Abstract
Background
Ethanol intoxication affects cognitive performance, contributing to attentional deficits and poor decision making, which may occur via actions in the medial prefrontal cortex (mPFC). mPFC function is modulated by the catecholamines dopamine and norepinephrine. In this study, we examine the acute effects of ethanol on electrically-evoked dopamine release and clearance in the mPFC of anaesthetized rats naïve to alcohol or chronically exposed to alcohol during adolescence.
Methods
Dopamine release and clearance was evoked by electrical stimulation of the VTA and measured in the mPFC of anaesthetized rats with fast-scan cyclic voltammetry. In Experiments 1 and 2, effects of a high dose of ethanol (4g/kg, i.p.) on dopamine neurotransmission in the mPFC of ethanol-naïve rats and rats given ethanol exposure during adolescence were investigated. Effects of cumulative dosing of ethanol (0.5–4g/kg) on the dopamine release and clearance were investigated in Experiment 3. Experiment 4 studied effects of ethanol locally applied to the ventral tegmental area (VTA) on the dopamine neurotransmission in the mPFC of ethanol-naïve rats.
Results
A high dose of ethanol decreased evoked dopamine release within 10 min of administration in ethanol-naïve rats. When tested via cumulative dosing from 0.5–4g/kg, both 2 and 4g/kg ethanol inhibited evoked dopamine release in the mPFC of ethanol-naïve rats, while 4g/kg ethanol also slowed dopamine clearance. A similar effect on electrically-evoked dopamine release in the mPFC was observed after infusion of ethanol into the VTA. Interestingly, intermittent ethanol exposure during adolescence had no effect on observed changes in mPFC dopamine release and clearance induced by acute ethanol administration.
Conclusions
Taken together, these data describe ethanol-induced reductions in the dynamics of VTA-evoked mPFC dopamine release and clearance, with the VTA contributing to the attenuation of evoked mPFC dopamine release induced by ethanol.
Keywords: dopamine, ethanol, prefrontal cortex, voltammetry, in vivo
Introduction
Alcohol addiction is characterized by loss of control over alcohol intake, and the mechanisms underlying the transition from occasional to escalated drinking are still unclear (Koob and Volkow, 2010). The medial prefrontal cortex (mPFC) is critical for reward, behavioral control and flexibility (Rogers et al., 2004, Rushworth et al., 2011). These mPFC functions are modulated by dopamine from ventral tegmental area (VTA) neurons, released in the mPFC and activating D1 receptors on cortical pyramidal neurons (Arnsten, 1997, Wang et al., 2006). Thus, investigation of ethanol-induced changes in dopamine release and clearance in the mPFC might elucidate mechanisms of ethanol actions and subsequent neuroadaptations leading to loss of control over intake.
Recently, Schier and colleagues (2013) used microdialysis to demonstrate increased extracellular dopamine in the mPFC induced by intravenous ethanol in rats (Schier et al., 2013), similar to the well-known effect of ethanol in the nucleus accumbens (Imperato and Di Chiara, 1986). Ethanol is thought to activate firing of VTA dopaminergic neurons to increase tonic and phasic dopamine release (Gessa et al., 1985, Robinson et al., 2009). However, alterations in the dynamics of dopamine release and clearance may also contribute to this effect. Real-time dopamine release and uptake has been measured with fast-scan cyclic voltammetry (FSCV) in rodent striatum. Recently, this technique was validated to evoke dopamine and not norepinephrine release in the mPFC (Shnitko and Robinson, 2014). In FSCV studies, acute ethanol blunted electrically-evoked dopamine release in striatum and sometimes slowed reuptake by the dopamine transporter (DAT) (Robinson et al., 2005, Jones et al., 2006). Ethanol may affect mesocortical dopamine transmission in a different manner, given that dopamine release and clearance dynamics in the mPFC differ from those in striatum (Garris et al., 1993, Mundorf et al., 2001). Specifically, mPFC dopamine clearance is slower and occurs via reuptake at the DAT and norepinephrine transporter (NET), as well as via metabolism by catechol-O-methyl transferase (COMT) and monoamine oxidase (Mundorf et al., 2001, Yavich et al., 2007).
Generally, we hypothesize that alcohol alters mesocortical dopamine release and this alteration contributes to the behavioral and cognitive effects of alcohol intoxication. To begin to address this hypothesis, the current pharmacological study presents the first examination of the effects of acute and chronic ethanol on mPFC dopamine transmission on a sub-second time scale. We used FSCV in anesthetized rats to measure endogenous dopamine release evoked by electrical stimulation of the VTA and subsequent clearance. The use of anesthetized rats provided low-noise signals and avoided artifacts associated with the behavioral or sensory response to ethanol or the stimulation. We predicted that electrically-evoked dopamine release in the mPFC would be diminished by ethanol, similar to its effect in striatum, and that this effect would be due to ethanol actions in the VTA. Finally, as the development of alcohol dependence is highly associated with early onset of drinking (Spear and Varlinskaya, 2005), and as dopamine circuitry and the mPFC mature across adolescence, adolescent ethanol intoxication might lead to permanent alteration of cortical dopaminergic neurotransmission (Trantham-Davidson et al., 2014). Therefore, we also made initial measurements of cortical dopamine release and clearance in rats exposed to intermittent ethanol during adolescence.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (390 ± 8g at recording) were purchased from Harlan Laboratories (Frederick, MD; Experiments 1, 3 and 4) or were bred in-house (Experiment 2). They were pair-housed in a temperature- and humidity-controlled room on a 12-h light-dark schedule with food and water ad libitum. All procedures complied with the Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of North Carolina.
Surgery
Rats were anesthetized (1.5g/kg urethane, i.p.) and placed in a stereotaxic frame on a heated pad for the surgery and experiment. Urethane is known to have minimal effects on dopaminergic neurotransmission (Maggi and Meli, 1986) and is widely used for the voltammetric measurements of dopamine and electrophysiological investigations of ethanol effects in the brain (Garris et al., 1993, Moisan and Rompre, 1998). Placements of the carbon-fiber, reference and stimulating electrodes were described previously (Shnitko and Robinson, 2014). Electrode placements (from bregma) were AP −5.2mm and ML −0.8mm for the stimulating electrode and AP +3.7mm and ML −2.0mm for the carbon-fiber electrode. The stimulating electrode (bipolar, parallel, stainless-steel, 0.2mm diameter/tip; Plastics One, Roanoke, VA) was lowered to the VTA at DV −8.5 or −8.6mm while the carbon-fiber electrode was initially placed in the mPFC at DV −3.0mm with 20° angle to the midline. An advantage of electrode placement in the anterior VTA is that it activates both neuronal cell bodies in this region and ascending fibers from the posterior VTA.
Voltammetry
Electrochemical measurement was performed as previously described (Shnitko and Robinson, 2014). The applied potential was ramped from −0.4V to +1.3V and back to −0.4V every 100ms (scan rate 400V/s). The carbon-fiber electrode was lowered into the mPFC (DV −3.0 to −3.5mm) to optimize the VTA-evoked dopamine signal to >5-fold higher than the root mean square of the background. The electrical stimulation (24 biphasic square-wave pulses, 2ms per phase, 125μA, 60Hz) is supraphysiological, although single-unit recordings demonstrate that dopamine neurons can burst with a transitory firing rate of >30Hz (Grace, 1987). This stimulation is self-administered via intracranial self-stimulation in awake rats (Sombers et al., 2009) and uses less current than typical for anesthetized preparations (Garris et al., 1993). It is used here to achieve appropriate signal-to-noise ratios that are required for analysis of endogenous dopamine dynamics while minimizing current spread (Shnitko and Robinson, 2014).
To estimate the sensitivity of the carbon-fiber to dopamine, electrodes were calibrated in vitro post-experiment (Robinson et al., 2009). When calibration was precluded due to electrode damage, an estimated calibration factor was used (76±0.002nM/nA, n=41).
Experiment 1: Single dose-response study in ethanol-naïve rats
The effects of 4g/kg ethanol on electrically-evoked dopamine release and clearance in the mPFC were determined, a dose that significantly and robustly affects striatal dopamine release (Robinson et al., 2005). After positioning the carbon-fiber and stimulating electrodes, three “baseline” evoked signals were collected 5 minutes apart in each rat. Then the animal was given an i.p. injection of saline and three “saline-pretreatment” signals evoked by VTA stimulation were collected, followed by i.p. injection of ethanol in the ethanol group or an equivalent volume of saline in the control group. Five evoked signals were collected. For the ethanol injections, 95% ethanol was diluted in saline to 30% (w/v). Note that the saline-pretreatment was an injection control performed to conduct within-subject analyses in Experiments 1, 2 and 3.
Experiment 2: Single dose-response study in rats exposed to ethanol during adolescence
A separate group of rats was given intermittent ethanol exposure during adolescence. Ethanol (25% w/v in a 0.125% saccharin/3% sucrose solution) was given intragastrically every other day from postnatal day (P) 25 to P45 at a dose of 2.5g/kg; control rats received the vehicle solution. The sweetened ethanol solution (Ji et al., 2008) was used in order to model self-administration procedures (Morales et al., 2014, Schindler et al., 2014). Following the exposure period, the rats were allowed to reach adulthood. FSCV was performed on P94±3 as described in Experiment 1, with both groups (ethanol-exposed, vehicle-exposed) receiving 4g/kg ethanol (i.p.).
Experiment 3: Cumulative dose–response study
The effects of cumulative ethanol dosing on electrically-evoked dopamine release and clearance in the mPFC were determined in a third group of rats. Three “baseline” evoked signals were collected 5 minutes apart in each rat. Next, all rats received saline (i.p.) and three “saline pretreatment” signals were collected. The first injection of ethanol (ethanol group) or saline (control group) was made immediately after collection of the last saline-pretreatment signal and three VTA-evoked voltammetric signals were collected. During a single experiment, each rat in the ethanol group was given four i.p. injections of ethanol: 0.5g/kg, 0.5g/kg (cumulative 1g/kg), 1g/kg (cumulative 2g/kg) and 2g/kg (cumulative 4g/kg). Injections were given 15 min apart and electrically-evoked voltammetric signals were recorded every 5 min for 60 min after the first 0.5g/kg injection. Control rats received equivalent volumes of saline.
Experiment 4: Microinfusion of ethanol to the VTA
In a fourth group of rats, we determined the effect of VTA ethanol infusion on evoked dopamine release in the mPFC. A 33-gauge infusion cannula was attached to the stimulating electrode such that the infusion needle was adjacent to the stimulating electrode tips (Plastics One). Three evoked “baseline” dopamine signals were collected, followed by infusions of either phosphate-buffered saline (PBS, pH 7.4) or ethanol in PBS at 20 and 40mM. Infusions were made via a 26-gauge injection needle attached by tubing to a 10μL syringe on a pump; the tip of the infusion needle protruded 0.5mm past the end of the cannula. Solutions were injected as 0.5μL/2 min. After each infusion, six stimulations were delivered to the VTA at 5-min intervals and evoked dopamine release was measured in the mPFC. In each rat, two infusions were performed, 30-min apart, using either 20mM and then 40mM ethanol or PBS/PBS. As a positive infusion control, 0.5μL of lidocaine (350μmol/2 min) was infused after the collection of last dopamine signals.
Histology
Detailed histological analysis of electrode placements in the mPFC were described previously (Shnitko & Robinson, 2014); we used identical coordinates in the present study. However, to keep the carbon-fiber electrode intact for post-experiment calibration, in this study we did not create a lesion at the carbon-fiber tip that would allow visualization of the electrode placement.
Data Analysis
The presence of dopamine in the voltammetric signals was identified by using both color plots and cyclic voltammograms (CVs). The voltammetric current resulting from oxidation of dopamine at ~0.65V vs the Ag/AgCl reference electrode was converted to concentration by using principal component regression (Heien et al., 2005). As previously described (Shnitko and Robinson, 2014), a parameter of dopamine release was obtained from the peak dopamine concentration ([DA]max) and clearance was obtained as T1/2, the time required for dopamine to decay to half of [DA]max (Yorgason et al., 2011). T1/2 was chosen based on prior work where we compared T1/2, slope from T20 to T80 and full width at 50% of height, and found that T1/2 was the most consistent within-subject and between-subject for VTA-evoked, mPFC dopamine (unpublished data).
For data analysis, the three baseline signals were averaged into one baseline measurement, and the three saline-pretreatment signals were averaged into one saline measurement. Summarized data are presented as a percent of the baseline measurement; the saline-pretreatment measurement is a within-subject injection control. Statistics were calculated on the raw data. Data were analyzed in a 2-way, repeated-measures (RM) ANOVA of group by time or dose (SigmaPlot 11.0, Systat Software, Inc., San Jose, CA). Posthoc comparisons were corrected for multiple comparisons to avoid type I errors. Only results with p<0.05 were considered significant. Data are presented as mean±SEM, and group n’s are stated in the figure captions.
Results
Single dose-response study in ethanol naïve rats
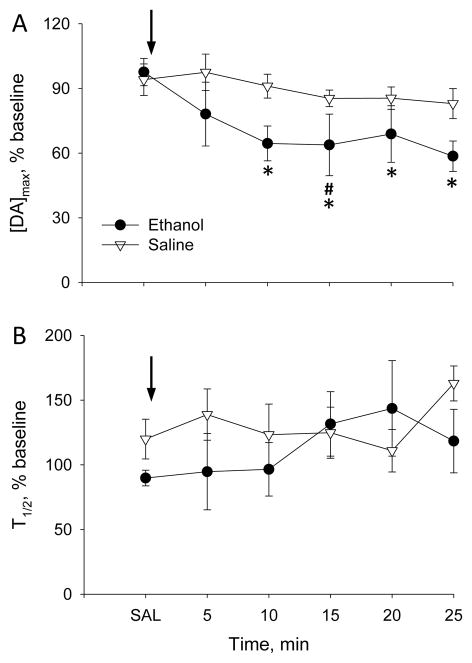
Previous studies revealed robust effects of 2.5–5g/kg ethanol on striatal dopamine release 10 min after i.p. injection, an effect that persisted for at least 60 min (Budygin et al., 2001, Robinson et al., 2005). To identify whether ethanol similarly alters evoked dopamine release and clearance in the mPFC, Experiment 1 investigated the acute effect of ethanol on mPFC dopamine release and clearance in ethanol-naïve rats. Evoked [DA]max at baseline was not statistically different between groups (ethanol group: 87±14nM; saline group:122±7nM). Similar to its effect in striatum (Budygin et al., 2001), ethanol decreased [DA]max in the mPFC by 36% from baseline and 34% from saline-pretreatment within 10 min after injection and this effect persisted for the recording period (Figure 1A). A 2-way, RM-ANOVA yielded a significant main effect of time (F6,66=5.9, p<0.001) with a group-by-time interaction (F6,66=3.0, p<0.05), but no main effect of group. Posthoc comparisons revealed that [DA]max in the ethanol group was significantly reduced from 10 to 25 min after injection relative to both baseline and saline-pretreatment (Bonferroni t-test, p<0.05); the 15-min timepoint was also significantly different (Bonferroni t-test, p<0.05) and the 10-min and 25-min timepoints were marginally different (p<0.08) when compared with the saline control group. In contrast, [DA]max did not vary over time within the saline group. Dopamine clearance (T1/2) in the mPFC was not significantly affected by 4g/kg ethanol (Figure 1B, no significant main effects or interactions).
Figure 1.
Effect of 4g/kg ethanol on (A) [DA]max and(B) T1/2 of VTA-evoked dopamine release recorded in the mPFC of ethanol-naïve rats. Arrows indicate time of ethanol or saline injection. Ethanol group, n=7 rats; saline group, n=6 rats. * P<0.05 vs baseline and saline pretreatment and # P<0.05 vs saline group.
Single dose-response study in rats exposed to ethanol during adolescence
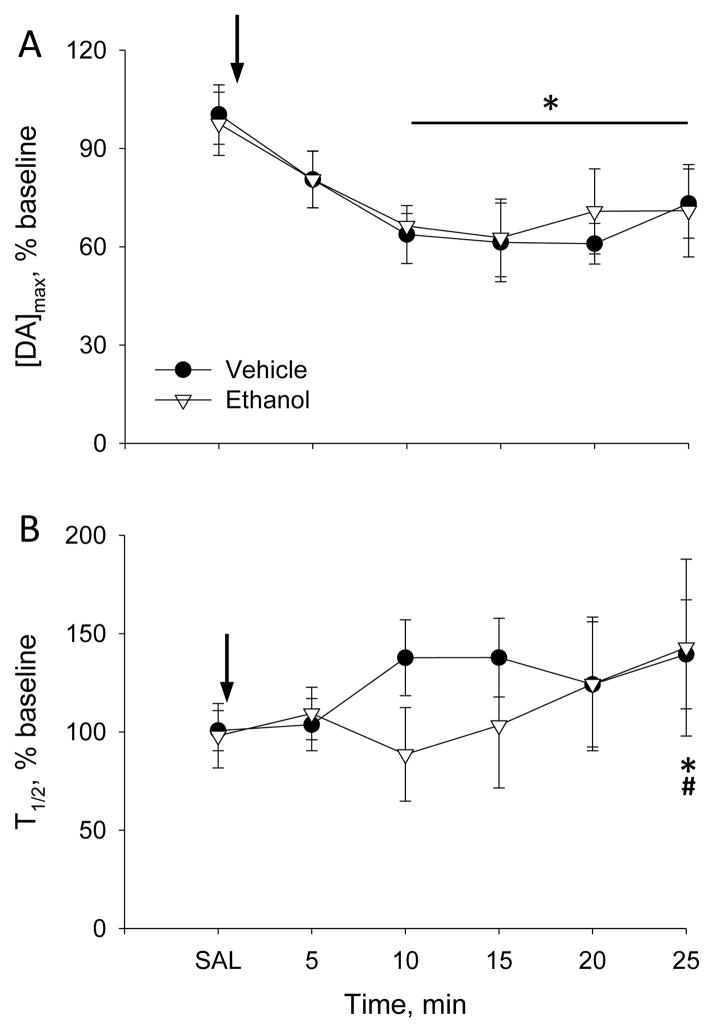
Experiment 2 evaluated the effect of 4g/kg ethanol challenge on dopamine release and clearance in the mPFC of adult rats exposed to ethanol during adolescence. Similar baseline [DA]max was recorded in both groups of rats: 133±20nM in vehicle-exposed and 144±30nM in ethanol-exposed rats. Similar to Experiment 1, ethanol decreased electrically-evoked [DA]max in both ethanol- and vehicle-exposed groups by 33% and 36% versus baseline and by 30% and 31% versus saline-pretreatment values, respectively (Figure 2A). A 2-way, RM-ANOVA yielded a significant main effect of time (F6,54=8.2, p<0.001) with no main effect of group or interaction. Collapsed across group, [DA]max was significantly reduced relative to baseline and saline pretreatment from 10 to 25 min after injection (post-hoc Bonferroni t-test, p<0.05).
Figure 2.
Effect of 4g/kg ethanol on (A) [DA]max and (B) T1/2 of VTA-evoked dopamine release recorded in the mPFC of rats given intermittent ethanol exposure during adolescence. Arrows indicate time of ethanol injection. Ethanol-exposed group, n=5 rats; vehicle-exposed group, n=6 rats. Collapsed across group, * P<0.05 vs baseline and saline pretreatment and # P<0.05 vs 5 min after injection.
In Experiment 2, ethanol also slowed the time required for dopamine clearance (Figure 2B). In the vehicle-exposed group, T1/2 was increased by 37% versus baseline and 32% versus saline pretreatment within 10 min after injection, while in the ethanol-exposed group it increased by 42% and 39% versus baseline and saline pretreatment, respectively, only at 25 min. However, this ethanol effect was quite variable. A 2-way, RM-ANOVA revealed a significant main effect of time (F6,53=3.7, p<0.005), without a main effect of group or group-by-time interaction. Collapsed across groups, T1/2 was significantly increased at 25 min relative to the baseline, saline-pretreatment and 5-min post-injection timepoints (Bonferroni t-test, p<0.05).
Cumulative dose–response study
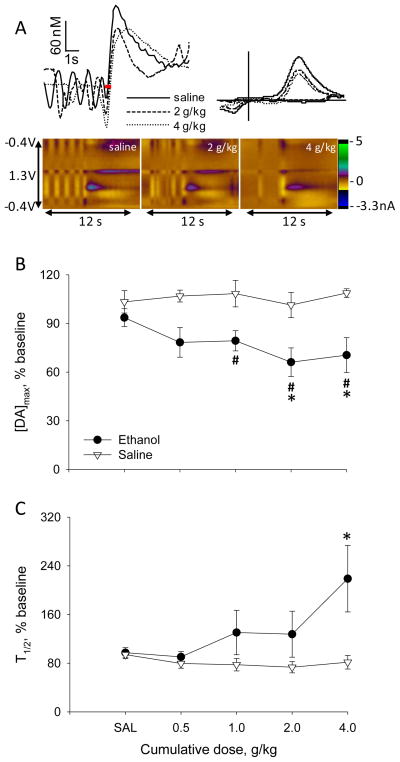
Experiment 3 investigated evoked dopaminergic responses in the mPFC to increasing doses of ethanol by using a cumulative-dose design. Figure 3A displays representative VTA-evoked dopamine signals after saline, 2 and 4g/kg ethanol injection obtained from an individual rat. Ethanol decreased the amplitude of the signals at both cumulative doses. Moreover, there was slower dopamine decay after 4g/kg, compared to the signals obtained after saline and 2g/kg ethanol. Summarized data on the effect of cumulative ethanol on [DA]max and T1/2 are shown in Figure 3B–C. [DA]max at baseline was 150±30nM in the ethanol group and 100±10nM in the saline group. As in Experiments 1 and 2, ethanol decreased electrically-evoked dopamine release. A 2-way, RM-ANOVA yielded a significant main effect of dose (F5,45=4.6, p<0.005) with a significant group-by-dose interaction (F5,45=6.2, p<0.001), but no main effect of group. The 0.5 and 1g/kg cumulative doses slightly decreased [DA]max by 17% and 22% versus baseline and 13% and 18% from saline pretreatment, but only 1g/kg was significantly different from baseline within the ethanol group (Bonferroni t-test, p<0.05). Cumulative 2 and 4g/kg ethanol significantly reduced [DA]max by 35% and 33% versus baseline and 31% and 30% versus saline pretreatment, respectively (Bonferroni t-test, p<0.001). [DA]max did not vary over time within the saline group. Interestingly, ethanol increased T1/2, demonstrating slower clearance of dopamine (Figure 3B). A 2-way, RM-ANOVA yielded a significant group-by-dose interaction (F5,45=2.8, p<0.05) with no significant main effects. Post-hoc comparisons revealed that T1/2 was significantly enhanced at 4g/kg ethanol relative to saline pretreatment (Bonferroni t-test, p<0.05), while T1/2 did not change over time in the saline group.
Figure 3.
Effect of cumulative doses of ethanol on VTA-evoked dopamine release and clearance recorded in the mPFC of ethanol-naïve rats. (A) Representation of cumulative doses of ethanol on dopamine release and clearance evoked by stimulation of the VTA and recorded in the mPFC of an individual rat is shown on the top. Voltammetric signals are presented after saline and ethanol injections at cumulative doses 2 and 4g/kg. In color plots, applied potential is indicated on the y-axis; time is indicated on the x-axis and current is expressed in color. In the traces above the color plots, [DA] is plotted as function of time and red bars indicate electrical stimulation delivered to the VTA. In the CVs (top right) obtained from the peaks of dopamine release, current is plotted versus potential applied to the carbon-fiber electrode. (B, C) Averaged (±SEM) effects of ethanol on [DA]max and T1/2. Ethanol group, n=5 rats; saline group, n=6 rats. #* P<0.05 vs baseline and saline pretreatment, respectively.
Microinfusion of ethanol to the VTA
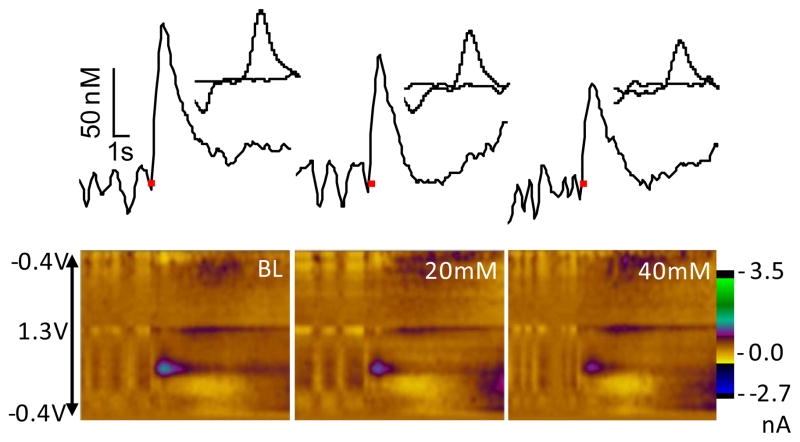
Experiment 4 aimed to localize the site of ethanol action on mPFC dopamine release by infusing consecutive infusions of 20 and 40mM ethanol directly into the VTA at the stimulation site; control rats received equivalent volumes of PBS. Figure 4 shows electrochemical signals recorded in the mPFC of an individual rat before and after ethanol infusion. The data demonstrate some reduction of the VTA-evoked dopamine signal 5 min after infusion of 20mM ethanol and greater reduction 5 min after the subsequent 40mM infusion.
Figure 4.
Effect of VTA-infusion of ethanol on evoked dopamine release observed in the mPFC of an individual rat. In color plots, applied potential is indicated on the y-axis; time is indicated on the x-axis and current is expressed in color. In corresponding traces above the color plots, [DA] is plotted as function of time and the red bars indicate electrical stimulation delivered to the VTA. CVs of the peak dopamine signal are inset.
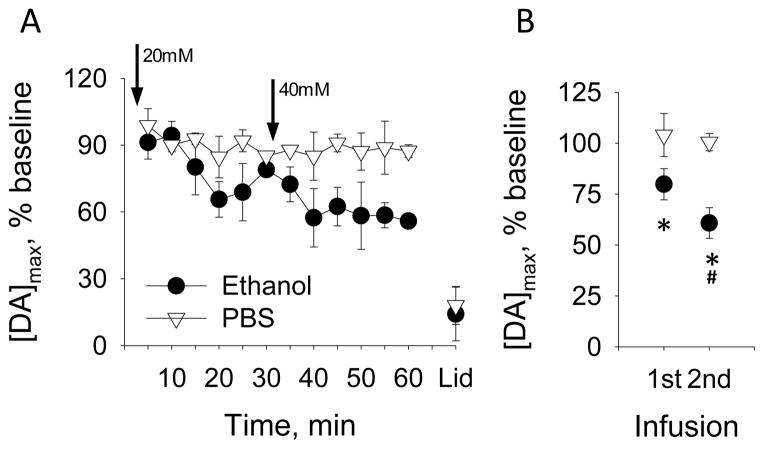
The composite data are presented in Figure 5. Figure 5A shows that 20mM ethanol decreased dopamine release, with the maximal reduction of 34% versus baseline occurring 20 min after the infusion. Next, 40mM ethanol reduced the evoked dopamine release in 10 min by 43% relative to baseline. For statistical analysis, we collapsed data into 3 epochs: baseline (3 signals over 15 min), first infusion and second infusion (each 6 signals over 30 min). Both 20 and 40mM ethanol decreased [DA]max in the mPFC by 20% and 40% from baseline, respectively (Figure 5B). A 2-way, RM-ANOVA yielded a significant main effect of time (F2,6=33.1 p<0.001) with a significant group-by-time interaction (F2,6=6.8, p<0.05) and no main effect of group. Post-hoc comparisons confirmed that [DA]max was significantly reduced by 20 and 40mM ethanol versus baseline; moreover, 40mM inhibited dopamine release twice as much as the 20mM infusion (Bonferroni t-test, p<0.05).
Figure 5.
Effect of ethanol locally applied to the VTA on [DA]max of VTA-evoked dopamine in the mPFC. (A) [DA]max evoked at 5-min intervals before and after infusions of either ethanol or PBS (arrows). (B) Data from Panels A are collapsed across time. * P<0.05 vs baseline for ethanol group, # P<0.05 vs 20mM ethanol.
To verify that the infusions reached the neurons that were electrically stimulated, we infused 350nM lidocaine after all recordings (Figure 5A). Lidocaine significantly decreased dopamine release within 10 min by 82% versus baseline in both ethanol and PBS groups (paired t-test, p<0.05).
Discussion
Acute ethanol increases firing of dopamine neurons (Gessa et al., 1985), resulting in higher dopamine concentrations in striatal regions as measured with microdialysis (Imperato and Di Chiara, 1986) and FSCV (Cheer et al., 2007, Robinson et al., 2009). Linked to this increase in spontaneous dopamine activity, the effect of ethanol on electrically-evoked dopamine release in striatum is to reduce the amount of release per action potential (Budygin et al., 2001), an effect that may be due to less vesicular loading or to dopamine D2 autoreceptor activation (Ludlow et al., 2009). We now extend this research to examine ethanol’s effects on VTA-evoked dopamine release and clearance measured in the mPFC. We found that ethanol reliably reduced [DA]max, consistent with its actions on dopamine release in striatum. Moreover, this effect was duplicated by ethanol infusion into the VTA, indicating ethanol action at the dopaminergic soma. In addition, ethanol slowed dopamine clearance in the mPFC under some conditions; while we have also reported slower uptake of dopamine in striatum after ethanol challenge (Robinson et al., 2005), the mechanism of action is likely to be different in the mPFC due to the paucity of the DAT in that region. Surprisingly, adolescent ethanol exposure did not alter VTA-evoked dopamine dynamics in the mPFC after acute ethanol challenge in adulthood. By describing ethanol actions on the real-time dynamics of phasic dopamine release in the mPFC, this study provides a neurobiological mechanism by which ethanol can alter the catecholaminergic modulation of mPFC function, possibly leading to cognitive impairment and loss of control over ethanol intake.
The most consistent effect of ethanol on VTA-evoked dopamine in the mPFC was to reduce [DA]max. For example, the 4g/kg ethanol used in Experiments 1 and 2 produced a 30–35% decrease in [DA]max from saline-pretreatment values, which is smaller but consistent with previously reported ethanol effects in the striatum. For example, 4g/kg ethanol reduced [DA]max by 55% in the olfactory tubercle (Robinson et al., 2005) and 2.5g/kg ethanol reduced [DA]max by 65% in the caudate (Budygin et al., 2001), effects that were apparent within 10-min post-injection and persisted for the 60-min recording period.
Importantly, ethanol acts in the VTA to reduce evoked dopamine release, as direct infusion of ethanol to the VTA stimulation site reproduced the reductions in [DA]max observed after systemic administration of ethanol. This conclusion is consistent with ethanol reductions of electrically-evoked striatal dopamine release observed in in vivo but absent in striatal slice preparations that do not include the soma (Budygin et al., 2001, Jones et al., 2006). A caveat is that the ethanol-induced decrease in dopamine release typically occurred ~10 min after infusion, suggesting that ethanol may be diffusing to its site of action. Indeed, our stimulating electrodes and attached guide cannula were placed relatively anterior in the VTA, while the posterior VTA may be more sensitive to ethanol effects (Ding et al., 2011) (but see (Ericson et al., 2008)). Interestingly, the effect of 40mM ethanol persisted for 30-min post-infusion, consistent with Ding et al. (2011), who reported increased extracellular dopamine levels in the mPFC which persisted for ~30min following a 44mM infusion of ethanol to the posterior VTA.
While ethanol increases extracellular dopamine levels in both striatum and mPFC (Robinson et al., 2009, Schier et al., 2013), when dopamine release is electrically-evoked and detected with FSCV, ethanol decreases release in striatum (Budygin et al., 2001, Robinson et al., 2005) and mPFC as demonstrated in the present study. This ethanol effect on electrically-evoked dopamine may at first appear contradictory with its effect on spontaneous neuronal firing and dopamine release. The difference may be explained by activity-dependent alterations on subsequent dopamine release per impulse, such as that evoked by electrical stimulation (Robinson et al., 2005). In striatum, dopamine release is regulated by autoinhibition via D2 receptors. For example, systemic injections of D2 agonists decreased electrically-evoked dopamine release in mouse striatum, while antagonists reversed this effect (Maina and Mathews, 2010). As ethanol increases spontaneous firing of dopamine neurons, the subsequent dopamine release might activate D2 autoreceptors; this regulation is one likely mechanism for reductions in evoked DA release when spontaneous activity increases. However, our previous study found that systemic administration of a D2-receptor antagonist reduced VTA-evoked dopamine release in the mPFC (Shnitko and Robinson, 2014), the opposite effect of that observed in striatum, suggesting that mesocortical and mesostriatal dopamine neurons are differentially regulated by D2 autoreceptors. Another possible explanation of ethanol’s effect on evoked dopamine release is that dopamine is less efficiently recycled in the mPFC as compared to striatum. This might occur due to less efficient dopamine reuptake via DAT, dopamine transport into noradrenergic terminals by NET, and metabolism of extracellular dopamine by COMT. Under saline conditions, the readily releasable pool of dopamine may be maintained with the current stimulation protocol, as indicated by stable [DA]max. However, when ethanol increases spontaneous firing of dopamine neurons, and consequently dopamine release, the readily releasable pool may be depleted. Future studies can investigate this possibility by pharmacologically manipulating dopamine synthesis/metabolism, vesicular packaging and release capability.
Previous studies demonstrated clear dose-dependent effects of ethanol on striatal dopamine release (Budygin et al., 2001, Robinson et al., 2005). In the present study, we used a cumulative dose-response procedure (Robinson et al., 2009, Schier et al., 2013) and found that 0.5g/kg ethanol did not alter dopamine release, consistent with previous studies in striatum where this dose had little effect (Budygin et al., 2001, Robinson et al., 2009). Furthermore, 1, 2 and 4g/kg ethanol doses reduced [DA]max by 20–30% relative to saline pretreatment with no statistical difference between doses. It is possible that the lack of more obvious dose-dependent effects of ethanol on [DA]max may result from the emergence of concurrent effects on cortical dopamine clearance; as extracellular dopamine is dependent on both release and clearance, slower clearance would amplify the evoked [DA]max (Wightman and Zimmerman, 1990). Specifically, changes in clearance can affect the concentration of dopamine that accumulates to achieve [DA]max, and significant changes in [DA]max will affect the time of T1/2 under constant clearance conditions. Thus, the finding that we observed slower T1/2 under conditions of smaller [DA]max suggests that ethanol exerted two opposing effects on dopamine transmission. This interpretation is strengthened by Experiment 4: VTA infusion of ethanol that was unlikely to alter clearance mechanisms produced a clear dose-dependent effect on [DA]max, with 40mM ethanol reducing [DA]max twice as much as 20mM ethanol.
The present study evaluated dopamine clearance by measuring T1/2, the time required for VTA-evoked dopamine to decay to the half of [DA]max, and found that high doses sometimes slowed dopamine clearance in the mPFC. While 4g/kg ethanol challenge did not reliably affect mPFC dopamine clearance in ethanol-naïve rats, there was between-rat variability in these data. When tested in rats previously exposed to ethanol or vehicle during adolescence, we found that clearance of evoked dopamine was significantly slower at 25 min after the same ethanol challenge in both groups of rats. Similar results were obtained in the cumulative-dose experiment, where T1/2 was doubled by ethanol at the 4g/kg dose. Interestingly, ethanol had a larger effect on dopamine clearance when its dose was cumulatively increased to 4g/kg than when the same dose was administered as a single injection. This may be due to the longer time of ethanol exposure in the brain during the cumulative dosing procedure and subsequent accumulation of ethanol metabolites, which themselves can also alter dopamine transmission (Foddai et al., 2004, Deehan et al., 2013). It may be noteworthy that such cumulative dosing better mimics human binge drinking than single-dose challenges.
Ethanol-induced reductions in dopamine uptake have been observed in ventral striatum, both in vivo and in vitro (Robinson et al., 2005), although this observation was not made in other studies (Jones et al., 2006). However, it is important to note that the mechanism of dopamine clearance in cortex differs from striatum, where dopamine is primarily cleared by the DAT. In the mPFC, although dopamine is removed from the extracellular space by the NET and, to a lesser extent, the DAT (Mundorf et al., 2001), a primary means of clearance is via extracellular degradation by COMT (Yavich et al., 2007). While COMT genotype has been linked to ethanol drinking in people and animals (Tammimaki and Mannisto, 2011), it is unknown whether ethanol challenge acutely alters COMT function in a way that may explain the present results. In contrast, ethanol can reduce exogenous norepinephrine uptake at the NET (Lin et al., 1997), and rodent strains bred for differences in ethanol sensitivity also differ in NET activity (Freund et al., 2003, Haughey et al., 2005). Thus, future studies are required to determine the mechanism(s) of ethanol’s ability to slow dopamine clearance in the mPFC, but both COMT and NET are plausible targets.
We investigated whether intermittent ethanol exposure during adolescence affects the described changes in VTA-evoked dopamine overflow. The dopaminergic system changes during adolescence (McCutcheon et al., 2012, Naneix et al., 2012), and ethanol exposure during this time might have lasting consequences on dopaminergic neurodevelopment and behavior (Spear and Varlinskaya, 2005, Pascual et al., 2009, Philpot et al., 2009). Nevertheless, surprisingly similar effects of ethanol challenge on VTA-evoked dopamine release were observed in the mPFC of adults exposed to ethanol as adolescents when compared to controls. While the consequences of adolescent ethanol treatment on real-time dopamine release and clearance in any brain region is unclear, chronic ethanol exposure in adulthood blunted ethanol-induced decreases of evoked dopamine release in caudate slices of monkeys (Budygin et al., 2003). Moreover, while chronic ethanol exposure did not affect baseline dopamine release evoked by electrical stimulation, it enhanced the rate of dopamine uptake in striatal slices of rats (Budygin et al., 2007). In the present study, it is possible that the adolescent exposure regimen was too brief or the doses were too low to produce a lasting effect. Another possibility is that a critical exposure period was missed, as the mPFC continues to mature past puberty (Gogtay et al., 2004), and cortical dopamine receptor expression progressively increases from P21–60 (Andersen et al., 2000, Tarazi and Baldessarini, 2000). Future studies can address these possibilities, considering such important parameters as age, duration of ethanol exposure, and acute effects of ethanol at a wide range of doses. Nevertheless, despite a lack of group differences, this experiment provided an important replication of the effects of ethanol challenge to diminish VTA-evoked dopamine release in the mPFC and sets a direction for future studies.
Few voltammetric measurements of dopamine dynamics have been made in prefrontal cortex due to technical concerns of selectivity between dopamine and norepinephrine. After validating the selectivity of VTA-evoked dopamine measurements in the mPFC (Shnitko and Robinson, 2014), the present study applied this approach to pharmacological effects of alcohol on cortical dopamine dynamics. While innovative, this study has important limitations. One limitation is the use of anesthesia, which was required to enhance the signal-to-noise of VTA-evoked dopamine and to avoid potential interference of spontaneous catecholamine release potentially triggered by the interceptive effects of the VTA stimulation. Ethanol can interact with urethane to produce different effects than other anesthetics on inferior olivary nucleus neurons (Rogers et al., 1986). However, this effect may be specific to certain brain circuits. Urethane is widely used in the study of dopaminergic mechanisms in vivo as it does not affect firing activity of dopamine neurons (Brischoux et al., 2009) and ethanol effects on electrically-evoked striatal dopamine release is similar in awake and urethane-anesthetized rats (Budygin et al., 2001, Robinson et al., 2005). A second limitation was the necessity to use electrical stimulation to evoke dopamine release. The parameters of the stimulation (24 pulses, 60Hz, 125μA) produced sufficient signal-to-noise ratio for data analysis and were previously shown to selectively evoke dopamine (Shnitko and Robinson, 2014), but they are supraphysiological. Moreover, our findings are currently limited to estimates phasic dopamine release as opposed to tonic release, which is typically modeled with frequencies of <10Hz. Therefore, it is not clear whether ethanol would have similar effects on dopamine release evoked by small stimulation, mimicking tonic release. As detection limits of the electrochemical instrumentation improve, mPFC dopamine can be investigated with smaller stimulation trains that better mimic burst firing as well as tonic firing of dopamine neurons. Finally, while this study employed single and cumulative dosing of ethanol, future studies can utilize additional dosing regimens to model alcohol drinking and various stages of intoxication. Nevertheless, the present study is an important initial step to understand the pharmacological effects of alcohol on cortical dopamine dynamics.
In conclusion, we show that acute ethanol alters real-time dopamine dynamics in the mPFC by reducing release and slowing clearance. While ethanol’s effects on dopamine release appear to occur through actions in the VTA, its effects on clearance are variable and the mechanism is yet unknown. Future studies can examine the roles of the NET and COMT in ethanol-induced reductions in dopamine clearance in the mPFC, of vesicular loading and autoreceptor regulation in the ethanol-induced inhibition of VTA-evoked dopamine release.
Acknowledgments
Completion of this project partially fulfilled honors thesis requirements for LCK in the UNC Department of Biology. This research was funded by the National Institute of Alcoholism and Alcohol Abuse (R01AA018008, U01AA019972 NADIA project) and the UNC Bowles Center for Alcohol Studies. Salary support for DLR was also provided by NIAAA grant U24AA020024.
References
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse (New York, NY. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology. 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001;297:27–34. [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Jr, Engleman EA, Ding ZM, McBride WJ, Rodd ZA. Microinjections of acetaldehyde or salsolinol into the posterior ventral tegmental area increase dopamine release in the nucleus accumbens shell. Alcohol Clin Exp Res. 2013;37:722–729. doi: 10.1111/acer.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hall SR, Engleman EA, Hauser SR, McBride WJ, Rodd ZA. The stimulating effects of ethanol on ventral tegmental area dopamine neurons projecting to the ventral pallidum and medial prefrontal cortex in female Wistar rats: regional difference and involvement of serotonin-3 receptors. Psychopharmacology (Berl) 2011;216:245–255. doi: 10.1007/s00213-011-2208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Chau P, Soderpalm B. Nicotinic acetylcholine receptors in the anterior, but not posterior, ventral tegmental area mediate ethanol-induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther. 2008;326:76–82. doi: 10.1124/jpet.108.137489. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Freund RK, Gerhardt GA, Marshall KE, Palmer MR. Differences in norepinephrine clearance in cerebellar slices from low-alcohol-sensitive and high-alcohol-sensitive rats. Alcohol. 2003;30:9–18. doi: 10.1016/s0741-8329(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Garris PA, Collins LB, Jones SR, Wightman RM. Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem. 1993;61:637–647. doi: 10.1111/j.1471-4159.1993.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. The regulation of dopamine neuron activity as determined by in vivo and in vitro intracellular recordings. In: Chiodo LA, Freeman AS, editors. The Neurophysiology of Dopamine Systems. Detroit: Lake Shore Publications; 1987. pp. 1–66. [Google Scholar]
- Haughey HM, Kaiser AL, Johnson TE, Bennett B, Sikela JM, Zahniser NR. Norepinephrine transporter: a candidate gene for initial ethanol sensitivity in inbred long-sleep and short-sleep mice. Alcohol Clin Exp Res. 2005;29:1759–1768. doi: 10.1097/01.alc.0000183009.57805.a6. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse (New York, NY. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Bickford PC, Palmer MR, Cline EJ, Gerhardt GA. Effects of ethanol and nomifensine on NE clearance in the cerebellum of young and aged Fischer 344 rats. Brain Res. 1997;756:287–292. doi: 10.1016/s0006-8993(97)00229-1. [DOI] [PubMed] [Google Scholar]
- Ludlow KH, Bradley KD, Allison DW, Taylor SR, Yorgason JT, Hansen DM, Walton CH, Sudweeks SN, Steffensen SC. Acute and chronic ethanol modulate dopamine D2-subtype receptor responses in ventral tegmental area GABA neurons. Alcohol Clin Exp Res. 2009;33:804–811. doi: 10.1111/j.1530-0277.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Maina FK, Mathews TA. A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem Neurosci. 2010;1:450–462. doi: 10.1021/cn100003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan J, Rompre PP. Electrophysiological evidence that a subset of midbrain dopamine neurons integrate the reward signal induced by electrical stimulation of the posterior mesencephalon. Brain Res. 1998;786:143–152. doi: 10.1016/s0006-8993(97)01457-1. [DOI] [PubMed] [Google Scholar]
- Morales M, Anderson RI, Spear LP, Varlinskaya EI. Effects of the kappa opioid receptor antagonist, nor-binaltorphimine, on ethanol intake: impact of age and sex. Dev Psychobiol. 2014;56:700–712. doi: 10.1002/dev.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM. Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem. 2001;79:130–142. doi: 10.1046/j.1471-4159.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci. 2012;32:16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcoholism, clinical and experimental research. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Volz TJ, Schenk JO, Wightman RM. Acute ethanol decreases dopamine transporter velocity in rat striatum: in vivo and in vitro electrochemical measurements. Alcoholism, clinical and experimental research. 2005;29:746–755. doi: 10.1097/01.alc.0000164362.21484.14. [DOI] [PubMed] [Google Scholar]
- Rogers J, Madamba SG, Staunton DA, Siggins GR. Ethanol increases single unit activity in the inferior olivary nucleus. Brain Res. 1986;385:253–262. doi: 10.1016/0006-8993(86)91071-1. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Schier CJ, Dilly GA, Gonzales RA. Intravenous Ethanol Increases Extracellular Dopamine in the Medial Prefrontal Cortex of the Long-Evans Rat. Alcohol Clin Exp Res. 2013;37:740–747. doi: 10.1111/acer.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, Clark JJ. Chronic Alcohol Intake During Adolescence, but not Adulthood, Promotes Persistent Deficits in Risk-Based Decision Making. Alcohol Clin Exp Res. 2014;38:1622–1629. doi: 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Robinson DL. Anatomical and pharmacological characterization of catecholamine transients in the medial prefrontal cortex evoked by ventral tegmental area stimulation. Synapse. 2014;68:131–143. doi: 10.1002/syn.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Tammimaki A, Mannisto PT. Effect of genetic modifications in the synaptic dopamine clearance systems on addiction-like behaviour in mice. Basic Clin Pharmacol Toxicol. 2011;108:2–8. doi: 10.1111/j.1742-7843.2010.00647.x. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci. 2014;34:3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]