Abstract
Glutamate receptors are ligand-gated ion channels that mediate fast excitatory synaptic transmission throughout the central nervous system. Functional receptors are homo- or heteromeric tetramers with each subunit contributing a re-entrant pore loop that dips into the membrane from the cytoplasmic side. The pore loops form a narrow constriction near their apex with a wide vestibule toward the cytoplasm and an aqueous central cavity facing the extracellular solution. This article focuses on the pore region, reviewing how structural differences among glutamate receptor subtypes determine their distinct functional properties.
Ionotropic glutamate receptors (iGluRs) are members of the pore loop superfamily of ion channels that also includes potassium channels, voltage-gated sodium and calcium channels, cyclic nucleotide gated (CNG) channels and transient receptor potential (TRP) channels (Hille, 2001). In contrast to most members of this family, in iGluRs the re-entrant pore loops dip into the plane of the membrane from the cytoplasmic side, while the central cavity and the inner helix bundle crossing, thought to form the gate for ion flow, face the extracellular side (Sobolevsky et al. 2009). Each iGluR subunit contributes exclusively to one of three distinct iGluR subtypes that are named for the agonists N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate (KA) (Hollmann & Heinemann, 1994). These subtypes serve distinct roles at excitatory synapses and aberrant activation of each has been implicated in pathologies of the nervous system (Traynelis et al. 2010).
Functional AMPA and KA receptors may be homo- or heteromeric tetramers, whereas NMDA receptors are obligate heteromers, requiring a GluN1 subunit combined with either a GluN2 or GluN3 subunit (Traynelis et al. 2010). Figure 1 displays a protein alignment for the transmembrane domain (TMD) of the four AMPA receptor subunits (GluA1–A4), five KA receptor subunits (GluK1-K5) and seven NMDA receptor subunits (GluN1, GluN2A-2D, GluN3A-3B) from rat. Each TMD comprises one pore loop (M2) that includes a short helix and random coil selectivity filter flanked by outer (M1) and inner (M3) transmembrane helices (Fig. 1A and C; Fig. 2A–C; Sobolevsky et al. 2009). All eukaryotic iGluR subunits also include an additional (M4) transmembrane helix (Fig. 1B and C; Fig. 2A–C) that is required for receptor assembly and function (Schorge & Colquhoun, 2003; Terhag et al. 2010; Salussolia et al. 2011, 2013). Opening of the pore is controlled by four extracellular ligand binding domains (LBD), with each LBD connected to the transmembrane helices by three short random coil linkers (Sobolevsky et al. 2009).
Figure 1. Sequence alignment of glutamate receptor TMDs.
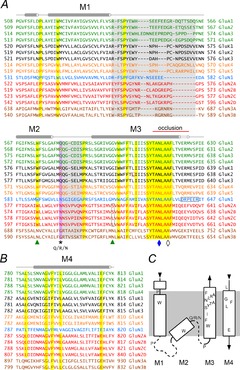
A, alignment of the M1–M3 segment for the four AMPA receptor subunits (green, UniProt numbers: P19490–19493), three low affinity KA receptor subunits capable of forming homomeric channels (black, P22756, P42260, P42264), two high affinity KA receptor subunits that do not form functional homomers (orange, Q01812, Q63273), the GluN1 subunit essential for all NMDA receptors (blue, P35439), four GluN2 subunits (red, Q00959–009561, Q62645) and two GluN3 subunits (brown, Q9R1M7, Q8VHN2). All sequences are from rat. Highly conserved residues are highlighted in yellow. Sequence that was not resolved in the GluA2 crystal structure is highlighted in grey. The asterisk indicates the Q/R/N site. GluA2, GluK1 and 2 are shown in the edited (R) form (red boxes). Green triangles indicate the M3 S/L site of GluN2 and the conserved M2 Trp of GluN1 that interact (green boxes). The GluN1 DRPEER motif is boxed in black. The conserved Asn (N5) and Lurcher site Ala (A8) in the SYTANLAAF motif are indicated by a filled blue diamond and open black diamond, respectively. Residue numbers in A and B are for the mature proteins after signal sequence removal. Secondary structure is shown as cylinders (α-helices) and lines (random coil loops) above the alignment. The red line above M3 indicates the occlusion at the inner helix bundle crossing in the GluA2 crystal structure. B, alignment of the M4 helix. C, diagram of TMD topology displaying the approximate position of the conserved residues highlighted in yellow in A and B. Dashed lines indicate segments that were not resolved in the GluA2 crystal structure.
Figure 2. TMD structure.
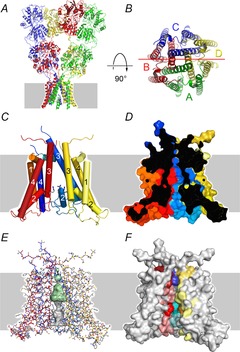
A, X-ray crystal structure of the homomeric GluA2 AMPA receptor (PDB 3KG2) from Sobolevsky et al. (2009). Grey background indicates the approximate position of the membrane. B, view down the pore axis from the extracellular membrane surface. The A/C (green/blue) and B/D (red/yellow) subunits are labelled. The horizontal red line indicates the plane of section through the TMD shown in side view in panel D. C–F, homology model of the rat GluK2(R) TMD from Lopez et al. (2013) based on the GluA2 crystal structure. For clarity, the A (green) subunit has been removed to provide a view into the pore. All four panels are shown in the same orientation. C, M1, M3 and M4 transmembrane helices and the M2 pore-loop helix shown as numbered cylinders. D, surface rendering sectioned along the axis of the pore as indicated in B. E, the pore cavity rendered as a surface generated by the program Hollow (Ho & Gruswitz, 2008). The central cavity is shaded green. The narrow constriction and cytoplasmic vestibule in grey are more speculative because residues in this region were disordered in the GluA2 crystal structure and were modelled without a template. F, coloured residues on this surface rendering are homologous to AMPA receptor locations that can be modified by extracellular or intracellular MTS reagents in the presence of agonist (Kuner et al. 2001; Sobolevsky et al. 2003). Darker colouration near the extracellular surface indicates residues within the bundle crossing occlusion zone.
Amino acids throughout the TMD contribute to determining the distinctive pore properties of NMDA, AMPA, and KA receptors but the Q/R/N site near the apex of the pore loop plays a particularly important role (Traynelis et al. 2010). Genes for all AMPA and KA receptor subunits encode a glutamine (Q) at this position, but mRNA for the GluA2 AMPA receptor subunit and GluK1 and GluK2 KA receptor subunits can undergo RNA editing that changes the amino acid at this location to arginine (R) (Traynelis et al. 2010). GluN1 and GluN2 NMDA receptor subunits all encode asparagine (N) at this position whereas rodent GluN3 subunits have a glycine (G) (Fig. 1A and C) and human GluN3B has an Arg.
The only crystal structure that includes an iGluR TMD is of the homomeric GluA2 AMPA receptor (Sobolevsky et al. 2009), which was generated with a competitive antagonist bound and is presumed to represent the closed state (Fig. 2A and B). In this structure the M2 pore helix and transmembrane M1, M3 and M4 helices are resolved; but, the random coil ‘turret’ domain connecting M1 to M2 and the selectivity filter connecting the M2 helix to the cytoplasmic end of M3 are disordered (Sobolevsky et al. 2009). Such disorder in the crystal structure suggests greater intrinsic mobility for these segments in iGluRs (see also Kuner et al. 1996), particularly as compared to the relatively rigid selectivity filter of potassium-selective channels (Doyle et al. 1998). In contrast to potassium channels (Hille, 2001), iGluRs are unlikely to be long, single file, multi-ion pores (Zarei & Dani, 1994, 1995). Instead, glutamate receptor pores have a short narrow constriction just below the Q/R/N site at the apex of the pore loop (Kuner et al. 1996; Wollmuth et al. 1996), with a relatively wide vestibule toward the cytoplasm and a water-filled central cavity facing the extracellular solution (Fig. 2D–F; Zarei & Dani, 1995; Kuner et al. 2001). In addition, the homomeric GluA2 structure exhibits 2-fold symmetry in the extracellular domains with distinct A/C and B/D subunit conformations arranged diagonally across from each other within the 4-fold symmetric TMD (Fig. 2B; Sobolevsky et al. 2009).
NMDA receptor pores
For conventional NMDA receptors, made up of GluN1 and GluN2 subunits, there is substantial evidence supporting an N1–N2–N1–N2 alternating arrangement (Sobolevsky et al. 2009; Salussolia et al. 2011; Riou et al. 2012), although a few studies instead favor an N1–N1–N2–N2 adjacent organization (Schorge & Colquhoun, 2003; Balasuriya et al. 2013). Detailed molecular architecture within the pore is currently lacking; however, inferences about structure can be made from the permeability of organic cations, from the accessibility of substituted cysteine residues to modification by methanethiosulfonate (MTS) reagents, and from the effects of amino acid substitutions on pore properties, all of which suggest that NMDA receptors have asymmetric pores. Based on the relative permeability of organic cations, NMDA receptors have a rectangular minimal cross-section estimated at 0.45 by 0.55 nm (Villarroel et al. 1995; Zarei & Dani, 1995), which is somewhat larger than the narrow selectivity filters defined for sodium (∼0.31 by 0.51 nm) and potassium (∼0.33 nm diameter) selective channels but smaller than the ∼0.65 nm diameter pore of pentameric acetylcholine receptors (Hille, 2001). Cysteine substitutions along the M3 helix have demonstrated asymmetric modification for homologous positions of GluN1 (Beck et al. 1999) versus GluN2 subunits (Sobolevsky et al. 2002), suggesting an offset and asymmetry of the A–C (GluN1) relative to B–D (GluN2) subunit pairs. For Cys substitutions in the pore loop, several positions can be modified by cytoplasmic application of MTS reagents, whereas modification of GluN1 and GluN2 by extracellular MTSET only occurs for Cys substitution at the Q/R/N site (Kuner et al. 1996), suggesting that the Asn side-chain at the Q/R/N position of each subunit extends up into the central cavity while other pore loop residues face away from the central cavity and in many cases contribute to the cytoplasmic vestibule.
NMDA receptor channels are permeable to Na+, K+ and Ca2+, but are subject to voltage-dependent block by Mg2+ (Traynelis et al. 2010). This unique permeation profile underlies their role as coincidence detectors in triggering synaptic plasticity (Bliss & Collingridge, 1993). Calcium entry through NMDA receptors activates cytoplasmic biochemical pathways that regulate synaptic strength (Lisman et al. 2012) but only when cells are sufficiently depolarized to relieve channel block by Mg2+ (Mayer et al. 1984; Nowak et al. 1984). Single amino acid substitutions have shown that calcium permeability and magnesium block both involve interactions at or near the Q/R/N site (Burnashev et al. 1992a,b1992b; Mori et al. 1992; Cavara et al. 2010), as does channel block by organic inhibitors such as MK-801 and memantine (Mori et al. 1992; Sakurada et al. 1993; Kashiwagi et al. 2002; Chen & Lipton, 2005). However, homologous substitutions in GluN1 and GluN2 indicate that the two subunits make asymmetric contributions. For example, Asn replacement with Gln in GluN1, when co-expressed with wild-type GluN2A or 2C, reduces calcium permeability and increases channel block by calcium. This substitution also slightly reduces block by extracellular magnesium (Burnashev et al. 1992b) and strongly decreases block by intracellular magnesium (Kupper et al. 1998; Wollmuth et al. 1998b). In contrast, substitutions at the Q/R/N site, or the adjacent N+1 Asn, of GluN2 cause only a modest decline in calcium permeability but substantially increase magnesium permeability and reduce block by magnesium applied extracellularly (Burnashev et al. 1992b; Sakurada et al. 1993; Wollmuth et al. 1996, 1998a) and, to a lesser extent, intracellularly (Kupper et al. 1998; Wollmuth et al. 1998b). Similar evidence for asymmetry at the pore loop comes from studies of channel block by memantine, which is reduced to a greater extent by substitutions at the Q/R/N site in GluN1 than by homolgous substitution in GluN2 or by substitutions at the GluN2 N+1 Asn (Kashiwagi et al. 2002; Chen & Lipton, 2005). Not all organic blockers display such asymmetry, however. For example, N to Q substitution at the Q/R/N site of GluN1 and GluN2B, as well as at the N+1 Asn of GluN2B all produce a similar reduction in potency of channel block by MK-801 (Kashiwagi et al. 2002).
The changes in minimal pore dimensions produced by substitutions at or near the Q/R/N site do not directly correlate with their effects on permeation and block (Wollmuth et al. 1998a), indicating that the dimensions of the narrow constriction put an upper limit on the size of ions that can pass through the pore but do not directly determine either unitary conductance or relative permeability. Indeed, single channel analysis of receptors with the Asn to Gln substitution at the Q/R/N site in GluN1 revealed two main conductance levels with distinct relative permeability to sodium and caesium (Schneggenburger & Ascher, 1997), suggesting that subtle differences in side-chain orientation may differentially affect the energy barriers for passage of each ion species. The Q/R/N site residue is critical to normal function in vivo, as mice engineered with pore loop N to Q or N to R point mutations in the GluN1 subunit exhibit drastic abnormalities in behaviour and physiology (Single et al. 2000).
In addition to the narrow constriction near the tip of the pore loop, there are additional sites along the NMDA receptor permeation pathway that influence ion flux (Premkumar & Auerbach, 1996; Antonov et al. 1998) as well as channel inhibition by organic (Kashiwagi et al. 2002; Chang & Kuo, 2008a; Jin et al. 2008; Nelson et al. 2009; Limapichat et al. 2013) and inorganic (Kupper et al. 1996; Sharma & Stevens, 1996; Williams et al. 1998) blockers. On the cytoplasmic side of the narrow constriction, substitutions of the negatively charged Glu located five residues C-terminal to the GluN1 Q/R/N site reduce calcium permeability (Schneggenburger, 1998) and block by internal Mg2+ (Kupper et al. 1996). In addition, mutation in GluN2 of the highly conserved M2 Trp residue reduces barium permeability and channel block by organic compounds (Kashiwagi et al. 2002) and by external Mg2+ (Williams et al. 1998).
Mutation experiments (Watanabe et al. 2002) on the extracellular side of the narrow constriction suggest that two components of GluN1 near the external end of the pore contribute to calcium permeation: the Asn in the conserved SYTANLAAF motif and residues in the DRPEER motif just past the end of M3 (Fig. 1A and C). Interestingly, analogous mutations in GluN2A either are non-functional or have little effect on calcium permeability (Watanabe et al. 2002). Such differences are consistent with an asymmetric contribution of pore-lining residues from GluN1 and GluN2 (Sobolevsky et al. 2007). An extensive study of mutations that influence pore block by spermine also reveals substantial differences between GluN1 and GluN2B (Jin et al. 2008). On the other hand, analysis of M3 residues essential for channel block by memantine (Limapichat et al. 2013) and felbamate (Chang & Kuo, 2008a) indicates a similar contribution to antagonist binding by specific homologous residues along M3 in both GluN1 and GluN2, which argues against a drastic asymmetry (see also Dai & Zhou, 2013). Final determination of how GluN1 and GluN2 subunits are arranged within the pore will likely require a high resolution NMDA receptor structure that includes the TMD.
Many aspects of NMDA receptor function depend on the identity of the GluN2 subunit (Paoletti et al. 2013). Properties thought to be determined by structural differences in the pore and surrounding transmembrane domain include unitary conductance, relative permeability to calcium, and potency of block by magnesium, all of which are quantitatively greater in channels formed by GluN1 plus GluN2A or GluN2B than in receptors made up of GluN1 with GluN2C or GluN2D (Burnashev et al. 1995; Schneggenburger, 1998; Cull-Candy & Leszkiewicz, 2004). Although a number of structural elements contribute to these differences (Kuner & Schoepfer, 1996; Wrighton et al. 2008), the main determinant is a single amino acid difference near the cytoplasmic end of the GluN2 M3 transmembrane helix (Siegler Retchless et al. 2012; Clarke et al. 2013). GluN2A and 2B have a polar serine at this location where 2C and 2D encode a more hydrophobic leucine (Fig. 1A). Substituting L for S in GluN2A reduces unitary conductance, calcium permeability, and the potency of Mg2+ block, thus converting the phenotype from GluN2A to that of GluN2C or 2D. Similarly, the reverse S for L substitution in GluN2D has the opposite effect, yielding conductance, permeability and block expected for receptors with GluN2A or 2B (Siegler Retchless et al. 2012). In addition, double mutant cycle analysis indicates that this location in M3 interacts with a conserved M2 helix tryptophan in the adjacent GluN1 subunit (Siegler Retchless et al. 2012; see Fig. 1A). Interestingly, substitution of the conserved tryptophan residue in M2 of GluN1 causes only modest changes in magnesium block. In contrast, as mentioned above mutations at the homologous tryptophan residue in GluN2B (or 2A) dramatically reduce both Mg2+ block and permeability to barium (Williams et al. 1998; Siegler Retchless et al. 2012). Substitution of the conserved GluN2B M2 Trp with unnatural amino acids provides evidence that this side-chain is unlikely to interact directly with divalent cations in the pore (McMenimen et al. 2006) but it remains to be determined whether the conserved M2 Trp of GluN2 forms an intersubunit interaction with residues along M3 of GluN1.
AMPA and KA receptor pores
Editing at the Q/R/N site alters many properties of AMPA and KA receptors (Traynelis et al. 2010). Channels that include an edited (R) subunit exhibit lower unitary conductance and reduced permeability to calcium as compared to channels made up only of unedited (Q) subunits (Sommer et al. 1991; Burnashev et al. 1992a, 1995, 1996; Dingledine et al. 1992; Howe, 1996; Swanson et al. 1996). In addition, recombinant homomeric edited (R) channels exhibit finite permeability to chloride ions, with PCl/PCs estimated from reversal potential measurements at 0.14 for GluA2(R) and 0.74 for GluK2(R) (Burnashev et al. 1996). Editing of GluA2 mRNA is highly efficient such that nearly all GluA2 subunits are in the edited form (Isaac et al. 2007). Indeed, mutant mice unable to make unedited GluA2 appear completely normal (Kask et al. 1998), whereas mice with defects in GluA2 editing are prone to seizures and die prematurely (Feldmeyer et al. 1999; Higuchi et al. 2000). By contrast, GluK1 and GluK2 editing increases over the course of development (Bernard et al. 1999) but even in the adult, reaches only ∼80–90%. Mutant animals with greatly reduced GluK2 editing exhibit enhanced synaptic plasticity and greater susceptibility to seizures but are otherwise normal (Vissel et al. 2001). In most brain cells, native AMPA and KA receptors are likely to be heteromeric combinations that include both edited and unedited subunits (Traynelis et al. 2010); however, calcium permeable receptors that only contain unedited subunits have been demonstrated in a number of cell types where they are postulated to play specific functional roles (Lee et al. 2001; Isaac et al. 2007).
As for NMDA receptors (Wollmuth et al. 1998a), the estimated minimal cross-section of non-NMDA receptor pores does not directly correlate with permeability or conductance (Burnashev et al. 1996). For example, both edited (R) and unedited (Q) KA receptors are estimated to have similar minimal pore diameters of 0.76 and 0.75 nm, respectively, comparable to the 0.78 nm diameter estimated for homomeric GluA1 AMPA receptors (Burnashev et al. 1996). Homomeric edited (R) channels are essentially impermeable to calcium (Burnashev et al. 1995) and have unitary conductance less than 1 pS (Howe, 1996; Swanson et al. 1996, 1997), whereas unedited (Q) channels exhibit multiple conductance levels greater than 1 pS (Swanson et al. 1996, 1997; Rosenmund et al. 1998) and are calcium permeable (Burnashev et al. 1995), with estimated fractional calcium current of 1.5 to 2% for KA receptors and 3 to 4% for unedited AMPA receptors (Burnashev et al. 1995; Wollmuth & Sakmann, 1998). In contrast, NMDA receptors have a smaller minimal pore constriction (0.45 by 0.55 nm; Villarroel et al. 1995; Zarei & Dani, 1995) but exhibit larger unitary conductance and even higher fractional calcium current 8 to 18% (Burnashev et al. 1995; Schneggenburger, 1998; Wollmuth & Sakmann, 1998). Thus, Q/R site editing, or differences in primary sequence between NMDA and non-NMDA receptor channels, present distinct energetic barriers to ion flux without necessarily constricting or enlarging the minimal dimensions of the pore.
In non-NMDA receptors, as in NMDA receptors (Kuner et al. 1996), evidence suggests that the narrowest constriction is just below the apex of the pore loop (Kuner et al. 2001) and only the Q/R/N site side-chain extends up from the pore loop into the central cavity. For example, MTSES in the extracellular solution only reacts with Cys substituted at the Q/R/N site of GluA4 AMPA receptors and not at any other pore loop location (Kuner et al. 2001). In addition, substantial permeability to chloride is only observed for homomeric edited GluK2(R) KA receptors (Burnashev et al. 1996), and for unedited (Q) channels with positively charged Arg or Lys substitutions along M3 at the level of the central cavity (Wilding et al. 2010) but not for Arg substitutions at any other position in the pore loop (Wilding et al. 2008). Thus, chloride permeability appears to depend on electrostatic interactions on the extracellular side of the narrowest pore constriction with the Q/R/N site being the only pore loop residue capable of influencing ion stability in the central cavity. As for NMDA receptors (Watanabe et al. 2002), calcium permeability through non-NMDA receptors depends on other amino acids lining the pore including the Asn residue in the conserved SYTANLAAF motif (Jatzke et al. 2003); however, residues homologous to the GluN1 DRPEER motif appear to play less of a role in AMPA and KA receptors (Jatzke et al. 2003).
In addition to permeability and conductance, Q/R site editing also determines susceptibility to inhibition by polyamines (Bowie & Mayer, 1995; Donevan & Rogawski, 1995; Isa et al. 1995; Kamboj et al. 1995; Koh et al. 1995) and cis-unsaturated fatty acids (Wilding et al. 2005). Voltage-dependent block of outward current by cytoplasmic polyamines underlies the strong inward rectification of fully unedited (Q) channels. With depolarization past approximately +50 mV, outward current increases, suggesting that extreme voltages force polyamines to pass through the channel and relieve the block (Bowie & Mayer, 1995; Koh et al. 1995). Cytoplasmic polyamines can also interact with closed channels (Bowie et al. 1998; Rozov et al. 1998), resulting in use-dependent unblocking that underlies short term plasticity at some synapses (Rozov & Burnashev, 1999). In contrast to open channels, blockade of closed channels exhibits very little voltage dependence (Bowie et al. 1998; Rozov et al. 1998), suggesting that in the closed state, the binding may be to superficial sites in the cytoplasmic vestibule followed by entry into the narrowest portion of the pore when channels open (Bowie et al. 1998). Consistent with this idea, polyamine block of GluK2(Q) is eliminated by neutral substitutions for a negatively charged Glu side-chain in the selectivity filter located four residues C-terminal to the Q/R site (Panchenko et al. 2001; see also Dingledine et al. 1992). Block is restored in double mutants that reintroduce a Glu residue at several nearby positions (Panchenko et al. 2001). A number of substitutions along the M2 helix and adjacent portions of M1 and M3 also reduce or eliminate polyamine block (Panchenko et al. 2001; Wilding et al. 2010), possibly by inducing subtle changes in pore helix orientation. In addition to changes in the pore loop, block by cytoplasmic polyamines of GluA1 AMPA receptors (Sobolevsky et al. 2005) and GluK2 KA receptors (Wilding et al. 2010; Lopez et al. 2013) can be influenced by mutations at several locations along M3 extracellular to the narrow pore loop constriction at the level of the central cavity. Introduction of positively charged side-chains reduces block of GluK2(Q) while negatively charged side-chains increase block of GluK2(R), suggesting a direct interaction between the Q/R site residue and the M3 helix (Lopez et al. 2013).
Lipid-derived modulators differentially regulate NMDA, AMPA, and kainate receptors. NMDA receptors are potentiated by long-chain cis-unsaturated Ω-3 and Ω-6 compounds including fatty acids and endocannabinoids (Miller et al. 1992; Nishikawa et al. 1994; Hampson et al. 1998), whereas neuronal AMPA (Kovalchuk et al. 1994) and KA (Wilding et al. 1998) receptors exhibit weak and strong inhibition, respectively. Interestingly, although native KA receptors are likely to be heteromeric and include both edited (R) and unedited (Q) subunits (Roche & Huganir, 1995), wild-type recombinant KA receptors expressed in heterologous cells only exhibit strong inhibition if all four subunits are edited (Wilding et al. 2005). Mutation studies have shown that in addition to the Q/R site, KA receptor modulation can be modified dramatically by amino acid substitutions throughout the TMD (Wilding et al. 2008, 2010; Lopez et al. 2013) including numerous gain of function mutations that render unedited channels susceptible to inhibition (Wilding et al. 2008) as well as loss of function mutations that eliminate or in some cases reverse the inhibition of fully edited channels (Lopez et al. 2013). Most gain of function positions are in the interface between the M2 pore loop helix and portions of the transmembrane M1 and M3 helices that contact M2 (Wilding et al. 2008, 2010) whereas M3 locations where substitution converts inhibition of fully edited channels into potentiation are at the level of the central cavity (Lopez et al. 2013). Recent work (Wilding et al. 2014) on chimeric subunits has shown that the GluK2 KA receptor TMD, fused to GluN1 and GluN2B NMDA receptor extracellular domains, is sufficient to recapitulate the modulation observed in homomeric GluK2 receptors, suggesting that cis-unsaturated compounds act directly on the TMD. Because modulation exhibits little or no voltage dependence (Wilding et al. 2005), negatively charged fatty acids are unlikely to enter the pore along the conduction pathway but instead may partition into the membrane and affect channel operation either by altering membrane mechanical properties (Patel et al. 2001; Bruno et al. 2007) or by direct interaction with the TMD, possibly via lateral fenestrations that may expose the pore to membrane constituents (Payandeh et al. 2011; Mayer, 2011).
Importantly, double mutant cycle experiments on both KA receptors (Lopez et al. 2013) and NMDA receptors (Siegler Retchless et al. 2012) provide support for the idea that channel opening may involve significant movement of the M2 helix relative to M1 and M3 (see Sobolevsky et al. 2005). In both cases, the strength of interaction from double mutant cycle analysis (Siegler Retchless et al. 2012; Lopez et al. 2013) predicts much closer contact between residue pairs than the 8–12 Å separation that is observed in homology models based on the GluA2 closed state (Sobolevsky et al. 2009) In NMDA receptors the interaction occurs across the boundary between adjacent subunits with the highly conserved M2 Trp in GluN1 interacting with an M3 Ser on the adjacent GluN2 subunit (green triangles in Fig. 1A; Siegler Retchless et al. 2012). In KA receptors, however, the Q/R site and central cavity M3 residues interact within the same subunit, with nearly equivalent interactions for subunits in the A–C and B–D configurations (Wilding et al. 2014).
Although native Q/R site editing, and other pore substitutions, clearly affect channel properties, the extent of regulation for any given parameter may vary in individual receptors (Swanson et al. 1997) depending on additional factors such as the subunit composition and stoichiometry, alternative splicing or editing at other locations, post-translational covalent modifications, or interactions with other proteins including the growing number of auxiliary subunits (Isaac et al. 2007). For example, although block by endogenous polyamines, or polyamine toxins, has been widely used to identify channels that are likely to exhibit high calcium permeability, there is increasing evidence that some cell types express calcium permeable AMPA receptors that are much less sensitive to polyamine block (see Bowie, 2012).
Opening the gate
As for other pore loop channel family members, the inner helix (M3) bundle crossing is thought to form the gate for ion flux through the pore (Sobolevsky, 2013). In the closed state GluA2 crystal structure occlusion of the pore involves several helical turns near the extracellular end of M3 (Sobolevsky et al. 2009), beginning in the highly conserved SYTANLAAF motif but extending six residues beyond, where there is structural divergence between the A–C and B–D subunit conformations as well as primary sequence divergence among the different iGluR subtypes (Figs 1A and 2C–F). Earlier studies had suggested that the gate for ion flow might involve a narrowing of the pore loop constriction rather than a tight seal at the bundle crossing because extracellularly applied MTS reagents were found to modify Cys residues substituted deep into the central cavity in the absence of added agonist (Beck et al. 1999; Sobolevsky et al. 2002, 2003). In retrospect, such modification may have resulted from entry and trapping of MTS reagents in the pore during brief channel openings (Phillips et al. 2003) that either occurred spontaneously (Turecek et al. 1997) or were triggered by trace agonist levels in control conditions (Johnson & Ascher, 1987; Kleckner & Dingledine, 1988).
Observation of tight occlusion at the bundle crossing is consistent with the fact that some pore blocking drugs only bind and unbind when channels are open (Huettner & Bean, 1988; Qian & Johnson, 2002). However, bundle crossing occlusion does not rule out the possibility that closed state constriction might also occur at the selectivity filter, which was not resolved in the crystal structure (Sobolevsky et al. 2009). One argument against occlusion at the pore loop selectivity filter comes from single channel analysis of NMDA receptors with the GluN1 Q/R/N site Asn mutated to Gln, which showed that cytoplasmic ions rapidly equilibrate with the apex of the pore loop in closed channels, (Schneggenburger & Ascher, 1997).
Mutations in the SYTANLAAF motif significantly alter normal iGluR gating (Klein & Howe, 2004; Blanke & VanDongen, 2008; Chang & Kuo, 2008b) as first observed for the Lurcher mutant mouse, which has an Ala to Thr (A8T) substitution in the orphan GluD2 subunit (Kohda et al. 2000). Mutations at A8 in AMPA and KA receptor subunits slow desensitization and deactivation and promote constitutive activation (Kohda et al. 2000; Taverna et al. 2000; Schwarz et al. 2001; Klein & Howe, 2004) whereas NMDA receptor gating is more strongly affected by A7 substitutions (Jones et al. 2002; Blanke & VanDongen, 2008; Chang & Kuo, 2008b; Murthy et al. 2012). Although a detailed description of iGluR gating is not yet available (Sobolevsky, 2013), analysis of spatial relations and timing of pore movements that underlie gating remains an active area of research, including both experimentation (Murthy et al. 2012; Kazi et al. 2013; Wilding et al. 2014) and modelling (Dong & Zhou, 2011; Dai & Zhou, 2013).
Conclusions
In almost every central neuron, iGluR activation at excitatory synapses leads to depolarization and in some cases entry of calcium. The ability of iGluRs to conduct monovalent cations, calcium or chloride ions depends on the specific amino acid side-chain at the apex of the pore loop as well as at several other critical positions along the pore axis. Better understanding of iGluR gating and permeation should aid in design of drugs and allosteric modulators that may have therapeutic benefit in the treatment of seizures, memory disorders and other pathologies. Much of the recent research on iGluRs has been fueled by insights gleaned from the homomeric GluA2 AMPA receptor crystal structure (Sobolevsky et al. 2009). Further progress toward understanding the operation of glutamate receptor pores will clearly benefit as additional iGluR structures are solved, including structures for NMDA and KA receptors as well as additional conformational states representing open and desensitized channels (Sobolevsky, 2013).
Glossary
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CNG
cyclic nucleotide gated
- iGluR
ionotropic glutamate receptor
- KA
kainate
- MTS
methanethiosulfonate
- NMDA
N-methyl-d-aspartate
- TMD
transmembrane domain
- TRP
transient receptor potential
Biography
James Huettner (Washington University School of Medicine) earned his PhD in Neurobiology from Harvard Medical School in 1987, working in the lab of Robert Baughman to analyse synaptic transmission by identified populations of central nervous system neurons in culture. He received a Junior Fellowship to remain at Harvard for postdoctoral research with Bruce Bean and then joined the faculty at Washington University in St Louis in 1991. His main research interests include glutamate receptor physiology, synaptic transmission, and in vitro neural differentiation of embryonic stem cells.
Additional information
Competing interests
None declared.
Funding
Work in my lab is supported by NIH grant NS30888.
References
- Antonov SM, Gmiro VE. Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat Neurosci. 1998;1:451–461. doi: 10.1038/2167. [DOI] [PubMed] [Google Scholar]
- Balasuriya D, Goetze TA, Barrera NP, Stewart AP, Suzuki Y. Edwardson JM. α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors adopt different subunit arrangements. J Biol Chem. 2013;288:21987–21998. doi: 10.1074/jbc.M113.469205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Wollmuth LP, Seeburg PH, Sakmann B. Kuner T. NMDAR channel segments forming the extracellular vestibule inferred from the accessibility of substituted cysteines. Neuron. 1999;22:559–570. doi: 10.1016/s0896-6273(00)80710-2. [DOI] [PubMed] [Google Scholar]
- Bernard A, Ferhat L, Dessi F, Charton G, Represa A, Ben-Ari Y. Khrestchatisky M. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci. 1999;11:604–616. doi: 10.1046/j.1460-9568.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- Blanke ML. VanDongen AM. The NR1 M3 domain mediates allosteric coupling in the N-methyl-d-aspartate receptor. Mol Pharmacol. 2008;74:454–465. doi: 10.1124/mol.107.044115. [DOI] [PubMed] [Google Scholar]
- Bliss TV. Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bowie D. Redefining the classification of AMPA-selective ionotropic glutamate receptors. J Physiol. 2012;590:49–61. doi: 10.1113/jphysiol.2011.221689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD. Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci. 1998;18:8175–8185. doi: 10.1523/JNEUROSCI.18-20-08175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Bruno MJ, Koeppe RE., 2nd Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci U S A. 2007;104:9638–9643. doi: 10.1073/pnas.0701015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH. Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992a;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Günther W, Seeburg PH. Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992b;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Villarroel A. Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol. 1996;496:165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E. Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavara NA, Orth A, Hicking G, Seebohm G. Hollmann M. Residues at the tip of the pore loop of NR3B-containing NMDA receptors determine Ca2+ permeability and Mg2+ block. BMC Neurosci. 2010;11:133. doi: 10.1186/1471-2202-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HR. Kuo CC. Molecular determinants of the anticonvulsant felbamate binding site in the N-methyl-d-aspartate receptor. J Med Chem. 2008a;51:1534–1545. doi: 10.1021/jm0706618. [DOI] [PubMed] [Google Scholar]
- Chang HR. Kuo CC. The activation gate and gating mechanism of the NMDA receptor. J Neurosci. 2008b;28:1546–1556. doi: 10.1523/JNEUROSCI.3485-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS. Lipton SA. Pharmacological implications of two distinct mechanisms of interaction of memantine with N-methyl-d-aspartate-gated channels. J Pharmacol Exp Ther. 2005;314:961–971. doi: 10.1124/jpet.105.085142. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Glasgow NG. Johnson JW. Mechanistic and structural determinants of NMDA receptor voltage-dependent gating and slow Mg2+ unblock. J Neurosci. 2013;33:4140–4150. doi: 10.1523/JNEUROSCI.3712-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG. Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;255:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dai J. Zhou HX. An NMDA receptor gating mechanism developed from MD simulations reveals molecular details underlying subunit-specific contributions. Biophys J. 2013;104:2170–2181. doi: 10.1016/j.bpj.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Hume RI. Heinemann SF. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992;12:4080–4087. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD. Rogawski MA. Intracellular polyamines mediate inward rectification of Ca2+-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. Zhou HX. Atomistic mechanism for the activation and desensitization of an AMPA-subtype glutamate receptor. Nat Commun. 2011;2:354. doi: 10.1038/ncomms1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT. MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Kask K, Brusa R, Kornau HC, Kolhekar R, Rozov A, Burnashev N, Jensen V, Hvalby O, Sprengel R. Seeburg PH. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat Neurosci. 1999;2:57–64. doi: 10.1038/4561. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X. Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Bornheim LM, Scanziani M, Yost CS, Gray AT, Hansen BM, Leonoudakis DJ. Bickler PE. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R. Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland, MA, USA: Sinauer Associates; 2001. [Google Scholar]
- Ho BK. Gruswitz F. HOLLOW: generating accurate representations of channel and interior surfaces in molecular structures. BMC Struct Biol. 2008;8:49. doi: 10.1186/1472-6807-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M. Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Bean BP. Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Iino M, Itazawa S. Ozawa S. Spermine mediates inward rectification of Ca2+-permeable AMPA receptor channels. Neuroreport. 1995;6:2045–2048. doi: 10.1097/00001756-199510010-00022. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC. McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jatzke C, Hernandez M. Wollmuth LP. Extracellular vestibule determinants of Ca2+ influx in Ca2+-permeable AMPA receptor channels. J Physiol. 2003;549:439–452. doi: 10.1113/jphysiol.2002.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Miyazaki M, Mizuno S, Takigawa M, Hirose T, Nishimura K, Toida T, Williams K, Kashiwagi K. Igarashi K. The pore region of N-methyl-d-aspartate receptors differentially influences stimulation and block by spermine. J Pharmacol Exp Ther. 2008;327:68–77. doi: 10.1124/jpet.108.140459. [DOI] [PubMed] [Google Scholar]
- Johnson JW. Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jones KS, VanDongen HM. VanDongen AM. The NMDA receptor M3 segment is a conserved transduction element coupling ligand binding to channel opening. J Neurosci. 2002;22:2044–2053. doi: 10.1523/JNEUROSCI.22-06-02044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT. Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K, Masuko T, Nguyen CD, Kuno T, Tanaka I, Igarashi K. Williams K. Channel blockers acting at N-methyl-d-aspartate receptors: differential effects of mutations in the vestibule and ion channel pore. Mol Pharmacol. 2002;61:533–545. doi: 10.1124/mol.61.3.533. [DOI] [PubMed] [Google Scholar]
- Kask K, Zamanillo D, Rozov A, Burnashev N, Sprengel R. Seeburg PH. The AMPA receptor subunit GluR-B in its Q/R site-unedited form is not essential for brain development and function. Proc Natl Acad Sci U S A. 1998;95:13777–13782. doi: 10.1073/pnas.95.23.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Siuda ER, Nisenbaum ES. Bredt DS. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59:986–996. doi: 10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Kazi R, Gan Q, Talukder I, Markowitz M, Salussolia CL. Wollmuth LP. Asynchronous movements prior to pore opening in NMDA receptors. J Neurosci. 2013;33:12052–12066. doi: 10.1523/JNEUROSCI.5780-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW. Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Klein RM. Howe JR. Effects of the lurcher mutation on GluR1 desensitization and activation kinetics. J Neurosci. 2004;24:4941–4951. doi: 10.1523/JNEUROSCI.0660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Burnashev N. Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda K, Wang Y. Yuzaki M. Mutation of a glutamate receptor motif reveals its role in gating and delta2 receptor channel properties. Nat Neurosci. 2000;3:315–322. doi: 10.1038/73877. [DOI] [PubMed] [Google Scholar]
- Körber C, Werner M, Hoffmann J, Sager C, Tietze M, Schmid SM, Kott S. Hollmann M. Stargazin interaction with alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors is critically dependent on the amino acid at the narrow constriction of the ion channel. J Biol Chem. 2007;282:18758–18766. doi: 10.1074/jbc.M611182200. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Miller B, Sarantis M. Attwell D. Arachidonic acid depresses non-NMDA receptor currents. Brain Res. 1994;643:287–295. doi: 10.1016/0006-8993(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Kuner T, Beck C, Sakmann B. Seeburg PH. Channel-lining residues of the AMPA receptor M2 segment: structural environment of the Q/R site and identification of the selectivity filter. J Neurosci. 2001;21:4162–4172. doi: 10.1523/JNEUROSCI.21-12-04162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T. Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Wollmuth LP, Karlin A, Seeburg PH. Sakmann B. Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron. 1996;17:343–352. doi: 10.1016/s0896-6273(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Kupper J, Ascher P. Neyton J. Probing the pore region of recombinant N-methyl-d-aspartate channels using external and internal magnesium block. Proc Natl Acad Sci U S A. 1996;93:8648–8653. doi: 10.1073/pnas.93.16.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper J, Ascher P. Neyton J. Internal Mg2+ block of recombinant NMDA channels mutated within the selectivity filter and expressed in Xenopus oocytes. J Physiol. 1998;507:1–12. doi: 10.1111/j.1469-7793.1998.001bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Kong H, Manzini MC, Albuquerque C, Chao MV. MacDermott AB. Kainate receptors expressed by a subpopulation of developing nociceptors rapidly switch from high to low Ca2+ permeability. J Neurosci. 2001;21:4572–4581. doi: 10.1523/JNEUROSCI.21-13-04572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limapichat W, Yu WY, Branigan E, Lester HA. Dougherty DA. Key binding interactions for memantine in the NMDA receptor. ACS Chem Neurosci. 2013;4:255–260. doi: 10.1021/cn300180a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R. Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MN, Wilding TJ. Huettner JE. Q/R site interactions with the M3 helix in GluK2 kainate receptor channels revealed by thermodynamic mutant cycles. J Gen Physiol. 2013;142:225–239. doi: 10.1085/jgp.201311000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenimen KA, Petersson EJ, Lester HA. Dougherty DA. Probing the Mg2+ blockade site of an N-methyl-d-aspartate (NMDA) receptor with unnatural amino acid mutagenesis. ACS Chem Biol. 2006;1:227–234. doi: 10.1021/cb6000944. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19:1370–1380. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Sarantis M, Traynelis SF. Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Mori H, Masaki H, Yamakura T. Mishina M. Identification by mutagenesis of a Mg2+-block site of the NMDA receptor channel. Nature. 1992;358:673–675. doi: 10.1038/358673a0. [DOI] [PubMed] [Google Scholar]
- Murthy SE, Shogan T, Page JC, Kasperek EM. Popescu GK. Probing the activation sequence of NMDA receptors with lurcher mutations. J Gen Physiol. 2012;140:267–277. doi: 10.1085/jgp.201210786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JK, Frølund SU, Tikhonov DB, Kristensen AS. Strømgaard K. Synthesis and biological activity of argiotoxin 636 and analogues: selective antagonists for ionotropic glutamate receptors. Angew Chem Int Ed Engl. 2009;48:3087–3091. doi: 10.1002/anie.200805426. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Kimura S. Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-d-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol. 1994;475:83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A. Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C. Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M. Honoré E. Lipid and mechano-gated 2P domain K+ channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Panchenko VA, Glasser CR. Mayer ML. Structural similarities between glutamate receptor channels and K(+) channels examined by scanning mutagenesis. J Gen Physio. 2001;117:345–360. doi: 10.1085/jgp.117.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N. Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LR, Enkvetchakul D. Nichols CG. Gating dependence of inner pore access in inward rectifier K+ channels. Neuron. 2003;37:953–962. doi: 10.1016/s0896-6273(03)00155-7. [DOI] [PubMed] [Google Scholar]
- Premkumar LS. Auerbach A. Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. Neuron. 1996;16:869–880. doi: 10.1016/s0896-6273(00)80107-5. [DOI] [PubMed] [Google Scholar]
- Qian A. Johnson JW. Channel gating of NMDA receptors. Physiol Behav. 2002;77:577–582. doi: 10.1016/s0031-9384(02)00906-x. [DOI] [PubMed] [Google Scholar]
- Riou M, Stroebel D, Edwardson JM. Paoletti P. An alternating GluN1-2-1-2 subunit arrangement in mature NMDA receptors. PLoS One. 2012;7:e35134. doi: 10.1371/journal.pone.0035134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW. Huganir RL. Synaptic expression of the high-affinity kainate receptor subunit KA2 in hippocampal cultures. Neuroscience. 1995;69:383–393. doi: 10.1016/0306-4522(95)00253-f. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y. Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Rozov A. Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP. Burnashev N. Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol. 1998;511:361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada K, Masu M. Nakanishi S. Alteration of Ca2+ permeability and sensitivity to Mg2+ and channel blockers by a single amino acid substitution in the N-methyl-d-aspartate receptor. J Biol Chem. 1993;268:410–415. [PubMed] [Google Scholar]
- Salussolia CL, Prodromou ML, Borker P. Wollmuth LP. Arrangement of subunits in functional NMDA receptors. J Neurosci. 2011;31:11295–11304. doi: 10.1523/JNEUROSCI.5612-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salussolia CL, Corrales A, Talukder I, Kazi R, Akgul G, Bowen M. Wollmuth LP. Interaction of the M4 segment with other transmembrane segments is required for surface expression of mammalian α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J Biol Chem. 2011;286:40205–40218. doi: 10.1074/jbc.M111.268839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salussolia CL, Gan Q, Kazi R, Singh P, Allopenna J, Furukawa H. Wollmuth LP. A eukaryotic specific transmembrane segment is required for tetramerization in AMPA receptors. J Neurosci. 2013;33:9840–9845. doi: 10.1523/JNEUROSCI.2626-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R. Altered voltage dependence of fractional Ca2+ current in N-methyl-d-aspartate channel pore mutants with a decreased Ca2+ permeability. Biophys J. 1998;74:1790–1794. doi: 10.1016/S0006-3495(98)77889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R. Ascher P. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- Schorge S. Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 2003;23:1151–1158. doi: 10.1523/JNEUROSCI.23-04-01151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz MK, Pawlak V, Osten P, Mack V, Seeburg PH. Köhr G. Dominance of the lurcher mutation in heteromeric kainate and AMPA receptor channels. Eur J Neurosci. 2001;14:861–868. doi: 10.1046/j.0953-816x.2001.01705.x. [DOI] [PubMed] [Google Scholar]
- Sharma G. Stevens CF. Interactions between two divalent ion binding sites in N-methyl-d-aspartate receptor channels. Proc Natl Acad Sci U S A. 1996;93:14170–14175. doi: 10.1073/pnas.93.24.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler Retchless B, Gao W. Johnson JW. A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nat Neurosci. 2012;15:406–413. doi: 10.1038/nn.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Single FN, Rozov A, Burnashev N, Zimmermann F, Hanley DF, Forrest D, Curran T, Jensen V, Hvalby O, Sprengel R. Seeburg PH. Dysfunctions in mice by NMDA receptor point mutations NR1(N598Q) and NR1(N598R) J Neurosci. 2000;20:2558–2566. doi: 10.1523/JNEUROSCI.20-07-02558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI. Structure and gating of tetrameric glutamate receptors. J Physiol. 2013 doi: 10.1113/jphysiol.2013.264911. DOI: 10.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rooney L. Wollmuth LP. Staggering of subunits in NMDAR channels. Biophys J. 2002;83:3304–3314. doi: 10.1016/S0006-3495(02)75331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP. Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Prodromou ML, Yelshansky MV. Wollmuth LP. Subunit-specific contribution of pore-forming domains to NMDA receptor channel structure and gating. J Gen Physiol. 2007;129:509–525. doi: 10.1085/jgp.200609718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Yelshansky MV. Wollmuth LP. Different gating mechanisms in glutamate receptor and K+ channels. J Neurosci. 2003;23:7559–7568. doi: 10.1523/JNEUROSCI.23-20-07559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Yelshansky MV. Wollmuth LP. State-dependent changes in the electrostatic potential in the pore of a GluR channel. Biophys J. 2005;88:235–242. doi: 10.1529/biophysj.104.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Köhler M, Sprengel R. Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M. Cull-Candy SG. Effect of RNA editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. J Physiol. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK. Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna F, Xiong ZG, Brandes L, Roder JC, Salter MW. MacDonald JF. The Lurcher mutation of an alpha-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid receptor subunit enhances potency of glutamate and converts an antagonist to an agonist. J Biol Chem. 2000;275:8475–8479. doi: 10.1074/jbc.275.12.8475. [DOI] [PubMed] [Google Scholar]
- Terhag J, Gottschling K. Hollmann M. The transmembrane domain C of AMPA receptors is critically involved in receptor function and modulation. Front Mol Neurosci. 2010;3:117. doi: 10.3389/fnmol.2010.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek R, Vlachová V. Vyklický L., Jr Spontaneous openings of NMDA receptor channels in cultured rat hippocampal neurons. Eur J Neurosci. 1997;9:1999–2008. doi: 10.1111/j.1460-9568.1997.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ. Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A, Burnashev N. Sakmann B. Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophys J. 1995;68:866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissel B, Royle GA, Christie BR, Schiffer HH, Ghetti A, Tritto T, Perez-Otano I, Radcliffe RA, Seamans J, Sejnowski T, Wehner JM, Collins AC, O'Gorman S. Heinemann SF. The role of RNA editing of kainate receptors in synaptic plasticity and seizures. Neuron. 2001;29:217–227. doi: 10.1016/s0896-6273(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Beck C, Kuner T, Premkumar LS. Wollmuth LP. DRPEER: a motif in the extracellular vestibule conferring high Ca2+ flux rates in NMDA receptor channels. J Neurosci. 2002;22:10209–10216. doi: 10.1523/JNEUROSCI.22-23-10209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Chai YH. Huettner JE. Inhibition of rat neuronal kainate receptors by cis-unsaturated fatty acids. J Physiol. 1998;513:331–339. doi: 10.1111/j.1469-7793.1998.331bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Chen K. Huettner JE. Fatty acid modulation and polyamine block of GluK2 kainate receptors analyzed by scanning mutagenesis. J Gen Physiol. 2010;136:339–352. doi: 10.1085/jgp.201010442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Fulling E, Zhou Y. Huettner JE. Amino acid substitutions in the pore helix of GluR6 control inhibition by membrane fatty acids. J Gen Physiol. 2008;132:85–99. doi: 10.1085/jgp.200810009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Lopez MN. Huettner JE. Radial symmetry in a chimaeric glutamate receptor pore. Nat Commun. 2014;5:3349. doi: 10.1038/ncomms4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Zhou Y. Huettner JE. Q/R site editing controls kainate receptor inhibition by membrane fatty acids. J Neurosci. 2005;25:9470–9478. doi: 10.1523/JNEUROSCI.2826-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Pahk AJ, Kashiwagi K, Masuko T, Nguyen ND. Igarashi K. The selectivity filter of the N-methyl-d-aspartate receptor: a tryptophan residue controls block and permeation of Mg2+ Mol Pharmacol. 1998;53:933–941. [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T. Sakmann B. Adjacent asparagines in the NR2-subunit of the NMDA receptor channel control the voltage-dependent block by extracellular Mg2+ J Physiol. 1998a;506:13–32. doi: 10.1111/j.1469-7793.1998.013bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T. Sakmann B. Intracellular Mg2+ interacts with structural determinants of the narrow constriction contributed by the NR1-subunit in the NMDA receptor channel. J Physiol. 1998b;506:33–52. doi: 10.1111/j.1469-7793.1998.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Seeburg PH. Sakmann B. Differential contribution of the NR1- and NR2A-subunits to the selectivity filter of recombinant NMDA receptor channels. J Physiol. 1996;491:779–797. doi: 10.1113/jphysiol.1996.sp021257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP. Sakmann B. Different mechanisms of Ca2+ transport in NMDA and Ca2+-permeable AMPA glutamate receptor channels. J Gen Physiol. 1998;112:623–636. doi: 10.1085/jgp.112.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighton DC, Baker EJ, Chen PE. Wyllie DJ. Mg2+ and memantine block of rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J Physiol. 2008;586:211–225. doi: 10.1113/jphysiol.2007.143164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM. Dani JA. Ionic permeability characteristics of the N-methyl-d-aspartate receptor channel. J Gen Physiol. 1994;103:231–248. doi: 10.1085/jgp.103.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM. Dani JA. Structural basis for explaining open-channel blockade of the NMDA receptor. J Neurosci. 1995;15:1446–1454. doi: 10.1523/JNEUROSCI.15-02-01446.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


