Abstract
Early-life experience strongly impacts neurodevelopment and stress susceptibility in adulthood. Maternal separation (MS), an established model of early-life adversity, has been shown to negatively impact behavioral and endocrine responses to stress in adulthood. However, the impact of MS in rats with heightened inborn stress susceptibility has not been fully explored. To address this issue we conducted MS in Wistar-Kyoto (WKY) rats, an animal model of comorbid depression and anxiety, and Wistar rats, which share a similar genetic background with WKYs. WKY and Wistar pups experienced either 180-min daily MS or 15-min separation (neonatal handling) during the first two postnatal weeks, and were tested for depressive- and anxiety- like behaviors in adulthood. Exposure to early-life MS in WKY rats decreased anxiety- and depressive- like behaviors, leading to increased exploration on the open field test (OFT), enhanced social interaction, and diminished immobility on the forced swim test. MS had an opposite effect in Wistar offspring, leading to enhanced anxiety-like behaviors, such as reduced OFT exploration and decreased social interaction. These findings are consistent with the match/mismatch theory of disease and the predictive adaptive response, which suggest that early life stress exposure can confer adaptive value in later life within certain individuals. Our data supports this theory, showing that early-life MS has positive and perhaps adaptive effects within stress-vulnerable WKY offspring. Future studies will be required to elucidate the neurobiological underpinnings of contrasting behavioral effects of MS on WKY vs. Wistar offspring.
Keywords: early life stress, depression, anxiety, maternal separation, predictive adaptive response
1. Introduction
Adverse life experiences during the early developmental period have long-term effects on the brain, stress-elicited behaviors, endocrine function, and physiological responses in a variety of species [26]. Early-life adversity in humans can predispose individuals to neuropsychiatric disorders and suicidality [16]. Childhood abuse and neglect are also associated with adverse behavioral risk factors in adulthood, including smoking, physical inactivity, obesity, depression, and cardiovascular disease [4].
Prolonged early-life maternal separation (MS) is a widely used rodent model of early-life adversity where pups are deprived of maternal contact for variable time periods during the early weeks of life [11]. Most reports document myriad negative effects of MS including: increased anxiety-like behavior [12], depression-like behavior [6], and exaggerated hypothalamic-pituitary adrenal (HPA) axis stress responses [22]. There are discrepancies in the MS literature, with certain studies unable to confirm such behavioral and neuroendocrine findings. For instance, some reports failed to see effects of MS on anxiety measures [8, 24], and another showed that MS decreased contextual and auditory fear conditioning rather than increasing it [2]. These conflicting results likely stem from a host of factors, including varied experimental procedures, gender, and rat strain that was used [11]. Importantly, these findings suggest that early-life stress is not uniformly deleterious and its long term effects may depend upon an organism's innate level of stress reactivity [18].
The current study tested the hypothesis that early-life MS elicits disparate effects on rat strains that exhibit innate differences in stress susceptibility. To do so, we applied the early-life MS paradigm to two rat strains: Wistar-Kyoto (WKY) rats, a well-established model animal of heightened depressive-/anxiety-like behavior and stress vulnerability [17], and genetically similar Wistar rats. WKY rats manifest heightened endocrine and physiological responses to stress [20], increased stress-induced ulcers and gastrointestinal dysfunction [21], and display robust behavioral despair and learned helplessness, a core feature of major depression in humans [21]. Given WKY rats' high baseline levels of anxiety/depression-like behavior and stress susceptibility, we initially hypothesized that they would be particularly vulnerable to the deleterious effects of early-life MS. On the other hand, Wistar rats typically display low levels of basal anxiety-/depressive-like behavior relative to WKY rats [17]. Our data indicate that MS elicits disparate effects on Wistar vs. WKY rats. Consistent with previous studies, early-life MS led to increased anxiety-like behavior in the Wistar offspring. However, MS elicited protective effects in the WKY offspring, improving their anxiety- and depression- like behaviors along with social behaviors.
2. Methods
All animal handling and experimental procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals.
2.1 Animals and Early-Life Manipulation
WKY and Wistar female and male rats (n=8/sex/strain) were purchased from Charles River Laboratories (Kingston, NY) and housed in a temperature-controlled facility (kept at 21-23 °C, 50-55% humidity) with a 12/12 h light-dark cycle (lights on at 6:00 a.m.). Male/female pairs were mated for 14 days, and at birth (P0) litters were randomly assigned to one of two groups: (a) neonatal handling group or (b) MS group (n = 4 litters/group/strain). Litters assigned to the MS group and neonatal handling group were separated from their dam daily for 180 min and 15 min, respectively, between 8:30 a.m. – 12:00 p.m. from P1-P14 as previously described [3]. Dams remained in the home cages while separated litters were transferred to a different room in a small cage placed on a heating pad (∼37°C). Littermates remained in close contact throughout the separation period and were returned to home cage after the conclusion of the 15- or 180-min period.
After the final separation on P14, litters remained undisturbed until weaning on P21. Only male pups were chosen for subsequent behavioral tests performed in adulthood. Rats of the same strain and same early-life experience (neonatal handling or MS) were housed together 3 per cage. Animals were left undisturbed except for weekly weighing and standard cage changes from weaning until P60+ when behavioral testing commenced.
2.2 Behavior Test Battery
Behavior tests were conducted under dim light conditions (30 lux) between 8:30-11:30 a.m.. Animals were placed in the testing room overnight for habituation before all tests except for forced swim test (FST). The following tests were conducted (in the order indicated): open field test (OFT), social interaction, and FST.
OFT and FST were conducted as previously described [17]. Social interaction testing was conducted in a rectangular black Plexiglas box (91 × 61×30 cm) with a black floor, which was divided into three chambers (zones) separated by two black Plexiglas dividers with openings in the center to allow animals to move freely between zones. Testing was conducted over two days (10 min/day). On Day 1, the test rat was placed in the neutral zone (middle chamber), while one of the other zones contained an empty cylindrical metal bar-cage in a corner of the zone. The third zone contained a male stimulus rat within an interaction cage placed in a corner. The metal bars of the interaction cage allowed rats to interact, but prevented any aggressive encounters between animals. On Day 2, the test rat was again placed in the neutral zone; one of the other zones contained a male stimulus rat within the interaction cage and the third zone contained a female stimulus rat within its interaction cage. Position of the male stimulus rat was switched between test days to eliminate side preference. Stimulus rats were age-matched and of the same strain as the test animals, and were previously habituated to interaction cages. An approximately 2-cm wide zone around each interaction cage was designated as the interaction zone. Behavior in all tests was recorded with a digital camera, and quantified utilizing Ethovision® XT 8.0 software (Noldus, Wageningen, The Netherlands).
2.3 Statistical Analysis
Data from the OFT, Social Interaction test Day 1, and FST were analyzed via two-way ANOVA, with strain and early-life treatment (MS/neonatal handling) as independent variables. When necessary, post-hoc analysis was performed using independent samples t-test within each strain independently and p-values were adjusted using Holm-Bonferroni correction. Data from Social Interaction Day 2 were analyzed separately for the two rat strains. Within each strain, sex of stimulus rat and early-life manipulation were used as independent variables with Bonferroni tests post-hoc. Statistical analysis was performed using GraphPad Prism 6.0. Significance was set at p < 0.05; results are presented as mean ± SEM.
3. Results
Both WKY and Wistar offspring gained weight from weaning through adulthood and there was no effect of early life experience (MS or neonatal handling) on their weight gain (data not shown).
3.1 Contrasting effects of MS
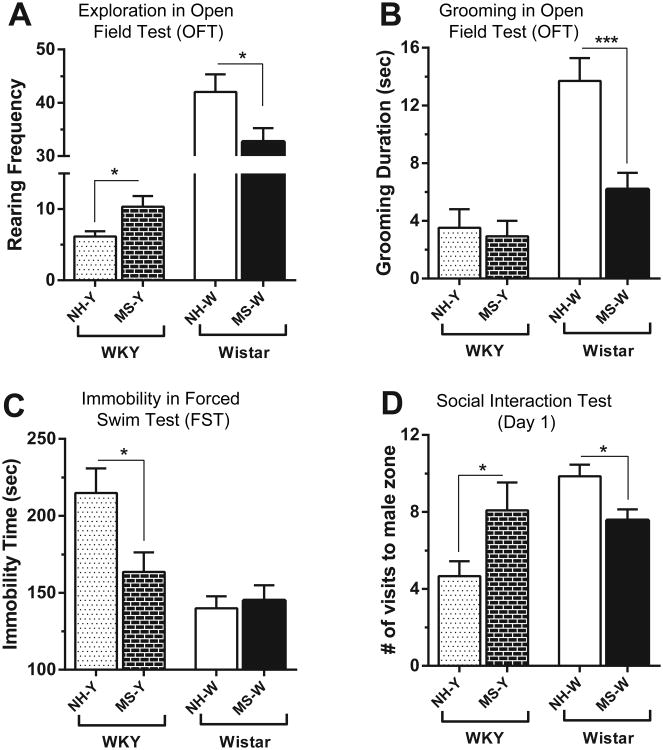
MS differentially affected OFT behavior in WKY vs. Wistar offspring. First, there was a significant main effect of strain (F(1, 61) = 106.4, p < 0.0001) with Wistar rats generally displaying greater amounts of rearing compared to WKYs (Fig. 1A). Interestingly, there was a significant strain × early-life treatment interaction (F(1, 61) = 5.728, p < 0.05). Post-hoc analysis showed that MS elicited disparate effects within each strain, with MS-exposed WKY rats showing increased rearing in the OFT compared to the neonatal handing-exposed WKY offspring (p < 0.05). On the other hand, Wistar offspring that were exposed to MS displayed decreased rearing compared to Wistars that been exposed to neonatal handling (p < 0.05; Fig. 1A). Similar to rearing, grooming duration in the OFT was also differentially impacted by MS, with significant effects of: strain (F(1, 62) = 21.67, p < 0.0001), early-life treatment (F(1, 62) = 7.907, p < 0.01), and strain × early-life treatment interaction (F(1, 62) = 5.774, p < 0.05). Post-hoc testing revealed a large decrease in the amount spent grooming in the MS-exposed Wistar rats (p < 0.001), but no change in the WKY rats, so that MS-exposed WKY and Wistar rats exhibited similar levels of grooming (Fig. 1B). MS also differentially impacted FST immobility in WKY versus Wistar offspring. First, there were significant main effects of strain (F(1, 62) = 17.05, p < 0.001) and MS (F(1, 62) = 4.171, p < 0.05), with WKY rats generally exhibiting greater FST immobility compared to Wistar rats (Fig. 1C). There was also a significant strain × early-life treatment interaction (F(1, 62) = 6.240, p < 0.05). Post-hoc analysis revealed that MS lead to reduced FST immobility selectively within WKY rats (p < 0.05), while Wistar rats' FST behavior was unaffected by MS exposure (Fig. 1C). MS exposure also elicited different effects in WKY and Wistar offspring during Day 1 of the Social Interaction test. There was a main effect of strain (F(1, 62) = 8.105, p < 0.01), but not of MS, on the frequency to visit the novel male social interaction zone on the first social interaction test day, with Wistar rats generally making more visits to the novel male compared to WKY rats (Fig. 1D). However, there was also a significant interaction of strain and early-life treatment (F(1, 62) = 12.04, p = 0.0010), and post-hoc analysis revealed disparate effects of MS on WKY and Wistar offspring's social behavior. MS-exposed WKY offspring showed more visits to the novel male compared to neonatal handling-exposed WKYs. MS had the opposite effect on Wistar offspring, with MS-exposed Wistars showing less social interaction with the novel male rat relative to Wistar offspring that had been exposed to neonatal handling (p < 0.05 for each comparison; Fig. 1D). There were no significant main effects of strain or early-life treatment and no strain × early-life treatment interaction on the rats' interaction with an inanimate novel object (a control used in the Social Interaction Day 1 test; data not shown).
Figure 1.
Contrasting effects of early-life experience on WKY vs. Wistar rats. Two-way ANOVA revealed significant early-life treatment × strain interactions for: rearing frequency (A) (A) and grooming in OFT (B); immobility in FST (C); frequency to male zone on social interaction testing (D). Abbreviations: NH-Y – neonatally-handled WKYs, MS-Y – maternally-separated WKYs, NH-W – neonatally-handled Wistars, MS-W – maternally-separated Wistars. * − p < 0.05, ** − p < 0.01, *** − p < 0.001.
3.2 MS and male vs. female social interaction
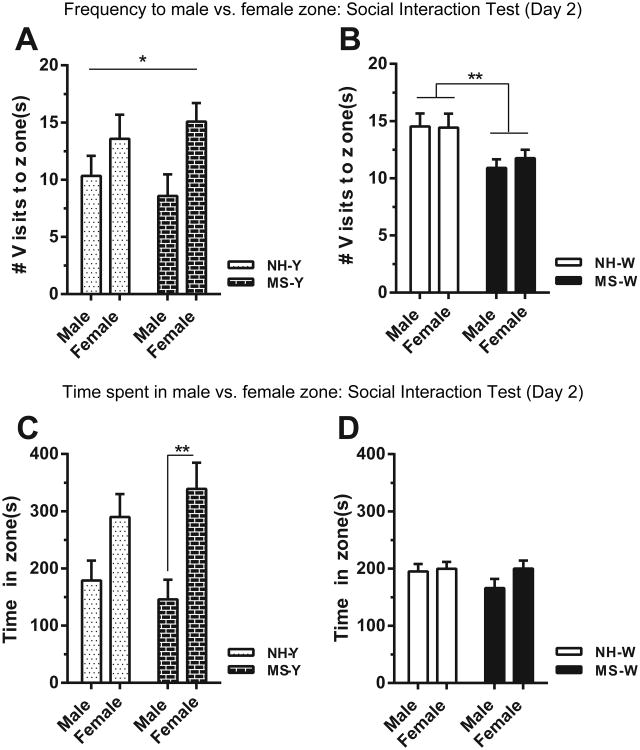
On Day 2 of social interaction testing, rats were given a choice between exploring a novel male or a novel female stimulus rat. WKY groups (MS- and neonatal handling-exposed offspring) showed a preference for visiting the female stimulus rat, with more frequent visits to the female interaction zone versus the male interaction zone (main effect of sex of stimulus rat: F(1,44) = 6.853, p < 0.05; Fig. 2A). There was no main effect of early-life treatment on this measure in WKY offspring, and no stimulus rat × early-life treatment interaction. We observed a different pattern of social behavior in the Wistar rats. MS-exposed Wistar offspring made fewer visits to both male and female interaction zones relative to neonatal handling-exposed Wistar offspring (main effect of MS: F(1,80) = 10.22, p < 0.01) and did not show a preference for visiting female over male stimulus rats (no main effect of stimulus rat and no interaction; Fig. 1B). We also examined the effect of stimulus rat and MS exposure on the amount of time WKY and Wistar rats spent in the male/female rat interaction zones. For WKY rats, there was a significant main effect of stimulus rat, with all WKY offspring spending more time in the female interaction zone (F(1, 44) = 15.05, p < 0.001). Post-hoc analysis revealed that this difference was significant in the maternally-separated, but not neonatally-handled, animals (p < 0.01; Fig. 2C). There was no main effect of early-life treatment or sex of the stimulus rat (and no interaction) for time spent in the male and female interaction zones was detected for Wistar rats (Fig. 2D).
Figure 2.
When given a choice between exploring a novel male or female on Day 2 of the social interaction test, significant main effect of sex of the stimulus rat was observed in WKY offspring (A). Wistars exhibited significant effect of early-life treatment, where maternally-separated rats made fewer visits to both male and female zones (B). Maternally-separated WKY rats spent more time interacting with the female stimulus rat (C). No differences in interaction times were observed in Wistar rats (D). Abbreviations as in Fig. 1. * − p < 0.05, ** − p < 0.01.
Discussion
The present study demonstrates contrasting effects of early-life MS in two strains of rats that exhibit innate differences in emotional behavior and stress susceptibility. In Wistar rats, MS increased anxiety-like behaviors in the OFT and decreased social behavior, which is consistent with several prior studies documenting adverse effects of MS. We were surprised to find that MS improved anxiety- and depression-like behavior in WKY offspring, leading to increased exploratory behavior (OFT), increased social interaction, and diminished FST immobility. We also observed a decrease in grooming in the Wistar, but not WKY, rats exposed to MS, which may be an indication of diminished novelty-induced exploration and increased anxiety-like behavior [15, 28]. These findings demonstrate contrasting effects of early life stress on adult behavior in rat strains with divergent endogenous stress susceptibility, an effect that has not been reported previously.
Although most work on early-life stress focuses on its harmful effects on current and future health, emerging evidence suggests a more nuanced view. For example, the match/mismatch hypothesis of disease posits that stress during early life is not necessarily pathological but can instead have adaptive value to an individual that faces later stressful environments or experiences during adulthood [25]. Examples from metabolic research report that human and rodent offspring exposed to malnutrition stress in utero develop metabolic dysfunction when raised under non-stressful food abundant conditions, but not under food scarce conditions [23]. The notion of beneficial stress inoculation has been suggested based on the observation that repeated stress exposure in childhood confers protective neuroendocrine effects in adulthood [14]. Consistent with this idea is the concept of the predictive adaptive response (PAR), which means that an individual will use experience of past stressors to augment coping with future stressors [7]. This idea is supported by data demonstrating that rat pups exposed to the stress of either poor maternal care or 24-hour maternal deprivation exhibited increased memory performance and long-term potentiation as adults when tested under stressful conditions but not under non-stressful conditions [19]. This PAR is thought to be strongest in stress susceptible individuals and may be evolutionarily conserved by rapidly changing environmental conditions across generations [7]. Our present data are consistent with this theory, showing that early-life MS has positive effects in the stress susceptible WKY rats.
Previous MS studies have utilized a variety of manipulations and comparison groups to ascertain effects of early life stress, including separation paradigms of single 24-hour separation, as well as repeated daily separations lasting 1-6 hours over the first 2-3 postnatal weeks. A variety of comparison groups have been used which include groups that were: 1) non-handled; 2) animal facility reared; or 3) brief handling (3-15 min), during the early postnatal period. In the wild, the dam must leave the nest on a regular basis to search for food, and inducing brief separations via daily neonatal handling is one way to mimic this effect. Extensive literature demonstrates the contrasting effects of neonatal handling vs. MS in terms of HPA axis reactivity and stress-elicited behaviors in adulthood [22], thus we decided to use these manipulations in the present study.
It's important to keep in mind that the effects of MS depend on the strain and genetic endowment of rats experiencing it. In our previous study we evaluated the effects of MS on behavior and endocrine function in the selectively-bred Low (bLR) and High (bHR) responder rats [3]. These rats were bred from the Sprague-Dawley strain and show striking differences in their depressive- and anxiety-like behavior, with bLRs showing a profile similar to the WKY rats and bHRs resembling Wistar rats [5]. While MS did not induce changes in the bLR/bHR behaviors on the OFT and FST, their endocrine responses were differentially impacted with the bLRs manifesting an augmentation of adrenocorticotropic hormone secretion in response to an anxiogenic stimulus (i.e. exposure to the light/dark box) and bHRs showing its blunting [3]. While beyond the scope of the current study, it will be important to determine the impact of MS on the HPA axis activity in the WKY and Wistar rats given that the activity of this system has the potential to shape to depression and anxiety [9]. Stress exposure, such as MS, leads to increased secretion of corticosterone, which has strong effects on brain development [1]. MS also leads to increases in mineralocorticoid and glucocorticoid receptors in the hippocampus, which mediate corticosterone signaling in the brain [10]. It is therefore likely that this system mediates the observed behavioral effects in our study. It is also important to note that MS may be a relatively mild stressor, and that more severe stressors, such as more prolonged maternal separation or chronic stress exposure, may have different consequences in both strains and may be detrimental to their development and behavior.
Previous studies reported decreased weight in maternally-separated rats before and after weaning, suggesting that the separation suppressed maternal milk availability or circulating growth hormone levels [27]. In the current study, we did not see an effect on Wistar or WKY offspring's physical development, suggesting the observed behavioral differences are not due to metabolic differences induced by MS. Instead, the contrasting effects of neonatal handling and MS on WKY vs. Wistar offspring may be mediated by alterations in maternal care induced by the manipulations. Variations in maternal care significantly impact stress-elicited behavioral and endocrine responses in adulthood, and contribute to the effects of brief early-life separation (as in the neonatal handling paradigm) [13]. These observations indicate a key role of maternal behavior in shaping the effects of early-life manipulations on adult offspring's behavior. A limitation of the present experiment is that we did not compare maternal behaviors under MS and neonatal handling; future studies will be required to address this.
In previous study we failed to see an effect of MS or neonatal handling on depressive/anxiety- like behaviors in WKY offspring [17]. However, in that study, pregnant WKY females were purchased and delivered to our animal housing facility around gestational day 15. Given the heightened sensitivity of WKY rats to stress and other environmental perturbations, it is very likely that the stress of transport significantly impacted offspring via prenatal stress and/or possible changes in maternal behavior, which could have interfered with effects of neonatal handling/MS. To circumvent this issue in the current study, we mated male/female WKY and Wistar rats in our own animal housing facility, thus eliminating the potential confound of prenatal stress. The current observations taken together with our previous report [17] suggest significant differences in the behavioral profiles of WKY rats that were: 1) reared under commercial conditions and shipped as adults; 2) shipped in utero; and 3) reared in our animal facility from conception through adulthood. These observations highlight the importance of minimizing exposure to external stressors throughout the lifespan of WKY rats, including during the prenatal period, in studies examining their behavioral alterations.
In conclusion, our current data suggest that the effects of early-life MS on anxiety- and depression-like behavior in adulthood may depend on innate stress reactivity. Future studies will be required to determine the role of maternal care and the neurobiological mechanisms that mediate these effects.
Highlights.
Wistar-Kyoto (WKY) and Wistar (W) pups were exposed to maternal separation (MS).
W-MS pups increased anxiety-like behavior and decreased social behavior as adults.
WKY-MS pups decreased anxiety- and depressive- like behavior in adulthood.
WKY-MS pups increased their social interaction behavior in adulthood.
Acknowledgments
The study was funded by MH081927 (IAK), NARSAD Young Investigator award (IAK), MH085859 (SMC), AHA 13PRE16940050 (SR). We thank Dr. Burel Goodin for statistical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- 2.Chocyk A, Przyborowska A, Makuch W, Majcher-Maslanka I, Dudys D, Wedzony K. The effects of early-life adversity on fear memories in adolescent rats and their persistence into adulthood. Behavioural brain research. 2014;264:161–172. doi: 10.1016/j.bbr.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Clinton SM, Watson SJ, Akil H. High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress. 2014;17:97–107. doi: 10.3109/10253890.2013.850670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 5.Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76 Pt B:425–436. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 8.Hulshof HJ, Novati A, Sgoifo A, Luiten PG, den Boer JA, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behavioural brain research. 2011;216:552–560. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Kathol RG, Jaeckle RS, Lopez JF, Meller WH. Pathophysiology of HPA axis abnormalities in patients with major depression: an update. Am J Psychiatry. 1989;146:311–317. doi: 10.1176/ajp.146.3.311. [DOI] [PubMed] [Google Scholar]
- 10.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 12.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. The European journal of neuroscience. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 14.Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys, J Trauma Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- 15.McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middlebrooks JS, Audage NC. In: The effects of childhood stress on health across the lifespan. C.o.D.C.a. Prevention, editor. National Center for Injury Prevention and Control; Atlanta, GA: 2008. [Google Scholar]
- 17.Nam H, Clinton SM, Jackson NL, Kerman IA. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Frontiers in behavioral neuroscience. 2014;8:109. doi: 10.3389/fnbeh.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann ID, Torner L, Toschi N, Veenema AH. Oxytocin actions within the supraoptic and paraventricular nuclei: differential effects on peripheral and intranuclear vasopressin release. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R29–36. doi: 10.1152/ajpregu.00763.2005. [DOI] [PubMed] [Google Scholar]
- 19.Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joels M, Lucassen PJ, Krugers H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 21.Pare WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989;46:993–998. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- 22.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 23.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 24.Roman E, Gustafsson L, Berg M, Nylander I. Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Hormones and behavior. 2006;50:736–747. doi: 10.1016/j.yhbeh.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Santarelli S, Lesuis SL, Wang XD, Wagner KV, Hartmann J, Labermaier C, Scharf SH, Muller MB, Holsboer F, Schmidt MV. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur Neuropsychopharmacol. 2014 doi: 10.1016/j.euroneuro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Talge NM, Neal C, Glover V T.R. Early Stress, F. Prevention Science Network, C. Neonatal Experience on, H. Adolescent Mental, Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? Journal of child psychology and psychiatry, and allied disciplines. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallee M, Mayo W, Maccari S, Le Moal M, Simon H. Long-term effects of prenatal stress and handling on metabolic parameters: relationship to corticosterone secretion response. Brain research. 1996;712:287–292. doi: 10.1016/0006-8993(95)01459-4. [DOI] [PubMed] [Google Scholar]
- 28.van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behavioural brain research. 1994;65:47–55. doi: 10.1016/0166-4328(94)90072-8. [DOI] [PubMed] [Google Scholar]