Abstract
Background
Varenicline, a partial nicotinic acetylcholine receptor (nAChR) agonist, is a promising new drug for the treatment of alcohol (ethanol) dependence. Varenicline has been approved by the Food and Drug Administration as a smoking cessation therapeutic and has also been found to reduce ethanol consumption in humans and animal models of alcohol use. The current studies examined the hypotheses that varenicline attenuates the stimulant and sensitizing effects of ethanol, and reduces the motivational effects of ethanol-associated cues. The goal was to determine if these effects of varenicline contribute to its pharmacotherapeutic effects for alcohol dependence. In addition, effects of varenicline on acute stimulation and/or on the acquisition of sensitization would suggest a role for nAChR involvement in these effects of ethanol.
Methods
Dose-dependent effects of varenicline on the expression of ethanol-induced conditioned place preference (CPP), locomotor activation, and behavioral sensitization were examined. These measures model motivational effects of ethanol-associated cues, euphoric or stimulatory effects of ethanol, and ethanol-induced neuroadaptation. All studies used DBA/2J mice, an inbred strain with high sensitivity to these ethanol-related effects.
Results
Varenicline did not significantly attenuate the expression of ethanol-induced CPP. Varenicline reduced locomotor activity and had the most pronounced effect in the presence of ethanol, with the largest effect on acute ethanol-induced locomotor stimulation and a trend for varenicline to attenuate the expression of ethanol-induced sensitization.
Conclusions
Because varenicline did not attenuate the expression of ethanol-induced CPP, it may not be effective at reducing the motivational effects of ethanol-associated cues. This outcome suggests that reductions in the motivational effects of ethanol-associated cues may not be involved in how varenicline reduces ethanol consumption. However, varenicline did have effects on locomotor behavior and significantly attenuated acute ethanol-induced locomotor stimulation. In humans who drink while taking varenicline, it might similarly reduce stimulant responses and have an impact on continued drinking. General sedative effects in such individuals should be carefully considered.
Keywords: Alcohol, chantix, CPP, DBA/2J, mice
Introduction
Excessive use of alcohol (ethanol) remains a leading preventable cause of death (Danaei et al., 2009). A significant obstacle in treating alcohol addiction is the lack of effective pharmaceutical options. Nicotinic acetylcholine receptors (nAChR) have been identified as potential pharmacological targets for the treatment of alcohol dependence (Chatterjee & Bartlett, 2010). One promising new drug is varenicline, an approved smoking cessation therapeutic, that acts as a partial agonist at α4β2 nAChR, with lower order of magnitude effects at other nAChR subtypes (Rollema et al., 2007) and 5-HT3 receptors (Lummis et al., 2011). Varenicline reduced ethanol consumption in heavy drinking smokers (Fucito et al., 2011; McKee et al., 2009; Mitchell et al., 2012), nonhuman primates (Kaminski & Weerts, 2013), mice (Hendrickson et al., 2010; Kamens et al., 2010) and rats (Chatterjee et al., 2010; Steensland et al., 2007), and had positive effects on number of heavy drinking days and other measures in a recently completed larger clinical trial (Litten et al., 2013).
Nicotinic-AChRs likely contribute to the actions of ethanol through both direct and indirect mechanisms (for review, see Davis & Fiebre et al., 2006). As a partial agonist, varenicline weakly activates certain nAChRs in the absence of other nAChR agonists (Rollema et al., 2007); it can also act as a competitive nAChR antagonist. In a previous study, pharmacological inhibition of nAChRs inhibited the conditioned rewarding effects of ethanol (Bhutada et al., 2012). We hypothesized that varenicline would attenuate the motivational effects of ethanol-associated cues (expression of CPP), and the stimulant and sensitizing effects of ethanol via two possible mechanisms: by weakly activating some of the same neurocircuitry as ethanol and/or by blocking actions of ethanol.
Two phases of investigation can be considered in CPP and sensitization studies: acquisition and expression. Acquisition refers to the period when ethanol-cue associations (in CPP studies) or sensitization are developing, and expression refers to a final response, measured after acquisition. Addiction treatment is not likely to occur at the same time that ethanol use is initiated; thus, our initial study focused on the effect of varenicline on the expression of an ethanol-induced CPP. CPP is a method for determining preference for environmental stimuli previously paired with the administration of a drug, and is used as a model of conditioned reward (Cunningham et al., 2006; Prus et al., 2009). By administering varenicline for the first time before the preference test we hypothesized that varenicline would decrease the preference for ethanol-paired cues, indicating an effect on the motivational processes that underly the expression of ethanol-induced CPP (Cunningham et al., 2011). Given the powerful influence of ethanol-paired cues on continued ethanol use, such an outcome would suggest that one reason why varenicline reduces ethanol consumption is by reducing the motivational effects of ethanol-paired stimuli.
The second and third experiments examined the effects of varenicline, first, on the expression of ethanol-induced locomotor sensitization and then on the acquisition of sensitization. In both studies, effects of varenicline on acute ethanol-induced stimulation were also examined. Ethanol has stimulant effects and sensitivity to such effects in humans has been proposed as a risk factor for increased alcohol use (Holdstock et al., 2000; King et al., 2002; 2011; Schuckit, 1994). Repeated exposure to ethanol can result in long-lasting sensitization, such that the same dose of ethanol induces a greater response than that seen initially, even after an extended period of abstinence (e.g., Lessov & Phillips, 1998). The altered neurochemical mechanisms underlying behavioral sensitization are thought to be related to the development of drug dependence and relapse; the behavioral changes reflect these underlying neuroadaptations (for reviews see Phillips et al., 2011; Steketee & Kalivas, 2011). Ethanol-induced sensitization has been most commonly studied in rodents, but also documented in humans (Newlin & Thomson, 1991; 1999). We hypothesized that varenicline would attenuate the expression and acquisition of ethanol-induced locomotor sensitization and would reduce the acute stimulant response to ethanol. An effect on the expression of sensitization would support the ability of varenicline to impact the altered response to ethanol established by chronic treatment. Attenuation of stimulation or acquisition of sensitization by varenicline would suggest the involvement of nAChRs in these ethanol-induced effects.
Materials and Methods
Animals
Male and female DBA/2J mice were purchased from The Jackson Laboratory (Sacramento, CA) or produced by breeders purchased from The Jackson Laboratory and housed (2–5 per cage) in same-sex groups. Shipped mice were allowed to acclimate for at least 2 weeks before testing. Produced mice were weaned at 20–22 days of age and group housed with same sex littermates. All mice were maintained in standard “shoebox” cages (28.5L x 17.5W x 12H cm) lined with Bed-o’Cobs® bedding (The Andersons, Inc., Maumee, OH, USA) and had ad libitum access to water and food (LabDiet® 5001, PMI Nutrition International LLC, St. Louis, MO, USA). DBA/2J mice were used in these studiesbecause of their high sensitivity to ethanol-induced CPP (Cunningham et al., 1992; 2003; 2006), locomotor stimulation (Crabbe et al., 1994; Dudek et al., 1994), and locomotor sensitization (Phillips et al., 1994; Lessov et al., 2001; Meyer et al., 2005). All mice were experiment- and drug-naïve prior to testing, and behavioral testing was conducted during the light phase of the 12:12 h light:dark cycle (lights on at 0600 h), between 0800 and 1600 h.
Drugs
Ethyl alcohol was purchased from Decon Laboratories, Inc. (King of Prussia, PA, USA). Varenicline tartrate used in the sensitization experiments was a generous gift from Pfizer (Groton, CT, USA); varenicline tartrate used in the CPP experiment was purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Doses of varenicline are expressed as the tartrate salt. All drugs were prepared in physiological (0.9%) saline (Baxter Healthcare Corp., Deerfield, IL, USA) and administered as IP (intraperitoneal) injections.
Procedures
Ethanol-induced CPP
The effect of varenicline on the expression of ethanol-induced CPP was measured using a standard procedure (Cunningham et al., 1992; 2003; 2006), shown in Table 1. The CPP chambers (San Diego Instruments, San Diego, CA, USA) consisted of clear plastic walls (30L x 15W x 15H in cm) and were equipped with exchangeable floor panels and housed in illuminated and ventilated sound attenuating chambers (AccuScan Instruments, Inc., Columbus, OH, USA). Three different floor types were used: a solid black plastic acrylic floor; a “grid” floor constructed of 2.3 mm stainless steel rods mounted 6.4 mm apart; and a “hole” floor constructed of a stainless steel panel with 6.4 mm round holes aligned with 9.5 mm staggered centers. Activity and location of the mouse were measured by photocell beam interruptions recorded by a fully automated system. This experiment was designed so that mice received the same handling and injection procedures; on all days mice received 2 injections that were spaced 15 min apart.
Table 1.
Outline of experimental groups and procedures for the effect of varenicline on the expression of CPP study
| Experimental Groups |
All mice received two injections spaced 15 min apart on all days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
Day 8 |
Day 9 |
Day 10 |
|
| Effect of Varenicline on the Expression of CPP | ||||||||||
| G+: 4 groups (VAR 0, 0.5, 1, and 1.5 mg/kg) | SAL, SAL (Black) | SAL, SAL (Hole) | SAL, E2 (Grid) | SAL, SAL (Hole) | SAL, E2 (Grid) | SAL, SAL (Hole) | SAL, E2 (Grid) | SAL, SAL (Hole) | SAL, E2 (Grid) | VAR, SAL (Grid+Hole) |
| G-: 4 groups (VAR 0, 0.5, 1, and 1.5 mg/kg) | SAL, SAL (Black) | SAL, SAL (Grid) | SAL, E2 (Hole) | SAL, SAL (Grid) | SAL, E2 (Hole) | SAL, SAL (Grid) | SAL, E2 (Hole) | SAL, SAL (Grid) | SAL, E2 (Hole) | VAR, SAL (Grid+Hole) |
Under Experimental Groups, the entry following G+: or G-: indicates the pretreatment received on the place preference test day (day 10) by 4 groups of mice in each case. Entries under Day 1 – Day 9 indicate the two injections received and the type of flooring in the chamber during the 5-min trial (habituation on Day 1; acquisition conditioning on Day 2 – Day 9). Entries under Day 10 indicate the two injections received and the floor types on which mice could choose to spend time. The effect of varenicline pretreatment on the expression of a place preference was assessed during a 30-min test on this day. Note that this table does not reflect all treatment conditions as the groups were also counterbalanced for side on which the grid floor was placed during the preference test, and whether ethanol or saline was the first conditioning session. CPP = conditioned place preference; G+ = mice conditioned with ethanol on the grid floor; G− = mice conditioned with ethanol on the hole floor; E2 = 2 g/kg ethanol; SAL = 0.9% saline; VAR = varenicline.
On day 1, to habituate mice to handling and the CPP apparatus, each was placed in the CPP chamber immediately after the second injection for a 5-min session with black plastic flooring on both sides of the chamber. This flooring was used to avoid exposing the miceto the floor types (grid and hole) that served as associative cues during conditioning sessions. On the next 8 days, 5-min conditioning sessions with 2 g/kg ethanol were alternated with sessions with saline, with a single floor type (e.g., grid with ethanol and hole with saline) on both sides of the chamber.Groups were counterbalanced for floor type associated with ethanol and whether ethanol or saline was the first conditioning session (only one order is shown in Table 1, but there were equal numbers of animals that received ethanol or that received saline as their first conditioning trial). The 2 g/kg dose of ethanol induces robust CPP in DBA/2J mice (Cunningham et al., 2006). Twenty-four hours after the last conditioning session, mice were given a 30-min floor preference test, during which both floor types (grid and hole) were present. Mice were pretreated with varenicline (0, 0.5, 1 or 1.5 mg/kg) 15 min before treatment with saline. A maximum dose of 1.5 mg/kg varenicline was chosen to avoid depressant effects of a higher dose on the locomotor activity of DBA/2J mice (pilot data) that might make interpretation of the CPP results problematic. Consistent with previous studies for ethanol (Cunningham et al., 1992; 2003; 2006), the dependent variable used to determine the expression of a CPP was second/min on the grid floor, with the expectation that mice for which ethanol was paired with the grid floor (G+ group) would spend more time on that floor compared to mice for which ethanol had been paired with the hole floor (G- group).
Ethanol-induced locomotor sensitization
Locomotor activity was measured using sixteen automated locomotor activity monitors (40W x 40L x 30H in cm) (AccuScan Instruments, Inc.; Columbus, OH, USA), each equipped with 8 pairs of photocell beams and detectors, mounted 2 cm above the chamber floor. Beam breaks were automatically converted to distance traveled, and each monitor was housed in a chamber that eliminated external noise and was ventilated with a fan and illuminated with a 3.3 W incandescent light bulb (Gubner et al., 2013).
A 14-day behavioral sensitization protocol was used. Treatments are summarized in Table 2. Mice received 2 injections spaced 15 min apart, each day. The 15-min pretreatment period for varenicline was based on the rationale given above for the CPP study. Day 1 testing familiarized the animals with handling, and apparatus; day 2 provided a habituated baseline activity measure. Days 3–12 were the sensitization acquisition phase. Day 13 was the sensitization expression test day. Day 14 was the drug-free test day to determine if there were remaining effects of prior treatments. On test days, mice were moved into the testing room 45 min prior to the start of testing to acclimate. A cohort of mice were then weighed, given their first injection (pretreatment) and placed back into home cages; 15 min later, the second injection (treatment) was given and locomotor activity was measured for 15 min. On non-test days, mice were weighed, given their first injection, placed back into their home cage for 15 min, given their second injection, and returned to their home cages.
Table 2.
Outline of experimental groups and procedures for the effect of varenicline on the expression and acquisition of ethanol-induced locomotor sensitization studies
| Experimental Groups | All mice received two injections spaced 15 min apart on all days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1–2 | Day 3 | Day 4–5 | Day 6 | Day 7–8 | Day 9 | Day 10–11 | Day 12 | Day 13 | Day 14 | |
| Effect of Varenicline on the Expression of Sensitization | ||||||||||
| SAL → VAR (4 groups: 0, 0.5, 1, and 2 mg/kg) + E2 | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | VAR, E2 | SAL, SAL |
| E2 → VAR (4 groups: 0, 0.5, 1, and 2 mg/kg) + E2 | SAL, SAL | SAL, E2 | SAL, E2 | SAL, E2 | SAL, E2 | SAL, E2 | SAL, E2 | SAL, E2 | VAR, E2 | SAL, SAL |
| SAL → VAR (4 groups: 0, 0.5, 1, and 2 mg/kg) + SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | SAL, SAL | VAR, SAL | SAL, SAL |
| Effect of Varenicline on the Acquisition of Sensitization | ||||||||||
| VAR (4 groups: 0, 0.5, 1, and 2 mg/kg) + SAL → E2 | SAL, SAL | VAR, SAL | VAR, SAL | VAR, SAL | VAR, SAL | VAR, SAL | VAR, SAL | VAR, SAL | SAL, E2 | SAL, SAL |
| VAR (4 groups: 0, 0.5, 1, and 2 mg/kg) + E2 → E2 | SAL, SAL | VAR, E2 | VAR, E2 | VAR, E2 | VAR, E2 | VAR, E2 | VAR, E2 | VAR, E2 | SAL, E2 | SAL, SAL |
| Activity test | Yes | Yes | No | Yes | No | Yes | No | Yes | Yes & Blood | Yes |
Under Experimental Groups, the entry prior to the arrow indicates the repeated treatment during the acquisition phase (Day 3 – Day 12) and the entry after the arrow indicates the treatment on the ethanol challenge day (Day 13). Entries under Day 1 – Day 14 indicate the two injections received. For the expression study, the effect of varenicline was assessed on day 13 by pretreating mice with varenicline (0, 0.5, 1 or 2 mg/kg) prior to ethanol challenge or saline treatment. For the acquisition study, the effect of varenicline (0, 0.5, 1 or 2 mg/kg) was assessed by administering it before ethanol or saline during the sensitization acquisition period (Day 3 – Day 12), and then assessing the response to ethanol challenge on Day 13. In both studies, mice were tested after saline treatment on Day 14 to assess whether prior treatments affected baseline activity levels that had been assessed on Day 1 (novel apparatus) and Day 2 (familiar environment). Information given in the Activity Test row of the table applies to both studies. E2 = 2 g/kg ethanol; SAL = 0.9% saline; VAR = varenicline; Yes = activity test was performed; No = no activity test was performed; Blood = a blood sample for blood ethanol concentration determination was obtained after activity testing on this day.
Our primary focus was on the effect of varenicline on the expression of ethanol-induced sensitization (top section of Table 2), most relevant to a clinical population that has developed dependence and is seeking treatment. However, to assess whether nAChR play a role in the acute stimulant response to ethanol and in acquisition of ethanol-induced locomotor sensitization, the effect of varenicline given prior to each ethanol treatment was examined (bottom section of Table 2).
Due to non-significant effects of varenicline in the CPP experiment, a slightly higher, 2 mg/kg dose of varenicline was used as the highest dose in the sensitization studies. For both studies, immediately after activity testing on day 13, a 20 μl periorbital sinus blood sample was obtained from ethanol-treated mice with a calibrated glass micro-Hematocrit capillary tube (Fisher Scientific, Pittsburgh, PA) and used to determine blood ethanol concentration (BEC). Blood samples were processed and analyzed, using an established gas chromatography method (Boehm et al., 2000).
Data analysis
Statistical analyses were performed using Statistica12 (StatSoft, Tulsa, OK, USA). Sex was first included as a factor and then follow-up analyses were performed with data from the sexes combined, when sex did not interact with other factors. Effects were considered significant at an alpha level of 0.05 or less. For analysis of preference test day data in the CPP study, sec/min on the grid floor and locomotor activity counts were dependent measures and conditioning group (G+ and G) and varenicline dose were independent variables in the analysis of variance (ANOVA). For the sensitization studies, data were first analyzed by repeated measures ANOVA, including baseline day 2 and all other activity test day data. Significant interaction effects can be difficult to detect when a large number of groups or days are present and effects are expected in only a small number of groups or on only a single day, as is the case for the sensitization studies (see Wahlsten, 1990). Therefore, for some analyses, we used composite drug treatment (varenicline plus ethanol group) as a factor. In addition, to correct for baseline activity differences, locomotor scores on days 3 and 13 were corrected for individual day 2 baseline activity scores and examined in separate analyses. Day 2 subtracted from day 3 provided a within-group acute ethanol stimulate score; similarly, day 3 subtracted from day 12 provided a within-group sensitization score. This method is consistent with our previous work (Phillips et al., 1995; Kamens & Phillips 2008; Palmer et al., 2002; Gubner et al., 2013). These drug response data were amenable to analysis by ANOVA with varenicline dose and ethanol dose as independent variables. Significant two-way interactions were interpreted using simple main effects analysis and pairwise mean comparisons, when appropriate. The design of the study examining the expression of sensitization was not fully balanced; groups treated with ethanol during acquisition and then saline on the ethanol challenge day were not included, because they would not address the experimental goal of determining the effect of varenicline on the expression of ethanol-induced sensitization. This also reduced animal usage. However, a control group treated with saline during acquisition and varenicline plus saline on the ethanol challenge day was included to provide information about non-specific effects of varenicline. Our approach to analysis of these data is described along with the results.
Results
Effect of varenicline on the expression of ethanol-induced CPP
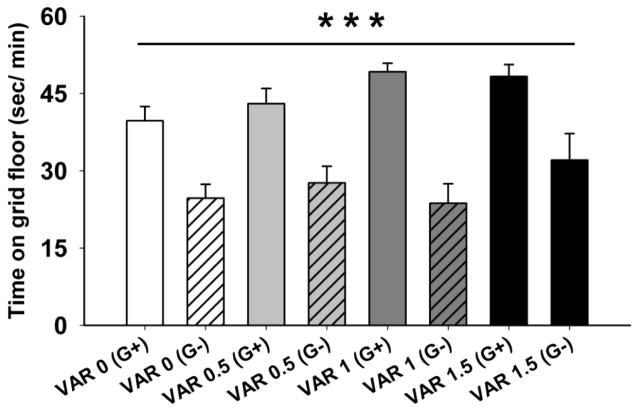
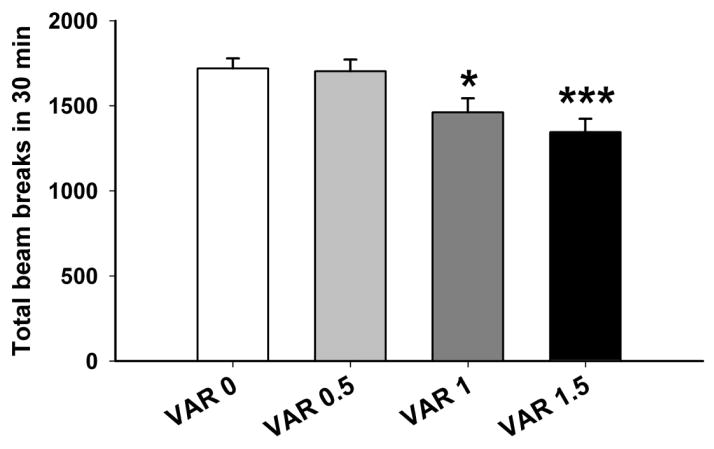
Mice were 69–95 days old (n=13–14 per varenicline dose group and conditioning floor type). There were no significant main or interaction effects involving sex. For the preference test on day 12 (Fig.1), there was a statistically significant difference between the G+ and G- groups for sec/min on the grid floor (F[1,102] = 62.9, p < 0.001), indicating that the DBA/2J mice developed a CPP to 2 g/kg ethanol, as expected. However, there was no significant pretreatment dose x conditioning group interaction. Thus, varenicline did not attenuate the expression of ethanol-induced CPP, indicating that varenicline did not reduce preference for the ethanol-paired cues. There was a significant effect of varenicline on locomotor activity during the preference test (F[3,106] = 6.5, p < 0.001); regardless of conditioning group (G+ or G-), the groups pretreated with 1 or 1.5 mg/kg of varenicline had lower locomotor activity, compared to the saline pretreated group (Fig. 2). This indicates that pharmacologically relevant doses of varenicline were used and that, although they reduced locomotor activity, these doses of varenicline did not significantly impact the ability of the animals to express a place preference.
Figure 1.
Varenicline did not affect the expression of an ethanol-induced CPP. Shown are mean ± SEM sec/min spent on the grid floor during a 30-min preference test after varenicline (0, 1 or 1.5 mg/kg) and then saline treatment, given 15 min later. VAR x = varenicline tartrate dose in mg/kg; G+ = ethanol paired with grid floor; G- = ethanol paired with hole floor. ***: p<0.001 for the main effect of floor type (G+, G−), indicating that ethanol induced a significant CPP.
Figure 2.
Varenicline reduced locomotor activity during the CPP preference test. Shown are total beam breaks (mean ± SEM), with data collapsed on conditioning group. VARx = varenicline tartrate dose in mg/kg. *: p<0.05; ***: p<0.001 for the comparison of the indicated group with the VAR 0 group.
Effect of varenicline on the expression of ethanol-induced locomotor sensitization
Mice were 55–71 days old (n=11–16 per varenicline+ethanol treatment group). Initial analyses identified no significant effects involving sex, but significant time-dependent effects. The largest ethanol effects on locomotor activity were seen during the first 5 min of the 15-min test, consistent with our previously published work (e.g., Shen et al., 1995), a time period which corresponds with the rising phase of the blood ethanol curve (Goldstein 1983). Prior analyses have indicated that the first 5 min after ethanol treatment represents a time when purely stimulant effects of ethanol are seen that are devoid of depressant responses to ethanol (Phillips et al., 1995). Examination of the time-course data from the current study determined that the first 5-min time point best represents the drug effects seen in this study. Therefore, data from this time period are presented here. Furthermore, when data accumulated for the entire 15-min period were analyzed, there were no significant effects of varenicline on saline-treated, repeated ethanol-treated, or acute ethanol-treated mice. This suggests that varenicline did not have greater generalized locomotor effects when assessed during this longer time-frame.
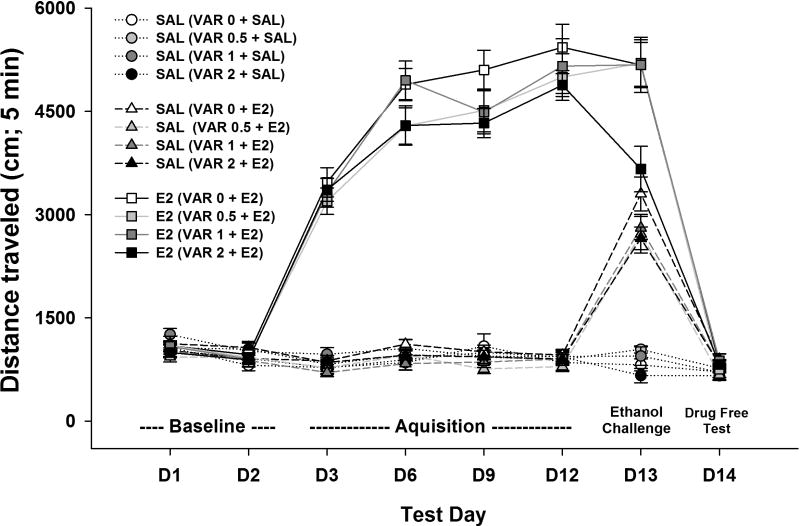
Locomotor activity data across days are shown in Fig. 3. The repeated measures ANOVA identified a significant day x combined dose group [acquisition pretreatment (varenicline 0, 0.5, 1 or 2 mg/kg) + acquisition treatment (ethanol or saline) + day 13 treatment (ethanol or saline)] interaction (F[66,918] = 38.2, p < 0.001), indicating that patterns of activity for the various treatment groups were different across days. Data were next examined for the presence of ethanol-induced sensitization and whether level of sensitization was similar for those groups that were subsequently treated with saline versus a dose of varenicline. The locomotor response on day 3 (initial ethanol response) was subtracted from that on day 12 (final ethanol response during the acquisition phase) for each individual animal to create a sensitization score (for the ethanol groups) and group means are shown as the first 4 bars in Fig. 4A; data for the saline groups are also shown, and show stability in level of locomotor activity after saline. A two-way ANOVA was performed that included data from these 8 groups (only those mice to be challenged with ethanol on day 13). There was a significant main effect of treatment (F[1,111] =69.6, p < 0.001), with the ethanol groups having larger scores than the saline groups, but there were no significant differences among the ethanol or among the saline groups, indicating that the groups were well-matched prior to varenicline treatment on day 13. The last 4 groups of mice shown in Fig. 4A were included in the study to provide data regarding effects of varenicline in mice to be treated with saline on day 13, compared to ethanol. A two-way ANOVA comparing these groups (the last 8 bars of data shown in Fig. 4A) identified no significant differences, indicating again that the groups were well-matched for general level of locomotor activity prior to challenge day 13 treatments.
Figure 3.
Locomotor activity levels across test days for the study examining the effect of varenicline on the expression of ethanol-induced locomotor sensitization. Shown are means ± SEM distance traveled in cm during the first 5 min of each 15-min activity session. All mice received only saline (2 injections) on days 1 and 2; groups represented by circles and triangles received only saline on days 3–12 (2 injections); groups represented by squares received saline then ethanol on days 3–12. Treatment of each group on day 13 is indicated within the parentheses (pretreatment + treatment). All mice received only saline (2 injections) on day 14. SAL = 0.9% saline; VARx = varenicline tartrate dose in mg/kg. E2 = ethanol 2 g/kg.
Figure 4.
Pretreatment with 2 mg/kg of varenicline attenuated the expression of ethanol-induced locomotor sensitization. Shown are means ± SEM distance traveled in cm during the first 5 min of the 15 min activity session. Labels along x-axis show acquisition group and day 13 group (pretreatment + treatment). (A) Ethanol-induced sensitization (day 12 minus day 3) during acquisition is shown in the first 4 bars; change in activity level in saline-group mice during the acquisition period is shown in the remaining bars. (B) Effect of varenicline on ethanol challenge response (first 8 bars) and activity level after saline treatment (last 4 bars); data are corrected for baseline activity level (day 13 minus day 2). SAL = 0.9% saline; VARx = x mg/kg varenicline tartrate. E2 = 2 g/kg ethanol.
To examine the effect of varenicline on acute stimulation and on the expression of ethanol-induced sensitization, locomotor activity data on day 13 were corrected for day 2 baseline activity for each animal and a two-way ANOVA was performed that included all animals challenged with ethanol on day 13. Group means are shown in Fig. 4B (first 8 bars). There was a significant main effect of acquisition group (F[1,111] =82.1, p<0.001); mice repeatedly treated with ethanol had larger locomotor activity scores on the challenge day compared to mice receiving ethanol for the first time, supporting the presence of sensitization. There was also a significant effect of pretreatment group (F[3,111] = 5.4, p<0.01), with reduced locomotor stimulation in the groups treated with varenicline, which was most pronounced for the 2 mg/kg dose of varenicline. There was a trend for a significant interaction (p = 0.14) between acquisition treatment (ethanol or saline) and day 13 pretreatment (varenicline 0, 0.5, 1.0, or 2.0 mg/kg). The data suggest that the 2 mg/kg dose of varenicline reduced the expression of sensitization to a greater extent than it reduced the acute response to ethanol; however, mean comparisons were not conducted due to the absence of a statistically significant interaction.
A similar analysis was performed to compare the effects of varenicline in mice given saline during acquisition and treated with saline or ethanol on the challenge day (last 8 bars of Fig. 4B). For this analysis, there was a significant effect of acute ethanol treatment on day 13 (F[3,93] = 221.3, p < 0.0001). There was also a significant effect of varenicline pretreatment (F[3,93] = 2.63.4, p = 0.05), but no interaction; thus, varenicline had significant locomotor depressant effects, regardless of treatment.
There was no significant treatment group effect for BEC on day 13; BEC was 1.35 ± 0.04 mg/ml for the overall average and group means ranged from 1.24 ± 0.11 to 1.58 ± 0.09 mg/ml. In addition, there were no significant differences in locomotor activity among the groups on day 14 (see Fig. 3), when all groups were treated with saline, indicating that there were no significant carryover effects of prior ethanol or varenicline exposure.
Effect of varenicline on the acquisition of ethanol-induced locomotor sensitization
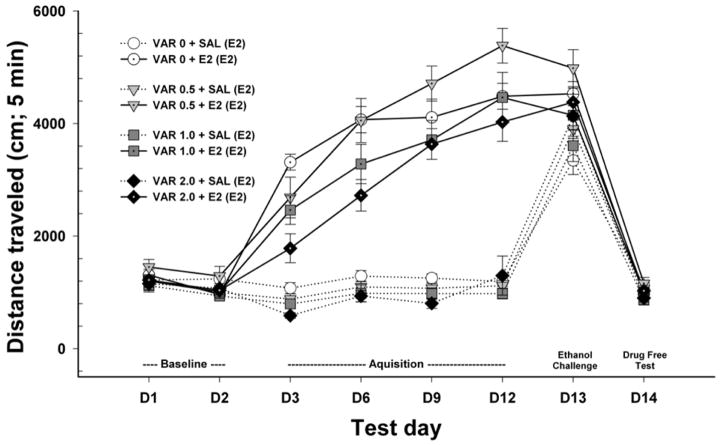
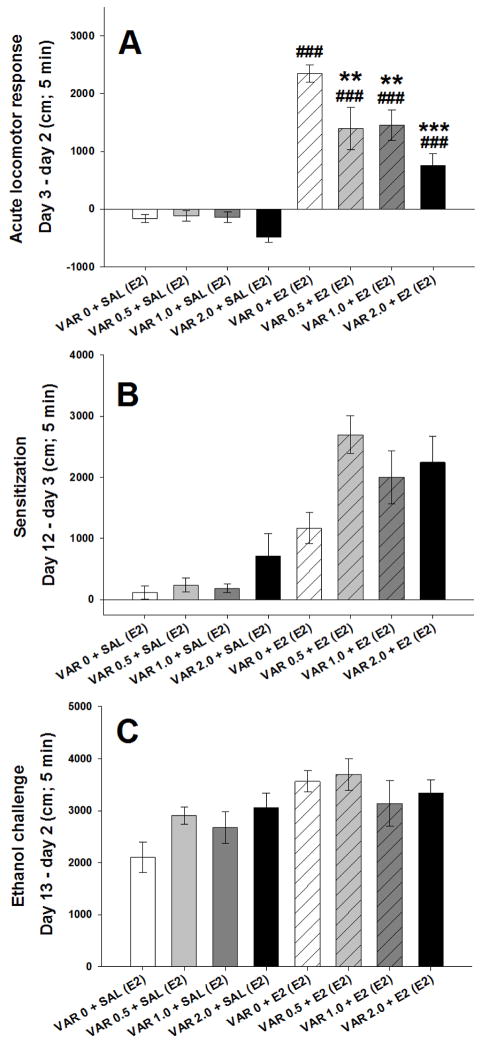
Mice were 55–71 days old (n = 14–17 per varenicline+ethanol treatment group). Initial analyses identified no significant effects involving sex, but significant time-dependent effects. Similar to the expression study, locomotor activity data are shown for the first 5 min of the 15-min sessions in Fig.5. All mice were treated with ethanol on the challenge day. A repeated measures ANOVA identified a significant 3-way interaction (F[21,819] = 1.6, p < 0.05) of day x acquisition pretreatment (varenicline 0, 0.5, 1, or 2 mg/kg) x acquisition treatment (ethanol or saline). Data were next examined for the day 3 locomotor response corrected for day 2 baselineto examine the effect of varenicline on acute ethanol-induced locomotor stimulation (Fig. 6A). There was a significant pretreatment (VAR 0, 0.5, 1.0, or 2.0 mg/kg) x treatment (ethanol or saline) interaction (F[3,117] = 4.3, p < 0.01). Varenicline pretreatment had a significant attenuating effect in the groups that had received ethanol (p < 0.001), but not in the groups that had received saline.
Figure 5.
Locomotor activity levels across test days for the study examining the effect of varenicline on the acquisition of ethanol-induced locomotor sensitization. Shown are means ± SEM distance traveled in cm during the first 5 min of each 15-min activity session. All mice received only saline (2 injections) on days 1 and 2; treatments received on days 3–12 (pretreatment + treatment) and day 13 (in parentheses) are shown in the figure legend. SAL = 0.9% saline; VARx = x mg/kg varenicline tartrate. E2 = 2 g/kg ethanol.
Figure 6.
The effect of varenicline on acute ethanol stimulation and the acquisition of ethanol-induced locomotor sensitization. Shown are means ± SEM distance traveled during the first 5 min of the 15 min session in cm. Labels along x-axis show acquisition group (pretreatment + treatment) and day 13 treatment (in parentheses). (A) Effect of varenicline in saline-treated mice (first 4 bars) and on the acute response to ethanol (last 4 bars); data are corrected for baseline activity level (day 3 minus day 2); (B) Effect of varenicline during the sensitization acquisition period in repeatedly saline-treated mice (first 4 bars) and repeatedly ethanol-treated mice (last 4 bars); change in response is represented as day 12 minus day 3; (C) Response to ethanol challenge in groups that had received various doses of varenicline during the acquisition period, but not prior to this ethanol challenge on day 13; data are corrected for baseline activity level (day 13 minus day 2). SAL= 0.9% saline; VARx = x mg/kg varenicline tartrate. E2 = 2 g/kg ethanol. **: p<0.01; ***: p<0.001 for the comparison between groups receiving ethanol and saline during acquisition for each varenicline dose. ###: p<0.001 for comparison to the VAR 0 + E2 (E2) group.
For level of sensitization during the acquisition period (day 3 data subtracted from day 12 data; Fig. 6B), there was a significant main effect of repeated saline vs. ethanol treatment (F[1,117] = 69.29, p < 0.001), supporting the presence of ethanol-induced sensitization. There was also a significant main effect of varenicline pretreatment (F[3,117] = 3.7, p < 0.05), but only a trend for a pretreatment x treatment interaction (p = 0.11). Groups repeatedly pretreated with 0.5, 1 or 2 mg/kg varenicline had larger sensitization scores during the acquisition period, compared to the 0 mg/kg varenicline pretreated mice, and there was a trend for this effect to be more pronounced in mice that had received ethanol. However, this trend should be cautiously interpreted, given the lower activity levels of the varenicline pretreated groups on day 3, compared to the 0 mg/kg varenicline group (see Fig. 5).
To determine whether varenicline given prior to ethanol during the acquisition period affected sensitization seen in the absence of varenicline, ethanol challenge day 13 data were corrected for day 2 baseline and analyzed (Fig. 6C). There was a significant main effect of repeated saline vs. ethanol treatment (F[1,117] = 13.2, p < 0.001) that reflected the presence of sensitization. However, there were no significant effects involving varenicline pretreatment. Thus, varenicline administered during acquisition did not disrupt ethanol-induced locomotor sensitization on the challenge day. Mice that had received repeated varenicline with saline during acquisition exhibited somewhat higher locomotor activity levels on day 13, but group differences were not examined because the ethanol vs saline by varenicline dose interaction was not significant. Thus, any lasting effects of prior varenicline exposure on the acute response to ethanol were subtle. There were no significant differences between the groups for BEC on day 13; BEC was 1.64 ± 0.05 mg/ml for the overall average and group means ranged from 1.51 ± 0.08 to 1.73 ± 0.07 mg/ml.
There were no significant differences in locomotor activity among the groups on day 14, when all groups were treated with saline, indicating the absence of significant carryover effects of prior ethanol or varenicline treatment.
Discussion
The present work determined the effect of varenicline on non-consummatory ethanol traits that may influence ethanol consumption. Such data are important for establishing the basis for varenicline as an effective pharmacotherapy for alcohol dependence. Contrary to our hypothesis, we found that varenicline did not attenuate the expression of ethanol-induced CPP. This indicates that varenicline may not be effective at reducing effects of ethanol-associated environmental cues that may impact ethanol drinking or relapse. Varenicline did attenuate acute ethanol-induced locomotor stimulation and there was a trend for attenuation of the expression of ethanol-induced locomotor sensitization with the higher dose of varenicline (2 mg/kg). These traits were examined partly because they have been implicated as risk factors for ethanol dependence (King et al., 2011; Newlin & Thompson, 1991; 1999). Drugs that block the stimulant effects of ethanol and ethanol-induced sensitization in mice have also shown promise for the treatment of alcohol dependence in humans. For example, baclofen, a γ-aminobutyric acid (GABA)-B receptor agonist, which has shown promise for the treatment of alcohol dependence in some human studies and case reports (see Agabio and Colombo 2014; Leggio et al., 2010 for reviews) was also found to attenuate the stimulant effects of ethanol (Holstein et al., 2009) and ethanol-induced sensitization (Pastor et al., 2010) in mice. Our data suggest that activation of nAChR using a partial agonist may attenuate the locomotor effects of ethanol, which may be important to ethanol addiction. However, reductions in locomotor activity by varenicline in non-ethanol treated mice suggest that effects of varenicline may be due to sedative effects that are more pronounced in the presence of ethanol.
In the acquisition of sensitization study, varenicline was found to attenuate the acute locomotor stimulant effects of ethanol. A trend for this effect was also seen in the expression of sensitization study in mice receiving ethanol and varenicline for the first time on day 13; however, for this study, the effect did not reach statistical significance. This suggests that varenicline had a less significant effect on locomotor stimulation in mice that had been exposed to the apparatus and handling procedures for an extended period of time (e.g., 10 additional days). Although the exact reason for this finding is unclear, it is possible that the varenicline effect interacts with the level of habituation to the procedures and test environment. We previously found that in DBA/2J mice, the acute locomotor stimulant effects of ethanol were accentuated by nicotine (Gubner et al., 2013), a full nAChR agonist and attenuated by mecamylamine (Kamens & Phillips, 2008), a nAChR antagonist. A partial agonist could have either agonist- or antagonist-like effects. The current effects of varenicline on acute ethanol-locomotor stimulation are more compatible with its actions as a nAChR antagonist. Overall, the doses of varenicline used in this study caused small to moderate reductions in locomotor activity when tested alone, not in the presence of ethanol. This effect reached significance in the CPP study after repeated ethanol exposure, and in the expression of sensitization study. However, our data suggest that varenicline has more pronounced locomotor depressant effects in the presence of ethanol. BEC was measured because changes by varenicline in the clearance of ethanol could provide an explanation for altered behavioral effects. However, there were no significant differences between the groups for BEC, indicating that varenicline did not alter the metabolism of ethanol.
In the acquisition of sensitization study, groups that received repeated varenicline exhibited greater changes in activity across days (Fig. 5), compared to the group pretreated with 0 mg/kg varenicline; this was most pronounced in the groups receiving ethanol (Fig. 6B). However, the differences in sensitization score across varenicline treatment groups largely reflected differences in locomotor response on day 3 (the first varenicline treatment day; see Fig. 5). That all of these groups had similar levels of locomotor response on day 12, indicates that mice developed tolerance to the locomotor reducing effects of varenicline. This suggests that varenicline did not affect the development of neuroadaptations established by repeated ethanol and that the mechanisms underlying the acquisition of ethanol-induced sensitization are not affected by administration of a partial nAChR antagonist. For established alcohol problems, taking varenicline over a longer period of time, or using a dose-escalation procedure, similar to that used for treatment with baclofen (de Beaurepaire, 2012), may lessen the potential sedating effects and lead to better outcomes, though future research is needed.
We hypothesized that varenicline is an effective ethanol pharmacotherapy because it decreases the motivational effects of ethanol-associated cues , but did not confirm such an effect. Varenicline did reduce the expression of nicotine-induced CPP (Biala et al., 2010), suggesting that nAChR partial agonists may be more effective treatments for conditioned rewarding effects of nicotine than ethanol. Mecamylamine did attenuate the expression of ethanol-induced CPP, suggesting a role for nAChR antagonists (Bhutada et al., 2012). However, varenicline could also enhance certain effects of ethanol which may be perceived as aversive. For example, varenicline has been found to increase the ataxic and sedative-hypnotic effects of ethanol in mice (Kamens et al., 2010). Varenicline was also found to increase the subjective dysphoric effects of ethanol in humans (Childs et al., 2012). Low sensitivity to the sedative effects and high sensitivity to the stimulating effects of alcohol have both been found to be risk factors for developing ethanol dependence (Schuckit, 1994; King et al., 2002; 2011). Our data showing attenuation of stimulation are consistent with a shift in the ethanol dose-response curve toward sedation. Additional research is needed to determine if the alternate hypothesis that varenicline reduces ethanol consumption by enhancing the intoxicating/sedating effects of ethanol is valid.
Genotype could have contributed to the limited effects of varenicline found in the current studies. DBA/2J mice were used because they have previously been shown to be highly sensitive to ethanol-induced CPP (Cunningham et al., 1992; 2003; 2006), acute stimulation (Crabbe et al., 1994; Dudek et al., 1994) and locomotor sensitization (Phillips et al., 1994; Lessov et al., 2001; Meyer et al., 2005). However, most of the preclinical mouse studies with varenicline have used C57BL/6J mice, an inbred strain that readily drinks ethanol (Belknap et al., 1993; Yoneyama et al., 2008), but shows little sensitivity to the conditioned rewarding (Cunningham et al., 1992), acute stimulating (Crabbe et al., 1994; Dudek et al., 1994) and locomotor sensitizing (Phillips et al., 1994) effects of ethanol. In addition, DBA/2J and C57BL/6J mice differ in sensitivity to some effects of nicotine (Grabus et al., 2006). It is possible that varenicline would have different effects on ethanol-induced CPP and sensitization in a different genotype of mouse.
Results from the current studies indicate that varenicline does not disrupt ethanol-induced CPP, does attenuate ethanol induced locomotor stimulation, and has modest effects on the response to ethanol in sensitized mice. It is possible that attenuation of the stimulant effects of ethanol by varenicline may impact further ethanol use.
Acknowledgments
Funded by the Department of Veterans Affairs, NIH NIAAA grants P60AA010760, T32AA007468, R24AA020245 and F31AA020732, a grant from the American Psychological Association, and a Tartar Trust Fellowship. We thank Chris Cunningham for assisting in the design and interpretation of the CPP study, and Pfizer for the generous gift of varenicline tartrate
References
- Agabio R, Colombo G. GABAB receptor ligands for the treatment of alcohol use disorder: preclinical and clinical evidence. Front Neurosci. 2014;8:140. doi: 10.3389/fnins.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bhutada P, Mundhada Y, Ghodki Y, Dixit P, Umathe S, Jain K. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of exposure to stress and modulation by mecamylamine. J Psychopharmacol. 2012;26:315–323. doi: 10.1177/0269881111431749. [DOI] [PubMed] [Google Scholar]
- Biala G, Staniak N, Budzynska B. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn-Schmiedebergs Arch Pharmacol. 2010;381:361–370. doi: 10.1007/s00210-010-0498-5. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Schafer GL, Phillips TJ, Browman KE, Crabbe JC. Sensitivity to ethanol-induced motor incoordination in 5-HT(1B) receptor null mutant mice is task-dependent: implications for behavioral assessment of genetically altered mice. Behav Neurosci. 2000;114:401–409. [PubMed] [Google Scholar]
- Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE. Partial Agonists of the α3β4(*) Neuronal Nicotinic Acetylcholine Receptor Reduce Ethanol Consumption and Seeking in Rats. Neuropsychopharmacology. 2010;36:603–615. doi: 10.1038/npp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC, de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res. 2012;36:906–1014. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher ES, Phillips TJ, Belknap JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behav Neurosci. 1994;108:186–195. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Groblewski PA, Voorhees CM. Place conditioning. In: Olmstead MC, editor. Animal models of drug addiction. Totowa, NJ: Humana Press; 2011. pp. 167–189. [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- De Beaurepaire R. Suppression of alcohol dependence using baclofen: a 2-year observational study of 100 patients. Front Psychiatry. 2012;3:103. doi: 10.3389/fpsyt.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek BC, Tritto T, Underwood KA. Genetic influences on locomotor activating effects of ethanol and sodium pentobarbital. Pharmacol Biochem Behav. 1994;48:593–600. doi: 10.1016/0091-3057(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. Pharmacology of Alcohol. New York: Oxford University Press; 1983. [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Gubner NR, McKinnon CS, Reed C, Phillips TJ. Accentuating effects of nicotine on ethanol response in mice with high genetic predisposition to ethanol-induced locomotor stimulation. Drug Alcohol Depend. 2013;127:108–114. doi: 10.1016/j.drugalcdep.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Holstein SE, Dobbs L, Phillips TJ. Attenuation of the stimulant response to ethanol is associated with enhanced ataxia for a GABA-A, but not a GABA-B, receptor agonist. Alcohol Clin Exp Res. 2009;33:108–120. doi: 10.1111/j.1530-0277.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–387. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Weerts EM. The effects of varenicline on alcohol seeking and self-administration in baboons. Alcohol Clin Exp Res. 2013;38:376–383. doi: 10.1111/acer.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. A Double-Blind, Placebo-Controlled Trial Assessing the Efficacy of Varenicline Tartrate for Alcohol Dependence. J Addict Med. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets. 2010;9:33–44. doi: 10.2174/187152710790966614. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacology (Berl) 1998;135:374–382. doi: 10.1007/s002130050525. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology (Berl) 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Lummis SC, Thompson AJ, Bencherif M, Lester HA. Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. J Pharmacol Exp Ther. 2011;339:125–131. doi: 10.1124/jpet.111.185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Palmer AA, McKinnon CS, Phillips TJ. Behavioral sensitization to ethanol is modulated byenvironmental conditions, but is not associated with cross-sensitization to allopregnanolone or pentobarbital in DBA/2J mice. Neuroscience. 2005;131:263–273. doi: 10.1016/j.neuroscience.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology. 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics. Alcohol Clin Exp Res. 1991;15:399–405. doi: 10.1111/j.1530-0277.1991.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol. 1999;7:234–243. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- Palmer AA, McKinnon CS, Bergstrom HC, Phillips TJ. Locomotor activity responses to ethanol, other alcohols, and GABA-A acting compounds in forward- and reverse-selected FAST and SLOW mouse lines. BehavNeurosci. 2002;116:958–967. doi: 10.1037//0735-7044.116.6.958. [DOI] [PubMed] [Google Scholar]
- Pastor R, Kamens HM, McKinnon CS, Ford MM, Phillips TJ. Repeated ethanol administration modifies the temporal structure of sucrose intake patterns in mice: effects associated with behavioral sensitization. Addict Biol. 2010;15:324–335. doi: 10.1111/j.1369-1600.2010.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S, Shen EH. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res. 1995;19:269–278. doi: 10.1111/j.1530-0277.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Pastor R, Scibelli AC, Reed C, Tarragon E. Behavioral sensitization to addictive drugs: Clinical relevance and methodological aspects. In: Raber J, editor. Animal Models of Behavioral Analysis. Humana Press; New York: 2011. pp. 267–305. [Google Scholar]
- Prus AJ, James JR, Rosecrans JA. Conditioned Place Preference. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Chapter 4. CRC Press; Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ. Bidirectional selective breeding for ethanol effects on locomotor activity: characterization of FAST and SLOW mice through selection generation 35. Alcohol Clin Exp Res. 1995;19:1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behavioral and Brain Sciences. 1990;13:109–120. [Google Scholar]