To the Editor:
Cow's milk (CM) allergy is the most common food allergy in young children, affecting 2-3% of infants.1 Most CM allergic patients are co-sensitized to casein and whey (alpha-lactalbumin, beta-lactoglobulin) proteins.1 In formula-fed infants with CM allergy, extensively hydrolyzed, amino acid-based, or soy formulas are typically recommended.2 Here we report a case where assessment for allergy to specific CM proteins allowed for formula choice beyond the typical recommendations.
A seven month-old female presented with concern for milk allergy. She had been breastfed until age ten weeks and then transitioned to a partially hydrolyzed whey formula (pHWF), Gerber Good Start Gentle®, which she was tolerating well. She had also been successfully introduced to fruits (including bananas), vegetables, and grains.
At age six months, she ingested banana yogurt. Within five minutes, she developed a dry cough, vomiting, and hives over 90% of her body. Her parents immediately brought her to the pediatrician, who administered oral steroids and antihistamines, and her symptoms resolved. No epinephrine was administered.
A local allergist performed skin testing with the following results (wheal mm/flare mm): plain yogurt 14/30, banana yogurt 12/22, CM 4/8, fresh banana 5/14, commercial banana extract 0/0. Serum specific IgE (sIgE) levels sent to an outside commercial laboratory were: milk 8.01 kUA/L, casein 23.4 kUA/L, alpha-lactoglobulin<0.35 kUA/L, beta-lactoglobulin<0.35 kUA/L, banana <0.35 kUA/L. The allergist advised that the patient be switched from pHWF to an amino acid-based formula, Nutricia Neocate®, and to avoid bananas. The patient did not like the taste of the amino acid-based formula, and her parents became concerned about insufficient caloric intake.
The patient was then evaluated in our clinic. Skin testing at our practice showed (wheal mm/flare mm): histamine 6/12, saline 0/0, commercial banana extract 4/0, pHWF 2/0. Based on her history, sIgE and skin test results, we diagnosed her with casein-specific CM allergy. We advised that the patient resume pHWF but to avoid all other CM. At follow-up several weeks later, she was back on pHWF and eating bananas with appropriate growth.
We performed a series of laboratory studies to further evaluate the basis for our patient's ability to tolerate pHWF but not CM. To directly compare the protein components of CM and pHWF, non-fat dry milk powder and pHWF were separated by SDS-PAGE and transferred to ImmobilonP (Figure 1, left panel). CM showed proteins at 14 kDa, 18 kDa, and >28kDa, corresponding to alpha-lactalbumin, beta-lactoglobulin, and casein proteins, respectively. Irrespective of the quantity used (25-150 μg), pHWF showed only very low molecular weight hydrolyzed product (3-14 kDa).
Figure 1. SDS-PAGE and Western blot results support casein-specific allergy.
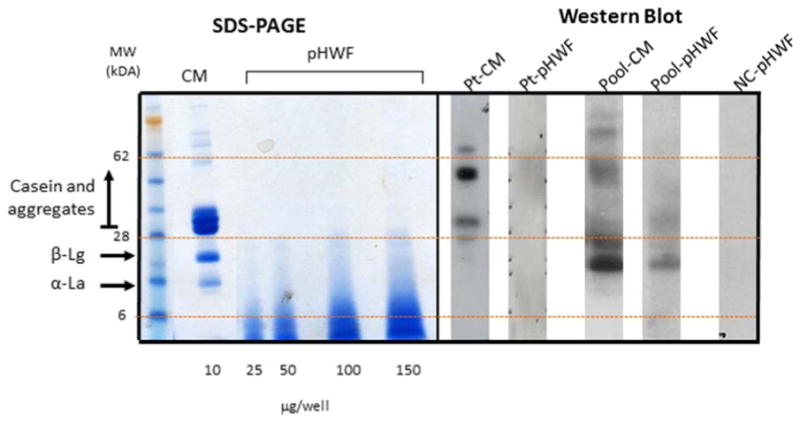
Casein and whey (α-lactalbumin and β-lactoglobulin) are the main proteins in CM (column CM). pHWF has no casein proteins (column pHWF).The case patient's serum showed binding to only casein proteins in CM (column Pt-pHWF), and no binding to any proteins in pHWF(column Pt-pHWF). Pooled sera from subjects with typical CM allergy showed binding to casein and whey proteins in CM (column pool-CM) and to whey proteins and residual casein in pHWF(column pool-pHWF). The negative control showed no binding.Pt= patient, CM=cow's milk, pHWF=partially hydrolyzed whey formula, NC=negative control, α-La=α-lactalbumin, β-Lg=β-lactoglobulin
Immunoblotting of the patient's serum against CM and pHWF was then performed using iodinated goat anti-human IgE as a secondary antibody. There was evidence of the patient's serum sIgE binding to higher molecular weight proteins in CM (≥ 28kDa, corresponding to casein and its aggregates) (Figure 1, column Pt-CM). In contrast, the patient's serum did not show any binding to proteins in pHWF (Figure 1, column Pt-pHWF). These results were consistent with her clinical picture of casein-specific allergy and tolerance of pHWF.
To compare our patient to subjects with more typical CM allergy, we then performed a Western blot against CM and pHWF using pooled serum from five children ≤ 10 years with more typical CM allergy (Table 1). The pooled serum showed binding to casein and whey proteins in CM (Figure 1, column Pool-CM). The pooled serum bound to lower molecular weight bands corresponding to whey proteins in pHWF (Figure 1, column Pool-pHWF). There was also evidence for some binding to ≥ 28 kDa proteins corresponding to casein, suggesting residual casein in pHWF that is consistent with previous studies showing presence of some high molecular weight peptides in partially hydrolyzed formulas.3
Table 1.
Clinical and immunologic features of the case patient and patients with more typical cow's milk allergy
| Subject | Presentation | Cow's milk sIgE (kUA/L) | Casein sIgE (kUA/L) | α-lactalbumin sIgE (kUA/L) | β-lactoglobulin sIgE (kUA/L) |
|---|---|---|---|---|---|
| Case patient | Banana yogurt → dry cough, vomiting, and hives | 8.01 | 23.40 | <0.35 | <0.35 |
| Typical CM allergy patient #1 | Milk → rash | 50.50 | 67.00 | 0.54 | 1.73 |
| Typical CM allergy patient #2 | CM formula → hives, nasal congestion, coughing | 37.30 | 37.90 | 3.38 | 8.31 |
| Typical CM allergy patient #3 | Ice cream → rash, vomiting | >100 | >100 | 69.90 | >100 |
| Typical CM allergy patient #4 | Milk → throat swelling | 49.80 | 29.70 | 16.50 | 23.90 |
| Typical CM allergy patient #5 | Milk → vomiting | 66.00 | 34.00 | 39.20 | 44.70 |
| Negative Control | Tolerates milk | <0.10 | <0.10 | <0.10 | <0.10 |
CM=cow's milk, sIgE=specific IgE
This case highlights the clinical utility of diagnosing allergy to individual CM proteins, as it allowed for liberalization of CM restrictions. Casein (alphaS1-, alphaS2-, beta-, and kappa-caseins) and whey (alpha-lactalbumin, beta-lactoglobulin, bovine lactoferrin, bovine serum albumin, and bovine immunoglobulins) account for 80 and 20 percent of CM protein, respectively.1 The major allergens in CM are casein, alpha-lactalbumin, and beta-lactoglobulin, and most patients with CM allergy are sensitized to multiple proteins.1 Some patients may be sensitized to individual milk proteins only. In this case, the patient had a casein-specific allergy and was able to tolerate pHWF, which was a more palatable and economical choice. As she had been tolerating pHWF for many months, the initial allergist's advice to switch her to an amino-acid based formula was not necessary and she could have continued on pHWF. A specific teaching point from this case is that patients should continue tolerated dietary exposures, and test results need to be appropriately interpreted.
While the immunoblots performed were not essential for her diagnosis, they confirmed her clinical presentation and further demonstrated the specificity of her allergy. Although some residual casein was apparent in the Western blot utilizing serum from CM allergic patients against pHWF, this residual casein had minimal clinical effects for our patient, as she tolerates pHWF.
We are not aware of prior studies that have examined formula selection for individuals with specific CM protein allergies. Prior work on specific CM protein allergies has focused on their role in predicting resolution of CM allergy overall, as high levels of casein-specific IgE as well as other individual CM proteins have been associated with prolonged CM allergy.4 Although Giampietro et al. observed that CM allergic patients can tolerate hydrolysate-based products to varying degrees, the subjects' sIgE profiles to specific CM proteins were not considered in this study.5 Other studies have addressed the potential effects of formula selection on the natural history of CM allergy, but pHWFs were not specifically examined.2, 6, 7 pHWF may carry unique immunoregulatory characteristics.8,9
In conclusion, this case highlights the clinical utility of testing for sensitization to individual CM proteins. Some patients may be sensitized to specific individual milk proteins only. When CM allergy is specific to selected proteins, formula selection may not have to be as restrictive and could be carefully considered with an allergist to allow for more economical and palatable options. If an allergist considers pHWF to be appropriate, then the first dose should be given under allergist supervision due to the possibility of residual casein proteins. Batch-to-batch variation in casein residue must also be considered. Further studies are needed to investigate if there are specific patient populations that would benefit from testing individual CM proteins.
Clinical Implications.
Testing for sensitization to individual cow's milk proteins can be clinically informative. When milk allergy is specific to selected cow's milk proteins, formula selection may not have to be as restrictive.
Acknowledgments
Funding: SB is funded by AI093538; HS is funded in part by AI44236 and AI66738.
Abbreviations
- CM
cow's milk
- pHWF
partially hydrolyzed whey formula
- sIgE
specific IgE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jarvinen-Seppo KM. Milk allergy: clinical features and diagnosis. 2014 UpToDate. www.uptodate.com. downloaded 6/4/2014.
- 2.Terracciano L, Bouyque GR, Sarratud T, Veglia F, Martelli A, Fiocchi A. Impact of dietary regimen on the duration of cow's milk allergy: a random allocation study. Clin Exp Allergy. 2010;40:637–42. doi: 10.1111/j.1365-2222.2009.03427.x. [DOI] [PubMed] [Google Scholar]
- 3.Catala-Clariana S, Benavente F, Gimenez E, Barbosa J, Sanz-Nebot V. Identification of bioactive peptides in hypoallergenic infant milk formulas by CE-TOF-MS assisted by semiempirical model of electromigration behavior. Electrophoresis. 2013;34:1886–94. doi: 10.1002/elps.201200547. [DOI] [PubMed] [Google Scholar]
- 4.Ahrens B, Lopes de Oliveira LC, Grabenhenrich L, Schulz G, Niggermann B, Wahn U, et al. Individual cow's milk allergens as prognostic markers for tolerance development? ClinExp Allergy. 2012;42:1630–7. doi: 10.1111/cea.12001. [DOI] [PubMed] [Google Scholar]
- 5.Giampietro PG, Kjellman NI, Oldaeus G, Wouters-Wesseling W, Businco L. Hypoallergenicity of an extensively hydrolyzed whey formula. Pediatri Allergy Immunol. 2001 Apr;12(2):83–6. doi: 10.1034/j.1399-3038.2001.012002083.x. [DOI] [PubMed] [Google Scholar]
- 6.Reche M, Pascual C, Fiandor A, Polanco I, Rivero-Urgell M, Chifre R, et al. The effect of a partially hydrolysed formula based on rice protein in the treatment of infants with cow's milk protein allergy. Pediatri Allergy Immunol. 2010;21:577–85. doi: 10.1111/j.1399-3038.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berni Canani R, Nocerino R, Terrin G, Frediani T, Lucarelli S, Cosenza L, et al. Formula selection for management of children with cow's milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163:771–7. doi: 10.1016/j.jpeds.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Gill HS, Doull F, Rutherfurd KJ, Cross ML. Immunoregulatory peptides in bovine milk. Br J Nutr. 2000;84(Suppl 1):S111–7. doi: 10.1017/s0007114500002336. [DOI] [PubMed] [Google Scholar]
- 9.Sandre C, Gleizes A, Forestier F, Gorges-Kergot R, Chilmonczyk S, Leonil J, et al. A peptide derived from bovine beta-casein modulates functional properties of bone marrow-derived macrophages from germfree and human flora-associated mice. J Nutr. 2001;131:2936–42. doi: 10.1093/jn/131.11.2936. [DOI] [PubMed] [Google Scholar]


