Abstract
The neural circuitry underlying the fear response is extremely well conserved across mammalian species, which has allowed for the rapid translation of research findings in rodent models of fear to therapeutic interventions in human populations. Many aspects of exposure-based psychotherapy treatments in humans, which are widely used in the treatment of PTSD, panic disorder, phobias, and other anxiety disorders, are closely paralleled by extinction training in rodent fear conditioning models. Here, we discuss how the neural circuitry of fear learning and extinction in rodent animal models may be used to understand the underlying neural circuitry of fear-related disorders, such as PTSD in humans. We examine the factors that contribute to the pathology and development of PTSD. Next, we will review how fear is measured in animal models using classical Pavlovian fear conditioning paradigms, as well as brain regions such as the amygdala, which are involved in the fear response across species. Finally, we highlight the following three systems involved in the extinction of fear, all of which represent promising avenues for therapeutic interventions in the clinic: (1) the role of the glutamatergic N-methyl-d-aspartate (NMDA) receptor, (2) the role of the brain-derived neurotrophic factor (BDNF)–tyrosine kinase B (TrkB) induced signaling pathway, and (3) the role of the renin-angiotensin system. The modulation of pathways underlying fear learning and extinction, such as the ones presented in this review, in combination with extinction-based exposure therapy, represents promising avenues for therapeutic intervention in the treatment of human fear related disorders.
Keywords: amygdala, fear memory, consolidation, extinction, biomarkers, PTSD
INTRODUCTION
Fear learning is an adaptive and evolutionarily advantageous response to traumatic events; however, dysregulated processes of fear regulation, such as sensitization and overgeneralization can be harmful to the individual. Normal fear responses involve both the consolidation and manifestation of fear memories in fearful situations and also the suppression and extinction of fear behaviors in safe situations. The extinction of fear memories involves the gradual decline in fear responses upon repeated presentations of the fearful cue in nonthreatening situations. The overgeneralization of fear and the inability to extinguish fear memories comprise two of the key symptoms of fear-related disorders such as pho-bias and posttraumatic stress disorder (PTSD).[1] This review will focus specifically on PTSD, a debilitating fear-related disorder in which fear memories of a traumatic incident become overgeneralized and are difficult to extinguish. Estimates indicate that PTSD occurs in 5– 10% of the general population[2] and populations that are exposed to chronic physical and emotional trauma experience even higher lifetime rates of PTSD of up to 20–30%.[3]
In DSM-IV, there are three main clusters of PTSD symptoms: re-experiencing, avoidance, and hyperarousal. Re-experiencing symptoms are provoked by reminders of the trauma and include intrusive and distressing thoughts of the traumatic incident, recurring nightmares, or experience of intense emotional upset or physiological reactivity following exposure to reminders of the trauma. Re-experiencing symptoms can be thought of in terms of classical Pavlovian fear conditioning, in that a cue associated with the trauma will trigger an often painful emotional and/or physiological response, in addition to concomitant nightmares and flashbacks. The avoidance symptom cluster may be thought of as a type of operant conditioning, in which the avoidance of reminders of the trauma in and of itself becomes a reinforcing process. Finally, hyperarousal symptoms include central and autonomic nervous system processes that lead to behaviors such as being easily startled or having trouble sleeping. Hyperarousal symptoms are the cluster most commonly targeted with currently available medications; however, these medications treat only the biological processes of hyperarousal, failing to address the specific trauma memory underlying the fear disorder.[4,5]
Underlying the three main clusters present in PTSD is a dysregulated fear response that characterizes most anxiety disorders including phobias and PTSD. A number of factors make it feasible to translate research on PTSD and other fear-related disorders conducted at the laboratory bench to clinical settings. First, the neural circuitry and phenotypic outputs of the fear response are well understood and have been studied since the time of Ivan Pavlov (in addition to appetitive conditioning, Pavlov had also studied aversive fear conditioning).[6] Second, the fear processing and behaviors following trauma exposure are highly evolutionarily conserved across mammalian species, thus allowing for translational research in rodent models toward the treatment of human fear disorders.[7,8] And finally, PTSD is the only psychiatric disorder in which the instigating event—the trauma—is known, and in many instances the traumatic incident leading to PTSD development may be recalled and is readily identifiable, leading to more efficacious and tailored prevention and intervention approaches.
This review will discuss the neurobiology of fear learning and extinction and will present several identified systems involved in the underlying circuitry of fear extinction. One of the primary goals of modern psychiatry related to fear disorders has been to identify therapies/drugs that modulate signaling pathways involved in synaptic plasticity of fear learning in associated brain regions such as the amygdala in order to enhance the neurocircuitry of extinction. The genetic, molecular, and behavioral literature on extinction processes is vast and complex and interested readers are directed to additional reviews for further and more comprehensive reading.[1,9]
FACTORS AND STAGES UNDERLYING THE DEVELOPMENT OF PTSD
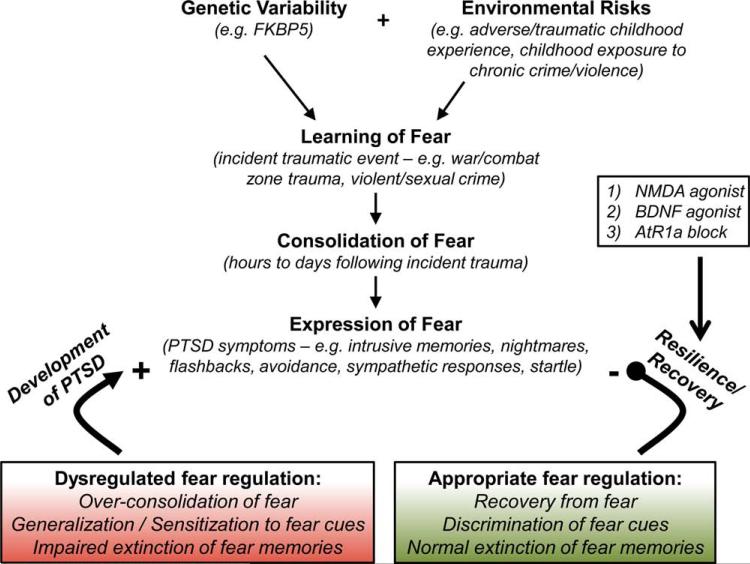
Although estimates suggest that 90% of individuals will be exposed to a significant traumatic event in their lifetime,[2] the rates of PTSD are relatively low at 5–20%,[3] depending on the cohort and trauma exposure levels. Thus, a critical question in the study of PTSD is why some trauma victims go on to develop PTSD, while others experiencing the same event do not; and they appear instead to be resilient to the effects of the trauma. A number of factors contribute to the variability in an individual's risk of developing PTSD[10] (Fig. 1). Preexisting genetic sensitivities or early life experience such as child abuse represent factors that contribute to risk for PTSD following exposure to trauma in adulthood. Twin studies indicate that risk for PTSD following trauma is in part genetically mediated.[11] Additionally, many studies have now demonstrated a strong interaction between genetics and early life environment in predicting adult PTSD.[12] Certain gene pathways (such as FKBP5) interact with early life trauma (but not adult trauma) to predict adult PTSD.[13–15] For example, FKBP5 regulates key aspects of the hypothalamic-pituitary-adrenal (HPA) axis and may play a role in stress and glucocorticoid-dependent critical periods of amygdala and hippocampal development.[13,16] Specifically, certain gene variants of FKBP5 when combined with exposure to early life stress may result in amygdala sensitization, thus affecting adult responses to trauma.[12,14,15,17] How genetic background and early environmental factors such as childhood trauma interact and impact adult vulnerability and resilience following trauma exposure is a critical area of ongoing research.
Figure 1.
Factors contributing to the development and pathology of posttraumatic stress disorder (modified from ref. 9). Variability in individual risk of developing PTSD may be attributed to a number of factors. Preexisting sensitivities such as genetic variability may interact with early life trauma to predict adult PTSD. Upon experience of the incident trauma in adulthood, the individual will undergo consolidation of the fear memory, which occurs in the minutes, hours, and days following the traumatic event. After the formation of the fear memory, individuals will express their fear in the form of PTSD symptoms that can include intrusive thoughts or memories related to the trauma, nightmares, flashbacks, avoidance of trauma cues that might trigger memories of the trauma event, or sympathetic responses. Individuals who go on to develop PTSD exhibit dysregulated fear regulation in the form of overconsolidation of fear, generalization of fear cues, and impaired extinction of fear memories. On the other hand, resilient individuals who recover from the trauma will display appropriate fear regulation consisting of recovery from the fear, discrimination of fear cues, and normal extinction of fear memories. Current therapeutic research approaches for PTSD seek to enhance the extinction learning that forms the basis of common cognitive behavioral therapies such as exposure therapy, thus enhancing the resilience and recovery of individuals susceptible to PTSD. This review will highlight three systems involved in the extinction of fear learning: (1) NMDA, (2) BDNF, and (3) the renin-angiotensin system.
The subjective severity of a traumatic experience can also contribute significantly to learning the fear memory. For example, a physical attack could be extremely traumatic for one individual and less so for another. In addition, the minutes, hours, and days directly following the traumatic event define the consolidation period of the trauma memory, representing the time frame in which the memory transitions from a labile state to a chronic stable state. Much current translational research is focused on therapeutic interventions that may be administered during the consolidation window to decrease the emotional memory that leads, long term, to the development of PTSD. Reconsolidation is also being increasingly explored as a process in which a fear memory may be reactivated and made to re-enter a labile state for therapeutic interventions[18,19] (however, the extent to which reconsolidation occurs in humans remains somewhat unclear).
Following the formation of the fear memory, individuals may retrieve and express their fear through intrusive memories, nightmares, flashbacks, and startle responses. In addition to individual variations in genetic makeup and consolidation effects, the ability of an individual to recover from the trauma is important. Individuals who go on to develop PTSD may generalize and sensitize these fears, whereas those who are able to recover will learn to discriminate and will extinguish the fear memory over time (Fig. 1). Generalization occurs when the fear responses occur to a broader range of cues that become stimulated in parallel with the initial trauma cue, leading to a more general fear-response (symptom triggering) pattern across cues and contexts. Sensitization occurs when fear symptoms in response to the trauma cue become worse over time. On the other hand, discrimination refers to the ability to differentiate between trauma cues and nontrauma cues, and extinction refers to the diminished fear response to cues over time or with repeated presentations of the trauma cue.
An understanding of the factors that account for resilience in certain individuals would contribute significantly to the development of targeted treatments as well as the prevention of PTSD in individuals with a predisposition to developing PTSD. In addition, the identification of vulnerable individuals could lead to specialized interventions that would prevent the development of disorders such as PTSD by enhancing resiliency. Furthermore, an understanding of the time course of the pathogenesis of PTSD may allow for therapeutic interventions at multiple time points across the progression and development of the fear disorder. As mentioned above, both pharmacological and behavioral interventions during consolidation are currently being explored. For example, recent studies have investigated the effects of exposure-based psychotherapy given in the hours directly following a trauma exposure and have found protective effects of treatment when PTSD symptoms were assessed 4 and 12 weeks following the intervention.[20] Unfortunately, therapeutic interventions may not always be possible during the consolidation window in the hours directly following the traumatic incident. Thus, recent efforts in the field have focused on therapies that may enhance the extinction of the fear memory.
HOW WE MODEL FEAR RESPONSES IN ANIMALS: CLASSICAL PAVLOVIAN FEAR CONDITIONING
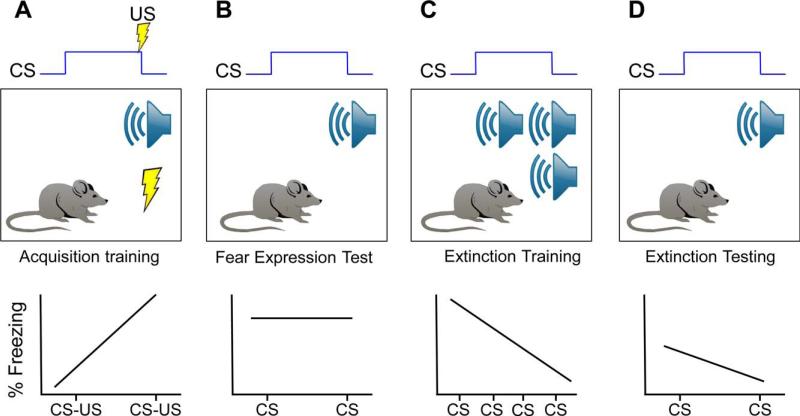
Classical Pavlovian fear conditioning paradigms have been used to dissect the neural mechanisms involved in the acquisition and extinction of learned fear responses. In this paradigm (Fig. 2), a conditioned stimulus (CS; e.g., a light or tone that is initially inoffensive) is paired with an aversive unconditioned stimulus (US; such as a mild foot shock), such that after several CS–US pairings the subject exhibits a conditioned response (CR) to presentations of the CS (tone, light, etc.).
Figure 2.
Basic procedure for fear conditioning in rodent models. Classic Pavlovian fear conditioning is used to study the acquisition and extinction of learned fear responses in rodent animal models. (A) During acquisition training, the conditioned stimulus (CS; tone) is paired with an aversive unconditioned stimulus (US; mild foot shock). After several CS–US pairings, the organism will learn the CS–US association and will exhibit a conditioned fear response (CR) to the CS (tone), as evidenced by increased levels of freezing over the course of several CS–US pairings as well as (B) after training during the presentation of the CS alone. (C) During extinction training, the previously fear conditioned organism is exposed the CS (tone) in the absence of the aversive US (shock). The repeated or prolonged exposure to the CS (tone) will result in a gradual decline in the CR as evidenced by decreasing levels of freezing both during extinction training as well as (D) after extinction training upon presentation of the CS.
Within Pavlovian fear conditioning paradigms, “fear” is defined operationally as freezing (the complete lack of all bodily movements except those involved in respiration), fear-potentiated startle responses (increases in acoustically elicited startle responses), increases in blood pressure, changes in respiration in the presence of the CS (tone, light, etc.), or avoidance of the fear-conditioning context. Rodent model systems commonly measure fear responses with freezing behavior and potentiated startle, which are easily quantified objectively and in an automated fashion by computer programs. The fear-potentiated startle assesses the increase (potentiation) in the acoustic startle reflex when the organism is presented with the CS (tone, light, etc.) that was previously paired with an aversive US (shock). The acoustic startle reflex is a three-synapse circuit between the ear and spinal cord, which activates a reflexive skeletal musculature contraction response and is directly modulated by the amygdala. In humans, acoustic startle response and skin conductance responses are physiological responses that are commonly used as behavioral measures of fear.
To study extinction of the learned fear, the previously fear conditioned organism is exposed to the CS (tone, light, etc.) in the absence of the aversive US (shock). The procedure of repeated or prolonged exposures to the fear-eliciting CS results in a gradual decline in the CR is referred to as fear extinction.[1] Notably, the diminished CRs following extinction training are often not permanent and are subject to reinstatement, renewal, and spontaneous recovery. Renewal consists of the re-emergence of the extinguished CR when animals are exposed to the CS in a novel context (separate from the one in which extinction training occurred). Spontaneous recovery refers to the reappearance of the extinguished CRs after enough time has passed following extinction training. Reinstatement occurs when the extinguished fear response is triggered and reappears upon exposure to the US (after the organism has undergone extinction training).
Extinction learning leading to active inhibition of the fear response forms the basis of most exposure-based psychotherapies in the treatment of human fear disorders. Extinction training in rodent fear conditioning paradigms parallels many aspects of exposure-based psychotherapy treatments used in human subjects and thus has high face validity. These parallels have fueled translational research, allowing therapeutic interventions for fear-related disorders to progress very rapidly from the research lab to the clinic.
THE FEAR RESPONSE IS A HARDWIRED PROCESS INVOLVING THE AMYGDALA
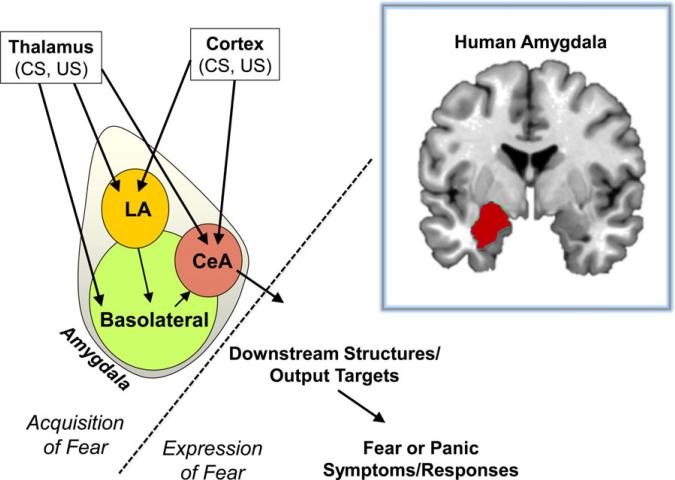
There are several brain regions involved in fear processing and fear-related disorders. The key brain regions belong to the limbic system (which regulates emotional processing across species) and include the hippocampus, amygdala, and prefrontal cortex (PFC; other regions such as the parahippocampal gyrus, orbitofrontal cortex, sensorimotor cortex, the thalamus, and anterior cingulate cortex are also involved).[1,21] Amygdala activation is a hallmark of all fear-related disorders (Fig. 3).[6] Functional brain imaging studies demonstrate increased amygdala activation during the presentation of fearful faces, fearful cues, as well as during fear acquisition and expression.[22] Individuals diagnosed with PTSD or other fear-related disorders exhibit hyperactive amygdala activity compared to normal subjects. The amygdala is composed of many subnuclei including the basolateral complex (lateral, basal, and accessory basal nuclei) and the central nuclei (CeA). The basolateral amygdala (BLA) is critical in the acquisition, expression, and extinction of fear.[23] In the context of rodent classical fear conditioning, multimodal sensory information from thalamic and sensory cortical areas (auditory, visual, somatosensory cortex, etc.) specific to the CS project to the lateral nucleus of the amygdala (LA).[24,25] In parallel, information specific to the US is relayed to the LA from somatosensory thalamic and cortical areas and the periaqueductal gray.[26,27] The LA is thus thought of as a site critical for synaptic plasticity and Hebbian learning that occurs during paired presentations of the CS and US during fear learning.[28–34] Broadly, the amygdala receives sensory input from the environment and serves as a “hub” to sample motifs of traumatic experience.
Figure 3.
Simplified diagram of amygdala nuclei and circuits underlying the acquisition and expression of fear. The amygdala is a key region involved in fear learning. The human amygdala is shown in the upper right box. Multimodal sensory information is first processed in thalamic and sensory cortical areas before converging on the amygdala. The lateral amygdala is critical for synaptic plasticity involved in fear acquisition. The central nucleus (CeA) has traditionally been viewed as the “fear output structure” that sends projections to brain regions, which activate downstream behavioral fear responses. Recent data support a role for the lateral division of the central amygdala (CEl) in the acquisition of fear (ref. 37 and 38); we direct the reader to a number of excellent reviews on the detailed microcircuitry underlying amygdala-dependent learning (ref. 9).
The central amygdala (CeA) has primarily been regarded as the fear output structure that sends projections to brain regions, which activate a host of downstream behavioral fear responses/symptoms.[35–38] For example, the CeA projects to the lateral hypothalamus (initiates increase heart rate and blood pressure), dorsal vagal nucleus (bradycardia and ulcers), parabrachial nucleus (panting and respiratory distress), basal fore-brain (arousal, vigilance, attention), reticular pontis caudalis (increased startle response), central gray area (freezing and social interactions), and paraventricular nucleus (corticosteroid release).[6] It is important to note that the amygdala is equally involved in appetitive learning processes that are not discussed in this review. Long-term potentiation (LTP) and synaptic plasticity at any point along this circuit contributes to alterations in pathways that underlie the fear response.[28–34] From a translational perspective, the hardwired fear reflex outputs following amygdala activation provide a specific set of neural circuits to understand the mechanisms underlying fear-related disorders.
The amygdala is in turn modulated by several brain regions including the PFC and hippocampus. In the context of classical fear conditioning models, the PFC has primarily been thought to provide inhibitory modulation to the amygdala. However, the complexity of the PFC is only recently starting to be understood.[39–44] Certain regions of the PFC appear to be positively correlated with amygdala activation of fear, whereas other regions are negatively correlated. The infralimbic (IL) cortex (ventromedial PFC in humans) is required for fear extinction but not fear acquisition, whereas the prelimbic (PL) cortex (thought to be similar to the dorsal anterior cingulate cortex in humans) is required for fear acquisition but not extinction.[45] Our understanding of the role of the hippocampus in spatial regulation and declarative learning and memory is vast. In the context of fear learning and memory, the hippocampus is crucial for the regulation and discrimination of contextual fear learning and extinction, and like the PFC, it also heavily modulates amygdala activity.[46,47] The current knowledge of the neural circuitry underlying the fear response is growing rapidly and its breadth lies beyond the scope of this review.[48] For the remainder of this review, we will specifically discuss three molecular ligand/receptor systems that play an important role in the extinction of fear responses: (1) the glutamatergic N-methyl-d-aspartate (NMDA) receptor, (2) the brain-derived neurotrophic factor (BDNF)–tyrosine kinase B (TrkB) induced signaling pathway, and (3) the renin-angiotensin system.
THE NMDA RECEPTOR AND AMYGDALA-DEPENDENT LEARNING
The role of the glutamatergic NMDA receptor in amygdala-dependent learning has been one of the most exciting and clinically successful areas in the study of fear extinction. NMDA receptors are highly expressed in the amygdala and are important for fear learning. Early studies found that fear learning requires NMDA receptor activation, and learning is blocked by antagonists at the NMDA receptor. In investigations of fear extinction, administration of NMDA receptor antagonists either systemically or directly into the BLA before fear extinction training were found to block the extinction of freezing and fear-potentiated startle, suggesting an important role for NMDA receptors in the consolidation of extinction memories.[49]
The NMDA receptor partial agonist d-cycloserine (DCS) activates the NMDA receptor glycine/serine modulatory site, resulting in increased calcium influx upon glutamate binding. DCS facilitates the extinction of fear-potentiated startle when given either systemically or by direct infusion into the amygdala prior to or immediately following extinction training.[50] In addition to enhancing extinction training, DCS-facilitated extinction is more resistant to reinstatement and also generalizes the inhibition of fear to a trained, yet nonextinguished cue. DCS was previously FDA-approved and had been prescribed as an antibiotic treatment for tuberculosis in the 1960s (the cyclic serine moiety acts to block translation in certain bacteria), which allowed for the quick translation from DCS-facilitated extinction in rodent models to clinical studies of DCS-assisted exposure psychotherapy in human subjects. A number of studies have now investigated the ability of DCS to enhance exposure-based psychotherapy for a number of phobia and fear-related disorders, with two recent positive meta-analytic analyses.[51,52]
Of particular clinical relevance has been the use of DCS-facilitated virtual reality exposure (VRE) therapies. Although exposure-based therapies for simple pho-bias rely on the repeated presentation of the feared object or situation (spiders, heights, etc.), exposure-based therapies for many fear disorders such as PTSD rely on self-recounting of the traumatic experience. VRE is an ideal therapy for clinical research because there is no variability in the exposure experience between patients, and the therapist is able to tightly control the exposure session, which takes place in a confined physical area.[53–55] VRE has been successful in the treatment of phobia disorders and PTSD, and has been shown to be significantly improved when administered in combination with DCS.[51,56] In a study examining the effects of DCS in the treatment of acrophobia (fear of heights) using VRE, the group receiving DCS prior to VRE showed significantly greater improvements in fear reduction at both 1-week and 3-month follow-ups. VRE specific to war experience in Iraq is now currently underway to investigate the DCS enhancement of VRE in Iraq war veterans. Rodebaugh and Lenze have developed a high-throughput rapid clinical assay as another recent approach to translating drugs that enhance fear extinction preclinically in clinical exposure therapy paradigms.[57]
The effect of DCS on the enhancement of extinction has now been replicated in several human trials across multiple fear disorders, though work remains to be done.[51,52,58–60] Together these data demonstrate that NMDA receptor dependent mechanisms are required for the consolidation and extinction of fear memories, and the enhancement of NMDA receptor activity with DCS enhances extinction in rodents and humans in a variety of paradigms. There are many other molecular systems that we are beginning to understand, which are directly involved in extinction processing. The identification of these systems has led to new ways of thinking about pharmacological targeting of memory formation or memory inhibition in the treatment of human fear disorders.
BDNF SIGNALING MECHANISMS IN FEAR LEARNING
BDNF and its action at the TrkB receptor plays a significant role in both the acquisition and extinction of fear learning in both human PTSD and in animal models of fear conditioning and extinction.[61] The Val66Met polymorphism is a single-nucleotide polymorphism (SNP) in the pro-region of BDNF that consists of a Met substitution for Val at position 66 and has been implicated in several psychiatric disorders including depression, schizophrenia, and PTSD.[62] Individuals with the Val66Met SNP release less BDNF peptide, have decreased hippocampal volume, and exhibit deficits in declarative memory and fear extinction.[63–65] These data suggest that this polymorphism in the BDNF gene may disrupt BDNF signaling and, in so doing, affect emotional learning and memory.
To investigate the importance of the Val66Met SNP experimentally in a rodent model, investigators generated mice with the knock-in allele of the human Val66Met allele. Knock-in mice display reduced hippocampal dendritic arborization, decreased hippocampal volume, and impaired LTP as well as deficits in declarative memory and decreased fear extinction,[62,66–71] indicating similar phenotypes between transgenic mice and human carriers of the Val66Met SNP. Furthermore, knock-in mice display impaired NMDA receptor dependent synaptic plasticity in the hippocampus, and DCS appears to rescue the BDNF Val66Met extinction deficit of conditioned aversive memories.[68]
In support of these findings, studies in rodent models have demonstrated that BDNF–TrkB signaling is necessary for the acquisition of fear conditioning and the consolidation of fear extinction in the amygdala, hippocampus, and PFC.[72,73] In the amygdala, BDNF transcription is upregulated in extinction-trained rats compared to nonextinction-trained controls. Furthermore, intra-BLA infusions of a TrkB.t1 dominant negative lentiviral vector that expresses a truncated form of the TrkB receptor prior to extinction training re sulted in deficits in extinction retention, suggesting an important role for TrkB in the consolidation of extinction memory.[72] BDNF deletion by injecting Cre recombinase expressing lentivirus into the brain of floxed BDNF transgenic mice provides a useful technique to investigate the regional dependent effects of BDNF.[46,74] BDNF deletion in the hippocampus results in deficits in fear extinction but has no effects on fear consolidation.[46] BDNF also plays a role in distinct regions of the PFC in fear extinction. Direct BDNF infusion in the IL region enhances fear extinction.[42] In contrast, BDNF deletion in the PL region of the PFC results in deficits in fear acquisition but does not affect fear extinction. BDNF deletion induced fear acquisition deficits in the PL may be rescued by administration of 7,8-dihydroxyflavone (7,8-DHF), a small molecule compound that activates the TrkB receptor, thus mimicking the actions of endogenous BDNF.[41] 7,8-DHF has been recently identified as a relatively specific TrkB receptor agonist which, when systemically administered in mice, crosses the blood brain barrier (BBB) to initiate TrkB receptor dimerization, auto-phosphorylation, and activation of downstream signaling.[75] Systemic administration of a single dose of 7,8-DHF has been found to activate TrkB receptors in the amygdala and also enhance the acquisition and extinction of fear in mice.[76] 7,8-DHF also rescues the extinction deficit present in a mouse model of stress using the immobilization on boards stressor paradigm (IMO)[76] as well as in mouse models of chronic stress.[61]
Together, these data suggest an important role for BDNF in the extinction of and recovery from fear. Other specific small molecule agonists and antagonists that target the BDNF–TrkB signaling pathway may provide other approaches in the enhancement of extinction and the treatment and prevention of fear-related disorders in humans.
A ROLE FOR THE RENIN-ANGIOTENSIN SYSTEM IN THE TREATMENT OF PTSD
Recent studies have also identified a potential role for hypertension and blood pressure medication in the treatment of PTSD.[77] The renin-angiotensin system has been shown to play a role in stress- and anxiety-related disorders, and has also been implicated in mechanisms of learning and memory. Of note, individuals diagnosed with PTSD are also more likely to suffer from hyperlipidemia, obesity, and hypertension and are also at an increased risk for stroke and heart attack. Drugs that target the renin-angiotensin system such as angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACE-I) are commonly prescribed to treat hypertension and cardiovascular diseases. Previous studies indicate that ARBs may be useful in the prevention of stress-related disorders, and may also provide protective effects for cognition and memory. A recent clinical study of over 500 traumatized human patients demonstrated a significant association between ACE-I/ARB medication and decreased PTSD symptoms, suggesting an important role for the renin-angiotensin system in PTSD.[77]
Unexpectedly, there are large amounts of the angiotensin receptor expressed in the amygdala and bed nucleus of the stria terminalis, which regulate fear behaviors. Furthermore, both acute and chronic administration of Losartan, an angiotensin receptor type 1 (AT1) antagonist, enhanced extinction consolidation in mice previously exposed to a fear-conditioning paradigm (as assessed by reduced freezing behavior during extinction retention tests when Losartan was given prior to extinction training).[113] Neither acute nor chronic administration of this low-dose Losartan-affected blood pressure or anxiety levels in mice, yet fear levels were decreased and extinction was increased at this drug level. These data from both mouse and human studies suggest that targeting the renin-angiotensin system may provide additional important potential therapies in the treatment of human PTSD.
CONCLUSION
Molecular mediators of synaptic plasticity, such as the ones discussed in this review, are required for the consolidation and extinction of fear memories. A critical question is whether the approach of enhancing the functional neurocircuitry for extinction holds for other mediators of synaptic plasticity and emotional memory. Recent work has identified additional systems/agents involved in extinction processes beyond the scope of this review (Table 1), which, when given in conjunction with exposure therapy may also enhance the reduction of fear which occurs with extinction learning. We direct the reader to a number of excellent reviews for more extensive overview of additional systems involved in fear extinction (ref. 1, 9, 115). The identification of such systems represents important and promising avenues toward the treatment of PTSD.
TABLE 1.
Summary of selected other studies to date (not discussed in this review) examining manipulation of neurotransmitter systems in the consolidation and extinction offear
| System | Function | Summary of supporting evidence | References |
|---|---|---|---|
| GABA | Consolidation | Auditory fear learning enhanced with genetic deletion of alpha-1 subunit of GABA-A receptor | 78 |
| Deficits in conditioned fear with GABAergic inactivation of amygdala, hippocampus, PFC, or striatum | 45,79,80 | ||
| Extinction | Impaired by inverse agonist of GABA-A receptor (FG-7142) | 81 | |
| Impaired by GABAergic inactivation of infralimbic cortex, BLA, or ventral hippocampus | 45 | ||
| Impaired by bilateral amygdala LV knockdown of GAD67 (glutamic acid decarboxylase 67, enzyme that catalyzes most GABA synthesis). | 116 | ||
| Enhanced by systemic administration of GABA antagonist picrotoxin | 82 | ||
| Enhanced extinction of contextual freezing by intra-BLA infusion of GABA receptor antagonist bicuculline | 83 | ||
| Dopamine (DA) | Consolidation | Enhanced by dopamine D2 receptor agonists in the ventral tegmental area (VTA) | 84-86 |
| Disrupted by dopamine receptor antagonists or lesions of midbrain dopamine systems | 87-89 | ||
| FPS impaired by D2 receptor antagonists in the BLA | 85 | ||
| Extinction | Impaired by loss of D1 receptor (genetic knock-out (KO) or siRNA in hippocampus) | 90 | |
| Impaired with systemic or intra-IL PFC infusion of D2 antagonist | 91 | ||
| Impaired with systemic D2 receptor agonist quinpirole | 92 | ||
| Facilitated with systemic administration of D2 receptor antagonist | 93 | ||
| Endocannabinoid | Consolidation | Enhanced with administration of CB1 inverse agonist in the CEA or BLA | 94 |
| Impaired by CB1 receptor agonist or anandamide transport inhibition in vmPFC | 94 | ||
| Extinction | Disrupted by genetic deletion or blockade of CB1 receptors | 95,114 | |
| Acetylcholine (Ach) | Consolidation | Impaired by alpha-7 nAch receptor antagonists | 96 |
| Enhanced with nicotinic Ach agonists in the hippocampus | 97-99 | ||
| Extinction | Impaired by systemic administration of scopolamine, muscarinic Ach receptor antagonist | 100 | |
| Norepinephrine (NE) | Consolidation | Enhanced with alpha-1 adrenergic receptor blockade/antagonists (LA) | 101 |
| Impaired by siRNA for beta-1 adrenergic receptors (BLA) | 102 | ||
| Extinction | Enhanced by systemic administration of yohimbine, alpha-2 autoreceptor antagonist | 103 | |
| Within session extinction facilitated by administration of propranolol (beta-adrenoreceptor antagonist) | 83 | ||
| Opioids | Consolidation | Facilitated acquisition of conditioned fear with administration of opiate antagonists (e.g., naloxone) | 104, 105 |
| Immediate morphine treatment following trauma may prevent PTSD development | 106-108 | ||
| The NOP-R agonist SR-8993 impairs cued-fear memory consolidation in mice | 110 | ||
| Extinction | Impaired by systemic administration of naloxone, opiate antagonist (before extinction training) | 111 | |
| Impaired by infusion of naloxone into the ventrolateral PAG | 112 | ||
| Enhanced by u-opioid pathway activation | 111 |
The treatment of fear-related disorders is increasingly moving away from treating just symptoms of disease to a more comprehensive approach that addresses the underlying causes of fear dysregulation. This shift has been heralded by the use of cognitive enhancers, such as DCS, to enhance the targeted approach of exposure-based psychotherapy. Such a strategy in the therapeutic treatment of PTSD seeks to improve the extinction learning mechanisms that form the basis of common cognitive behavioral therapies. The extremely well conserved neural circuitry underlying the fear response across mammalian species has allowed for the rapid translation of research investigating the pharmacological targeting of memory formation and extinction in rodent models to the treatment of human fear disorders. The modulation of synaptic plasticity pathways underlying fear learning in combination with extinction-based exposure therapy represents promising avenues for therapeutic intervention in fear-related disorders. A major goal of modern psychiatry is thus to enhance the brain's endogenous mechanisms of fear extinction in order to enhance flexible and appropriate responses following extinction learning.
Acknowledgments
Support was provided by NIH (R01MH071537, R01MH096764), the Burroughs Wellcome Fund, NIH/NCRR base grant (P51RR00-0165) to Yerkes National Primate Research Center.
Footnotes
Conflict of interest. Dr. Ressler is a founding member of Extinction Pharmaceuticals/Therapade Technologies, which exist to develop d-cycloserine for use to augment the effectiveness of psychotherapy. He has received no equity or income from this relationship within the last 3 years.
REFERENCES
- 1.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62(Suppl 17):16–22. [PubMed] [Google Scholar]
- 4.Steckler T, Risbrough V. Pharmacological treatment of PTSD—established and new approaches. Neuropharmacology. 2012;62:617–627. doi: 10.1016/j.neuropharm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cain CK, Maynard GD, Kehne JH. Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs. 2012;21:1323–1350. doi: 10.1517/13543784.2012.704020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 7.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 8.Belzung C, Philippot P. Anxiety from a phylogenetic perspective: is there a qualitative difference between human and animal anxiety? Neural Plast. 2007;2007:1–17. doi: 10.1155/2007/59676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013;16:146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, Orr SP, Lasko NB, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta D, Binder EB. Gene × environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology. 2012;62:654–662. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Binder EB, Bradley RG, Epstein MP, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of post-traumatic stress disorder symptoms in adults. J Am Med Assoc. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Klengel T, Mehta D, Anacker C, et al. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat Neurosci. 2012:1–12. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriceau S, Wilson Da, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 18.Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci. 2006;63:999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agren T, Engman J, Frick A, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337:1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 20.Rothbaum BO, Kearns MC, Price M, et al. Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry. 2012;72(11):957–963. doi: 10.1016/j.biopsych.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2012;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanselow MS, Ledoux JE. Why we think Pavlovian fear conditioning occurs in the basolateral amygdala. Neruon. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 24.Ledoux JE, Romanski M, Xagoraris A. The lateral amygdaloid in fear conditioning nucleus: sensory interface amygdala. J Neurosci. 1990;10(4):1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanuza E, Nader K, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience. 2004;125:305–315. doi: 10.1016/j.neuroscience.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Pape H-C, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90(2):419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sah P, Westbrook RF, Lüthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann N Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- 30.Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Blair HT, Schafe GE, Bauer EP, et al. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- 33.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 34.Tsvetkov E, Carlezon Wa, Benes FM, et al. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 35.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciocchi S, Herry C, Grenier F, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 38.Haubensak W, Kunwar PS, Cai H, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 41.Choi DC, Maguschak Ka, Ye K, et al. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci USA. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phelps Ea, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 44.Milad MR, Wright CI, Orr SP, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heldt Sa, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight DC, Smith CN, Cheng DT, et al. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- 48.Orsini Ca, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falls Wa, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bontempo A, Panza KE, Bloch MH. D-cycloserine augmentation of behavioral therapy for the treatment of anxiety disorders: a meta-analysis. J Clin Psychiatry. 2012;73(4):533–537. doi: 10.4088/JCP.11r07356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Rothbaum BO, Hodges LF, Kooper R, et al. Effectiveness of computer-generated (virtual reality) graded exposure in the treatment of acrophobia. Am J Psychiatry. 1995;152(4):626–628. doi: 10.1176/ajp.152.4.626. [DOI] [PubMed] [Google Scholar]
- 54.Rothbaum BO, Hodges L, Smith S, et al. A controlled study of virtual reality exposure therapy for the fear of flying. J Consult Clin Psychol. 2000;68(6):1020–1026. doi: 10.1037//0022-006x.68.6.1020. [DOI] [PubMed] [Google Scholar]
- 55.Rothbaum BO, Hodges LF, Ready D, et al. Virtual reality exposure therapy for Vietnam veterans with posttraumatic stress disorder. J Clin Psychiatry. 2001;62(8):617–622. doi: 10.4088/jcp.v62n0808. [DOI] [PubMed] [Google Scholar]
- 56.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 57.Rodebaugh TL, Lenze EJ. Lessons learned from D-cycloserine: the promise and limits of drug facilitation of exposure therapy. J Clin Psychiatry. 2013;74(4):415–416. doi: 10.4088/JCP.13ac08464. [DOI] [PubMed] [Google Scholar]
- 58.de Kleine Ra, Hendriks G-J, Kusters WJC, et al. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 59.Kushner MG, Kim SW, Donahue C, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 60.Cukor J, Spitalnick J, Difede J, et al. Emerging treatments for PTSD. Clin Psychol Rev. 2009;29:715–726. doi: 10.1016/j.cpr.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frielingsdorf H, Bath KG, Soliman F, Difede J. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications or posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 65.Bueller JA, Aftab M, Sen S, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59(9):812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 66.Pattwell SS, Bath KG, Perez-Castro R, et al. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012;32:2410–2421. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bath KG, Chuang J, Spencer-Segal JL, et al. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012;72:499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu H, Wang Y, Pattwell S, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spencer JL, Waters EM, Milner Ta, et al. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci USA. 2010;107:4395–4400. doi: 10.1073/pnas.0915105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ninan I, Bath KG, Dagar K, et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. 2010;30(26):8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao L, Dhilla A, Mukai J, et al. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol. 2007;17(11):911–921. doi: 10.1016/j.cub.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heldt Sa, Ressler KJ. The use of lentiviral vectors and Cre/loxP to investigate the function of genes in complex behaviors. Front Mol Neurosci. 2009;2(22):1–13. doi: 10.3389/neuro.02.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang S-W, Liu X, Yepes M, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andero R, Ph D, Heldt SA, et al. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168(2):163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khoury NM, Marvar PJ, Gillespie CF, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiltgen BJ, Godsil BP, Peng Z, et al. The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci. 2009;3(37):1–12. doi: 10.3389/neuro.08.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PloS One. 2011;6:e15982. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corbit LH, Janak PH. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur J Neurosci. 2010;31(7):1312–1321. doi: 10.1111/j.1460-9568.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delamater AR, Campese V, Westbrook RF. Renewal and spontaneous recovery, but not latent inhibition, are mediated by gamma-aminobutyric acid in appetitive conditioning. J Exp Psychol Anim Behav Process. 2009;35(2):224–237. doi: 10.1037/a0013293. [DOI] [PubMed] [Google Scholar]
- 82.McGaugh JL, Castellano C, Brioni J. Picrotoxin enhances latent extinction of conditioned fear. Behav Neurosci. 1990;104(2):264–267. doi: 10.1037//0735-7044.104.2.264. [DOI] [PubMed] [Google Scholar]
- 83.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Borowski TB, Kokkinidis L. Contribution of ventral tegmental area dopamine neurons to expression of conditional fear: effects of electrical stimulation, excitotoxin lesions, and quinpirole infusion on potentiated startle in rats. Behav Neurosci. 1996;110(6):1349–1364. doi: 10.1037//0735-7044.110.6.1349. [DOI] [PubMed] [Google Scholar]
- 85.de Oliveira MR, da Rocha RF, Stertz L, et al. Total and mitochondrial nitrosative stress, decreased brain-derived neurotrophic factor (BDNF) levels and glutamate uptake, and evidence of endoplasmic reticulum stress in the hippocampus of vitamin A-treated rats. Neurochem Res. 2011;36(3):506–517. doi: 10.1007/s11064-010-0372-3. [DOI] [PubMed] [Google Scholar]
- 86.Biojone C, Casarotto PC, Resstel LB, et al. Anti-aversive effects of the atypical antipsychotic, aripiprazole, in animal models of anxiety. J Psychopharmacol. 2011;25(6):801–807. doi: 10.1177/0269881110376690. [DOI] [PubMed] [Google Scholar]
- 87.Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- 88.Guarraci Fa, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- 89.Greba Q, Kokkinidis L. Peripheral and intraamygdalar administration of the dopamine D1 receptor antagonist SCH 23390 blocks fear-potentiated startle but not shock reactivity or the shock sensitization of acoustic startle. Behav Neurosci. 2000;114(2):262–272. doi: 10.1037//0735-7044.114.2.262. [DOI] [PubMed] [Google Scholar]
- 90.Ortiz O, Delgado-Garcia JM, Espadas I, et al. Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a−/− mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci. 2010;30(37):12288–12300. doi: 10.1523/JNEUROSCI.2655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol Psychiatry. 2010;68:1055–1060. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nader K, LeDoux J. The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behav Neurosci. 1999;113(1):152–165. doi: 10.1037//0735-7044.113.1.152. [DOI] [PubMed] [Google Scholar]
- 93.Ponnusamy R, Nissim Ha, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lisboa SF, Reis DG, da Silva AL, et al. Cannabinoid CB1 receptors in the medial prefrontal cortex modulate the expression of contextual fear conditioning. Int J Neuropsychopharmacol. 2010;13(9):1163–1173. doi: 10.1017/S1461145710000684. [DOI] [PubMed] [Google Scholar]
- 95.Chhatwal JP, Gutman AR, Maguschak Ka, et al. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- 96.Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201(2):325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 97.Andre JM, Leach PT, Gould TJ. Nicotine ameliorates NMDA receptor antagonist-induced deficits in contextual fear conditioning through high-affinity nicotinic acetylcholine receptors in the hippocampus. Neuropharmacology. 2011;60(4):617–625. doi: 10.1016/j.neuropharm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kenney JW, Wilkinson DS, Gould TJ. The enhancement of contextual fear conditioning by ABT-418. Behav Pharmacol. 2010;21(3):246–249. doi: 10.1097/FBP.0b013e32833a5b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davis JA, Gould TJ. Beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190(3):343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prado-Alcalá Ra, Haiek M, Rivas S, et al. Reversal of extinction by scopolamine. Physiol Behav. 1994;56:27–30. doi: 10.1016/0031-9384(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 101.Lazzaro SC, Hou M, Cunha C, et al. Antagonism of lateral amygdala alpha1-adrenergic receptors facilitates fear conditioning and long-term potentiation. Learn Mem. 2010;17(10):489–493. doi: 10.1101/lm.1918210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fu AL, Yan XB, Sui L. Down-regulation of beta1-adrenoceptors gene expression by short interfering RNA impairs the memory retrieval in the basolateral amygdala of rats. Neurosci Lett. 2007;428(2-3):77–81. doi: 10.1016/j.neulet.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 103.Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fanselow MS. Opiate modulation of the active and inactive components of the postshock reaction: parallels between naloxone pretreatment and shock intensity. Behav Neurosci. 1984;98(2):269–277. doi: 10.1037//0735-7044.98.2.269. [DOI] [PubMed] [Google Scholar]
- 105.Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol. 1979;93(4):736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- 106.Holbrook TL, Galarneau MR, Dye JL, et al. Morphine use after combat injury in Iraq and post-traumatic stress disorder. New Engl J Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 107.Saxe G, Stoddard F, Courtney D, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry. 2001;40(8):915–921. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 108.Good AJ, Westbrook RF. Effects of a microinjection of morphine into the amygdala on the acquisition and expression of conditioned fear and hypoalgesia in rats. Behav Neurosci. 1995;109(4):631–641. doi: 10.1037//0735-7044.109.4.631. [DOI] [PubMed] [Google Scholar]
- 109.Van't Veer A, Yano JM, Carroll FI, et al. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the κ-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37:2809–2816. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andero R, Brothers SP, Jovanovic T, et al. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med. 2013;5:188ra73. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117(6):1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- 112.McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of Pavlovian fear conditioning. J Neurosci. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marvar PJ, Goodman J, Fuchs S, Choi DC, Banerjee S, Ressler KJ. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.024. [Epub online ahead of print]. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 115.Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol Behav. 2012;107(5):699–710. doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heldt SA, Mou L, Ressler KJ. In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl Psychiatry. 2012;2:e181. doi: 10.1038/tp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]