Abstract
Background
Civilian posttraumatic stress disorder (PTSD) and combat PTSD are major public health concerns. Although a number of psychosocial risk factors have been identified related to PTSD risk, there are no accepted, robust biological predictors that identify who will develop PTSD or who will respond to early intervention following trauma. We wished to examine whether genetic risk for PTSD can be mitigated with an early intervention.
Method
65 emergency department patients recruited in 2009–2010 at Grady Memorial Hospital in Atlanta, Georgia, who met criterion A of DSM-IV PTSD received either 3 sessions of an exposure intervention, beginning in the emergency department shortly after trauma exposure or assessment only. PTSD symptoms were assessed 4 and 12 weeks after trauma exposure. A composite additive risk score was derived from polymorphisms in 10 previously identified genes associated with stress-response (ADCYAP1R1, COMT, CRHR1, DBH, DRD2, FAAH, FKBP5, NPY, NTRK2, and PCLO), and gene x treatment effects were examined. The intervention included 3 sessions of imaginal exposure to the trauma memory and additional exposure homework. The primary outcome measure was the PTSD Symptom Scale-Interview Version or DSM-IV–based PTSD diagnosis in patients related to genotype and treatment group.
Results
A gene x intervention x time effect was detected for individual polymorphisms, in particular the PACAP receptor, ADCYAP1R1, as well as with a combined genotype risk score created from independent SNP markers. Subjects who did not receive treatment had higher symptoms than those who received intervention. Furthermore, subjects with the “risk” genotypes who did not receive intervention had higher PTSD symptoms compared to those with the “low-risk” or “resilience” genotypes or those who received intervention. Additionally, PTSD symptoms correlated with level of genetic risk at week 12 (P < .005) in the assessment-only group, but with no relationship in the intervention group, even after controlling for age, sex, race, education, income, and childhood trauma. Using logistic regression, the number of risk alleles was significantly associated with likelihood of PTSD diagnosis at week 12 (P < .05).
Conclusions
This pilot prospective study suggests that combined genetic variants may serve to predict those most at risk for developing PTSD following trauma. A psychotherapeutic intervention initiated in the emergency department within hours of the trauma may mitigate this risk. The role of genetic predictors of risk and resilience should be further evaluated in larger, prospective intervention and prevention trials.
Trial Registration
ClinicalTrials.gov identifier: NCT00895518
Unlike for other psychiatric disorders, the precipitant for posttraumatic stress disorder (PTSD) is a known event, allowing for immediate intervention with the potential to prevent PTSD’s occurrence. Currently, there are limited treatments to prevent PTSD in the acute aftermath of a trauma. One recently reported promising intervention1 compared an immediate intervention to assessment only in emergency department patients presenting after a DSM-IV criterion A traumatic event. The immediate intervention was aimed at preventing the development of PTSD and utilized components of prolonged exposure (PE), an empirically supported treatment for chronic PTSD that has not been tested immediately after trauma exposure. Results of this study indicated that the early intervention was effective at reducing the severity of reactions in those receiving the intervention compared to the control group.1 While identifying an intervention that can effectively prevent PTSD represents important progress, many individuals exposed to traumatic events will recover spontaneously and may not require intervention.2 An important goal of future research should be to determine who is at high risk for developing chronic PTSD so that efficacious interventions can be applied in a targeted fashion.3
Many research studies have sought to identify predictors of PTSD, including 2 meta-analyses4,5 that identified significant risk factors for PTSD such as prior trauma, family psychiatric history, peritraumatic emotionality and dissociation, and posttrauma life stress and social support. While these findings are important to attend to, it is difficult to identify which patients need early intervention and which will recover naturally. Furthermore, the components of posttraumatic life stress and social support generally cannot be determined prospectively at the time of trauma. Even more importantly, none of these risk factors were necessary or sufficient in consistently predicting who will develop PTSD following trauma exposure. More recent work has broadened the possible risk factors and performed factor analytic approaches to obtain overall risk indices,6–8 but further work remains important in the area of prediction of PTSD following trauma exposure.
Better understanding of the mechanisms involved in predicting PTSD development is greatly needed. One area of great promise is the role of genetic biomarkers that may influence how an individual responds to and copes with trauma exposure. Research suggests that genetic factors account for approximately one-third of the overall risk for developing PTSD following exposure to traumatic events.9–11 This overall genetic risk is likely accounted for by many genetic markers, each carrying a small effect size. Several studies have demonstrated, for instance, a gene-environment interaction whereby several single-nucleotide polymorphisms (SNPs) of the FKBP5 gene interact with severity of child abuse to predict adult PTSD symptoms.12–16 Other research has identified higher concentrations of corticotropin-releasing factor (CRF), an important influence on responses to acute and chronic stress, among patients with PTSD.17 Specific polymorphisms within the corticotropin-releasing hormone type 1 receptor gene (CRHR1) have been shown to interact with a history of child abuse in predicting depression symptoms in adulthood,18 and prospectively to predict acute PTSD symptoms among pediatric injury patients.19 A recent study identified a sex-specific association between the function of the pituitary adenylate cyclase–activating polypeptide (PACAP) receptor (ADCYAP1R1) and abnormal stress responses underlying PTSD.20
These findings of genetic biomarkers of PTSD risk have great potential not only in advancing our understanding of the neurobiological underpinnings of PTSD, but also in improving our ability to provide early intervention to trauma-exposed individuals who are at highest risk for developing chronic PTSD. No research to date has examined the influence of genetic risk factors on response to early intervention for PTSD in the immediate aftermath of a traumatic event.
The objectives of this study were first to determine the effect of our prior identified PTSD-related risk ADCYAP1R1 polymorphism in a separate, prospective study, and second, to examine whether response to an immediate exposure intervention would be associated with a combined potential “genetic risk score” across a number of formerly individually identified genes (ADCYAP1R1, COMT, CRHR1, DBH, DRD2, FAAH, FKBP5, NPY, NTRK2, and PCLO). We predicted that immediate psychotherapeutic intervention would decrease the likelihood of those with high-risk genotypes from developing PTSD following an index trauma.
METHOD
Study Design
A detailed description of the original study design has been reported previously.1 Eligible civilian patients presenting to the emergency department of the Grady Memorial Hospital in Atlanta, Georgia, following a DSM-IV criterion A trauma completed an initial assessment soon after the index trauma (mean = 11.8 hours, median = 6.92). Patients were randomly assigned to receive a modified PE intervention in the emergency department or assessment only. Patients separately consented to provide for DNA analysis a saliva sample that was collected during the initial assessment. Participants returned for follow-up at 4 and 12 weeks after trauma and were assessed for symptoms of PTSD. The study was approved by the university institutional review board and the hospital research oversight committee. The study was registered with ClinicalTrials.gov as “Examining the Effectiveness of an Early Psychological Intervention to Prevent Post-Traumatic Stress Disorder,” NCT00895518.
Setting
Participants were recruited from the emergency department of an inner city level I trauma center. This trauma center is the largest in the state, and one of the largest in the United States, treating > 100,000 patients annually.
Participant Sample
Of 137 patients who agreed to participate in the original study, salivary DNA was obtained from 94 subjects, of whom 65 (34 assessment only, 31 intervention) completed the 12-week follow-up assessment. Data were available for 62 subjects for the first analysis and 65 subjects for the remaining 12-week analyses. Table 1 contains demographic characteristics of the assessment and intervention participants. No differences in demographics or baseline measures were detected between the completer subsample of patients who provided a saliva sample and the total original sample.
Table 1.
Sample Demographic Information (N = 65 12-week completers with genotype data)
| Demographics | Intervention (n = 31) | Assessment (n = 34) | ANOVAa F, P Values |
|---|---|---|---|
| Men, n (%) | 15 (48.4) | 10 (29.4) | F1,65 = 2.49, P = .12 |
| Age, y, mean (SD) | 31.3 (11.6) | 34.7 (11.5) | F1,65 = 1.39, P = .24 |
| Ethnicity, n (%) | F1,65 = 1.19, P = .28 | ||
| White | 1 (3.2) | 5 (14.7) | |
| African American | 26 (83.9) | 28 (82.4) | |
| Native American | 1 (3.2) | 0 (0) | |
| Other | 3 (9.7) | 1 (2.9) | |
| Trauma type, n (%) | F1,65 = 0.47, P = .50 | ||
| Rape | 9 (29.0) | 9 (26.5) | |
| Nonsexual assault | 10 (32.3) | 8 (23.5) | |
| Motor vehicle accident | 10 (32.3) | 14 (41.2) | |
| Other | 2 (6.5) | 3 (8.8) |
One-way ANOVA is shown, comparing intervention to assessment group, for number of subjects in each analysis examining: sex, age, ethnicity, and trauma type, showing no differences between groups in these categories.
Abbreviation: ANOVA = analysis of variance.
Intervention
Therapists were trained in PE1,21 and also in the modified PE protocol. Therapists had a master’s or doctoral degree in psychology or social work. Patients received 3 hour-long sessions of a modified PE intervention distributed 1 week apart. See Table 1 of Rothbaum et al1 for a detailed description of the intervention. At both follow-up sessions, approximately 85% of participants were compliant with all homework assignments, or were missing only 1 component. Blinded assessors administered the PTSD Symptom Scale-Interview Version (PSS-I)22 and the Posttraumatic Stress Diagnostic Scale (PDS)23 at 4 and 12 weeks following enrollment in the emergency department. Approximately 88% of 4-week follow-ups and 84% of 12-week follow-ups were conducted in person. In cases where a participant was unable to return for follow-up in person, the option to conduct the interview by phone was offered to minimize missing data. Patients meeting DSM-IV criteria for PTSD at the 3-month follow-up were offered the full 9-session PE treatment1,21 at no charge.
Measures
Childhood Trauma Questionnaire (CTQ)
This self-report measure includes 28 items rated on a 1–5 scale (1 = never true, 5 = always true) and screens for 5 categories of childhood maltreatment: emotional neglect, emotional abuse, physical neglect, physical abuse, and sexual abuse.24 The CTQ has excellent psychometric properties.25–27
PTSD Symptom Scale-Interview Version (PSS-I).22
This semistructured interview consists of 17 items directly corresponding to the DSM-IV PTSD symptoms. Symptoms are rated by a clinician on a 0–3 scale (0 = not at all, 3 = 5 or more times per week/very much). The PSS-I has excellent psychometric properties and shows moderate to high agreement with the Structured Clinical Interview for DSM-IV Axis I Disorders and the Clinician-Administered PTSD Scale for DSM-IV.28 In the current study, interrater agreement was 0.99.
Posttraumatic Diagnostic Scale (PDS).23
The PDS is a 49-item self-report measure to assess severity of PTSD symptoms related to a single identified traumatic event. In this study, the PDS was used at the time of the emergency department assessment to determine the subject’s PTSD symptoms from traumas prior to the index trauma for which they presented.
Genetics Methods
DNA was extracted from saliva collected in Oragene tubes (DNA Genotek, Inc, Ontario Canada) using the Agencourt DNAdvance isolation kit (Beckman Coulter, Inc, Brea, California). DNA samples were normalized to a concentration of 10 ng/μL then plated and dried in a polymerase chain reaction (PCR) plate at 20 ng. Multiplex PCR followed by single base extensions at each SNP was performed using Sequenom iPlex chemistries (Sequenom, Inc, San Diego, California), followed by mass spectrometry using the Sequenom MassArray.
For quality control measures, each DNA sample was extracted and genotyped in duplicate at separate time points, with multiple quality controls. Call rates exceeded 95% for all SNP assays. All SNPs passed Hardy Weinberg equilibrium (HWE) at P > .01 (corrected for multiple testing) for African Americans only (shown in Table 2). Note that in this small cohort, the ADCYAP1R1 SNP was at a trend significant level when corrected for multiple testing (P = .04); however, with larger sample sizes this SNP has consistently been found to be in HWE.
Table 2.
Genes Comprising the Multigenotype Risk
| Gene | SNP | Risk Genotype | HWE P Value | References for SNP and Stress-Related Disorders |
|---|---|---|---|---|
| ADCYAP1R1 | rs2267735 | CC | .04 | Ressler et al, 201120 Jovanovic et al, 201229 |
| COMT | rs4680 | GG | .82 | Boscarino et al, 201230 Amstadter et al, 200931 |
| CRHR1 | rs7209436 | CC | .76 | Bradley et al, 200818 Polanczyk et al, 200932 Laucht et al, 201233 |
| DBH | rs1611115 | CC | .91 | Cubells et al, 200034 Tang et al, 201035 Mustapić et al, 200736 |
| DRD2 | rs6277 | TT | .17 | Huuhka et al, 200837 Jutras-Aswad et al, 201238 Huertas et al, 201039 |
| FAAH | rs324420 | CC | .79 | Sipe et al, 200240 Monteleone et al, 201041 Gunduz-Cinar et al, 201242 |
| FKBP5 | rs1360780 | TT | .15 | Binder et al, 200812 Xie et al, 201013 |
| NPY | rs16147 | GG | .95 | Zhou et al, 200843 Amstadter et al, 201044 |
| NTRK2 | rs1867283 | GG | .96 | Kohli et al, 201045 |
| PCLO | rs2522833 | AA | .34 | Sullivan et al, 200946 Hek et al, 201047 Kuehner et al, 201148 |
Sequenom MassArray technology was used to analyze a panel of 32 previously identified psychiatry-relevant SNPs representing 23 genes within 1 multiplex, 10 of which had previously been associated with PTSD or depression in traumatized populations. We focused on these 10 SNPs for this multigenic analysis. One SNP was chosen (due to its prior association with PTSD or its prior robust association with other psychiatric disorders in addition to its individual P < .05 association with PTSD within this dataset) from each of these 10 genes to be examined together within this analysis (Table 2).
Data Analysis
Throughout these analyses we either examined main effects of genotype on PTSD diagnosis (categorical based on DSM-IV from PSS interview), or we used continuous PTSD symptom scores with a dominant/recessive model for the SNPs examined. All included subjects had been exposed to a criterion A trauma. Data were examined with SPSS Statistics software (Version 20; IBM Corporation, Armonk, New York) using general linear model for treatment × genotype interactions and split either by treatment group or by defined genotype risk, followed by univariate ANOVA, bivariate correlations, and linear regression for continuous outcomes, and χ2 statistics and logistic regression for categorical outcomes. All analyses used 2-tailed tests.
RESULTS
Summary of Study Patients
Of the 65 participants who completed the 12-week assessment and for whom we had genetic data, 54 (83.1%) were African American, 40 (61.5%) were female, and the mean age was 33.0 years (SD = 11.6). See Table 1 for more details.
ADCYAP1R1, Genetic Risk, and Intervention Effects
We first examined the genotype of the PACAP receptor (ADCYAP1R1) allele that we previously found to be associated with PTSD (rs2267735, CC genotype)20 for its effect on PTSD symptoms at 4 and 12 weeks following the index trauma in intervention and assessment groups. We identified a gene × intervention × time-of-analyses effect (Figure 1, repeated measures ANOVA, 3-way interaction: F1,62 = 5.48, P < .02). For participants in the assessment group, those who were carriers of homozygous risk (CC) allele (PSS-I12-week [CC] = 27.83; 95% CI, 17.89 to 37.78) had higher levels of PTSD symptoms (PSS-I scores > 25) than those who were not (G-carriers), at week 12 (PSS-I12-week [G] = 16.32; 95% CI, 11.13 to 21.51). In contrast, for participants in the intervention group, we found no difference in PSS-I scores across those with risk genotype status (PSS-I12-week [CC] = 16.40; 95% CI, 8.70 to 24.10) and those without (PSS-I12-week [G] = 16.88; 95% CI, 10.98 to 22.79). There were no significant differences at the 4-week time point for the intervention (P = .22) and the assessment groups (P = .32). Given that the initial report of rs2267735 was in females, it is important to note that the above findings hold when sex and race are included as covariates.
Figure 1. ADCYAP1R1 Is Associated With Higher PTSD Symptoms in the Assessment Group Only, Not the Intervention Group at Follow-Up.
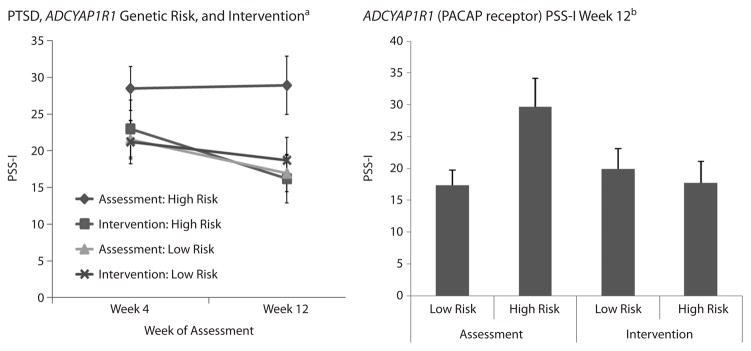
aPTSD symptoms (PSS-I total score) are graphed at the 4-week and 12-week follow-up time points after the index trauma as a function of assessment only vs intervention group as well as high-risk ADCYAP1R1 (CC) vs low-risk ADCYAP1R1 (GG, GC) genotypes. The number of subjects included in both 4- and 12-week assessment periods are as follows: assessment group, low-risk ADCYAP1R1 genotype (n = 25), high-risk genotype (n = 8); intervention group, low-risk genotype (n = 18), high-risk genotype (n = 11). There is a gene × intervention × time of analyses effect (repeated measures ANOVA, 3-way interaction: F1,62 = 5.48, P < .05).
bThe data are shown for the week 12 time point, in which those who were carriers of homozygous risk (CC) allele had higher levels of PTSD symptoms than those who were not carriers (F1,34 = 5.6, P < .05) for the assessment group only.
In addition to the association of ADCYAP1R1 on continuous PTSD symptoms, we examined whether the risk genotype was associated with PSS-I–based diagnosis of PTSD at week 12. Subjects with the risk genotype (CC) were more likely to be diagnosed with PTSD at week 12 (87.5%) compared to carriers of the low-risk allele (12.5%) if they were assessed-only (χ21 = 5.85, P = .02), whereas there was no difference between genotype risk groups in the intervention cohort (χ2 = 0.04, P = .84).
Multigenotype Risk and PTSD Symptoms
We next assessed whether a combination of genes previously associated with PTSD and other stress-related disorders would provide more power to detect risk versus resilience following a trauma. Ten SNPs from 10 different genes were selected from previously published data on the basis of association with PTSD and other stress-related disorders (see Table 2). Using these 10 genotypes, we defined for each subject a genetic risk score of 0–10 based on the number of homozygous risk genotypes. Notably, the direction of the risk variants utilized in the combined risk analyses was determined based on (1) their prior association with PTSD or (2) their prior robust association with other psychiatric disorders in addition to their individual P < .05 association with PTSD within this dataset. Seven genes were associated in the same direction as previously reported in PTSD and fear- or stress-related phenotypes (ADCYAP1R1, FKBP5, CRHR1, DBH, DRD2, NPY, and NTRK2). However, several genes appeared to be associated with risk in the opposite direction as previously reported (COMT, FAAH, and PCLO), which may be related to both the differing racial makeup of our sample as well as different stress-related phenotypes.
We then examined whether the level of putative genetic risk from these 10 markers would associate with PTSD symptoms at 12 weeks, and we found effects similar, but more robust, to those above with a single gene. We first examined whether there was a relationship between level of multigentotype risk and PSS-I symptoms in assessed groups versus intervention groups. Whereas there was no relationship in the intervention groups (Figure 2: PSS-I12-week: r < −0.01, P > .5), there were significant positive relationships between genetic risk and total PSS-I symptoms in the assessment group (Figure 2: PSS-I12-week: r = 0.55, P < .005). Of note, although we did not see the week-4 effect with the ADCYAP1R1 gene alone, when using the genetic risk score, we did find a similar positive relationship between genetic risk and total PSS-I symptoms in the assessment group (PSS-I4-week: r = 0.36, P < .05), but not in the intervention group (PSS-I12-week: r = 0.09, P > .5).
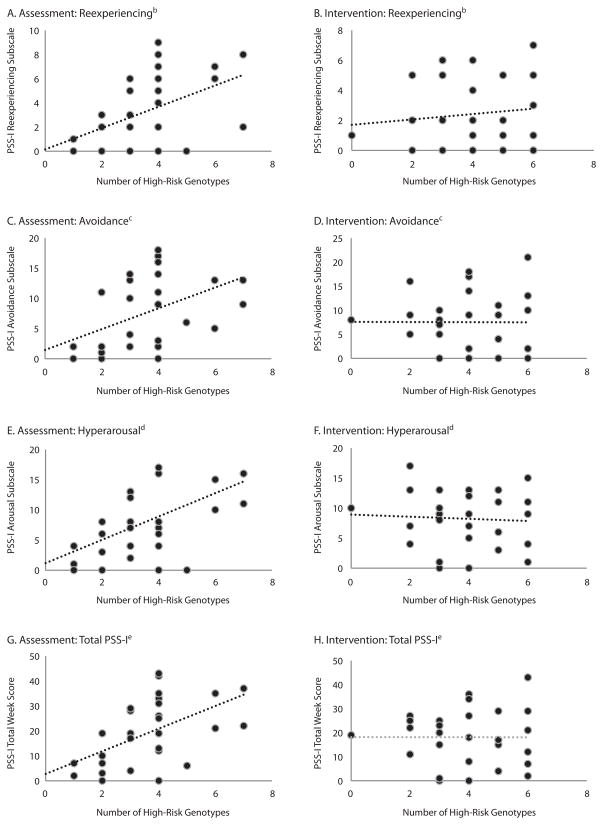
Figure 2. Number of Homozygous Risk Genotypes Are Correlated With Higher PTSD Symptoms in the Assessment Group but Not the Intervention Group at 12-Week Follow-Upa.
aNumber of high-risk genotypes are graphed relative to PSS-I total score and subscale scores across assessment and intervention groups.
bReexperiencing subscale symptoms are correlated with number of high-risk genotypes in the assessment group (A, n = 30, r = 0.49, P = .007), but not in the intervention group (B, n = 28, r = 0.12, P > .5).
cAvoidance subscale symptoms are correlated with number of high-risk genotypes in the assessment group (C, n = 30, r = 0.46, P = .011), but not in the intervention group (D, n = 28, r = −0.005, P > .5).
dHyperarousal subscale symptoms are correlated with number of high-risk genotypes in the assessment group (E, n = 30, r = 0.52, P = .003), but not in intervention group (F, n = 28, r = −0.06, P > .5).
eTotal PSS-I symptoms are correlated with number of high-risk genotypes in the assessment group (G, n = 34, r = 0.55, P = .002), but not in the intervention group (H, n = 31, r = −0.03, P > .5).
This relationship held across all 3 subscales of PTSD symptoms assessed at week 12 (Reexperiencing: r = 0.49, P < .01; Avoidance: r = 0.46, P < .01; Hyperarousal: r = 0.52, P = .003). In contrast, there were no significant relationships between number of risk homozygous genotypes with any subscales in the intervention group. The 4-week measure demonstrated an effect of Reexperiencing (PSS-I4-week: r = 0.44, P < .01), but again, no differences were found in the intervention group.
We also performed a logistic regression analysis in which the continuous variable of number of high-risk alleles (0–10) was used to predict PTSD diagnosis (based on PSS-I) at week 12. We found that number of risk alleles was significantly associated with the likelihood of PTSD diagnosis (OR = 1.53; 95% CI, 1.06 to 2.02).
Multigenotype Risk Effects
Survive Regression Analyses
Due to the limited sample size, all of the preceding analyses were performed by examining main effects of genotype and treatment not including any covariates. To ensure that other factors associated with PTSD symptom risk were not driving the genetic or intervention effects found, a linear regression analysis was next performed in 4 steps, examining the effect of the multigenotype risk after controlling for effects of age, sex, race, education, income, and childhood trauma levels on PTSD symptoms at week 12. As with the above analyses, there was no association of genotype risk with PTSD symptoms in the intervention group. In contrast, in the assessment group, multigenotype risk was still associated with PTSD symptoms at week 12, when controlling for these other risk factors (Table 3; P = .026).
Table 3.
Multigenotype Risk Is Associated With Increased PTSD Symptoms in the Assessment Group and Not the Intervention Group at Week 12a
| Treatment | Model Summary
|
Change Statistics
|
||
|---|---|---|---|---|
| Modelb | R2 | R2 Change |
F Change P Value |
|
| Assessment | 1 | 0.158 | 0.158 | .222 |
| 2 | 0.276 | 0.118 | .176 | |
| 3 | 0.405 | 0.129 | .040 | |
| 4 | 0.533 | 0.128 | .026 | |
| Intervention | 1 | 0.299 | 0.299 | .055 |
| 2 | 0.409 | 0.111 | .195 | |
| 3 | 0.517 | 0.107 | .061 | |
| 4 | 0.551 | 0.034 | .273 | |
Bolded values were significant at P < .05.
Model 1: age, sex, race. Model 2: age, sex, race, education, income. Model 3: age, sex, race, education, income, childhood trauma level (Childhood Trauma Questionnaire [CTQ]). Model 4: age, sex, race, education, income, childhood trauma level (CTQ), with combined genetic risk.
DISCUSSION
The current findings extend earlier work that genetic risk contributes to the relationship between exposure to traumatic events and the development of PTSD. More importantly, here we report that this risk may be mitigated by an early intervention. Our initial analyses showed that immediately following a traumatic event, survivors seeking medical care for their injuries were found to be at risk for increased PTSD symptoms 12 weeks after trauma if they had the risk allele of the rs2267735 SNP from the PACAP receptor. These data suggest that in the absence of intervention, the prior association of the PACAP receptor and PTSD20 is replicated in a prospective study of traumatized civilians, as it has been similarly replicated in other cohorts49–51 (but also see Chang et al52). The administration of an immediate therapeutic intervention following the index trauma appears to mitigate this effect in the treatment group. Further analyses found that this same relationship was even more robust when we examined the combined risk genotypes outlined in Table 2.
A 1-hour exposure-based intervention administered first in the emergency department, and then again 1 and 2 weeks later, for a total of 3 sessions, was associated with significantly fewer symptoms at 12 weeks in those with the risk genotypes compared to those with the risk genotypes who received assessment only. Overall, patients with the low-risk genotypes fared well in both the assessment and intervention groups. Symptoms appeared to be cumulative (ie, more risk genotypes, more PTSD) for those in the assessment group, but not for those in the intervention group. The multigenotype risk predicted PTSD severity at week 12 in the assessment group, but not in the intervention group. The presence of therapeutic intervention following the index trauma appears to mitigate the effect of genetic risk in the treatment group.
These results have meaningful clinical implications. Approximately 70% of individuals will undergo a potentially traumatic event in their lifetimes.53 Interventions can be costly, time consuming, and not necessary in all cases. If those at greatest risk can be identified and offered a brief intervention commencing before the memory is fully consolidated,54,55 the risk of that exposure may be mitigated. It is important to note that in this pilot trial a large percentage of patients seen in the emergency department were ineligible for enrollment,56 and that 65%–70% were seen at the 12-week follow-up, which is less than the 70%–80% follow-up rates that have been seen in some other acute medical injury PTSD intervention trials. As previously discussed, it is important that broad generalizations are not made based on this pilot study from small, select samples.57 Finally, future studies with improved sampling and follow-up rates, as have been attained by Cunningham and colleagues,58,59 are indicated.
It is important to consider whether identifying genetic biomarkers is realistic in an emergency department setting and whether such biomarkers can be readily interfaced with implementable electronic medical record databases that are already being examined in population-level screening to identify those at risk for PTSD. Our current study does not address these issues, and it is important to treat these results as pilot findings suggesting that such polygenic factors may eventually be powerful additional biomarkers to consider. Although it is not currently routine to examine genetic data in emergency department settings, the technology clearly exists, as a number of rapid PCR-based tests are routinely used in primary and emergency department settings for other diagnostic approaches. As the era of personalized medicine continues to expand, it is quite likely that rapid, inexpensive genetic tests will be introduced for some aspects of biomarker-based risk across medical disciplines.
Some strengths of this research include prospective screening and entering of high-risk patients presenting at a level I trauma center, innovative early intervention administered commencing in the emergency department, assessment and follow-up at time points when PTSD could be diagnosed, blind assessment, and rational exploration of the most promising genes for conferring risk. Limitations of this study include a small sample size for genetic research, lack of an attentional control group, utilization of common SNP variants identified primarily from candidate and not whole-genome studies, post hoc decisions regarding which genes to study, and loss of approximately 25% of the sample for the genetic analysis. Additionally, the sample was ethnically heterogeneous, although our findings survived covariation for race, as described. Future studies should rely on larger, in-progress Genome Wide Association Studies for PTSD risk and prospectively determine genotype prior to treatment assignment, as well as examine larger and more varied samples.60
The SNPs selected here are a very small percentage of the likely large and varied genetic risk involved in stress-related disorders that have yet to be identified. However, these data serve as a proof-of-principle, that as we obtain biomarkers for psychiatric risk, we may be better able to understand, prevent, and treat stress-related disorders. Although this was a small, pilot intervention study, these data suggest that genetic markers may serve to stratify and predict those who are most at risk for developing PTSD in the aftermath of severe trauma. Importantly, we find that utilizing a psychotherapeutic intervention within hours of the trauma may be successful at mitigating this potential genetic risk.
Clinical Points.
Psychosocial factors are related to differential PTSD risk, but biological markers are also needed to predict who is most at-risk for PTSD following trauma and who would respond to early intervention.
This pilot study suggests that combined genetic variants associate with increased risk for PTSD in a prospective emergency department cohort.
A psychotherapeutic intervention initiated within hours of the index trauma may mitigate this genetic risk.
Genetic risk markers should be further evaluated in larger prospective trials of PTSD.
Acknowledgments
Funding/support: This study was supported by National Institute of Mental Health Grant Numbers R34 MH083078 and R01 MH071537, and the Emory Center for Injury Control, Center for Disease Control Grant Number 5R49CE001494 to Dr Rothbaum.
Role of the sponsors: The funding agencies had no role in the conduct or publication of the study.
Footnotes
Potential conflicts of interest: None reported.
References
- 1.Rothbaum BO, Kearns MC, Price M, et al. Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry. 2012;72(11):957–963. doi: 10.1016/j.biopsych.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothbaum BO, Foa EB, Riggs D, et al. A prospective examination of post-traumatic stress disorder in rape victims. J Trauma Stress. 1992;5(3):455–475. doi: 10.1002/jts.2490050309. [DOI] [Google Scholar]
- 3.Kearns MC, Ressler KJ, Zatzick D, et al. Early interventions for PTSD: a review. Depress Anxiety. 2012;29(10):833–842. doi: 10.1002/da.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozer EJ, Best SR, Lipsey TL, et al. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 5.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68(5):748–766. doi: 10.1037/0022-006X.68.5.748. [DOI] [PubMed] [Google Scholar]
- 6.Richmond TS, Ruzek J, Ackerson T, et al. Predicting the future development of depression or PTSD after injury. Gen Hosp Psychiatry. 2011;33(4):327–335. doi: 10.1016/j.genhosppsych.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih RA, Schell TL, Hambarsoomian K, et al. Prevalence of posttraumatic stress disorder and major depression after trauma center hospitalization. J Trauma. 2010;69(6):1560–1566. doi: 10.1097/TA.0b013e3181e59c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donnell ML, Creamer MC, Parslow R, et al. A predictive screening index for posttraumatic stress disorder and depression following traumatic injury. J Consult Clin Psychol. 2008;76(6):923–932. doi: 10.1037/a0012918. [DOI] [PubMed] [Google Scholar]
- 9.Koenen KC, Amstadter AB, Nugent NR. Gene-environment interaction in posttraumatic stress disorder: an update. J Trauma Stress. 2009;22(5):416–426. doi: 10.1002/jts.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein MB, Jang KL, Taylor S, et al. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159(10):1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 11.True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 12.Binder EB, Bradley RG, Liu W, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie P, Kranzler HR, Poling J, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boscarino JA, Erlich PM, Hoffman SN, et al. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Res. 2011;188(1):173–174. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarapas C, Cai G, Bierer LM, et al. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Dis Markers. 2011;30(2–3):101–110. doi: 10.1155/2011/328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta D, Gonik M, Klengel T, et al. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch Gen Psychiatry. 2011;68(9):901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154(5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amstadter AB, Nugent NR, Yang BZ, et al. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30(2–3):89–99. doi: 10.1155/2011/928497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ressler KJ, Mercer KB, Bradley B, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foa EB, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 22.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. J Trauma Stress. 2000;13(2):181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 23.Foa EB, Cashman L, Jaycox L, et al. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychol Assess. 1997;9(4):445–451. doi: 10.1037/1040-3590.9.4.445. [DOI] [Google Scholar]
- 24.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein DP, Ahluvalia T, Pogge D, et al. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein D, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 27.Fink LA, Bernstein DP, Handelsman L, et al. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152(9):1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- 28.Foa EB, Riggs DS, Dancu CV, et al. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6(4):459–473. doi: 10.1002/jts.2490060405. [DOI] [Google Scholar]
- 29.Jovanovic T, Norrholm SD, Davis J, et al. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children [published online ahead of print July 10, 2012] Mol Psychiatry. 2013;18(7):742–743. doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boscarino JA, Erlich PM, Hoffman SN, et al. Higher FKBP5, COMT, CHRNA5, and CRHR1 allele burdens are associated with PTSD and interact with trauma exposure: implications for neuropsychiatric research and treatment. Neuropsychiatr Dis Treat. 2012;8:131–139. doi: 10.2147/NDT.S29508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amstadter AB, Nugent NR, Koenen KC, et al. Association between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic sample. Psychiatry. 2009;72(4):360–369. doi: 10.1521/psyc.2009.72.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polanczyk G, Caspi A, Williams B, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laucht M, Treutlein J, Blomeyer D, et al. Interactive effects of corticotropin-releasing hormone receptor 1 gene and childhood adversity on depressive symptoms in young adults: Findings from a longitudinal study. Eur Neuropsychopharmacol. 2013;23(5):358–367. doi: 10.1016/j.euroneuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Cubells JF, Kranzler HR, McCance-Katz E, et al. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry. 2000;5(1):56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- 35.Tang YL, Li W, Mercer K, et al. Genotype-controlled analysis of serum dopamine β-hydroxylase activity in civilian post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1396–1401. doi: 10.1016/j.pnpbp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mustapić M, Pivac N, Kozarić-Kovacić D, et al. Dopamine beta-hydroxylase (DBH) activity and -1021C/T polymorphism of DBH gene in combat-related post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1087–1089. doi: 10.1002/ajmg.b.30526. [DOI] [PubMed] [Google Scholar]
- 37.Huuhka K, Anttila S, Huuhka M, et al. Dopamine 2 receptor C957T and catechol-o-methyltransferase Val158Met polymorphisms are associated with treatment response in electroconvulsive therapy. Neurosci Lett. 2008;448(1):79–83. doi: 10.1016/j.neulet.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Jutras-Aswad D, Jacobs MM, Yiannoulos G, et al. Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: case-control study. PLoS One. 2012;7(6):e39243. doi: 10.1371/journal.pone.0039243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huertas E, Ponce G, Koeneke MA, et al. The D2 dopamine receptor gene variant C957T affects human fear conditioning and aversive priming. Genes Brain Behav. 2010;9(1):103–109. doi: 10.1111/j.1601-183X.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 40.Sipe JC, Chiang K, Gerber AL, et al. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99(12):8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteleone P, Bifulco M, Maina G, et al. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol Res. 2010;61(5):400–404. doi: 10.1016/j.phrs.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Gunduz-Cinar O, MacPherson KP, Cinar R, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity [published online ahead of print June 12, 2012] Mol Psychiatry. 2013;18(7):813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z, Zhu G, Hariri AR, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452(7190):997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amstadter AB, Koenen KC, Ruggiero KJ, et al. NPY moderates the relation between hurricane exposure and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2010;27(3):270–275. doi: 10.1002/da.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohli MA, Salyakina D, Pfennig A, et al. Association of genetic variants in the neurotrophic receptor-encoding gene NTRK2 and a lifetime history of suicide attempts in depressed patients. Arch Gen Psychiatry. 2010;67(4):348–359. doi: 10.1001/archgenpsychiatry.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan PF, de Geus EJ, Willemsen G, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14(4):359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hek K, Mulder CL, Luijendijk HJ, et al. The PCLO gene and depressive disorders: replication in a population-based study. Hum Mol Genet. 2010;19(4):731–734. doi: 10.1093/hmg/ddp529. [DOI] [PubMed] [Google Scholar]
- 48.Kuehner C, Huffziger S, Witt SH, et al. PCLO rs2522833 impacts HPA system activity in healthy young adults. Transcult Psychiatry. 2011;1(5):e10. doi: 10.1038/tp.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almli LM, Mercer KB, Kerley K, et al. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(3):262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Cao C, Wang R, et al. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. J Affect Disord. 2013;150(1):156–159. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Uddin M, Chang SC, Zhang C, et al. ADCYAP1R1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress Anxiety. 2013;30(3):251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang SC, Xie P, Anton RF, et al. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Mol Psychiatry. 2012;17(3):239–241. doi: 10.1038/mp.2011.118. [DOI] [PubMed] [Google Scholar]
- 53.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 54.Johansen JP, Cain CK, Ostroff LE, et al. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cain CK, Maynard GD, Kehne JH. Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs. 2012;21(9):1323–1350. doi: 10.1517/13543784.2012.704020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malcoun E, Houry D, Arndt-Jordan C, et al. Feasibility of identifying eligible trauma patients for posttraumatic stress disorder intervention. West J Emerg Med. 2010;11(3):274–278. [PMC free article] [PubMed] [Google Scholar]
- 57.Zatzick DF, Galea S. An epidemiologic approach to the development of early trauma focused intervention. J Trauma Stress. 2007;20(4):401–412. doi: 10.1002/jts.20256. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham RM, Resko SM, Harrison SR, et al. Screening adolescents in the emergency department for weapon carriage. Acad Emerg Med. 2010;17(2):168–176. doi: 10.1111/j.1553-2712.2009.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cunningham RM, Chermack ST, Zimmerman MA, et al. Brief motivational interviewing intervention for peer violence and alcohol use in teens: one-year follow-up. Pediatrics. 2012;129(6):1083–1090. doi: 10.1542/peds.2011-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koenen KC, Duncan LE, Liberzon I, et al. From candidate genes to genome-wide association: the challenges and promise of posttraumatic stress disorder genetic studies. Biol Psychiatry. 2013;74(9):634–636. doi: 10.1016/j.biopsych.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]