Abstract
Breathing in mammals is a seemingly straightforward behaviour controlled by the brain. A brainstem nucleus called the preBötzinger Complex sits at the core of the neural circuit generating respiratory rhythm. Despite the discovery of this microcircuit almost 25 years ago, the mechanisms controlling breathing remain elusive. Given the apparent simplicity and well-defined nature of regulatory breathing behaviour, the identification of much of the circuitry, and the ability to study breathing in vitro as well as in vivo, many neuroscientists and physiologists are surprised that respiratory rhythm generation is still not well understood. Our view is that conventional rhythmogenic mechanisms involving pacemakers, inhibition or bursting are problematic and that simplifying assumptions commonly made for many vertebrate neural circuits ignore consequential detail. We propose that novel emergent mechanisms govern the generation of respiratory rhythm. That a mammalian function as basic as rhythm generation arises from complex and dynamic molecular, synaptic and neuronal interactions within a diverse neural microcircuit highlights the challenges in understanding neural control of mammalian behaviours, many (considerably) more elaborate than breathing. We suggest that the neural circuit controlling breathing is inimitably tractable and may inspire general strategies for elucidating other neural microcircuits.
Introduction
‘A few of the earlier natural philosophers have dealt with respiration; some of them have offered no explanation why this phenomenon occurs in living creatures; others have discussed it without much insight, and with insufficient experience of the facts.’
Aristotle, On Respiration, ∼350 BC
‘Hopefully, not us.’
The authors, AD 2014
The complexity of the mammalian brain spans the entire scale of biological function from molecules to behaviour (Koch, 2012). Least understood are the mechanisms transforming the activity of networks of neurons into the vast array of distinct behaviours, each typically a set of movements in response to environmental stimuli or internal state (BRAIN Working Group, 2013). We consider the network of all neurons that participate in a given behaviour to be its neural circuit. In mammals, neural circuits are composed of large, heterogeneous populations of hundreds to thousands of neurons dynamically interacting with each other over time scales of milliseconds to years within and across brain regions, in topologically complex (and, at present, computationally refractory) networks (Fig. 1; Koch, 2012). The necessity of generating and synchronizing activity and coordinating signal processing within and across populations presents novel and perhaps unique challenges for understanding mammalian neural circuits (Ainsworth et al. 2012; Kumar et al. 2013).
Figure 1. Abstract representations of the respiratory CPG.
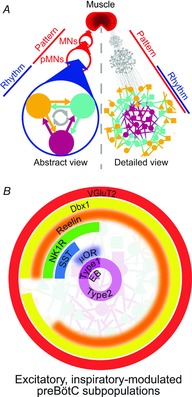
A, left, a common wiring diagram for the minimal respiratory neural circuit from rhythmogenesis to muscle involving several interconnected subpopulations, e.g. SST+, NK1R+, Type 1, within the rhythmogenic preBötC, projecting to premotoneuronal (pMN) and motoneuronal (MN) populations innervating respiratory muscles, e.g. tongue and diaphragm. Rhythm- and pattern-generating functions common to all CPGs are assumed to be segregated. Right, these representations obscure the microcircuit properties required for understanding mechanisms of neural dynamics and may overlook microcircuit connectivity, neuronal heterogeneity (represented by different shapes) and multifunctionality, such as the overlap of rhythm- and pattern-generating functions within the preBötC. B, proportional representations of molecularly and functionally defined excitatory, inspiratory-modulated preBötC populations. Radially overlapping concentric arcs represent populations with multiple properties. Blurred arcs, e.g. Reelin, are estimated or assumed. Data are extrapolated from rat and mouse. Functional categories, e.g. Types 1 and 2, and EB (endogenous bursters), are described in the text.
One approach to managing this complexity is to break the neural circuit into computational modules (Carandini, 2012; Koch, 2012), now often referred to as neural microcircuits (Grillner et al. 2005). This modular approach maps a signal-processing function to a smaller population of neurons within a defined region of brain that is, de facto, more amenable to analysis and perturbation (Koch, 2012). Commonly, since even small brain regions have thousands of neurons, microcircuits are further abstracted by lumping the properties of presumptively homogeneous categorical neuronal populations into representative ‘neurons’ (Fig. 1A; Grillner et al. 2005; Kumar et al. 2013). These ‘neurons’ are connected together and depicted in wiring diagrams that convey in a conceptual model how categorical neuronal elements might interact (Grillner et al. 2005). Such abstractions facilitate an intuitive and easy to grasp understanding of the role of microcircuits in behaviour.
Despite the advantages of such abstractions, compromises in detail can mask consequential aspects of microcircuit physiology, disconnecting microcircuits from underlying molecular and cellular mechanisms that implement the computation and breaking the link that microcircuits form between genes, single neurons and behaviour. Determining which details are consequential and which can be safely discarded is a fundamental issue (Abbott, 2008). To what extent can the microcircuit and the computation it performs be delimited as a module (Carandini, 2012; Koch, 2012) if there are lateral and recurrent, excitatory and inhibitory connections that cross modular boundaries (Horton & Adams, 2005)? Does the microcircuit perform different neural computation(s) across behaviours and states (Briggman & Kristan, 2008; Carr & Frank, 2012)? Is there consequential diversity in neuronal properties, e.g. protein expression, physiology, morphology, or connectivity, within apparently homogeneous populations, and are these identities stable over time (Fig. 1; Markram et al. 2004; Grillner et al. 2005)? How are results from in vivo and reduced preparations, different developmental time points, or different strains or species compared and reconciled across experimental conditions? Can microcircuit dynamics, i.e. the flow of activity that underlies signal generation and processing, be deduced from (static) wiring diagrams with lumped categorical elements (Carandini, 2012; Morgan & Lichtman, 2013; Kopell et al. 2014)? How sensitive is the microcircuit to the spatiotemporal pattern of inputs (Briggman & Kristan, 2008; Marder et al. 2014)? How is microcircuit output distributed during behaviour (Kumar et al. 2013)?
Recent studies of the neural circuits controlling breathing and, particularly, the microcircuit generating respiratory rhythm, are unmasking the limitations of common abstractions and assumptions applied to microcircuits. We describe the multidimensional problem of defining the rhythmogenic microcircuit in breathing and its constituent elements and functions; how hypotheses and models based on conceptually straightforward mechanisms are unlikely to explain respiratory rhythmogenesis; and the downstream transformations of rhythmic activity that can obscure the role of the microcircuit in behaviour. These issues invalidate the simplistic view of respiratory rhythm generation as the product of axiomatic mechanisms, such as pacemakers, inhibition and bursting, in ‘neurons’ that stand in for well-defined, clearly categorized populations. Instead, the breathing rhythm is an emergent property of the microcircuit (Suki et al. 2011), dependent on neuronal heterogeneity, recurrent and lateral connections, and other consequential details. The minimal neural circuit controlling breathing therefore permits close examination of the physiology of mammalian microcircuits in a simpler (but not simple) system that nonetheless preserves emergent properties that may be unique to mammals.
Why breathing?
Behaviour provides a critical, but broad constraint for adjudicating the relevance of neural activity, identifying regions constituting the neural circuit, and parsing out the component neural computations (Carandini, 2012). Basic rhythmic movements, such as breathing, walking and chewing, are among the simplest behaviours, with the capacity to operate independently of sensory input and with limited degrees of freedom in a stereotyped, well-defined, and measurable motor output (Feldman & Grillner, 1983; Grillner, 2006; Grillner & Jessell, 2009). The neural computations performed by circuits controlling rhythmic movements, known as central pattern generators (CPGs), are essentially limited to: (1) rhythm generation – the transformation of tonic drive (without the need for rhythmic afferent input) into the production of repetitive signals that determine the period of the cycle; and (2) pattern generation – the distribution and modulation of this signal to activate participating muscles with appropriate force and timing. Rhythmic motor patterns can range from the basic, bilaterally symmetric, finely tuned (for energy efficiency) breathing pattern with an active inspiratory phase and passive expiratory phase at rest, to more complex patterns, such as quadrapedal locomotion involving gait-dependent left–right, forelimb–hindlimb and flexor–extensor alternation or synchrony as well as coordination of proximal and distal muscles of each limb around multiple joints, i.e. hip, knee and ankle (Grillner, 2006; Andersson et al. 2012; Bachmann et al. 2013; Talpalar et al. 2013).
Separable microcircuits are hypothesized to mediate rhythm generation and patterning in mammalian CPGs (Feldman, 1986; McCrea & Rybak, 2008). While great progress has been made in illuminating patterning microcircuits (Grillner & Jessell, 2009; Garcia-Campmany et al. 2010; Kiehn, 2011), for most mammalian rhythmic behaviours, including locomotion and mastication, rhythmogenic microcircuits have resisted discovery (Grillner & Jessell, 2009). The coarse neuroanatomy of the CPGs for these behaviours is incomplete, and where, beyond being in spinal cord or brainstem, the rhythmic kernel is localized, if it is localized at all, has not yet been definitively determined (Grillner & Jessell, 2009). Rhythmogenic microcircuits in mammals should meet the following four criteria.
Inhibition, silencing, or destruction of key elements of the microcircuit significantly perturbs or even stops rhythm.
The microcircuit projects oligosynaptically to appropriate motoneuronal populations.
Modulatory afferents affect frequency.
The isolated microcircuit (when sufficiently driven) generates rhythmic activity.
Unique among mammalian CPGs, the respiratory CPG has a localized (<1 mm3/side in rodents; ∼1.5 mm3/side in humans) rhythmogenic microcircuit, the preBötzinger Complex (preBötC; Feldman et al. 1990; Smith et al. 1991; Schwarzacher et al. 2011). Acute silencing of a targeted subpopulation preBötC neurons abolishes the breathing rhythm in awake, behaving rodents (criterion 1; Tan et al. 2008). A well-established in vitro slice preparation containing the preBötC, when sufficiently driven (usually with elevated extracellular K+), recapitulates key aspects of rhythmic inspiratory behaviour, including motor nerve output, and rhythmic activity persists in the preBötC when it is further isolated by microdissection from surrounding tissue (criterion 4; Smith et al. 1991; Johnson et al. 2001). preBötC neurons generate rhythmic bursts of action potentials (APs) transmitted via premotoneurons to motoneurons to produce muscle contraction leading to inspiratory airflow and modulation of airflow resistance (criterion 2; Smith et al. 1991; Dobbins & Feldman, 1994, 1995; Koizumi et al. 2008). Neuromodulatory, suprapontine, cerebellar, and sensory inputs can modify the frequency and pattern of breathing to regulate blood gases and pH and to coordinate breathing with other movements and behaviours (criterion 3; Heywood et al. 1996; Huckstepp & Dale, 2011; Feldman et al. 2013; Moore et al. 2013, 2014).
As basic breathing behaviour is conserved across mammals (Milsom, 2010), translating findings from healthy and respiratory-related disease model organisms to humans should be straightforward. Conditions in which neural circuits controlling breathing are disturbed (recently reviewed in Feldman et al. 2013), e.g. in single gene disorders (Amir et al. 1999; Amiel et al. 2003; Weese-Mayer et al. 2003; Gallego, 2012), with sleep apnoea (Javaheri & Dempsey, 2013), following opiate intoxication (Stuth et al. 2012), or in sudden infant death syndrome (Lavezzi & Matturri, 2008; Weese-Mayer et al. 2008) have severe consequences for human health. Despite the development of rodent models for these conditions (Dubreuil et al. 2008; Calfa et al. 2011; Davis & O'Donnell, 2013), our current understanding of such disturbances of respiratory behaviour is insufficient for developing effective therapies or treatments and will depend on a better grasp of basic mechanisms underlying the neural control of breathing, including respiratory rhythmogenesis.
How the preBötC generates rhythm is, perhaps surprisingly, still unknown (Feldman et al. 2013). Localization and isolation of the kernel from the extensive regulatory, sensory and state-dependent feedback associated with respiratory physiology in vivo has not led to a straightforward mechanism analogous to any found in invertebrate CPGs or other biological oscillators (Monfredi et al. 2010; Selverston, 2010; Feldman et al. 2013). Nonetheless, the accessibility and tractability of in vitro experiments in slice (Smith et al. 1991), brainstem–spinal cord (‘en bloc’; Smith & Feldman, 1987; Thoby-Brisson et al. 2009; Bouvier et al. 2010), and in situ (Paton, 1996) perinatal rodent preparations constitutes the best opportunity with present technology for revealing cellular and microcircuit mechanisms that can be tested/verified in vivo in intact awake, sleeping, anaesthetized or decerebrate mammals (often rodents).
An important caveat
The literature on respiratory rhythmogenesis is full of disparate, even contradictory, data and associated interpretations. An obvious but non-trivial explanation is that divergent results are due to differences in experimental preparation or condition. Details of experimental protocol, both conspicuous, e.g. species differences, in vitro vs. in vivo, neonate vs. adult, and less conspicuous, e.g. type of anaesthetic, strain of mouse, presence/absence of peripheral nerves, indeed count, and we describe some examples below. Our advice is to read the label before consuming the literature and be careful when you mix and match!
Defining the preBötC
The preBötC evaded identification until 1990 (Feldman et al. 1990), eluding even Ramón y Cajal (Ramón y Cajal, 1904). A functionally and molecularly heterogeneous bilateral population (∼1000–3000 neurons in rodents) within the ventral respiratory column (VRC), a rostrocaudal column of respiratory-related neurons in the ventrolateral medulla, comprises the rodent preBötC (Feldman et al. 2013). The preBötC is ventrolateral to nucleus ambiguus with enriched inspiratory-modulated propriobulbar neurons (Smith et al. 1991; Monnier et al. 2003), caudal to the Bötzinger complex (BötC), which is populated primarily with expiratory-modulated glycinergic neurons, and rostral to the rostral ventral respiratory group (rVRG), which contains glutamatergic bulbospinal premotor neurons (Smith et al. 1991; Stornetta et al. 2003; Tan et al. 2012). A homologous structure is present in humans (Schwarzacher et al. 2011). In mice, the preBötC becomes rhythmically active late in embryonic development (embryonic day 15.5; Thoby-Brisson et al. 2009), coincident with the onset of fetal breathing movements that are necessary for proper development of lung, respiratory muscles, and the respiratory CPG itself (Kobayashi et al. 2001; Feldman et al. 2009); in humans this happens in the third trimester (Greer, 2012).
While these landmarks localize the general region of the preBötC, the lack of a cytoarchitectonically distinct nucleus and the neuronal heterogeneity in this area makes determination of constituent neurons and borders challenging (perhaps unsurprising for an area bounded dorsally by the nucleus ambiguus). Indeed, within this region are neurons that are not respiratory-modulated (Johnson et al. 1994; Ramirez et al. 1997; Shao & Feldman, 1997), and neurons from adjacent areas, including C1 (Guyenet et al. 2013), BötC (Schwarzacher et al. 1995), and rVRG (Tan et al. 2012) are probably intercalated among inspiratory-modulated preBötC neurons at their overlapping borders.
The preBötC is, nonetheless, a distinct area, functionally separable from surrounding neuronal populations. Perturbations within the preBötC produce markedly different responses compared with when they are targeted to adjacent regions (McCrimmon et al. 1986; Monnier et al. 2003), and rostrocaudal boundaries where rhythm is maintained in slices from perinatal rodents are now well-defined (Ruangkittisakul et al. 2006, 2011, 2014). We can therefore describe respiratory-related neurons in this region by their anatomy, physiology, and/or molecular identity, fully acknowledging that we are referencing the core of the preBötC and not necessarily its full real-estate.
Several functionally defined neuronal subtypes comprise the preBötC. Limited electrophysiological samples of brainstem neurons yield several classifications of neurons with firing patterns phase-locked to respiratory motor outflow. Early experiments categorized preBötC neurons based on peak activity during the respiratory cycle, i.e. inspiratory, expiratory, pre-inspiratory, post-inspiratory and phase-spanning (Smith et al. 1990; Schwarzacher et al. 1991; Johnson et al. 1994; Bianchi et al. 1995; Sun et al. 1998). Further subtypes are distinguished based on incrementing or decrementing firing patterns (Richter, 1996; Lindsey et al. 2012; Richter & Smith, 2014). Inspiratory preBötC neurons are also classified into Type 1 and Type 2 neurons, which differ in the duration of pre-inspiratory firing, membrane properties, including the expression of the hyperpolarization-activated cation current (Ih) and the A-type K+ current (IA), and voltage trajectory following the inspiratory burst in vitro (Fig. 1B; Rekling et al. 1996). Inspiratory-modulated preBötC neurons may also be divided into those with or without endogenous bursting (EB; pacemaker) properties (Fig. 1B; Smith et al. 1991; Johnson et al. 1994; Del Negro et al. 2001; Thoby-Brisson & Ramirez, 2001). These EB properties primarily depend on either of two well-studied currents, the Ca2+-activated non-specific cation current (ICAN) or the persistent Na+ current (INaP), that are not exclusively expressed by EB neurons and are, in fact, present in many, if not all, preBötC inspiratory neurons in vitro (Thoby-Brisson & Ramirez, 2001; Del Negro et al. 2002a, 2005; Rybak et al. 2003; Pace et al. 2007a).
A unique molecular marker or transcriptional lineage defining the preBötC has not yet been discovered, but expression patterns of some proteins differentiate the preBötC from surrounding regions. Molecular markers, first the neurokinin 1 receptor (NK1R; Gray et al. 1999, 2001), and subsequently the peptide somatostatin (SST; Stornetta et al. 2003; Wei et al. 2012), and the glycoprotein Reelin (Tan et al. 2012) identify subpopulations of preBötC glutamatergic neurons, which express vesicular glutamate transporter 2 (VGluT2; Fig. 1B; Wallen-Mackenzie et al. 2006). Neurons expressing μ opioid receptors (μORs), which overlap significantly with NK1R-expressing neurons and processes (Fig. 1B; Gray et al. 1999), the somatostatin 2a receptor (SST2aR; Gray et al. 2010), and tyrosine kinase B (Thoby-Brisson et al. 2003; Bouvier et al. 2008) are also found in the preBötC. These neurons are derived from precursors expressing the transcription factor developing brain homeobox 1 (Dbx1; Bouvier et al. 2010; Gray et al. 2010). Dbx1-derived (Dbx1+) neurons populate the entire nervous system, but are prominent in the VRC (Gray, 2013). Glycine transporter 2 (GlyT2) identifies preBötC glycinergic neurons, which are about half of all preBötC neurons (Winter et al. 2009), some with inspiratory-modulated activity in vitro (Winter et al. 2009; Morgado-Valle et al. 2010; Koizumi et al. 2013). A small subset of preBötC neurons also express glutamic acid decarboxylase 67 (GAD67), which specifies γ-aminobutyric acid (GABA)ergic neurons, and some of these are also weakly inspiratory and overlap with glycinergic neurons (Kuwana et al. 2006; Koizumi et al. 2013; Rahman et al. 2013).
In most cases, the molecular identities or firing pattern of preBötC neurons do not uniquely map to functionally definable subpopulations. In vitro, both glutamatergic and glycinergic preBötC neurons can have inspiratory-modulated activity, which can be variable from cycle to cycle, and a small percentage of both can be EBs (Morgado-Valle et al. 2010; Carroll & Ramirez, 2013; Carroll et al. 2013; Kam et al. 2013a; Picardo et al. 2013). Dbx1+ glutamatergic neurons are more functionally and morphologically homogeneous, most have a pre-inspiratory firing pattern and commissural connections and lack dendritic spines (Picardo et al. 2013). However, even Dbx1+ neurons are divided into Type 1 (some of which are EBs) or Type 2 (Rekling et al. 1996; Picardo et al. 2013), which may correlate with some Dbx1+ neurons serving a rhythmogenic role and others a premotor role (Wang et al. 2014). Additionally, neurons with inspiratory- or expiratory-modulated firing patterns, throughout the VRC from the rostral pons to the spinomedullary junction, can have very different roles in the production of respiratory pattern (Segers et al. 2008; Mellen & Mishra, 2010). In particular, bulbospinal and other premotoneurons proximal to the preBötC have inspiratory-modulated firing patterns but are not rhythmogenic. A recent attempt to find discrete classes of inspiratory-modulated preBötC neurons from multicellular recordings of firing patterns was unsuccessful, suggesting that inspiratory-modulated neurons fall along a continuum of firing behaviour (Carroll et al. 2013, but see section ‘preBötC connectivity – a critical microcircuit parameter’ below, for caveats).
Whether molecular markers are sufficient to determine functional classes of preBötC neurons remains to be established. Neurons with differing levels of expression of the same channels can have distinct electrophysiological properties (Golowasch et al. 2002; Schulz et al. 2006) and different combinations of channels can produce similar electrophysiological properties, e.g. ICAN- and INaP-dependent EBs (Del Negro et al. 2002b, 2005). Second order parameters, e.g. variability in expression level of a protein across a population, may be critical for unravelling the relationship between molecular and functional categories (Rybak et al. 2004; Marder & Taylor, 2011). Receptor markers such as NK1R or SST2aR confer unique input specificity to a subpopulation of preBötC neurons, but, in the absence of such input, the function of these neurons may be indistinguishable from neurons lacking such receptors (Hayes & Del Negro, 2007). Indeed, preBötC neurons probably participate in multiple signal processing functions, and distinct roles for many subpopulations may only be elicited under specific conditions or for behaviours other than normal breathing at rest (Lieske et al. 2000; Briggman & Kristan, 2008).
While the neuronal heterogeneity and ambiguity of cytoarchitectonic boundaries of the preBötC may be considerable, such issues are not unique to the preBötC (Markram et al. 2004; Horton & Adams, 2005; DeFelipe et al. 2013; Huang, 2014; Seung & Sumbul, 2014), and are greatly mitigated by the context provided by breathing behaviour. By bounding the circuit and utilizing a presumptively straightforward, spatiotemporally constant tonic excitatory drive as input with behaviourally relevant respiratory rhythm as output, we can focus specifically on how this rhythm emerges from the confederation of molecularly and functionally heterogeneous preBötC neurons. Until recently, most research has focused on a few likely mechanisms, and without success. In what follows, we discuss how several of these mechanisms, first pacemakers, then inhibition, and finally even bursts, are, in fact, unlikely to be critical for rhythmogenesis.
Pacemaker properties are not necessary for respiratory rhythmogenesis
Shortly after the preBötC was discovered (Feldman et al. 1990), excitatory preBötC neurons with conditional EB properties were hypothesized to serve as pacemakers driving rhythm (Smith et al. 1991), analogous to the cardiac sinoatrial node (Monfredi et al. 2010) and some invertebrate CPGs (Selverston, 2010). While the pacemaker hypothesis is compelling, easy to grasp, and, for a long time, widely considered the favoured mechanism for rhythmogenesis, neither EBs nor their associated conductances are obligatory for rhythmogenesis (recently reviewed in Feldman et al. 2013). Briefly, the loss of rhythmic activity observed during widespread application of pharmacological blockers of EB conductances in vitro or in vivo can be attributed to depression of tonic excitatory drive into the preBötC and to non-specific effects on excitability (Del Negro et al. 2005; Pace et al. 2007b; Montandon & Horner, 2013). When blockade of EB conductances is restricted to preBötC or excitability is increased, rhythmic activity persists (Pace et al. 2007b; Montandon & Horner, 2013).
What then are EBs and the associated ‘pacemaker’ currents doing? While EB neurons are a limited subset of all preBötC neurons, INaP and ICAN are ubiquitous (Thoby-Brisson & Ramirez, 2001; Del Negro et al. 2002a, 2005; Rybak et al. 2003; Pace et al. 2007a). Their widespread expression suggests that these conductances serve as one of many mechanisms for controlling excitability, possibly to ensure either the stability (Purvis et al. 2007) or the robustness of preBötC rhythmogenesis, or to amplify activity to ensure transmission of preBötC signals to other neuronal populations (Mellen, 2008, 2010). The specific role of these and other conductances and these neurons may depend on the state of the network, e.g. during sleep, rest, or exercise, or the developmental stage (Del Negro et al. 2005; Montandon & Horner, 2013). How EBs might function under in vivo conditions and in adult mammals is unknown; at present EB preBötC neurons have not been identified or characterized in vivo, at any age. Even if present, how such neurons would operate within a heterogeneous network is unlikely to be straightforward (Boyett et al. 2000). With pacemaker neurons unlikely to drive rhythmogenesis, we next consider another likely suspect, inhibition.
Fast inhibitory synaptic transmission is not necessary for rhythmogenesis
The earliest proposals for the neural basis of rhythmic mammalian movements were based on mutually inhibitory populations underlying locomotion (Brown, 1914). This ‘half-centre’ model was later modified to a threshold hypothesis for breathing, where inspiratory activity grows until it reaches a threshold to trigger massive inhibition that resets it to zero with a refractory interval before it can grow again (Rubio, 1972; Bradley et al. 1975; Feldman & Cowan, 1975). A contemporary extension of this idea postulates that normal breathing rhythm relies on an ‘inhibitory ring’ of three populations of inhibitory neurons in the preBötC and neighbouring BötC (Smith et al. 2007; Richter & Smith, 2014).
Brainstem respiratory neurons receive fast inhibitory synaptic input mediated by GABA and glycine (Richter, 1982; Ballantyne & Richter, 1984; Paton & Richter, 1995; Shao & Feldman, 1997). In an in situ perfused neonatal rodent preparation, blocking inhibition throughout the entire neuraxis with GABAA and glycine receptor antagonists or low extracellular Cl− abolishes respiratory-related activity (Hayashi & Lipski, 1992; Smith et al. 2007). This observation cannot exclusively be interpreted as demonstration of the necessity of inhibition for rhythmogenesis, since systemic blockade of inhibition will disinhibit preBötC neurons (Shao & Feldman, 1997), with the potential to shift the excitability of almost any type of rhythm generator into a tonic, even inactive, regime (Del Negro et al. 2001).
In contrast, in rhythmic medullary slices, a normal rhythm persists following blockade of fast synaptic inhibition (Feldman & Smith, 1989; Shao & Feldman, 1997). Similarly, a largely normal respiratory rhythm persists following well-controlled pharmacological blockade of GABAA and glycine receptors limited to preBötC and BötC in spontaneously breathing adult rats with vagus and carotid sinus nerves intact, preserving normal lung reflexes and chemo-/baroreception (Fig. 2; Janczewski et al. 2013). The changes induced by block of inhibition in these rats, increased inspiratory amplitude and decreased respiratory frequency, are similar to the effects of cutting the vagus nerve, which contains pulmonary afferents. Moreover, this blockade is profound as it abolishes the potent pulmonary afferent-mediated lung inflation reflex that under control conditions can produce apnoea (Fig. 2, compare top and bottom traces). Following subsequent vagotomy, block of inhibition does not change rhythm. Therefore, postsynaptic inhibition within the preBötC and/or BötC is NOT required for a normal breathing rhythm in intact rodents. The ‘inhibitory ring’ model (see section ‘Can models show us the way?’ below; Smith et al. 2007), as well as any other model requiring postsynaptic inhibition within the preBötC (e.g. Feldman, 1976) fails this critical test of necessity in the intact rat. The relevance of the ‘inhibitory ring’ model may therefore be limited to the reduced in situ preparations that provide the experimental basis for this concept (Smith et al. 2007) and to highly reduced in vivo preparations. This model cannot, however, be representative of basic mechanisms for generation of respiratory rhythm in intact mammals.
Figure 2. Inhibition is not essential for normal rhythmogenesis in adult rat.
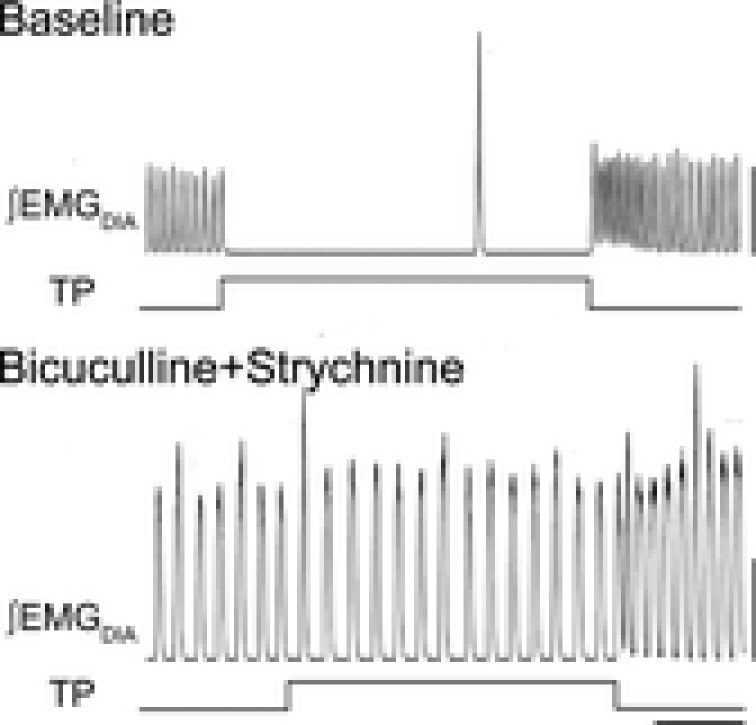
Block of postsynaptic inhibition within preBötC and BötC in vagus intact adult rat slows breathing frequency to that of vagotomized rat and completely blocks the Breuer–Hering inflation reflex. Top, lung inflation resulting from increased tracheal pressure (TP; 10 cmH2O) causes apnoea as reflected in loss of activity in diaphragmatic EMG (∫EMGDIA). Bottom, after microinjection of bicuculline and strychnine, GABAA and glycine antagonists, rhythm persists but lung inflation no longer has an effect on rhythm. Scale bars, 100 arbitrary units, 10 s. Adapted from Janczewski et al. (2013).
What then is inhibition doing in the respiratory CPG? Fast synaptic inhibition is generally accepted to play a key role in shaping motor nerve burst pattern and coordinating activity between motoneuron pools (Feldman & Smith, 1989; Grillner, 2006), and certainly this is the case for breathing, as fine tuning the motor output is a key element in the incredible mechanical efficiency of breathing (at rest ∼7% of total body metabolism, close to the minimal estimated energy for optimal breathing movements; Otis et al. 1950). In addition to being essential for the lung inflation reflex (Ezure & Tanaka, 2004; Janczewski et al. 2013), inhibition in the preBötC appears important for modulating the breathing frequency (Paton & Richter, 1995), for ensuring that spurious signals do not inappropriately affect ongoing respiratory activity (Busselberg et al. 2001), and in producing apnoeas (Lawson et al. 1991) required by such behaviours as swallowing and breath holding (Saito et al. 2002; Janczewski et al. 2013; Bautista & Dutschmann, 2014; Richter & Smith, 2014). Rhythmogenesis therefore does NOT depend on inhibition or pacemaker neurons. Next, we consider whether bursting is necessary.
Burstlets, not bursts, are rhythmogenic
In invertebrates, coordinated bursts of APs, either in phase with motor output in pacemaker-driven CPGs or out-of-phase with motor output in inhibitory network-driven CPGs, are essential for rhythmogenesis (Getting, 1989; Marder & Calabrese, 1996; Selverston, 2010). Elucidating the mechanisms initiating and terminating preBötC neuronal bursts that result in inspiratory motor nerve bursts in vivo, in situ and in vitro has been a major focus in the search for how preBötC generates rhythm (Feldman et al. 2013). While revealing cellular mechanisms underlying burst amplitude, duration and firing pattern (Pace et al. 2007a; Del Negro et al. 2009; Richter & Smith, 2014), this approach has so far been less successful in illuminating rhythmogenic mechanisms (Feldman et al. 2013).
The presumption that preBötC burst-generating mechanisms are rhythmogenic, while generally accepted, is not warranted. In many intact mammals and in vitro, burst shape parameters, such as burst amplitude and duration that reflect AP frequency and/or number of active neurons, may stay the same despite large changes in breathing frequency; conversely, changes in integrated burst shape parameters in motor output can occur without significantly affecting frequency (Clark & von Euler, 1972; Del Negro et al. 2009). In vitro, preBötC burst shape parameters, including the amplitude and duration of the inspiratory drive potential recorded in individual preBötC neurons, can be substantially altered without concomitant changes in frequency, e.g. blockers of metabotropic glutamate receptors and inositol 1,4,5-trisphosphate (IP3) receptors that significantly change preBötC burst shape do not affect frequency in vitro (Pace et al. 2007a). The separate modulation of burst shape and frequency suggests that determination of the time to the onset of the next burst is (at least partially) independent of the pattern-generating mechanisms that underlie bursting per se (Del Negro et al. 2009).
We recently discovered that bursts of preBötC population activity are not unitary events, but consist of two separable components: large amplitude inspiratory bursts (I-bursts) that are ultimately transmitted to hypoglossal (XII) motoneurons in vitro and all inspiratory motoneurons in vivo, and burstlets, of much smaller amplitude, that appear as pre-inspiratory activity and are rhythmogenic (Fig. 3). Burstlets are revealed by systematically altering respiratory CPG excitability in vitro and in vivo (Kam et al. 2013a). At conventional levels of extracellular K+ and Ca2+ in vitro, there is a 1:1 correspondence between preBötC bursts and XII nerve (XIIn) motor bursts (Fig. 3A; Ramirez et al. 1996; Kam et al. 2013a). The XIIn interburst interval (IBI), unimodal under baseline conditions in vitro, becomes longer and, somewhat surprisingly, multimodal when excitability is lowered through decreases in extracellular K+. These modes are multiples of the shortest IBI, i.e. ‘quantized’ (Fig. 3B; Kam et al. 2013a), so that, at times when a XIIn burst is expected but absent, burstlets occur in preBötC, and the number of preBötC burstlets during a XIIn IBI correlates linearly to the duration of the IBI (Kam et al. 2013a). Importantly, burstlets are neither an in vitro nor developmental epiphenomenon and can be elicited under specific conditions in vivo (Kam et al. 2013a). When preBötC bursts occur, burstlets appear as the low-level pre-inspiratory activity immediately preceding I-bursts (Fig. 3C; Kam et al. 2013a). Furthermore, a preBötC rhythm comprising only burstlets is possible when Cd2+ is bath-applied (Fig. 3D; Kam et al. 2013a). That rhythmic burstlet activity persists following the disappearance of bursts is incompatible with bursts, an essential element of many, if not most, models of rhythmogenesis (e.g. Butera et al. 1999; Rybak et al. 2004; Purvis et al. 2007), being necessary for generation of rhythmic activity (Fig. 4).
Figure 3. Bursts are not essential for rhythmogenesis in transverse medullary slices in vitro.
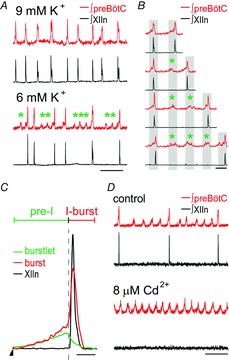
A, rhythmic burstlets are distinct from bursts in medullary slices in vitro. In 9 mm K+/1.5 mm Ca2+, XIIn and preBötC bursting are synchronous and regular. In 6 mm K+/1.5 mm Ca2+, XIIn bursting is highly variable, while preBötC rhythm with both bursts and burstlets (*), which do not produce XIIn bursts, is more regular. Integrated preBötC and XII activity is shown, which represents suprathreshold, i.e. action potential, activity. Scale bar, 10 s. B, traces showing interburst intervals (IBIs) with different numbers of burstlets. Scale bar, 2 s. C, superimposed average waveforms of XIIn bursts (black) and preBötC burstlets (green) and bursts (red) aligned by the start of burstlet/pre-inspiratory preBötC activity (arrowhead). Vertical dashed line represents start of XIIn burst. preBötC bursts can be divided into a pre-inspiratory (pre-I) phase and an inspiratory burst (I-burst) phase. Scale bar, 0.5 s. D, Cd2+ selectively eliminates preBötC and XII bursts, but burstlets persist (bottom). Scale bar, 10 s. Adapted from Kam et al. (2013a).
Figure 4. The burstlet hypothesis for rhythmogenesis fundamentally breaks with the burst hypothesis.
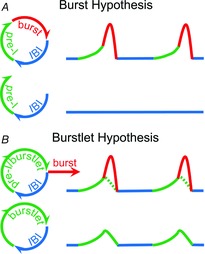
A, top, in the burst hypothesis, the interburst interval (IBI; blue) is a quiescent phase that leads to pre-inspiratory (pre-I) activity (green) and then the necessary generation of a high amplitude burst (red). Bottom, blockade of bursts in this model would lead to interruption of the rhythmogenic cycle and cessation of rhythmic activity. B, top, in the burstlet hypothesis, the IBI (blue) leads to burstlets (green), which are sufficient for rhythmogenesis. Generation of high amplitude bursts (red) is a distinct step and not essential for generation of rhythmic activity. When bursts occur, burstlets appear as pre-I activity (dotted green line) preceding bursts. Bottom, by lowering excitability or adding Cd2+, bursts disappear but rhythmic preBötC burstlets remain, contradicting the burst hypothesis.
The difference in burstlet and burst amplitude (which are population measures) is primarily determined by significantly increased AP frequency in neurons during I-bursts compared to burstlets and much less so by recruitment of more neurons (Kam et al. 2013a). Ninety per cent of inspiratory preBötC neurons are active during both, with recruitment of the remaining ∼10% during I-bursts (Kam et al. 2013a). preBötC neuron I-burst APs are preceded by a period of low frequency pre-inspiratory APs, comparable in duration and frequency to their activity during burstlets, suggesting that burstlets are the pre-inspiratory activity that triggers bursts (Kam et al. 2013a).
The generation of I-bursts from burstlets is a threshold process, akin to AP generation from EPSPs in single neurons (Fitzhugh, 1955). We speculate that when a burstlet exceeds an as yet undefined threshold, distinct mechanisms, probably involving both the activation of persistent inward conductances, e.g. INaP and ICAN, and the recruitment of additional neurons, generates an I-burst (Feldman et al. 2013; Kam et al. 2013a). Following each burst, the activity of the network is reset, and a refractory period is observed (Del Negro et al. 2009; Kam et al. 2013a). However, this refractory period, which may set a minimum interval between bursts, is only a fraction of the IBI in vitro and does not determine burstlet or burst timing (Del Negro et al. 2009; Kam et al. 2013b). We liken rhythmogenic burstlet activity to a preBötC ‘metronome’ maintaining an underlying beat, with preBötC I-bursts and downstream premotoneuronal (that could include preBötC neurons) and motoneuronal networks transforming this timekeeping signal into a physiologically appropriate pattern for breathing and other orofacial behaviours (Fig. 4; Moore et al. 2013, 2014).
Importantly, the burstlet hypothesis suggests a unitary rhythmogenic signal underlying inspiration over a wide dynamic range of frequencies and considerably constrains the space of possible rhythmogenic mechanisms to those not requiring the high levels of neuronal activity seen during bursting. The lower firing frequency of individual neurons during burstlets compared to bursts probably precludes the involvement of conductances with high voltage or high Ca2+ thresholds (Richter & Smith, 2014). Indeed, the relative paucity of activity that is nonetheless distributed throughout the preBötC microcircuit suggests an emergent mechanism for rhythmogenesis.
What constitutes an emergent mechanism in the preBötC? Single EPSPs in preBötC neurons (∼3 mV) are too small to produce an AP, since the typical threshold depolarization is ∼10 mV (Rekling et al. 2000). Temporal summation of EPSPs arising from a single input neuron may also be insufficient to generate an AP, being limited by the typical maximum firing frequency of preBötC neurons (∼30–40 Hz in vitro; Rekling et al. 1996; Kam et al. 2013b), since the resultant interspike intervals (∼30 ms) are too long. Instead, the activity of a few convergent neurons could depolarize a target neuron sufficiently to produce APs – a classic ‘emergent’ property (Ratte et al. 2013). As network activity grows, APs of individual neurons may become more synchronized, increasing temporal convergence of synaptic inputs leading to greater postsynaptic depolarization that (more readily) crosses the AP threshold. Increased synchrony could accelerate the collective growth of activity amongst already active neurons and facilitate the triggering of bursts by burstlets. The novelty of this mechanism is that it would not require either increased firing in active neurons or recruitment of quiescent neurons for burstlet growth or burst triggering. Whatever the mechanism, how this emergent growth process plays out at different breathing frequencies (10-fold range in humans from rest to extreme exercise, and 100-fold range across species: 3–5 Hz in mice to 0.05 Hz in whales; Mortola & Limoges, 2006; Forster et al. 2012; Berndt et al. 2014) or behaviours, e.g. eupnoea, sighs, gasps, cough, speech (Bartlett & Leiter, 2012), particularly when firing frequencies of individual neurons are low (Kam et al. 2013a), critically depends on microcircuit connectivity parameters (Feldt et al. 2011).
preBötC connectivity – a critical microcircuit parameter
Basic parameters of connectivity, such as the number of inputs and outputs for each neuronal subtype and how neuron subtypes are interconnected, can be described anatomically, i.e. based on structural contacts between two neurons, functionally, i.e. based on a statistical association between the firing of neurons, or effectively, i.e. based on physiological, electrical or chemical synaptic connection between neurons (Feldt et al. 2011). While the influence of neurons is not restricted to proximate synaptic connections, e.g. paracrine release of neuromodulators or peptides (Zoli et al. 1999), anatomical and functional connectivity are necessary parameters for constraining models, and effective connectivity parameters, such as the strength of connectivity and synaptic properties, e.g. probability of release, number of synapses, receptor kinetics, are essential data for fully understanding how dynamics arise from network connectivity (Song et al. 2005; Feldt et al. 2011). The network structure as determined by the connectivity distribution, e.g. random, small world, local, or all-to-all, has profound impact on how activity propagates through the network (Sporns et al. 2004); once determined it allows the application of graph theoretical approaches for network analysis (Feldt et al. 2011, but see Mitra, 2014).
Excitatory preBötC neurons are recurrently connected (Rekling et al. 2000; Hayes & Del Negro, 2007) with fast excitatory synaptic transmission necessary for rhythmogenesis (Greer et al. 1991). In a small sample of dual whole cell patch recordings in preBötC in vitro, a subset of pre-inspiratory neurons are effectively connected to 13% of other pre-inspiratory neurons by one-way excitatory chemical synaptic connections and to a distinct 13% of other pre-inspiratory neurons by weak gap-junction connections (Rekling et al. 2000). However, in a larger sample of spike time correlations obtained from multielectrode extracellular recordings in preBötC in vitro, a much lower functional connectivity of 1% is estimated (Carroll & Ramirez, 2013). Yet another different, more complex network structure is observed in medullary slice cultures, where neurons show small world anatomical connectivity with neurons grouped into local, highly connected (∼4 connections per neuron) clusters (Hartelt et al. 2008).
What might account for these divergent observations? The laboriousness of dual patch recordings, which provides direct physiological evidence of effective connectivity, limits the estimates of effective connectivity to small samples that may not be representative of the overall network. The multielectrode extracellular approach, in contrast, is indirect (Schwindel et al. 2014) and relies on proper assignment of APs to each stipulated neuron (Lewicki, 1998). High temporal synchrony and changes in spike shape (Lewicki, 1998; Carroll et al. 2013; Kam et al. 2013a; Picardo et al. 2013) can confound clustering and dimensionality reduction techniques such as independent components analysis (Valmianski et al. 2010), and complicate the parcelling of APs among different stipulated neurons (Lewicki, 1998). We suggest that the conclusion of 1% connectivity is too low, perhaps by an order of magnitude; we cannot readily reconcile such low connectivity with the successful electrophysiological detection of synaptically connected pairs from random sampling (Rekling et al. 2000). Finally, connectivity in slice cultures is unlikely to reflect network connectivity in acute in vitro preparations and in vivo due to immense sprouting and regrowth in cultured slices and the significant flattening of the three-dimensional structure (Zimmer & Gähwiler, 1984; Frotscher & Gähwiler, 1988). While the notion of clustering is intriguing, serious consideration should require validation in more intact networks.
Recent efforts to establish anatomical connectivity in mammalian networks at the electron microscopic (Kleinfeld et al. 2011) or light microscopic (Oh et al. 2014) levels will provide necessary information for understanding neural microcircuits. However, microcircuit structure is not sufficient for deducing network dynamics that emerge from the interplay of the electrophysiological and synaptic properties of neurons and the connectivity (Kopell et al. 2014). As the non-linearities of emergent behaviour typically defy intuition, we consider next the role of simulations in providing insight into how cellular and network properties interact to generate rhythmic population activity.
Can models show us the way?
Computational simulations of biologically plausible models can be useful for exploring rhythmogenic mechanisms (Abbott, 2008). Models of the respiratory CPG range from abstract models that test the feasibility of hypothetical mechanisms to attempts at biologic-ally realistic models based on large datasets. The broader utility of these models, however, ultimately depends on their ability to motivate novel, non-trivial experimental tests of proposed mechanisms.
Some models for breathing, including the aforementioned ‘inhibitory ring’ model (Smith et al. 2007), incorporate large amounts of data related to cellular parameters, and crude (because that is the best we have) and largely incomplete measures of connectivity between respiratory-related brainstem areas. However, the considerable amount of data necessary for a biologically realistic model (Markram, 2006; Marder & Taylor, 2011) is not yet available for the respiratory CPG. Many critical parameters have yet to be experimentally determined, so these parameters are typically fitted to a specific dataset, i.e. the values are chosen so that the simulation resembles some limited range of experimental phenomenology. Unfortunately, these fits are done without any analysis of whether the chosen parameters represent unique solutions, or whether the model is stable with physiologically reasonable changes in the parameter set (Marder & Taylor, 2011), as may be associated with experimental perturbations, development, or increased ventilatory demand such as during exercise or changes in sleep–wake state.
In the absence of values derived from experimental data, parameterization of models is necessary to explore their dynamic range and sensitivity to given parameter sets (Nowotny et al. 2007; Marder & Taylor, 2011). Such parameterization should be accompanied by detailed comparison and analysis of model output across parameter sets and with experiments (Nowotny et al. 2007). Many regions of multidimensional parameter space inherent in a complex model can produce similar behaviours, i.e. solutions are highly degenerate (Prinz et al. 2004; Nowotny et al. 2007; Mellen, 2010), and there is no a priori guarantee that any given solution is stable to small perturbations in parameters. Not all rhythmic outputs are equivalent (Nowotny et al. 2007), and model behaviour that resembles experimental results with apparently subtle differences may be satisfactorily close or may, instead, reflect significant flaws in the model. Rigorous protocols for evaluating models, such as the ‘verification, validation, and uncertainty quantification’ (VVUQ) paradigm (Pathmanathan & Gray, 2014), are needed to determine the success of these large, multineuronal simulations in representing biological reality. We should recognize the limits of models that reproduce some phenomenology, but stipulate parameters or mechanisms that have yet to be or cannot realistically be determined or validated experimentally (Mitra, 2014).
Smaller models focused on the preBötC have been developed. As with the more comprehensive models, their plausibility is based on whether they reproduce some set of experimental data. For example, several models can produce burstlet/burst patterns, or ‘irregular bursting,’ when the variance of the distribution of INaP conductances or synaptic strengths are adjusted (Butera et al. 1999; Rybak et al. 2004; Purvis et al. 2007). A major caveat for these particular models is their reliance on pacemaker conductances, specifically the slow deactivation kinetics of INaP, to generate rhythmic bursting, which is not consistent with the pharmacological data demonstrating that such conductances are not necessary (Pace et al. 2007b; Feldman et al. 2013). A recent model, incorporating both INaP and ICAN, explores the effects of these and other various cellular parameters on network dynamics and can reproduce rhythmic bursting independent of these conductances (Jasinski et al. 2013). However, the network connectivity in this model is all-to-all (Jasinski et al. 2013), which does not reflect the limited experimental data on preBötC connectivity (see previous section ‘preBötC connectivity – a critical microcircuit parameter’), and its stipulation can significantly affect computed network dynamics (Butera et al. 1999; Shao et al. 2006).
In contrast to biologically realistic models, abstract, or ‘toy’ models of the preBötC can explore how a specific parameter or mechanism might generate or affect rhythmic bursting, permitting formal dynamical systems analysis of network behaviour (Weiss et al. 2003; Sherman, 2011). In one example, simplified model neurons placed in a network with small world connectivity can produce network-wide bursting under some conditions without invoking conductances with slow kinetics (Shao et al. 2006). In another model, networks of simplistic threshold bursting neurons with a slow adaptation process that terminates firing demonstrate that complex dynamics can arise solely from particular connectivity patterns (Schwab et al. 2010). Here, a network with all-to-all connectivity fails to reproduce the complex quasiperiodic dynamics observed in higher excitability regimes in vitro (Del Negro et al. 2002c). However, in a network with heterogeneous connections, these dynamics are observed, with the transition from a stable oscillatory state to a more complex dynamical state governed by a discontinuous function of network size and excitability, a novel, non-trivial and testable prediction.
Rhythmogenesis may also emerge from interactions between preBötC neurons if synaptic drive dynamically modifies neuronal conductances to generate bursts (Rekling & Feldman, 1998) or burstlets. This group pacemaker hypothesis (Rekling & Feldman, 1998) is most relevant when considering the interaction between synaptic input and the membrane properties of individual neurons (Rubin et al. 2009). How this mechanism might apply in a preBötC neuronal microcircuit was explored later in a network simulation where the size of the population and the degree of connectivity were varied (Wang et al. 2014). In this model, a rhythmic bursting regime that overlapped with the experimentally determined size of the rhythmogenic Dbx1+ population and the 13% effective connectivity identified by paired recordings was found, demonstrating the plausibility of the group pacemaker mechanism (Wang et al. 2014). Importantly, when model results were compared with experimental data, discrepancies motivated further experiments that identified a novel premotor subpopulation of Dbx1+ neurons (Wang et al. 2014).
Critical data for testing this model was obtained from dynamic, targeted perturbations that imposed critical constraints on neural circuit dynamics (Wang et al. 2014). New techniques that permit more complex interrogation of network properties with single cell resolution and high spatiotemporal specificity show great promise in addressing the functional aspects of microcircuits. We describe such experiments aimed at understanding the preBötC.
Targeted photoablation and patterned photostimulation can uniquely reveal microcircuit properties
With sparse information on neuronal heterogeneity and connectivity, perturbations with high spatiotemporal resolution can illuminate the underlying circuitry and constrain models (Wang et al. 2014). In rodents, bulk manipulations of preBötC neurons are limited in their ability to reveal critical microcircuit properties since functionally diverse, anatomically intercalated, and molecularly heterogeneous preBötC neurons are lumped together. A precise understanding of microcircuit mechanisms requires perturbations targeted to limited subsets of neurons, defined molecularly, functionally and/or spatially (Huang & Zeng, 2013).
One novel approach for preBötC microcircuit dissection is sequential targeted removal of neurons by laser photoablation (Hayes et al. 2012). Sequential ablation of ∼120 inspiratory-modulated neurons in vitro results in the loss of rhythmic motor output (Hayes et al. 2012; Wang et al. 2013). When lesioning is restricted to Dbx1+ (glutamatergic) preBötC neurons, rhythm stops after ablating ∼85 neurons, suggesting that rhythmogenic circuits are more sensitive to the specific loss of Dbx1+ neurons than to generic inspiratory-modulated neurons (Wang et al. 2013, 2014), half of which are inhibitory (Winter et al. 2009). A more detailed analysis of how preBötC activity changes during photoablation may shed light on how the dynamics degrade (Gray et al. 2001; Wenninger et al. 2004; McKay et al. 2005; Tan et al. 2008) to provide additional constraints for preBötC models (e.g. Schwab et al. 2010; Wang et al. 2014). Moreover, further controls addressing damage to neighbouring neurons due to ATP or free radical release following photoablation (Shah & Jay, 1993) would eliminate the possibility that the effects are due to spread of the perturbation beyond the targeted neuron. Nonetheless, the preBötC is resistant to moderate lesioning, whether by cumulative single cell or targeted subpopulations (Gray et al. 2001; Wenninger et al. 2004; McKay et al. 2005; Tan et al. 2008), robustly maintaining proper network function in the face of limited neuronal loss, as may occur throughout life.
A potentially significant advance for investigation of rhythmogenic mechanisms is the ability to rapidly activate/inhibit functionally and/or molecularly defined neurons, perturbing the underlying microcircuits with single neuron and millisecond resolution. Optogenetic techniques partially achieve such control (Yizhar et al. 2011); however, axonal trafficking, an advantage for inter-regional circuit mapping (Petreanu et al. 2007), limits its utility in the brainstem where fibres of passage and somatodendritic compartments intermingle. An advanced optical technique, holographic photostimulation (Lutz et al. 2008; Zahid et al. 2010), permits simultaneous, spatiotemporally precise individual activation of small numbers of preBötC neurons (Kam et al. 2013b) that may mimic one way the network can be physiologically activated to trigger an inspiratory burst. Here, a laser beam is reflected off a spatial light modulator displaying an algorithmically generated hologram that produces the desired light pattern in the target plane, i.e. small (10 μm diameter) laser spots over designated (∼1–10) neuronal somas, that excite the target neuron by the highly localized release of caged glutamate without significant effects on surrounding neurons (Lutz et al. 2008; Zahid et al. 2010).
We utilized holographic photostimulation to address how preBötC network activity evolves from relative silence during the IBI to generate bursts by determining the threshold behaviour (de la Prida et al. 2006), i.e. the response of the network when the minimal number of neurons required to produce an inspiratory burst are activated. We found that simultaneous excitation of just 4–9 targeted preBötC inspiratory neurons during the IBI, ∼1% of the population, reliably initiates ectopic, network-wide bursts resulting in XIIn activity (Fig. 5A and B) that resemble endogenous bursts in amplitude, duration and shape (Fig. 5C; Kam et al. 2013b). Evoked XIIn bursts are substantially delayed relative to excitation onset, ∼255 ms (up to 500 ms; Fig. 5D), much longer than expected solely from oligosynaptic spread of activity (Kam et al. 2013b). This latency is dependent on the number of neurons excited (Fig. 5D). The relatively low threshold balances stability, buffering the network against spurious activation due to the non-synchronous activity of single neurons, with lability, permitting rapid changes in frequency or acute burst generation in response to environmental or metabolic changes, or cortical or other suprapontine input. As inspiratory-modulated activity was the only criterion for target neuron selection and with such a low threshold number for burst initiation, we consider it unlikely that the capacity for burst initiation is restricted to a specific subpopulation of preBötC neurons. Instead, we suggest that almost any excitatory, inspiratory-modulated preBötC neuron can participate in triggering bursts, which in network terms provides functional redundancy and robustness, particularly to modest neuron loss (Gray et al. 2001; McKay et al. 2005).
Figure 5. Activation of <10 inspiratory preBötC neurons during their silent phase can induce an inspiratory burst.
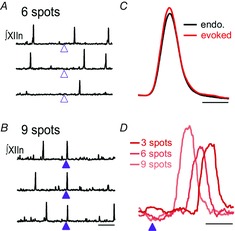
XIIn activity in 3 patterned photostimulation trials targeting 6 (A) or 9 (B) neurons. Laser stimulation produced (filled arrowheads) or failed to produce (open arrowheads) a burst within 1 s of the pulse. Scale bar, 5 s. C, evoked bursts (averaged, red) do not differ from endogenous (endo.) bursts (black). Scale bar, 0.2 s. D, evoked XIIn bursts as a function of number of spots (N) in a different experiment where threshold is 3 neurons reveal a substantial N-dependent delay between laser stimulation (filled arrowhead) and burst generation. Scale bar, 0.2 s. Adapted from Kam et al. (2013b).
We speculate that the similarities in latency to bursting onset (∼255 ms), and burstlet and pre-inspiratory (100–400 ms) duration, indicate a common mechanism (Kam et al. 2013a,b2013a). These data suggest a model whereby the rhythmogenic microcircuit, initially quiescent, yields to spontaneous activity (due to an as yet undetermined post-burstlet process) occurring in a small number of neurons that have convergent inputs to other preBötC neurons and fire APs in a cluster tight enough to evoke APs in their targets (see section ‘Burstlets, not bursts, are rhythmogenic’ above), leading to a slowly growing phenomenon that appears as a burstlet or pre-inspiratory activity. A simple model for many natural processes that describes phase transitions, such as the transition from quiescence to bursting, in networks is percolation (Suki et al. 2011). The initial spread of activity may therefore correspond to a percolation-like phenomenon, constrained by the long latency to bursting observed with threshold stimulation, which may illuminate preBötC dynamics through analogy with other physical systems (Breskin et al. 2006). Microcircuit properties, such as connectivity, that govern the hypothesized dynamic percolation of activity through preBötC neurons that generates burstlets remain to be determined.
After rhythm: generation of pattern by the respiratory CPG
While rhythmogenesis, which we postulate is expressed as burstlets, is the exclusive purview of the preBötC, preBötC bursts serve as only the first stage in a process that spans several regions in shaping the temporal pattern, strength and distribution of activity to other microcircuits and ultimately to motoneuron pools (Fig. 1A; Dobbins & Feldman, 1994, 1995; Koizumi et al. 2008; Kam et al. 2013a). The progression of this activity through even the few regions separating the preBötC from motor output involves the non-linear transformation of activity through neuronal populations (Dobbins & Feldman, 1994, 1995; Koizumi et al. 2008; Kam et al. 2013a). Little is known about whether there are specialized projection neurons in the preBötC, how these neurons converge or diverge onto premotoneurons, and how premotoneurons then transmit activity to motoneurons. Instead, we illustrate the complexity that can arise when activity is passed across microcircuits with three potential pitfalls: (1) rhythmogenic microcircuit activity may not be transmitted faithfully to the motor output; (2) motor output may be generated independently from the activity of the rhythmogenic microcircuit(s); and (3) microcircuit activity in vitro may not squarely map to behaviour in vivo.
In addition to a threshold for burst generation within the preBötC, a threshold for transmission of rhythmic activity probably mediated at multiple microcircuit levels, i.e. preBötC, premotoneuronal and motoneuronal, regulates the activity that reaches motor output (Dobbins & Feldman, 1994, 1995; Koizumi et al. 2008; Kam et al. 2013a). During normal breathing, every beat produces motor output; however, under lower excitability conditions, a failure in transmission of this timekeeping signal can occur when a preBötC burst is not triggered (Fig. 4). Raising excitability can lower this transmission threshold, permitting even smaller burstlet activity to appear in XIIn output in vitro or diaphragm activity and airflow in vivo (Kam et al. 2013a). Activity recorded in preBötC may therefore not result in motor output (Ramirez et al. 1996; Kam et al. 2013a). Conversely, motor output may not faithfully reflect activity occurring even two synapses upstream (Kam et al. 2013a).
Burst failure produces longer IBIs at integer multiples of the basic burstlet frequency, observed in the respiratory CPG as ‘quantal slowing’ in vitro (Mellen et al. 2003) and in vivo (Janczewski & Feldman, 2006) and, we speculate, in other CPGs as ‘deletions’ (Stein, 2005; Zhong et al. 2012). Occurrences of these skipped breaths may underlie the occasional prolonged absence of inspiration that defines intermittent apnoea, such as is characteristic of central sleep apnoea (Davis & O'Donnell, 2013). While ‘deletions’ are commonly interpreted as indicative of separate circuits or layers governing rhythm and pattern generation (Feldman, 1986; McCrea & Rybak, 2008), for breathing, distinct preBötC burstlet and I-burst-generating mechanisms (Fig. 3C) indicate that separable rhythm- and pattern-generating mechanisms may co-exist within a rhythmogenic microcircuit (Figs 1 and 4). In a more intact preparation, interactions with a second respiratory oscillator for ‘active expiration’ (Mellen et al. 2003; Janczewski & Feldman, 2006) can be another mechanism for skipped bursts.
The significance of ‘deletions’ deduced from discrepant activity between motor outputs implicitly assumes that all motor activity arises from the same underlying CPG (Mellen et al. 2003; Janczewski & Feldman, 2006; McCrea & Rybak, 2008). In embryonic or early neonatal in vitro preparations lacking more rostral brain areas or in transgenic mice with mutations that may affect other neural circuits (Gray et al. 2010; Tupal et al. a,b2014), nerve activity may be highly variable, and its provenance may be unclear. Activity in respiratory motor nerves may be driven by reflexes in vivo, e.g. during emesis or Valsalva manoeuvres, or by spinal locomotor circuits in wild-type rodents in vitro, and not by brainstem respiratory circuits (Viala et al. 1979; Schomburg et al. 2003; Bartlett & Leiter, 2012). Moreover, motoneurons themselves may burst spontaneously without premotor input, especially when neuromodulators are added or excitability is increased (Cifra et al. 2009; Manuel et al. 2012). Simultaneous recording or silencing of the preBötC or other oscillators can ensure that nerve activity is respiratory-related and preBötC in origin.
preBötC activity is not limited to producing normal breathing, and the preBötC appears to generate multiple burst types that underlie the distinct inspiratory airflow (and associated motor activity) of sighing, gasping, gagging, coughing, sneezing and sniffing (Bartlett & Leiter, 2012). When studied in vitro, preBötC burst types are sometimes mapped to in vivo behaviour based primarily on similarities in shape (Lieske et al. 2000). Assignment of a particular burst shape in even the most slightly altered or reduced preparation to a given function or behaviour in vivo can be problematic (Kam et al. 2013a). Sensory or neuromodulatory afferents and efferent patterning microcircuits can dramatically alter how breath types and preBötC burst types appear when inputs and outputs are removed in reduced preparations (Janczewski et al. 2013; Kam et al. 2013a).
As burst shape is variable and malleable and shape-based criteria for burst types are poorly constrained, differentiating between burst types across experiments, or even across conditions within the same experiment, is hardly unambiguous. Treating distinct burst types interchangeably (e.g. Rybak et al. 2004; Ruangkittisakul et al. 2008; Rose et al. 2009; Jasinski et al. 2013; Tupal et al. 2014a) may confound mechanistically distinct processes. Concomitant recordings of motor output, lacking in many studies (Lieske et al. 2000; Ruangkittisakul et al. 2008; Chapuis et al. 2014), would help determine the relationship between preBötC activity in vitro and motor output and airflow in vivo. For example, simultaneous recording of both preBötC and XIIn activity clearly delineates burstlets from bursts, and a distinct burst type, which we call doublets, closely resembles the double breaths that represent sighs in vagotomized animals (Kam et al. 2013a).
Beyond breathing: lessons for other neural microcircuits
Approaches for studying microcircuits were pioneered in invertebrates (see Getting, 1989; Marder & Calabrese, 1996; Selverston, 2010). The tremendous progress in elucidating specific network mechanisms in invertebrate CPGs has not, however, translated to a deep understanding of vertebrate, particularly mammalian, microcircuits or CPGs (Selverston, 2010). A major difference limiting the application of invertebrate mechanisms to mammalian microcircuits is the evolutionary increase in the number of neurons from invertebrate to mammalian CPGs (Katz et al. 2013). This expansion ultimately results in the dispersion of function and control from one or a few invertebrate neurons to tens or hundreds of mammalian neurons (Ainsworth et al. 2012), allowing for differential activity amongst these neurons and redundancy across neurons. With such networks, the vulnerability of the network to damage or disease is reduced, perhaps obviating the need for degenerate mechanisms to maintain robustness (Edelman & Gally, 2001; Marder & Taylor, 2011), while network lability is preserved. Where the invertebrate work continues to inform mammalian microcircuits, including respiratory microcircuits generating rhythm, is in relating biophysical detail to behaviour (Schulz et al. 2006), utilizing and testing models (Prinz et al. 2004; Nowotny et al. 2007), evaluating the role of neuromodulation (Marder et al. 2014), and confronting issues associated with biological complexity (Selverston, 2010; Marder & Taylor, 2011) that presage the challenges facing our efforts to understand the larger microcircuits found in mammals.
The CPG controlling breathing uniquely offers many of the advantages of invertebrate CPGs, i.e. smaller size, complete regional identification, well-defined function, in vitro accessibility, and behavioural simplicity, while retaining properties specific to mammalian brain. We hope we have made clear that understanding the neural control of breathing is still a tough problem. Nonetheless, the respiratory CPG remains a rich and inimitably tractable model system for establishing general approaches for understanding complex, emergent mechanisms in mammalian neural microcircuits.
One need not look far to appreciate the importance of respiratory rhythmogenesis for understanding other microcircuits and behaviours. As a continuous behaviour, breathing is constantly modulated, interacting with and sometimes controlling other behaviours, including respiratory-related behaviours, e.g. sniffing or coughing, orofacial movements, e.g. chewing, whisking or suckling, emotional behaviours, e.g. crying or sighing, and volitional movements, e.g. vocalization (Heywood et al. 1996; Bartlett & Leiter, 2012; Feldman et al. 2013; Moore et al. 2013, 2014). How these and other microcircuits interact depends critically on their interconnectivity and other details and properties described above, elaborating upon the basic emergent mechanisms suggested here for the preBötC.
The effort to elucidate rhythmogenic mechanisms in the preBötC may also inform the study of other rhythmic microcircuits, including locomotor CPGs (Grillner & Jessell, 2009) and cortical, thalamic and hippocampal microcircuits generating physiological and pathophysiological rhythmic activity (Buzsaki, 2006). The different mechanisms proposed for these various rhythmic microcircuits demonstrate that the same neural computation, namely rhythm generation, can be implemented in a number of ways (Buzsaki, 2006). However, issues common to rhythmic mammalian microcircuits can, nonetheless, be identified. How is activity synchronized across a population? How does the degree of connectivity affect rhythmogenesis? How do changes in drive affect frequency? Does rhythmic activity remain intrinsic to the microcircuit, serving as background activity to support microcircuit function or is the rhythmic activity transmitted to other microcircuits?
Finally, for these and other mammalian microcircuits, caveat experimentor. As with the respiratory CPG, axiomatic mechanisms derived from apparently parsimonious, heuristic, or phenomenological understandings of microcircuits may not withstand rigorous experimental testing and commonly abstracted biological details may in fact be consequential for a mechanistic understanding of the microcircuit (Kumar et al. 2013; Mitra, 2014). The lesson here is that defining microcircuit boundaries and functionality (Horton & Adams, 2005; Briggman & Kristan, 2008), classifying neuronal populations (Sharpee, 2014), comparing results across experimental conditions and preparations, deducing dynamics from connectivity (Kopell et al. 2014), and extracting how the microcircuit transforms the spatiotemporal activity patterns of physiological input into output appropriate for downstream microcircuits during behaviour are far from clear cut or straightforward.
Conclusion
Understanding neural circuits constitutes the next great challenge in basic neuroscience (BRAIN Working Group, 2013), and many novel concepts and principles related to mammalian microcircuit function remain to be discovered. For most behaviours, the lack of understanding at the microcircuit level is a roadblock for determining mechanisms by which genes, molecules, synapses and individual neurons initiate, produce, modulate and integrate behaviour. Microcircuits underlying respiratory rhythm generation offer an opportunity to bridge the gap between the dynamics of a mammalian behaviour and biological mechanisms that span the scale from protein expression to synapses, intrinsic neuronal properties to connectivity in populations, and microcircuits to behaviour.
Acknowledgments
The authors would like to thank Drs P. A. Gray, C. A. Del Negro and J. W. Worrell, as well as members of the Feldman laboratory, particularly Drs R. T. R. Huckstepp and C. F. Yang, for critical readings of the manuscript and helpful discussion.
Glossary
- AP
action potential
- BötC
Bötzinger complex
- CPG
central pattern generator
- Dbx1
developing brain homeobox 1
- EB
endogenous bursting/burster
- IBI
interburst interval
- I-burst
inspiratory burst
- ICAN
Ca2+-activated non-specific cation current
- INaP
persistent Na+ current
- NK1R
neurokinin 1 receptor
- preBötC
preBötzinger Complex
- SST
somatostatin
- VRC
ventral respiratory column
- XII
hypoglossal
- XIIn
hypoglossal nerve
Additional information
Competing interests
The authors declare that neither of the authors has any conflicts of interest.
Funding
This work was supported by NIH grant HL40959.
References
- Abbott LF. Theoretical neuroscience rising. Neuron. 2008;60:489–495. doi: 10.1016/j.neuron.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Ainsworth M, Lee S, Cunningham MO, Traub RD, Kopell NJ. Whittington MA. Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron. 2012;75:572–583. doi: 10.1016/j.neuron.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C. Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U. Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjalm G, Imsland F, Petersen JL, McCue ME, Mickelson JR, Cothran G, Ahituv N, Roepstorff L, Mikko S, Vallstedt A, Lindgren G, Andersson L. Kullander K. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann LC, Matis A, Lindau NT, Felder P, Gullo M. Schwab ME. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci Transl Med. 2013;5:208ra146. doi: 10.1126/scitranslmed.3005972. [DOI] [PubMed] [Google Scholar]
- Ballantyne D. Richter DW. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D., Jr Leiter JC. Coordination of breathing with nonrespiratory activities. Compr Physiol. 2012;2:1387–1415. doi: 10.1002/cphy.c110004. [DOI] [PubMed] [Google Scholar]
- Bautista TG. Dutschmann M. Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J Physiol. 2014;592:2605–2623. doi: 10.1113/jphysiol.2014.272468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Leme AS, Paigen B, Shapiro SD. Svenson KL. Unrestrained plethysmograph and anesthetized forced oscillation methods of measuring lung function in 29 inbred strains of mice. MPD:Berndt2. Mouse Phenome Database web site, The Jackson Laboratory, Bar Harbor, Maine USA. 2014. & http://phenome.jax.org [Cited 07 Oct, 2014]
- Bianchi AL, Denavit-Saubié M. Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Autran S, Dehorter N, Katz DM, Champagnat J, Fortin G. Thoby-Brisson M. Brain-derived neurotrophic factor enhances fetal respiratory rhythm frequency in the mouse preBötzinger complex in vitro. Eur J Neurosci. 2008;28:510–520. doi: 10.1111/j.1460-9568.2008.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A. Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Honjo H. Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Bradley GW, von Euler C, Marttila I. Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biol Cybern. 1975;19:105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- BRAIN Working Group. Interim report. Brain Research through Advancing Innovative Neurotechnologies (BRAIN) web site, National Institutes of Health, Bethesda, MD, USA. 2013. http://www.braininitiative.nih.gov [Cited 07 Oct, 2014]
- Breskin I, Soriano J, Moses E. Tlusty T. Percolation in living neural networks. Phys Rev Lett. 2006;97:188102. doi: 10.1103/PhysRevLett.97.188102. [DOI] [PubMed] [Google Scholar]
- Briggman KL. Kristan WB. Multifunctional pattern-generating circuits. Annu Rev Neurosci. 2008;31:271–294. doi: 10.1146/annurev.neuro.31.060407.125552. [DOI] [PubMed] [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol. 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Paton JF. Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J. Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol. 1999;82:398–415. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- Calfa G, Percy AK. Pozzo-Miller L. Experimental models of Rett syndrome based on Mecp2 dysfunction. Exp Biol Med (Maywood) 2011;236:3–19. doi: 10.1258/ebm.2010.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M. From circuits to behavior: a bridge too far. Nat Neurosci. 2012;15:507–509. doi: 10.1038/nn.3043. [DOI] [PubMed] [Google Scholar]
- Carr MF. Frank LM. A single microcircuit with multiple functions: state dependent information processing in the hippocampus. Curr Opin Neurobiol. 2012;22:704–708. doi: 10.1016/j.conb.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MS. Ramirez JM. Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J Neurophysiol. 2013;109:296–305. doi: 10.1152/jn.00830.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MS, Viemari JC. Ramirez JM. Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J Neurophysiol. 2013;109:285–295. doi: 10.1152/jn.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis C, Autran S, Fortin G, Simmers J. Thoby-Brisson M. Emergence of sigh rhythmogenesis in the embryonic mouse. J Physiol. 2014;592:2169–2181. doi: 10.1113/jphysiol.2013.268730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifra A, Nani F, Sharifullina E. Nistri A. A repertoire of rhythmic bursting produced by hypoglossal motoneurons in physiological and pathological conditions. Philos Trans R Soc Lond B Biol Sci. 2009;364:2493–2500. doi: 10.1098/rstb.2009.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FJ. von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EM. O'Donnell CP. Rodent models of sleep apnea. Respir Physiol Neurobiol. 2013;188:355–361. doi: 10.1016/j.resp.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R. Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Prida LM, Huberfeld G, Cohen I. Miles R. Threshold behavior in the initiation of hippocampal population bursts. Neuron. 2006;49:131–142. doi: 10.1016/j.neuron.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Johnson SM, Butera RJ. Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. III. Experimental tests of model predictions. J Neurophysiol. 2001;86:59–74. doi: 10.1152/jn.2001.86.1.59. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Kam K, Hayes JA. Feldman JL. Asymmetric control of inspiratory and expiratory phases by excitability in the respiratory network of neonatal mice in vitro. J Physiol. 2009;587:1217–1231. doi: 10.1113/jphysiol.2008.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ., Jr Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002a;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C. Feldman JL. Respiratory rhythm: an emergent network property. Neuron. 2002b;34:821–830. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA. Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Wilson CG, Butera RJ, Rigatto H. Smith JCc. Periodicity, mixed-mode oscillations, and quasiperiodicity in a rhythm-generating neural network. Biophys J. 2002;82:206–214. doi: 10.1016/S0006-3495(02)75387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG. Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dobbins EG. Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF. Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM. Gally JA. Degeneracy and complexity in biological systems. Proc Natl Acad Sci U S A. 2001;98:13763–13768. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K. Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Feldman JL. A network model for control of inspiratory cutoff by the pneumotaxic center with supportive experimental data in cats. Biol Cybern. 1976;21:131–138. doi: 10.1007/BF00337420. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology, Section I, The Nervous System, vol. 4, Intrinsic Regulatory Systems of the Brain. Bethesda, MD: American Physiological Society; 1986. pp. 463–524. [Google Scholar]
- Feldman JL, Connelly CA, Ellenberger HH. Smith JC. The cardiorespiratory system within the brainstem. Eur J Neurosci. 1990;3:171. (Suppl), [Google Scholar]
- Feldman JL. Cowan JD. Large-scale activity in neural nets II: A model for the brainstem respiratory oscillator. Biol Cybern. 1975;17:39–51. doi: 10.1007/BF00326708. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL. Grillner S. Control of vertebrate respiration and locomotion: a brief account. Physiologist. 1983;26:310–316. [PubMed] [Google Scholar]
- Feldman JL, Kam K. Janczewski WA. Practice makes perfect, even for breathing. Nat Neurosci. 2009;12:961–963. doi: 10.1038/nn0809-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL. Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann N Y Acad Sci. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Feldt S, Bonifazi P. Cossart R. Dissecting functional connectivity of neuronal microcircuits: experimental and theoretical insights. Trends Neurosci. 2011;34:225–236. doi: 10.1016/j.tins.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Fitzhugh R. Mathematical models of threshold phenomena in the nerve membrane. Bull Math Biol. 1955;17:257–278. [Google Scholar]
- Forster HV, Haouzi P. Dempsey JA. Control of breathing during exercise. Compr Physiol. 2012;2:743–777. doi: 10.1002/cphy.c100045. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Gähwiler BH. Synaptic organization of intracellularly stained CA3 pyramidal neurons in slice cultures of rat hippocampus. Neuroscience. 1988;24:541–551. doi: 10.1016/0306-4522(88)90348-x. [DOI] [PubMed] [Google Scholar]
- Gallego J. Genetic diseases: congenital central hypoventilation, Rett, and Prader-Willi syndromes. Compr Physiol. 2012;2:2255–2279. doi: 10.1002/cphy.c100037. [DOI] [PubMed] [Google Scholar]
- Garcia-Campmany L, Stam FJ. Goulding M. From circuits to behaviour: motor networks in vertebrates. Curr Opin Neurobiol. 2010;20:116–125. doi: 10.1016/j.conb.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Golowasch J, Goldman MS, Abbott LF. Marder E. Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol. 2002;87:1129–1131. doi: 10.1152/jn.00412.2001. [DOI] [PubMed] [Google Scholar]
- Gray PA. Transcription factors define the neuroanatomical organization of the medullary reticular formation. Front Neuroanat. 2013;7:7. doi: 10.3389/fnana.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J. Del Negro CA. Developmental origin of preBötzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR. Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM. Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ. Control of breathing activity in the fetus and newborn. Compr Physiol. 2012;2:1873–1888. doi: 10.1002/cphy.c110006. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC. Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grillner S. Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Markram H, De Schutter E, Silberberg G. LeBeau FE. Microcircuits in action–from CPGs to neocortex. Trends Neurosci. 2005;28:525–533. doi: 10.1016/j.tins.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG. Abbott SB. C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–R204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartelt N, Skorova E, Manzke T, Suhr M, Mironova L, Kugler S. Mironov SL. Imaging of respiratory network topology in living brainstem slices. Mol Cell Neurosci. 2008;37:425–431. doi: 10.1016/j.mcn.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Hayashi F. Lipski J. The role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respir Physiol. 1992;89:47–63. doi: 10.1016/0034-5687(92)90070-d. [DOI] [PubMed] [Google Scholar]
- Hayes JA. Del Negro CA. Neurokinin receptor-expressing pre-Bötzinger complex neurons in neonatal mice studied in vitro. J Neurophysiol. 2007;97:4215–4224. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Wang X. Del Negro CA. Cumulative lesioning of respiratory interneurons disrupts and precludes motor rhythms in vitro. Proc Natl Acad Sci USA. 2012;109:8286–8291. doi: 10.1073/pnas.1200912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood P, Murphy K, Corfield DR, Morrell MJ, Howard RS. Guz A. Control of breathing in man; insights from the ‘locked-in’ syndrome. Respir Physiol. 1996;106:13–20. doi: 10.1016/0034-5687(96)00060-6. [DOI] [PubMed] [Google Scholar]
- Horton JC. Adams DL. The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci. 2005;360:837–862. doi: 10.1098/rstb.2005.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ. Toward a genetic dissection of cortical circuits in the mouse. Neuron. 2014;83:1284–1302. doi: 10.1016/j.neuron.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ. Zeng H. Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT. Dale N. Redefining the components of central CO2 chemosensitivity – towards a better understanding of mechanism. J Physiol. 2011;589:5561–5579. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA. Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y. Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski PE, Molkov YI, Shevtsova NA, Smith JC. Rybak IA. Sodium and calcium mechanisms of rhythmic bursting in excitatory neural networks of the pre-Bötzinger complex: a computational modelling study. Eur J Neurosci. 2013;37:212–230. doi: 10.1111/ejn.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S. Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3:141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N. Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Bötzinger complex ‘island’. J Neurophysiol. 2001;85:1772–1776. doi: 10.1152/jn.2001.85.4.1772. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk GD. Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Kam K, Worrell JW, Janczewski WA, Cui Y. Feldman Jl. Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci. 2013a;33:9235–9245. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K, Worrell JW, Ventalon C, Emiliani V. Feldman JL. Emergence of population bursts from simultaneous activation of small subsets of preBötzinger complex inspiratory neurons. J Neurosci. 2013b;33:3332–3338. doi: 10.1523/JNEUROSCI.4574-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P, Grillner S, Wilson R, Borst A, Greenspan R, Buzsáki G, Martin K, Marder E, Kristan W, Friedrich R. Chklovskii D. Vertebrate versus invertebrate neural circuits. Curr Biol. 2013;23:R504–506. doi: 10.1016/j.cub.2013.05.039. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol. 2011;21:100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Bharioke A, Blinder P, Bock DD, Briggman KL, Chklovskii DB, Denk W, Helmstaedter M, Kaufhold JP, Lee WC, Meyer HS, Micheva KD, Oberlaender M, Prohaska S, Reid RC, Smith SJ, Takemura S, Tsai PS. Sakmann B. Large-scale automated histology in the pursuit of connectomes. J Neurosci. 2011;31:16125–16138. doi: 10.1523/JNEUROSCI.4077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Lemke RP. Greer JJ. Ultrasound measurements of fetal breathing movements in the rat. J Appl Physiol (1985) 2001;91:316–320. doi: 10.1152/jappl.2001.91.1.316. [DOI] [PubMed] [Google Scholar]
- Koch C. Systems biology. Modular biological complexity. Science. 2012;337:531–532. doi: 10.1126/science.1218616. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Koshiya N, Chia JX, Cao F, Nugent J, Zhang R. Smith JC. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Bötzinger complex respiratory microcircuits. J Neurosci. 2013;33:2994–3009. doi: 10.1523/JNEUROSCI.4427-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N. Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell NJ, Gritton HJ, Whittington MA. Kramer MA. Beyond the connectome: The dynome. Neuron. 2014;83:1319–1328. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Vlachos I, Aertsen A. Boucsein C. Challenges of understanding brain function by selective modulation of neuronal subpopulations. Trends Neurosci. 2013;36:579–586. doi: 10.1016/j.tins.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Kuwana S, Tsunekawa N, Yanagawa Y, Okada Y, Kuribayashi J. Obata K. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Bötzinger complex. Eur J Neurosci. 2006;23:667–674. doi: 10.1111/j.1460-9568.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM. Matturri L. Functional neuroanatomy of the human pre-Bötzinger complex with particular reference to sudden unexplained perinatal and infant death. Neuropathology. 2008;28:10–16. doi: 10.1111/j.1440-1789.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- Lawson EE, Richter DW, Czyzyk-Krzeska MF, Bischoff A. Rudesill RC. Respiratory neuronal activity during apnea and other breathing patterns induced by laryngeal stimulation. J Appl Physiol (1985) 1991;70:2742–2749. doi: 10.1152/jappl.1991.70.6.2742. [DOI] [PubMed] [Google Scholar]
- Lewicki MS. A review of methods for spike sorting: the detection and classification of neural action potentials. Network. 1998;9:R53–78. [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P. Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Rybak IA. Smith JC. Computational models and emergent properties of respiratory neural networks. Compr Physiol. 2012;2:1619–1670. doi: 10.1002/cphy.c110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Otis TS, DeSars V, Charpak S, DiGregorio DA. Emiliani V. Holographic photolysis of caged neurotransmitters. Nat Methods. 2008;5:821–827. doi: 10.1038/nmeth.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA. Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Feldman JL. Speck DF. Respiratory motoneuronal activity is altered by injections of picomoles of glutamate into cat brain-stem. J Neurosci. 1986;6:2384–2392. doi: 10.1523/JNEUROSCI.06-08-02384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA. Feldman JL. Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Li Y, Elbasiouny SM, Murray K, Griener A, Heckman CJ. Bennett DJ. NMDA induces persistent inward and outward currents that cause rhythmic bursting in adult rodent motoneurons. J Neurophysiol. 2012;108:2991–2998. doi: 10.1152/jn.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Marder E, O'Leary T. Shruti S. Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci. 2014;37:329–346. doi: 10.1146/annurev-neuro-071013-013958. [DOI] [PubMed] [Google Scholar]
- Marder E. Taylor AL. Multiple models to capture the variability in biological neurons and networks. Nat Neurosci. 2011;14:133–138. doi: 10.1038/nn.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H. The blue brain project. Nat Rev Neurosci. 2006;7:153–160. doi: 10.1038/nrn1848. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G. Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mellen NM. Belt-and-suspenders as a biological design principle. Adv Exp Med Biol. 2008;605:99–103. doi: 10.1007/978-0-387-73693-8_17. [DOI] [PubMed] [Google Scholar]
- Mellen NM. Degeneracy as a substrate for respiratory regulation. Respir Physiol Neurobiol. 2010;172:1–7. doi: 10.1016/j.resp.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM. Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM. Mishra D. Functional anatomical evidence for respiratory rhythmogenic function of endogenous bursters in rat medulla. J Neurosci. 2010;30:8383–8392. doi: 10.1523/JNEUROSCI.5510-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom WK. Adaptive trends in respiratory control: a comparative perspective. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1–R10. doi: 10.1152/ajpregu.00069.2010. [DOI] [PubMed] [Google Scholar]
- Mitra PP. The circuit architecture of whole brains at the mesoscopic scale. Neuron. 2014;83:1273–1283. doi: 10.1016/j.neuron.2014.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfredi O, Dobrzynski H, Mondal T, Boyett MR. Morris GM. The anatomy and physiology of the sinoatrial node–a contemporary review. Pacing Clin Electrophysiol. 2010;33:1392–1406. doi: 10.1111/j.1540-8159.2010.02838.x. [DOI] [PubMed] [Google Scholar]
- Monnier A, Alheid GF. McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G. Horner RL. State-dependent contribution of the hyperpolarization-activated Na+/K+ and persistent Na+ currents to respiratory rhythmogenesis in vivo. J Neurosci. 2013;33:8716–8728. doi: 10.1523/JNEUROSCI.5066-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Deschenes M, Furuta T, Huber D, Smear MC, Demers M. Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature. 2013;497:205–210. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Kleinfeld D. Wang F. How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci. 2014;37:370–380. doi: 10.1016/j.tins.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Baca SM. Feldman JL. Glycinergic pacemaker neurons in preBötzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL. Lichtman JW. Why not connectomics. Nat Methods. 2013;10:494–500. doi: 10.1038/nmeth.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP. Limoges MJ. Resting breathing frequency in aquatic mammals: a comparative analysis with terrestrial species. Respir Physiol Neurobiol. 2006;154:500–514. doi: 10.1016/j.resp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Nowotny T, Szucs A, Levi R. Selverston AI. Models wagging the dog: are circuits constructed with disparate parameters. Neural Comput. 2007;19:1985–2003. doi: 10.1162/neco.2007.19.8.1985. [DOI] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR. Zeng H. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis AB, Fenn WO. Rahn H. Mechanics of breathing in man. J Appl Physiol. 1950;2:592–607. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL. Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007a;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL. Del Negro CA. Role of persistent sodium current in mouse preBötzinger complex neurons and respiratory rhythm generation. J Physiol. 2007b;580:485–496. doi: 10.1113/jphysiol.2006.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathmanathan P. Gray RA. Verification of computational models of cardiac electro-physiology. Int J Numer Method Biomed Eng. 2014;30:525–544. doi: 10.1002/cnm.2615. [DOI] [PubMed] [Google Scholar]
- Paton JF. A working heart–brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JF. Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. J Physiol. 1995;484:505–521. doi: 10.1113/jphysiol.1995.sp020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A. Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Picardo MC, Weragalaarachchi KT, Akins VT. Del Negro CA. Physiological and morphological properties of Dbx1-derived respiratory neurons in the pre-Bötzinger complex of neonatal mice. J Physiol. 2013;591:2687–2703. doi: 10.1113/jphysiol.2012.250118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Bucher D. Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Purvis LK, Smith JC, Koizumi H. Butera RJ. Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. J Neurophysiol. 2007;97:1515–1526. doi: 10.1152/jn.00908.2006. [DOI] [PubMed] [Google Scholar]
- Rahman J, Latal AT, Besser S, Hirrlinger J. Hulsmann S. Mixed miniature postsynaptic currents resulting from co-release of glycine and GABA recorded from glycinergic neurons in the neonatal respiratory network. Eur J Neurosci. 2013;37:1229–1241. doi: 10.1111/ejn.12136. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ. Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol. 1996;491:799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ. Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respir Physiol. 1997;110:71–85. doi: 10.1016/s0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histology of the Nervous System of Man and Vertebrates. New York: Oxford University Press; 1904. [Google Scholar]
- Ratte S, Hong S, De Schutter E. Prescott SA. Impact of neuronal properties on network coding: roles of spike initiation dynamics and robust synchrony transfer. Neuron. 2013;78:758–772. doi: 10.1016/j.neuron.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling J, Champagnat J. Denavit-Saubié M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brainstem in vitro. J Neurophysiol. 1996;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Rekling JC. Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Shao XM. Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger complex. J Neurosci. 2000;20:RC113. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Richter DW. Windhorst U. Neural regulation of respiration: rhythmogenesis and afferent control. In: Gregor R, editor. Comprehensive Human Physiology. Berlin: Springer-Verlag; 1996. pp. 2079–2095. [Google Scholar]
- Richter DW. Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 2014;29:58–71. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MF, Ren J, Ahmad KA, Chao HT, Klisch TJ, Flora A. Greer JJ. Zoghbi HY. Math1 is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron. 2009;64:341–354. doi: 10.1016/j.neuron.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Kottick A, Picardo MC, Ballanyi K. Del Negro CA. Identification of the pre-Bötzinger complex inspiratory center in calibrated ‘sandwich’ slices from newborn mice with fluorescent Dbx1 interneurons. Physiol Rep. 2014;2:e12111. doi: 10.14814/phy2.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Panaitescu B. Ballanyi K. K+ and Ca2+ dependence of inspiratory-related rhythm in novel ‘calibrated’ mouse brainstem slices. Respir Physiol Neurobiol. 2011;175:37–48. doi: 10.1016/j.resp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD. Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci. 2008;28:2447–2458. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD. Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL. Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci USA. 2009;106:2939–2944. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio JE. A new mathematical model of the respiratory center. Bull Math Biophys. 1972;34:467–481. doi: 10.1007/BF02476709. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Ptak K, Shevtsova NA. McCrimmon DR. Sodium currents in neurons from the rostroventrolateral medulla of the rat. J Neurophysiol. 2003;90:1635–1642. doi: 10.1152/jn.00150.2003. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Ptak K. McCrimmon DR. Intrinsic bursting activity in the pre-Bötzinger complex: role of persistent sodium and potassium currents. Biol Cybern. 2004;90:59–74. doi: 10.1007/s00422-003-0447-1. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ezure K. Tanaka I. Swallowing-related activities of respiratory and non-respiratory neurons in the nucleus of solitary tract in the rat. J Physiol. 2002;540:1047–1060. doi: 10.1113/jphysiol.2001.014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H. Dembowsky K. Rhythmic phrenic, intercostal and sympathetic activity in relation to limb and trunk motor activity in spinal cats. Neurosci Res. 2003;46:229–240. doi: 10.1016/s0168-0102(03)00062-2. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM. Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci. 2006;9:356–362. doi: 10.1038/nn1639. [DOI] [PubMed] [Google Scholar]
- Schwab DJ, Bruinsma RF, Feldman JL. Levine AJ. Rhythmogenic neuronal networks, emergent leaders, and k-cores. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;82:051911. doi: 10.1103/PhysRevE.82.051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzacher SW, Rub U. Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134:24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC. Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Wilhelm Z, Anders K. Richter DW. The medullary respiratory network in the rat. J Physiol. 1991;435:631–644. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindel CD, Ali K, McNaughton BL. Tatsuno M. Long-term recordings improve the detection of weak excitatory-excitatory connections in rat prefrontal cortex. J Neurosci. 2014;34:5454–5467. doi: 10.1523/JNEUROSCI.4350-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF. Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol. 2008;100:1749–1769. doi: 10.1152/jn.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston AI. Invertebrate central pattern generator circuits. Philos Trans R Soc Lond B Biol Sci. 2010;365:2329–2345. doi: 10.1098/rstb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung HS. Sumbul U. Neuronal cell types and connectivity: lessons from the retina. Neuron. 2014;83:1262–1272. doi: 10.1016/j.neuron.2014.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah EM. Jay DG. Methods for ablating neurons. Curr Opin Neurobiol. 1993;3:738–742. doi: 10.1016/0959-4388(93)90146-p. [DOI] [PubMed] [Google Scholar]
- Shao XM. Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Shao J, Tsao TH. Butera R. Bursting without slow kinetics: a role for a small world. Neural Comput. 2006;18:2029–2035. doi: 10.1162/neco.2006.18.9.2029. [DOI] [PubMed] [Google Scholar]
- Sharpee TO. Toward functional classification of neuronal types. Neuron. 2014;83:1329–1334. doi: 10.1016/j.neuron.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A. Dynamical systems theory in physiology. J Gen Physiol. 2011;138:13–19. doi: 10.1085/jgp.201110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA. Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW. Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC. Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu GS. Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Song S, Sjöström PJ, Reigl M, Nelson S. Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M. Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stein PS. Neuronal control of turtle hindlimb motor rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:213–229. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC. Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Stuth EA, Stucke AG. Zuperku EJ. Effects of anesthetics, sedatives, and opioids on ventilatory control. Compr Physiol. 2012;2:2281–2367. doi: 10.1002/cphy.c100061. [DOI] [PubMed] [Google Scholar]
- Suki B, Bates JH. Frey U. Complexity and emergent phenomena. Compr Physiol. 2011;1:995–1029. doi: 10.1002/cphy.c100022. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Goodchild AK, Chalmers JP. Pilowsky PM. The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A. Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature. 2013;500:85–88. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM. Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Sherman D, Turesson J, Shao XM, Janczewski WA. Feldman JL. Reelin demarcates a subset of pre-Bötzinger complex neurons in adult rat. J Comp Neurol. 2012;520:606–619. doi: 10.1002/cne.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Cauli B, Champagnat J, Fortin G. Katz DM. Expression of functional tyrosine kinase B receptors by rhythmically active respiratory neurons in the pre-Bötzinger complex of neonatal mice. J Neurosci. 2003;23:7685–7689. doi: 10.1523/JNEUROSCI.23-20-07685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J. Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M. Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Tupal S, Huang WH, Picardo MC, Ling GY, Del Negro CA, Zoghbi HY. Gray PA. Atoh1-dependent rhombic lip neurons are required for temporal delay between independent respiratory oscillators in embryonic mice. Elife. 2014;3:e02265. doi: 10.7554/eLife.02265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Rieger MA, Ling GY, Park TJ, Dougherty JD, Goodchild AK. Gray PA. Testing the role of preBötzinger complex somatostatin neurons in respiratory and vocal behaviors. Eur J Neurosci. 2014;40:3067–3077. doi: 10.1111/ejn.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmianski I, Shih AY, Driscoll JD, Matthews DW, Freund Y. Kleinfeld D. Automatic identification of fluorescently labeled brain cells for rapid functional imaging. J Neurophysiol. 2010;104:1803–1811. doi: 10.1152/jn.00484.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viala D, Vidal C. Freton E. Coordinated rhythmic bursting in respiratory and locomotor muscle nerves in the spinal rabbit. Neurosci Lett. 1979;11:155–159. doi: 10.1016/0304-3940(79)90119-8. [DOI] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygard A, Enjin A, Fujiyama F, Fortin G. Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hayes JA, Picardo MC. Del Negro CA. Automated cell-specific laser detection and ablation of neural circuits in neonatal brain tissue. J Physiol. 2013;591:2393–2401. doi: 10.1113/jphysiol.2012.247338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hayes JA, Revill AL, Song H, Kottick A, Vann NC, LaMar MD, Picardo MC, Akins VT, Funk GD. Del Negro CA. Laser ablation of Dbx1 neurons in the pre-Bötzinger complex stops inspiratory rhythm and impairs output in neonatal mice. Elife. 2014;3:e03427. doi: 10.7554/eLife.03427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I. Rand CM. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008;164:38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME. Marazita ML. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet A. 2003;123A:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- Wei XY, Zhao Y, Wong-Riley MT, Ju G. Liu YY. Synaptic relationship between somatostatin- and neurokinin-1 receptor-immunoreactive neurons in the pre-Bötzinger complex of rats. J Neurochem. 2012;122:923–933. doi: 10.1111/j.1471-4159.2012.07862.x. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Qu Z. Garfinkel A. Understanding biological complexity: lessons from the past. FASEB J. 2003;17:1–6. doi: 10.1096/fj.02-0408rev. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S. Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol (1985) 2004;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J. Hulsmann S. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458:459–469. doi: 10.1007/s00424-009-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M. Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zahid M, Velez-Fort M, Papagiakoumou E, Ventalon C, Angulo MC. Emiliani V. Holographic photolysis for multiple cell stimulation in mouse hippocampal slices. PLoS One. 2010;5:e9431. doi: 10.1371/journal.pone.0009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Shevtsova NA, Rybak IA. Harris-Warrick RM. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol. 2012;590:4735–4759. doi: 10.1113/jphysiol.2012.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J. Gahwiler BH. Cellular and connective organization of slice cultures of the rat hippocampus and fascia dentata. J Comp Neurol. 1984;228:432–446. doi: 10.1002/cne.902280310. [DOI] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Sykova E, Agnati LF. Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]


