Abstract
BACKGROUND
Health care-associated infections (HAIs) are the most common noncardiac complications after cardiac surgery and are associated with increased morbidity and mortality. Current information about their economic burden is limited.
OBJECTIVES
To determine the cost associated with major types of HAIs during the first 2 months after cardiac surgery.
METHODS
Prospectively collected data from a multicenter observational study of the Cardiothoracic Surgery Clinical Trials Network, in which patients were monitored for infections for 65 days after surgery, were merged with related financial data, routinely collected by the University HealthSystem Consortium. Incremental length of stay (LOS) and cost associated with HAIs were estimated using generalized linear models, adjusting for patient demographics, clinical history, baseline laboratory values, and surgery type.
RESULTS
Among 4,320 cardiac surgery patients, mean age of 64 ± 13 years, 119 (2.8%) experienced a major HAI during the index hospitalization. The most common HAIs were pneumonia (48%), sepsis (20%) and C. Difficile colitis (18%). On average, the estimated incremental cost associated with a major HAI was nearly $38,000, of which 47% was related to intensive care unit services. The incremental LOS was 14 days. Overall, there were 849 readmissions, among these, 8.7% were attributed to major HAIs. The cost of readmissions due to major HAI was on average nearly three times as much as readmissions not related to HAI.
CONCLUSIONS
Hospital cost, length of stay, and readmissions are strongly associated with HAIs. These associations suggest the potential for large reductions in costs if HAIs following cardiac surgery can be reduced.
Introduction
Patients undergoing cardiac surgery are at risk of developing major postoperative infections (1–3), which carry devastating, if not lethal, clinical consequences and substantial costs, as reflected by prolonged hospitalizations and more frequent readmissions (4,5). Reducing the risk of health care-associated infections (HAI) is a key priority for improving surgical care. HAIs are of increasing concern given that patients undergoing cardiac surgery are older and have multiple co-morbidities, which further increases their infection risk. Some HAIs have recently decreased, due to the identification and successful implementation of best practices. For example, the national rate of catheter-related bloodstream infections in the intensive care unit (ICU), decreased by 46% between 2008 and 2012, and the rate of surgical site infections after cardiac procedures decreased by 30% over the same period (6–8). On the other hand, other important infections, such as pneumonia and sepsis, remain common and other previously uncommon infections, such as Clostridium difficile colitis have been increasing in recent years (9,10). Although significant achievements have been made, there remains a close to 5% risk of major post-operative infections in patients in the first 2 months following cardiac surgery, which is associated with a 10-fold higher risk of mortality (10).
Health care payers and policy makers have developed mechanisms aimed at preventing HAIs, including denial of reimbursement for the extra cost of treating HAIs considered preventable, mandatory public reporting of institutions’ HAI rates, and transparency regarding the level of adherence to national quality measures (11,12). However, detailed data regarding the economic impact of postoperative complications, particularly complications related to infectious processes, remain scarce. Measuring the economic impact of HAIs – especially in the context of invasive cardiac procedures, during which the patient is particularly vulnerable to infection – is essential for understanding the contribution of HAIs to rising health care costs and for developing sustainable approaches for infection prevention.
Research on the cost of HAIs has primarily focused on specific infection sites or pathogens, or has been limited to the index hospitalization. Few studies capture the full economic impact of a broad range of possible HAIs or the impact of these infections on hospital readmissions (13,14). Those studies that have addressed the economic impact of a broader spectrum of HAIs in cardiac surgery patients have typically relied on billing datasets (5,15) that have important limitations in identifying HAIs due to their reliance on ICD-9 infection codes (16,17). Only 1 of 4 HAIs, as detected by ICD-9 codes, meets standard Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network (NHSN) definitions and criteria (17). Billing data alone may lack the degree of clinical detail needed to adequately adjust for patients’ baseline clinical status. This study, however, combines 2 data sources: clinical data from a prospective multicenter observational cohort study, evaluating the occurrence of major HAIs and economic data obtained from the University HealthSystem Consortium (UHC), and it addresses the economic burden of the major types of HAIs acquired within 65 days after cardiac surgery.
METHODS
Data Sources
Between February 2010 and October 2010, a prospective, multicenter, observational study (n = 5,158) was conducted by the National Heart, Lung, and Blood Institute (NHLBI)-sponsored Cardiothoracic Surgical Trials Network (CTSN) (Online Supplement) to assess occurrence of HAIs after cardiac surgery. This study was approved by participating clinical site institutional review boards and was compliant with the Health Insurance Portability and Accountability Act. All adult (≥18 years old) cardiac surgery patients without active infections on admission were eligible to participate. Recruitment of all eligible patients continued until a pre-specified number of infectious events was reached. Data collected from this cohort are used in the present study and include demographics, body habitus, baseline laboratory results, comorbid conditions, type of surgery performed, infections, and readmissions within 60 ± 5 days of surgery. Patients had a follow-up visit or phone call at 30 and 60 ± 5 days after surgery to collect information about their health status and hospital readmission history. Discharge summaries and medical records were obtained and reviewed to determine the reason of the readmission for patients hospitalized at out-of-network institutions.
An independent committee of infectious disease experts using definitions adapted from the CDC/NHSN reviewed and adjudicated all major postoperative infections. The last infectious event was reviewed on June 2012. The quality and completeness of data were monitored as described elsewhere (10). Clinical data collected in the study were linked to patient-level economic data obtained directly from the sites or from the UHC, which is an alliance of U.S. academic medical centers. Data linkage was done using a combination of variables, including: sex, date of birth, procedure date and type, admission and discharge dates, and hospital identification number. In addition to the index hospitalization, data up to 65 days after surgery were extracted from the UHC database, including cost data, revenue codes, and ICD-9 codes. Costs were obtained by multiplying charges by the cost-center-specific cost-to-charge ratios for each institution. Such ratios are based on the annual Medicare cost reports submitted by individual hospitals. This method of approximating cost is widely used and provides reasonably accurate estimates of actual costs (18). For out-of-network readmissions (readmissions in hospitals not participating in the study), only length of stay (LOS) was available. Because the CTSN study protocol did not include readmissions for rehabilitation and emergency room visits, these types of events were filtered from UHC using the ICD-9 code for rehabilitation, the revenue code for emergency room visits, and direct verification with the clinical site. Of the 10 centers (9 American and 1 Canadian) participating in the CTSN study, only the U.S. centers were included in this study, in order to use more homogeneous cost data. Of 4,614 patients undergoing cardiac surgery in the U.S., 4,320 (93.6%) were matched to their corresponding financial records. Patients that could not be matched (6.4%) were distributed across all hospitals and did not differ in baseline characteristics, including demographics, laboratory values, and comorbidities from patients who could be matched. These unmatched patients were excluded from the analysis.
Endpoints and Analysis
The endpoints for this study were incremental hospital LOS and infection cost.
Cost during the Index Hospitalization
Direct hospital costs associated with major HAIs were calculated separately for the index hospitalization and for subsequent readmissions. For the index hospitalization, extra costs associated with major HAIs were estimated using a generalized linear model (GLM), with a log link function and a gamma distribution. This method adjusts for patient-related confounders, while accounting for the nature of cost data, which are often skewed to the right with heteroskedasticity. Factors found to be associated with cost (at p <0.15) were assessed in the multivariable analysis. To generate the final multivariable model, we removed statistically non-significant variables and refitted the model until all variables in the model had a p value of 0.05 or less. The final model included the baseline factors age, sex, race, ethnicity, body mass index (BMI), white blood cells count, diabetes, hemoglobin, creatinine, pulmonary disease, ejection fraction, hypertension, hypercholesterolemia, congestive heart failure, peripheral vascular disease, history of cerebrovascular accidents, prior cardiac surgery, and use of corticosteroids, and type of procedure and its duration. Covariates and parameter estimates are shown in the Online Supplement.
The incremental cost – the additional cost associated with major HAIs – was then calculated using the recycled prediction method (19). Put simply, via the parameters estimated through the GLM approach, we estimated the incremental cost due to major HAIs by varying the infection status while the other parameters were held constant. The mean difference of the 2 predictions – with and without major HAI – provided the incremental cost attributable to major HAI. Standard errors and confidence intervals were derived by 1,000 bootstrap resampling runs. In each run, we randomly drew patients with replacement from each group separately (infection and non-infection), thereby creating 1,000 pairs of bootstrap samples. GLM modeling was repeated for each pair to get the estimated predicted mean differences. By ordering all the 1,000 random estimates, the 2.5 and 97.5 percentiles were used as the confidence interval boundaries.
Cost of Re-hospitalizations
Hospital readmissions were stratified into 2 categories, depending on whether their occurrence was attributable to HAI or not. Descriptive statistics were then calculated. Cost data were available only for readmissions to hospitals participating to the CTSN study.
Length of Stay
Duration of hospital stay was obtained for all index hospitalizations and all readmissions. For the index hospitalizations, incremental LOS was calculated using GLM with a log link and a gamma distribution, and adjusting for the same variables that were used in the cost model. For re-hospitalizations, the incremental LOS associated with infections was considered equivalent to the mean LOS of the re-hospitalizations attributed to infections.
All analyses utilized SAS statistical software (SAS v9.2; Cary, NC). Descriptive analyses were performed using the Wilcoxon-Mann-Whitney test for all continuous variables, and using Chi-squared or Fisher’s exact tests for all categorical variables.
RESULTS
Characteristics of the Study Population
Among the 4,320 patients in this cohort, the average age was 64 ± 13 years, 66% were male, and the average BMI was 29 ± 6 (Table 1). Among patients with major HAIs, there was a higher prevalence of prior cardiac surgery, congestive heart failure, hypertension, and a history of stroke. Patients with major HAIs also had lower ejection fractions and lower levels of hemoglobin, and marginally higher levels of creatinine. Major infections were more common in patients who had longer, urgent or emergent surgeries. Transplant patients and patients receiving a ventricular assist device (VAD) were more likely to develop major HAIs during the index hospitalization as compared with patients receiving other types of cardiac procedures (15.4% vs. 2.4%, p <0.0001).
Table 1.
Patients Baseline Characteristics*
| Major Infection (N=119) | Control (N=4,201) | P-Value † | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 65.8 (14.1) | 64.3 (13.4) | 0.23 |
| Male | 83 (69.8) | 2,771 (66.0) | 0.39 |
| White | 88 (74.0) | 3,455 (82.2) | 0.02 |
| BMI (kg/m2) | 28.6 (25.1, 34.2) | 28.2 (25.1, 32.2) | 0.40 |
| Baseline Laboratories | |||
| White blood cells (×103/ml) | 7.3 (5.8, 8.6) | 6.9 (5.7, 8.3) | 0.21 |
| Creatinine (mg/dL) | 1.2 (0.9, 1.6) | 1.0 (0.8. 1.2) | <0.001 |
| Hemoglobin (g/dL) | 12.4 (11.0, 13.9) | 13.3 (12.0, 14.5) | 0.0004 |
| Cardiovascular morbidity | |||
| Congestive heart failure | 58 (48.7) | 1,104 (26.3) | <0.001 |
| Ejection fraction (%) | 50 (35.0, 60.0) | 55 (48.0, 60.0) | 0.0008 |
| Prior cardiac surgery | 42 (35.3) | 819 (19.5) | <0.001 |
| Peripheral Vascular disease | 16 (13.5) | 426 (10.1) | 0.24 |
| Cerebrovascular Accident | 18 (15.1) | 400 (9.5) | 0.04 |
| Corticosteroids | 12 (10.1) | 123 (2.9) | <0.0001 |
| Other morbidity | |||
| Diabetes | 31 (26.1) | 941 (22.4) | 0.35 |
| Lung Disease | 24 (20.2) | 606 (14.4) | 0.08 |
| Hypertension | 99 (83.2) | 3,138 (74.7) | 0.04 |
| Operative | |||
| Surgery duration (hours) | 5.4 (4.0, 6.9) | 4.3 (3.5, 5.3) | <0.0001 |
| Sternotomy | 112 (94.1) | 3,779 (90.0) | 0.13 |
| Surgery Type | 0.0003 | ||
| Elective | 66 (55.4) | 3,021 (71.9) | |
| Urgent | 46 (38.7) | 1,068 (25.4) | |
| Emergent | 7 (5.9) | 112 (2.7) | |
| Procedure | <0.0001 | ||
| Isolated CABG | 20 (16.8) | 1,213 (28.9) | |
| Isolated valve | 30 (25.2) | 1,316 (31.3) | |
| CABG + valve | 15 (12.6) | 492 (11.7) | |
| Transplant or VAD | 17 (14.3) | 93 (2.2) | |
| Thoracic aortic | 8 (6.7) | 213 (5.1) | |
| Other | 29 (24.4) | 874 (20.8) | |
Continuous variables, except age, are expressed as median (IQR) and categorical variables as count (%).
Chi-squre test is conducted for categorical variables and Wilcoxon-Mann-Whitney test is conducted for continuous variables.
CABG=coronary artery bypass grafting surgery; VAD=ventricular assist device
During the course of the index hospitalization and of ensuing readmissions, 250 major HAIs developed in 194 patients (4.5% of the cohort). Of these, 119 patients (2.7%) acquired 1 or more major HAIs during their index hospitalization, and 88 patients (2%) had a major HAI associated with their readmission (Table 2). Among patients with HAI-related readmissions, 9 (10.2%) had received a transplant or VAD during the index hospitalization. The most common type of major HAI was pneumonia, followed by bloodstream, Clostridium difficile, and surgical site infections.
Table 2.
Types of major infection during index hospitalization and re-hospitalization
| Type of infection | Index | Re-hospitalization | ||
|---|---|---|---|---|
| # of Events | Patients ‡ N (%) |
# of Events | Patients ‡ N (%) |
|
| Pneumonia | 73 | 72 (1.67) | 33 | 33 (0.76) |
| Bloodstream Infection | 31 | 28 (0.65) | 14 | 14 (0.32) |
| Clostridium difficile | 28 | 28 (0.65) | 17 | 15 (0.35) |
| Deep Incision Surgical site infect. (Chest)* | 6 | 6 (0.15) | 18 | 17 (0.44) |
| Deep Incision Surgical site infect. (Groin)* | 2 | 2 (0.05) | 5 | 5 (0.13) |
| Mediastinitis | 5 | 5 (0.12) | 5 | 5 (0.12) |
| Myocarditis or pericarditis | 3 | 3 (0.07) | 2 | 2 (0.05) |
| Empyema | 2 | 2 (0.05) | 1 | 1 (0.02) |
| Pocket infection† | 1 | 1 (1.27) | 1 | 1 (1.27) |
| Device-related percutaneous site infection | 0 | 0 (0) | 1 | 1 (0.02) |
| Endocarditis | 0 | 0 (0) | 2 | 2 (0.05) |
| Total | 151 | 119 (2.7) | 99 | 88 (2.0) |
Denominator for patients with a deep surgical site infection is patients having a sternotomy (n =3,891).
Denominator for patients with pocket infection is patients who had VAD placed, replaced, or removed for heart transplant (n = 79)
Denominator for other patients is entire population (n = 4,320)
Cost during the Index Hospitalization
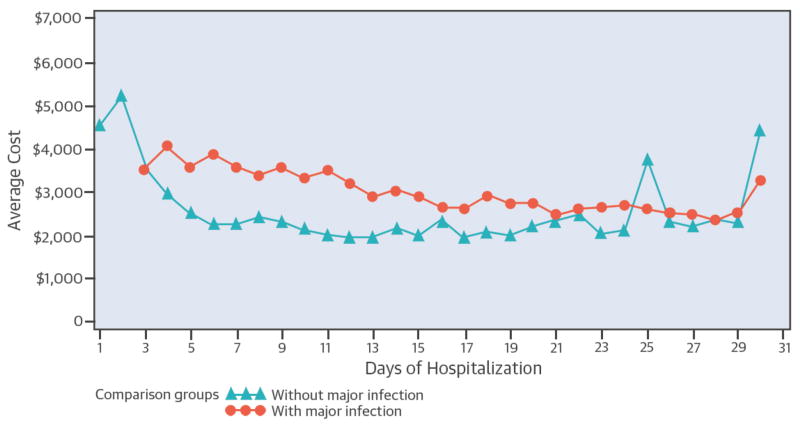
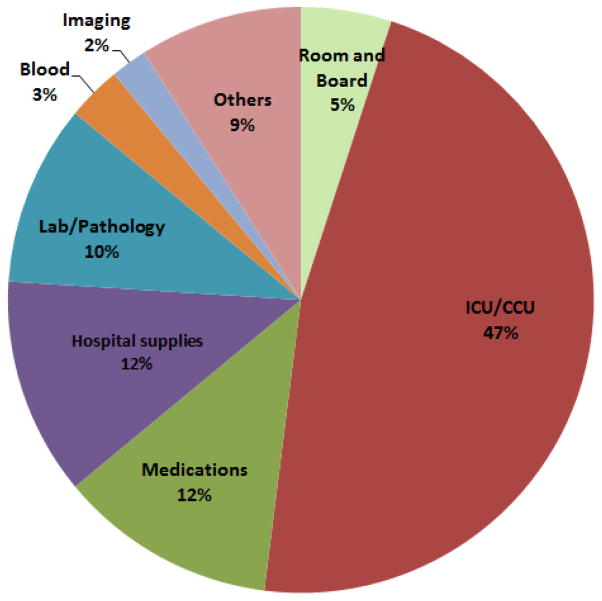
On average, patients who developed major HAI during their index hospitalization had a longer LOS than patients who did not (mean, 33 days versus 9 days). Patients with infections also had a more costly hospital stay than non-infected patients (mean $110,155 vs. $31,530). During the course of a hospitalization, the level of resource utilization changed daily. For patients without major HAIs, the mean cost of care per day peaked on the first 2 days around $5,000, and then declined sharply, leveling off at about $2,000 per day within a week (Central Illustration). In contrast, in patients who acquired a major HAI, the mean cost per day remained sustained for a longer period of time, gradually declining in roughly 3 weeks to a level comparable to non-infected patients. After adjusting for patient-related confounders, the additional cost per major HAI amounted to almost $38,000 (Table 3). On average, the LOS of patients who had major HAIs was 14 days longer than that of patients who did not acquire a major HAI. The adjusted incremental cost of major infections was $37,513 for the entire cohort and $39,264 when VAD and transplant patients were excluded (Table 3). ICU expenditures constituted almost half of the total incremental cost, whereas, hospital supplies, laboratory and pharmacy costs together contributed about one-third of the extra cost (Figure 1).
Central Illustration. Average cost per day in patients with and without infections.
The non-infection group includes all patients from admission until the date of discharge or the date of infection diagnosis. The infection group includes all patients who developed infection from the diagnosis of infection to discharge. The distribution of expenses overtime was derived using the dates of the hospital charges during each hospitalization.
Table 3.
Extra Mean Cost and Length of Stay During Index Hospitalization
| INDEX HOSPITALIZATIONS | ||||||
|---|---|---|---|---|---|---|
| Patients | Infection | COST (95%CI) | LOS (95%CI) | |||
| Median | Unadjusted mean | Adjusted* Incremental | Unadjusted mean | Adjusted* Incremental | ||
| VAD and Transplant Included | No (n = 4,201) | $24,513 | $31,530 ($30654, $32407) | (Ref) | 9.4 days (9.2, 9.7) | (Ref) |
| Yes (n = 119) | $83,833 | $110,155 ($94,664, $12,5646) | $37,513 ($30,403, $45,318) | 33.4 days (29.4, 37.5) | 14 days (11, 17) | |
| VAD and transplant Excluded | No (n = 4,108) | $24,308 | $28,577 ($27,980, $29,174) | (Ref) | 8.9 days (8.8, 9.1) | (Ref) |
| Yes (n = 102) | $73,268 | $93,363 ($80,215, $106,513) | $39,264 ($32532, $49,700) | 30.0 days (26.4, 33.7) | 14.1 days (11.8, 16.8) | |
Adjusted using generalized linear model.
CI=confidence interval; HAI=health care-associated infection; LOS=length of stay; VAD=ventricular assist device
Figure 1. Distribution of incremental costs by type of resource utilized.
The different types of resources were defined by selected revenue code categories.
Cost of Re-hospitalizations
In this cohort, 657 (15% of the index hospitalizations) readmissions occurred within 30 days. The reason for 60 (9.1%) of these readmissions was attributed to major infections. Because patients were monitored for a period of 60 ± 5 days after surgery, we were able to observe that the high incidence of readmissions persisted beyond the traditional 30 day cut off. During the entire follow-up period, after excluding rehabilitations and visits to the emergency department, there were 849 readmissions (19.7% of the index hospitalizations) of which 545 (64%) were to the same hospital where the initial surgery was done. Among these readmissions, 137 (16.1%) were infection related (including major and minor infections, such as urinary tract infections and superficial wound infections) and 74 (8.7%) were attributed to major HAIs alone. Readmissions due to major HAI had 2.6 times higher costs than readmissions due to other causes, and their LOS was twice the LOS of readmissions unrelated to major HAI (Table 4). Moreover, patients who had a major HAI during their index hospitalization had a higher rate of all-cause readmissions as compared with patients who did not experience any major HAI at index (33% vs 20%). Based on the observed difference in the rate of readmissions between these groups, we estimated 1 extra readmission for every 10 major HAIs developed during the index hospitalization. The corresponding extra cost per major HAI at index is reported in Table 4.
Table 4.
Readmission Cost
| READMISSION COST | ||||
|---|---|---|---|---|
| Reason of readmission | Mean Cost* of readmission (95%CI) | Mean Cost due to HAI | Mean LOS of readmission (95%CI) | Mean LOS due to HAI |
| Infection | $33,512 (20903, 46121) (n = 52) |
$33,512† | 11.5 days (8.7, 14.4) (n = 74) |
11.5 days |
| Other | $12,742 (10488, 14996) (n = 493) |
$1,285‡ | 6 days (5.6, 6.6) (n = 760) |
0.6 days |
N is different between cost and LOS because cost data were available only for readmissions within network.
The entire cost of these readmissions is considered fully attributable to HAI.
Patients who had an HAI during their index hospitalization have an increased risk of all-cause readmissions. The corresponding cost was calculated by: mean cost of readmissions not due to infection × number of extra readmissions due to HAI at index/number of HAI at index=12742 × 0.1 =$1,285
CI=confidence interval; HAI=health care-associated infection; LOS=length of stay
DISCUSSION
HAIs are a large impediment to achieving the full benefits of modern medicine, in that they affect 1.7 million patients annually and are associated with nearly 100,000 deaths (20). In cardiac surgery patients, HAIs are the most common non-cardiac complication which have been associated with increased morbidity, mortality, prolonged hospitalizations, and higher costs (4,5). Over the past decade, the field of cardiac surgery has undergone profound changes, which have likely affected the burden, clinical and financial, brought about by HAIs. This has stimulated the need for accurate, up-to-date evidence of such burden. For example, a general trend toward shorter LOS and a corresponding shift of care toward non-acute care facilities or the patient’s home (21) has partially shifted resource use to outpatient settings and readmissions. Studies on the effects of HAI, however, have almost exclusively focused on the index hospitalization, analyzing with only a few exceptions the occurrence of surgical site infections after discharge (22,23). Additionally, patients undergoing cardiac surgery are increasingly older and affected by comorbidities, such as diabetes and obesity (24). These demographic and epidemiological changes, along with modifications of appropriateness criteria (25,26), have reshaped the characteristics of the patient population undergoing cardiac surgery, and, therefore, the potential consequences of HAIs on both clinical and economic outcomes.
Among the type of major HAIs encountered in this study, during both index hospitalizations and readmissions, pneumonia was by far the most frequent (48% of major HAIs), followed by bloodstream infections (21%) (Table 2). These data are consistent with other reports from cardiac surgery and from ICU patients (4,27). Over the past decade, quality improvement efforts have gained ground against HAIs such as catheter-associated bloodstream infections and SSIs (28,29). Given the lack of standardization in making the diagnosis of pneumonia, it is unclear how much progress has been made toward reduction in pneumonia-related HAIs (30). Our results leave little doubt that pneumonia is the HAI with the highest economic impact in cardiac surgery patients. The rigorous definition of HAIs in general is crucial for valid comparisons across hospitals and over time. All major HAIs in our study were identified using CDC definitions and adjudicated by an independent committee of infectious disease experts. However, this level of monitoring and adjudication does not always reflect clinical decision-making. For example, a patient who was treated for pneumonia may not have met the diagnostic criteria for pneumonia. Therefore, by using stricter criteria in this study than the criteria used for clinical decisions, the economic burden of HAIs estimated in this study may be a conservative estimate.
Our results show that the increase in LOS and cost associated with major HAIs during the index hospitalization remains substantial. Our findings are consistent with previous estimates of cost and LOS reported for postoperative infection in coronary artery bypass graft patients and with the results of a recent meta-analysis (5,15,31). However, they are higher than the estimated upper bound of attributable hospital cost due to major HAIs in the U.S. inpatient population, ($25,903 per major HAI in 2007 dollars) (32).
Estimates of the incremental use of resources associated with HAIs vary significantly in the literature (33,34). Such variations are partly a result of the different settings and patient populations from which data were collected and partly a result of the different economic models used for each study. Infections acquired in surgical settings, for example, may have on average a higher impact on resource utilization than those acquired by medically managed patients (35). Moreover, estimates from single center studies will reflect the local case mix and practice of the center. The choice of statistical methodology can also lead to significant variation in the estimates of HAI-attributable cost and LOS (36–38). Regression analyses, such as GLM, are commonly the preferred method to account for heterogeneity of a patient population. Matching strategies, which control for confounders in the design stage, rather than in the analysis stage, present a tradeoff that is related to the number of variables used for matching infected patients with uninfected controls (39). Too few variables may not be sufficient to account for important variation (bias from omitted variables), whereas more variables may reduce the number of patients that can be compared in the cohort (selection bias). Regression models permit avoiding selection bias that results from the exclusion of unmatched cases and controls while adjusting for a high numbers of confounders (36,38,39).
Readmissions related to HAI are frequent and more expensive than non-HAI-related readmissions, costing on average 2.7 times more. As a result, the cost of HAI- related readmissions represents about one-third of the total cost attributable to HAIs. Although there is evidence that higher readmission rates may not be associated with worse outcomes (and could even be associated with lower mortality), readmissions have been recently targeted as an indicator of presumed low-quality care that is associated with high costs, (40). In particular, the Centers for Medicare & Medicaid Services (CMS) now impose financial penalties on hospitals that have higher than expected 30-day readmission rates (risk-standardized) after treatment for acute myocardial infarction, congestive heart failure, and pneumonia (41). Sometime this year, such penalties are expected to include readmissions for patients undergoing coronary artery bypass graft surgery and percutaneous coronary interventions (42). The expansion of these policies will increase the importance of HAI prevention in cardiac surgery patients (43,44).
Limitations
This study has several limitations. First, we only captured inpatient costs. The economic burden on patients and their family or other informal caregivers, as well as treatments provided in the outpatient setting, are not included. Second, cost data were available only for readmissions to the same hospital, which represented 64% of all readmissions. It might be possible that the cost of readmissions to non-index hospitals was systematically different from readmission to the same hospital, especially if the reason for change was dictated by emergency, or if the readmitting hospital lacked a cardiac surgery program, which could potentially lead to costlier and inferior care. However, the estimates of LOS, an important surrogate of resource utilization, which are reported in this study, also include out-of-network hospitalizations. Third, we did not examine the relationship between HAI-associated costs and specific types of cardiac procedures. However, the incremental cost and LOS data generated for a cohort of patients that excludes transplant and VAD patients were not significantly different from those generated using the entire cohort. This suggests that our estimates of HAI-associated cost and LOS may be broadly applicable to cardiac surgery patients. Finally, our choice of model to adjust for measured confounders (GLM) does not account for the time-dependent nature of HAIs (45). However, GLM does allow for adjustment of those baseline variables that had been measured in the observational study, which is an advantage over multistate models that adjust exclusively for time dependency (36,39).
Conclusions
This study identifies the types of major HAIs in cardiac surgery patients, and the effect of these HAIs on readmissions and the utilization of specific hospital resources. It supports the widely held belief that reducing the enormous infection-related toll of mortality and morbidity is not only a clinical imperative, but, especially in the current economic environment, an economic necessity. Every major HAI on average increases LOS by 2 weeks, adding nearly $38,000 to the cost of the index hospitalization. Moreover, readmissions associated with major infections have a LOS twice as long as other readmissions (11.5 days vs. 6.0 days), and cost nearly three times as much ($33,512 vs. $12,742). This study, therefore, substantiates the economic argument for preventive interventions and specifies the possible economic returns from such strategies. This information may help drive quality improvement initiatives to reduce HAIs and ultimately improve patient outcomes.
PERSPECTIVES.
Competency in Patient Care
Knowledge of the costs of health care-associated infections can align clinical and economic decisions to facilitate efficient, cost-effective health care.
Translational Outlook
Although considerable cost savings can accrue from prevention of health care-associated infections, additional research is necessary to evaluate the cost-effectiveness of various strategies for infection control.
Acknowledgments
Funding Sources: The infection prospective cohort study was supported by the National Heart Lung and Blood Institute, Bethesda, MD, the National Institute of Neurological Diseases and Stroke, Bethesda, MD (Grant no. 7U01 HL088942), the Canadian Institutes of Health Research, Ottawa, ON, and by The Institute for Health Technology Studies (InHealth), Washington, D.C.
Abbreviations
- GLM
generalized linear model
- HAI
health care-associated infections
- ICU
intensive care unit
- LOS
length of stay
- VAD
ventricular assist device
Footnotes
CLINICAL TRIAL: ClinicalTrials.gov at http://www.clinicaltrials.gov/ct2/show/NCT01089712?term=MANAGEMENT+PRACTICES+AND+THE+RISK+OF+INFECTIONS+FOLLOWING+CARDIAC+SURGERY&rank=1
ClinicalTrials.gov Identifier: NCT01089712
There is no relationship with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2001;20:1168–75. doi: 10.1016/s1010-7940(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 2.Fowler VG, Jr, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112:I358–65. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 3.Edwards FH, Ferguson TB. The Society of Thoracic Surgeons Practice Guidelines. Ann Thorac Surg. 2004;77:1140–1. doi: 10.1016/j.athoracsur.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Kollef MH, Sharpless L, Vlasnik J, Pasque C, Murphy D, Fraser VJ. The impact of nosocomial infections on patient outcomes following cardiac surgery. Chest. 1997;112:666–75. doi: 10.1378/chest.112.3.666. [DOI] [PubMed] [Google Scholar]
- 5.Brown PP, Kugelmass AD, Cohen DJ, et al. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg. 2008;85:1980–6. doi: 10.1016/j.athoracsur.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Dudeck MA, Weiner LM, Allen-Bridson K, et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am J Infect Control. 2013;41:1148–66. doi: 10.1016/j.ajic.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2012 National and State Healthcare-Associated Infections Progress Report. Centers for Disease Control and Prevention (CDC); 2014. [Google Scholar]
- 9.Flagg A, Koch CG, Schiltz N, et al. Analysis of Clostridium difficile infections after cardiac surgery: Epidemiologic and economic implications from national data. The Journal of thoracic and cardiovascular surgery. 2014 doi: 10.1016/j.jtcvs.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Gelijns AC, Moskowitz AJ, Acker MA, et al. Management practices and major infections after cardiac surgery. Journal of the American College of Cardiology. 2014;64:372–81. doi: 10.1016/j.jacc.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon RE. Control of health-care-associated infections, 1961–2011. MMWR Surveill Summ. 2011;60 (Suppl 4):58–63. [PubMed] [Google Scholar]
- 12.Fry DE. Surgical site infections and the surgical care improvement project (SCIP): evolution of national quality measures. Surg Infect (Larchmt) 2008;9:579–84. doi: 10.1089/sur.2008.9951. [DOI] [PubMed] [Google Scholar]
- 13.Laupland KB, Lee H, Gregson DB, Manns BJ. Cost of intensive care unit-acquired bloodstream infections. J Hosp Infect. 2006;63:124–32. doi: 10.1016/j.jhin.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312–7. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
- 15.Hall RE, Ash AS, Ghali WA, Moskowitz MA. Hospital cost of complications associated with coronary artery bypass graft surgery. Am J Cardiol. 1997;79:1680–2. doi: 10.1016/s0002-9149(97)00223-3. [DOI] [PubMed] [Google Scholar]
- 16.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. American journal of medical quality: the official journal of the American College of Medical Quality. 2005;20:319–28. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson KB, Khan Y, Dickman J, et al. Administrative coding data, compared with CDC/NHSN criteria, are poor indicators of health care-associated infections. Am J Infect Control. 2008;36:155–64. doi: 10.1016/j.ajic.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. American heart journal. 2003;145:452–8. doi: 10.1067/mhj.2003.3. [DOI] [PubMed] [Google Scholar]
- 19.Fu AZ, Qiu Y, Radican L, Wells BJ. Health care and productivity costs associated with diabetic patients with macrovascular comorbid conditions. Diabetes care. 2009;32:2187–92. doi: 10.2337/dc09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S hospitals, 2002. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowper PA, DeLong ER, Hannan EL, et al. Trends in postoperative length of stay after bypass surgery. American heart journal. 2006;152:1194–200. doi: 10.1016/j.ahj.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerging infectious diseases. 2003;9:196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183–9. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 24.Etzioni DA, Liu JH, O’Connell JB, Maggard MA, Ko CY. Elderly patients in surgical workloads: a population-based analysis. The American surgeon. 2003;69:961–5. [PubMed] [Google Scholar]
- 25.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110:e340–437. [PubMed] [Google Scholar]
- 26.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–53. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Morris AC, Hay AW, Swann DG, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med. 2011;39:2218–24. doi: 10.1097/CCM.0b013e3182227d52. [DOI] [PubMed] [Google Scholar]
- 28.Pronovost PJ, Marsteller JA, Goeschel CA. Preventing bloodstream infections: a measurable national success story in quality improvement. Health Aff (Millwood) 30:628–34. doi: 10.1377/hlthaff.2011.0047. [DOI] [PubMed] [Google Scholar]
- 29.Berenguer CM, Ochsner MG, Jr, Lord SA, Senkowski CK. Improving surgical site infections: using National Surgical Quality Improvement Program data to institute Surgical Care Improvement Project protocols in improving surgical outcomes. J Am Coll Surg. 2010;210:737–41. 741–3. doi: 10.1016/j.jamcollsurg.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Klompas M, Magill S, Robicsek A, et al. Objective surveillance definitions for ventilator-associated pneumonia. Crit Care Med. 2012;40:3154–61. doi: 10.1097/CCM.0b013e318260c6d9. [DOI] [PubMed] [Google Scholar]
- 31.Zimlichman E, Henderson D, Tamir O, et al. Health Care-Associated Infections: A Meta-analysis of Costs and Financial Impact on the US Health Care System. JAMA internal medicine. 2013 doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 32.Scott DR. Prevention CfDCa, editor. The direct medical costs of healthcare associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 33.Stone PW, Braccia D, Larson E. Systematic review of economic analyses of health care-associated infections. Am J Infect Control. 2005;33:501–9. doi: 10.1016/j.ajic.2005.04.246. [DOI] [PubMed] [Google Scholar]
- 34.Graves N, Barnett AG, Halton K, et al. The importance of good data, analysis, and interpretation for showing the economics of reducing healthcare-associated infection. Infect Control Hosp Epidemiol. 2011;32:927–8. doi: 10.1086/661600. author reply 928–30. [DOI] [PubMed] [Google Scholar]
- 35.Roberts RR, Hota B, Ahmad I, et al. Hospital and Societal Costs of Antimicrobial-Resistant Infections in a Chicago Teaching Hospital: Implications for Antibiotic Stewardship. Clinical Infectious Diseases. 2009;49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda H, Lee J, Imanaka Y. Variations in analytical methodology for estimating costs of hospital-acquired infections: a systematic review. J Hosp Infect. 2011;77:93–105. doi: 10.1016/j.jhin.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Roberts RR, Scott RD, Hota B, et al. Costs Attributable to Healthcare-Acquired Infection in Hospitalized Adults and a Comparison of Economic Methods. Medical Care. 2010;48:1026–1035. doi: 10.1097/MLR.0b013e3181ef60a2. [DOI] [PubMed] [Google Scholar]
- 38.De Angelis G, Murthy A, Beyersmann J, Harbarth S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin Microbiol Infect. 2010;16:1729–35. doi: 10.1111/j.1469-0691.2010.03332.x. [DOI] [PubMed] [Google Scholar]
- 39.Graves N, Weinhold D, Tong E, et al. Effect of healthcare-acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol. 2007;28:280–92. doi: 10.1086/512642. [DOI] [PubMed] [Google Scholar]
- 40.Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med. 2010;363:297–8. doi: 10.1056/NEJMc1001882. [DOI] [PubMed] [Google Scholar]
- 41.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–5. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 42.Fontanarosa PB, McNutt RA. Revisiting hospital readmissions. JAMA. 2013;309:398–400. doi: 10.1001/jama.2013.42. [DOI] [PubMed] [Google Scholar]
- 43.Hannan EL, Racz MJ, Walford G, et al. Predictors of readmission for complications of coronary artery bypass graft surgery. JAMA. 2003;290:773–80. doi: 10.1001/jama.290.6.773. [DOI] [PubMed] [Google Scholar]
- 44.Hannan EL, Zhong Y, Krumholz H, et al. 30-day readmission for patients undergoing percutaneous coronary interventions in New York state. JACC Cardiovascular interventions. 2011;4:1335–42. doi: 10.1016/j.jcin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Beyersmann J, Gastmeier P, Wolkewitz M, Schumacher M. An easy mathematical proof showed that time-dependent bias inevitably leads to biased effect estimation. J Clin Epidemiol. 2008;61:1216–21. doi: 10.1016/j.jclinepi.2008.02.008. [DOI] [PubMed] [Google Scholar]