studies are accumulating. For example, a recent study exposed F0 rats to cocaine for 60 days and allowed that population to segregate into high and low cocaine self-administering cohorts. The inheritance of behavior toward cocaine in rats sired by these ancestral populations was then measured. Interestingly, the F1 male offspring of F0 high self-administering animals showed a delayed acquisition to self-administer cocaine themselves [19].
Most recently, we subjected F0 mice to olfactory fear conditioning and then asked how descendant generations perceive and process a specific environmental cue after the ancestral population had been conditioned with that cue [20]. Olfactory fear conditioning results in fearful behavior toward the odor that was paired with the foot-shocks. When Acetophenone (an odor that activates the M71 expressing OSN population in the nose) presentations are paired with foot-shocks, more M71 neurons are found in the nose of these trained animals and more axons converge into a larger glomerulus in the olfactory bulb. These initial data suggested that there is sensory plasticity at the level of the primary olfactory system to allow for variable responses to salient olfactory environmental cues. Next, we mated F0 conditioned animals and then assayed behavior and neuroanatomy in descendant generations that had no prior exposure to the Acetophenone except at the time of behavioral testing. Remarkably, F1 males sired by F0 males conditioned to Acetophenone showed an enhanced behavioral sensitivity to Acetophenone. Complementing this behavioral sensitivity to Acetophenone, an increased number of M71 expressing OSNs was counted in the nose of the F1 animals resulting in larger glomeruli in the olfactory bulbs. To establish whether either social transmission or biological inheritance was the cause of these effects, we extended these studies to the F2 generation, performed in vitro fertilization (IVF) with F0 sperm, and incorporated cross-fostering studies into our experimental design. The persistence of the behavioral and neuroanatomical effects after all these approaches led us to conclude that information about the salient odor was being inherited via sperm. Using Acetophenone allowed us to focus on the epigenetic landscape around the M71 gene in the sperm of F0 and F1 males, and ask whether any epigenetic alterations around this gene might correlate with our effects. Analysis of bisulfite converted M71 DNA sequence revealed that the M71 locus is less methylated in F0 sperm as well as F1 sperm when the F0 generation had been conditioned with Acetophenone. We posited that this decreased methylation, which is generally associated with increased gene transcription, could potentially set up the M71 locus to be transcribed in more quantity in the descendant F1 and F2 generations.
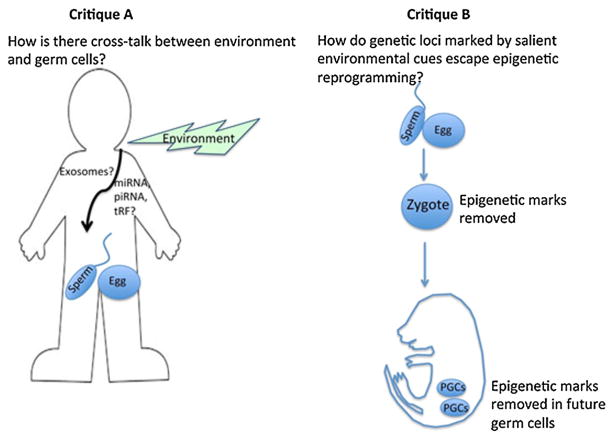
All these data, and those unfortunately not cited due to space constraints, make a case for both the inter- and trans-generational influence of ancestral environments on descendant generations. Constructive critiques of this field of research often center around two main issues [21] (Fig. 2). One, how does information from the environment make its way to the germ cells so as to result in the inheritance previously discussed? Two, if this information does indeed make its way to the germ cells as a consequence of epigenetic modifications, how do these modifications escape the phenomenon of epigenetic reprogramming? Applied to our study [20], these questions then pertain to (1) how does information about the salience of the odor used to condition the F0 generation reach the sperm of that generation, and (2) how would the DNA methylation status around the M71 receptor in the sperm of the F0 and F1 generations escape reprogramming after fertilization.
Figure 2.
Two avenues of scientific inquiry that need to be addressed to lend further credence to the idea of inter- and trans-generational influences on descendant populations. A: How does environmental information reach germ cells for inheritance to occur? One promising prospect is a non-coding RNA mediated mechanism that sees these RNA molecules (microRNA, PIWI-associated RNA, and tRNA derived fragments) carried to the sperm and egg via exosomes through the circulatory system. B: How do epigenetic signatures of genes that are marked as a consequence of ancestral environments escape reprogramming? The reprogramming of epigenetic marks occurs in two waves: soon after fertilization, and again in the germ cells of the developing fetus, and any genetic loci marked by the environment would need to escape these waves.
The first criticism stems from August Weismann’s theory of the germplasm that talks about the barrier that exists between the germ cells and circulation. This suggests that there exists an immunization of the germ cells from any environmental information that the somatic cells might have been privy to. However, the discovery of small RNA species such as microRNA, piRNA, and tRNA-derived RNA fragments (tRFs) at high levels in sperm [22–25], and that of cargo-containing exosomes traveling through circulation [26] present potential conduits between the environment and the germ-cells. In keeping with this idea, miRNA have been shown to be involved in the trans-generational inheritance of the Kit phenotype [10]. There are also the recent data indicating that an increase in non-coding RNA in sperm of a manipulated F0 generation results in an inter-generational effect in the descendants [27]. The question of how any marked loci escape epigenetic reprogramming that occurs soon after fertilization and then again in the primordial germ cells of the developing fetus is a trickier one to answer. Parent-of-origin-allele-specific resistance to reprogramming provides evidence for escape of genetic loci from epigenetic reprogramming [28, 29]. As more epi- and molecular genetic mechanisms of reprogramming come to light, more molecular candidates would come to light as avenues by which this epigenetic reprogramming might be escaped at genetic loci that have been tagged to be salient imprints of ancestral experience.
While one should certainly question this field of research and revise our impression of it as more light is shone on its mechanisms, one should also appreciate the phenomenology of inter- and trans-generational inheritance and the potential dynamic nature of epigenetic modifications that might underlie it. Just as one environment is laying down epigenetic marks at a specific genetic locus, so could another environment strip those marks or lay down an antagonistic set of marks at that same locus. Such malleability in the control of gene expression could well be harnessed to ameliorate the effects of detrimental influences of the ancestral environment, and examination of such processes will be an important practical trajectory of future research.
That an ancestral environment can influence descendant generations is slowly being accepted and debated in light of the accumulating evidence [30]. The mechanisms by which this occurs, and any potential avenues by which descendant generations could be buffered from such influences will undoubtedly see a plethora of continued scientific debate and inquiry.
Acknowledgments
Funding for [20] was provided by the Howard Hughes Medical Institute and the Burroughs Wellcome Fund to K. J. R. In addition, this project was partially funded by the National Center for Research Resources P51RR000165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD011132 to Yerkes National Primate Research Center.
Footnotes
The authors have declared no conflict of interest.
References
- 1.Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci USA. 2010;107:16757–8. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yehuda R, Engel SM, Brand SR, Seckl J, et al. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90:4115–8. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, et al. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. Am J Psychiatry. 2000;157:1252–9. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]
- 4.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13:153–62. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 5.Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84:131–76. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 6.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–8. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–8. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 9.Rakyan VK, Chong S, Champ ME, Cuthbert PC, et al. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci USA. 2003;100:2538–43. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–74. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 11.Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–35. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Weaver ICG, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–5. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anway MD. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews D, Gore AC, Hsu TS, Dangleben NL, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104:5942–6. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz DM, Laplant Q, Watts EL, Hodes GE, et al. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–14. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin TB, Russig H, Weiss IC, Gräff J, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–15. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todrank J, Heth G, Restrepo D. Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proc R Soc B Biol Sci. 2011;278:1949–55. doi: 10.1098/rspb.2010.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, et al. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16:42–7. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heard E, Martienssen RA. Trans-generational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–14. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawano M, Kawaji H, Grandjean V, Kiani J, et al. Novel small noncoding RNAs in mouse spermatozoa, zygotes and early embryos. PLoS One. 2012;7:e44542. doi: 10.1371/journal.pone.0044542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krawetz SA, Kruger A, Lalancette C, Tagett R, et al. A survey of small RNAs in human sperm. Hum Reprod. 2011;26:3401–12. doi: 10.1093/humrep/der329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng H, Shi J, Zhang Y, Zhang H, et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 2012;22:1609–12. doi: 10.1038/cr.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119:646–8. doi: 10.1182/blood-2011-11-389478. [DOI] [PubMed] [Google Scholar]
- 27.Gapp K, Jawaid A, Sarkies P, Bohacek J, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–9. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgel J, Guibert S, Li Y, Chiba H, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nat Genet. 2010;42:1093–100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 29.Lane N, Dean W, Erhardt S, Hajkova P, et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- 30.Gapp K, von Ziegler L, Tweedie-Cullen RY, Mansuy IM. Early life epigenetic programming and transmission of stress-induced traits in mammals: how and when can environmental factors influence traits and their transgenerational inheritance? BioEs-says. 2014;36:491–502. doi: 10.1002/bies.201300116. [DOI] [PubMed] [Google Scholar]