Abstract
Following incomplete spinal cord injury (SCI), collaterals sprout from intact and injured axons in the vicinity of the lesion. These sprouts are thought to form new synaptic contacts that effectively bypass the lesion epicentre and contribute to improved functional recovery. Such anatomical changes are known to be enhanced by exercise training; however, the mechanisms underlying exercise-mediated plasticity are poorly understood. Specifically, we do not know how SCI alone or SCI combined with exercise alters the intrinsic and synaptic properties of interneurons in the vicinity of a SCI. Here we use a hemisection model of incomplete SCI in adult mice and whole-cell patch-clamp recording in a horizontal spinal cord slice preparation to examine the functional properties of deep dorsal horn (DDH) interneurons located in the vicinity of a SCI following 3 or 6 weeks of treadmill exercise training. We examined the functional properties of local and descending excitatory synaptic connections by recording spontaneous excitatory postsynaptic currents (sEPSCs) and responses to dorsal column stimulation, respectively. We find that SCI in untrained animals exerts powerful effects on intrinsic, and especially, synaptic properties of DDH interneurons. Plasticity in intrinsic properties was most prominent at 3 weeks post SCI, whereas synaptic plasticity was greatest at 6 weeks post injury. Exercise training did not markedly affect intrinsic membrane properties; however, local and descending excitatory synaptic drive were enhanced by 3 and 6 weeks of training. These results suggest exercise promotes synaptic plasticity in spinal cord interneurons that are ideally placed to form new intraspinal circuits after SCI.
Key points.
Exercise training after spinal cord injury (SCI) enhances collateral sprouting from axons near the injury and is thought to promote intraspinal circuit reorganisation that effectively bridges the SCI. The effects of exercise training, and its duration, on interneurons in these de novo intraspinal circuits are poorly understood.
In an adult mouse hemisection model of SCI, we used whole-cell patch-clamp electrophysiology to examine changes in the intrinsic and synaptic properties of deep dorsal horn interneurons in the vicinity of a SCI in response to the injury, and after 3 and 6 weeks of treadmill exercise training.
SCI alone exerted powerful effects on the intrinsic and synaptic properties of interneurons near the lesion.
Importantly, synaptic activity, both local and descending, was preferentially enhanced by exercise training, suggesting that exercise promotes synaptic plasticity in spinal cord interneurons that are ideally placed to form new intraspinal circuits after SCI.
Introduction
Injury-induced plasticity within spinal circuits after incomplete spinal cord injury (SCI) is well documented. Regenerative sprouting of collaterals from both damaged and intact axons in the vicinity of a SCI is known to occur and results in synaptogenesis and synaptic remodelling (reviewed in Edgerton et al. 2004; Cai et al. 2006; Dunlop, 2008; Fouad & Tetzlaff, 2012). These spared and newly sprouted axons can form reorganised intraspinal circuits that effectively bridge a lesion site and contribute to improved functional recovery (Goldstein et al. 1997; Fouad et al. 2001; Bareyre et al. 2004; Courtine et al. 2008; Goldshmit et al. 2008; Flynn et al. 2011a,b2011b; Onifer et al. 2011).
Recent studies have highlighted the role of exercise training in enhancing the anatomical changes that follow SCI (Engesser-Cesar et al. 2007; Goldshmit et al. 2008). In fact, harnessing the plasticity that accompanies exercise training is increasingly recognised as an effective strategy to enhance functional recovery after SCI in humans (de Leon et al. 1998; Behrman et al. 2006; Dunlop, 2008; Galea, 2012). Surprisingly, the functional mechanisms underlying these exercise-induced improvements are poorly understood. In addition, the extent to which these newly formed intraspinal circuits function to bridge a lesion is unknown, as is the effect of exercise training (and its duration) on interneurons in these circuits (Battistuzzo et al. 2012; Flynn et al. 2013).
Electrophysiological changes in spinal interneurons and networks after SCI have been reported. Most of this work has focused on plasticity in motoneuron pools that innervate hindlimb musculature in rats and cats (Barbeau & Rossignol, 1987; Lovely et al. 1990; Beaumont & Gardiner, 2002, 2003; Petruska et al. 2007; Beaumont et al. 2008). In contrast, few data are available on the effects of exercise on the properties of spinal cord interneurons, even though they play a critical role in conveying locomotor drive from descending pathways to motoneuron pools (Cote et al. 2003; Cote & Gossard, 2004; Husch et al. 2012). This is particularly the case for interneurons in the deep dorsal horn (DDH) of the spinal cord. These interneurons lie in the terminal field of the corticospinal tract in rodents and include short and long propriospinal neurons (Tracey, 2004; Flynn et al. 2011a). They are therefore ideally placed to form new intraspinal circuits and bridge a SCI in rodents (Bareyre et al. 2004; Courtine et al. 2008).
Recently we developed a horizontal slice preparation of the adult mouse spinal cord, which maintains descending pathways and significant rostro-caudal spinal cord circuitry (Flynn et al. 2011a). We subsequently combined this preparation with whole-cell patch-clamp electrophysiology in a hemisection model of SCI to investigate intrinsic and synaptic plasticity in interneurons in the vicinity of a SCI after exercise training in adult mice. We showed that 3 weeks of treadmill training selectively increases the efficacy of synaptic connections from descending pathways to interneurons located in the vicinity of a spinal cord lesion (Flynn et al. 2013). This initial work established a hemisection model of SCI in mice and an effective treadmill exercise training regime. What remains unanswered is how longer durations of exercise training affect plasticity, and how this compares with the properties of neurons from age-matched uninjured animals. We hypothesise: (1) that 6 weeks of treadmill training will further strengthen local and descending synaptic connections; and (2) that both intrinsic and synaptic properties of neurons from age-matched uninjured control mice will differ from those of either trained or untrained SCI mice. Here we use our horizontal slice preparation to undertake a comprehensive analysis of the intrinsic and synaptic changes occurring within DDH interneurons after SCI following different durations of treadmill exercise training. Importantly, the inclusion of an age-matched uninjured control group in this study provides the opportunity to analyse neuronal changes occurring as a result of the SCI itself over time, as well as those resulting from exercise training.
By our inclusion of an uninjured control group we demonstrate that a lesion to the spinal cord has powerful effects on intrinsic, and especially, synaptic properties of DDH interneurons. These effects are observed equally in neurons as many as two spinal segments away from the lesion epicentre. We also show a distinct timeline of changes after SCI, with the 3 week time point being characterised by limited plasticity of intrinsic membrane properties and the 6 week time point showing distinct changes to synaptic drive and the strength of synaptic connections. Importantly we also show that although intrinsic properties of DDH interneurons are relatively resistant to the effects of our exercise training protocol, synaptic drive and activity in local synaptic networks are preferentially affected by both 3 and 6 weeks of exercise training. This study therefore provides a comprehensive analysis of detailed electrophysiological properties of interneurons in the vicinity of a SCI and how these properties are changed by the injury alone and by different durations of exercise training.
Methods
Ethical approval
The University of Newcastle Animal Care and Ethics Committee approved all procedures performed. Approval was issued by the University of Newcastle under the NSW Animal Research Act, 1985 (Approval Number A-2009-154).
The primary aim of the study was to compare the effects of 3 and 6 weeks of exercise training on the intrinsic and synaptic properties of interneurons in the vicinity of a SCI. We also made similar measurements from age-matched animals that did not receive a SCI to account for changes in neuron properties that are known to occur throughout development (Baccei & Fitzgerald, 2005; Walsh et al. 2009; Tadros et al. 2012). These animals are hereafter termed controls. Thus, the experimental groups in our study were: untrained and trained SCI groups and control animals. Most of the procedures for SCI and training have been described previously (Flynn et al. 2013). Animals were randomly assigned to untrained and trained groups, and all data were obtained by investigators blinded to the training status of each animal.
Hemisection surgery and exercise training procedures
Adult male C57/BL6 mice (aged 9–10 weeks) received a left T10 spinal cord hemisection (between T10 and T11 spinal nerves) under isoflurane anaesthesia. Buprenorphine (0.1 mg kg−1 s.c., every 8 h for 48 h) and carprofen (5 mg kg−1 s.c., every 24 h for 5 days) were administered for post-surgical analgesia. The same surgeon performed all surgeries. All SCI animals received 2 weeks of treadmill training prior to surgery to familiarise them with the equipment.
Following 1 week of post-surgical recovery, animals showing left hindlimb paralysis were randomly assigned to untrained or trained groups. Treadmill-trained animals completed two 10 min sessions of enforced treadmill exercise each day (5 days week−1) for a period of 3 (n = 12 mice) or 6 weeks (n = 14 mice). Untrained animals remained in their cages during this time (3 weeks, n = 14 mice; 6 weeks, n = 13 mice). Treadmill exercise was completed at speeds commensurate with each animal's ability (beginning at 6 m min−1 at 1 week after SCI surgery and progressing to 10–12 m min−1 over the training period). This treadmill-training regime has been used previously by our group to demonstrate that exercise training improves locomotor recovery after spinal cord hemisection and increases axonal regrowth and collateral sprouting in the vicinity of a lesion (Goldshmit et al. 2008). Animals allocated to the control group were age matched to mice having completed 3 weeks (∼13 weeks old; n = 19 mice) or 6 weeks (∼16 weeks old; n = 13 mice) of treadmill training. Control animals were uninjured and received no treadmill training.
Tissue preparation
Untrained and trained mice were killed for in vitro whole-cell patch-clamp electrophysiology at the completion of their treadmill training or matched passive recovery times. Investigators were blinded to the training status of each SCI animal. Control mice were killed at the appropriate age. Under deep ketamine anaesthesia (100 mg kg−1 i.p.) animals were decapitated and horizontal spinal cord slices were prepared as previously described in Flynn et al. (2013). Briefly, the mouse torso was submerged in ice-cold, oxygenated, sucrose-substituted artificial cerebrospinal fluid (sACSF containing (in mm): 250 sucrose, 25 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 6 MgCl2, and 1 CaCl2; pH 7.3) and a length of spinal cord (T5–T13) was isolated using a ventral approach. Isolation of the spinal cord was more challenging at the 6 week time point because of the presence of scar tissue along the dorsal surface of the spinal cord near the hemisection. Removal of this scar tissue may have resulted in some stretching and subsequent damage near the lateral T10 hemisection. Accordingly, it was often difficult to obtain electrophysiological recordings in this region (see Fig. 1C). The spinal cord was then mounted on an agar cutting stage using cyanoacrylate glue. Horizontal slices (250 μm thick) were cut using a vibrating microtome (HM 650V; Microm, Walldorf, Germany or VT1000s, Leica Microsystems, Nuslock, Germany). A slice containing the deep dorsal horn and a continuous strip of the dorsal columns (DCs) was selected (Fig. 1A) and transferred to a recording bath for whole-cell patch-clamp electrophysiology. Slices were perfused with oxygenated ACSF (containing (in mm): 118 NaCl, 25 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 1 MgCl2 and 2.5 CaCl2). The spinal cord slice was secured in the bath underneath a custom-made platinum and nylon net and allowed to equilibrate for 30–45 min at room temperature (22–25°C) before beginning experiments. After the equilibration period, a bipolar stimulating electrode was placed in the DCs (Fig. 1B and C) rostral to the transection to study DC evoked synaptic responses.
Figure 1. Horizontal spinal cord slice preparation and location of recorded neurons in SCI mice.
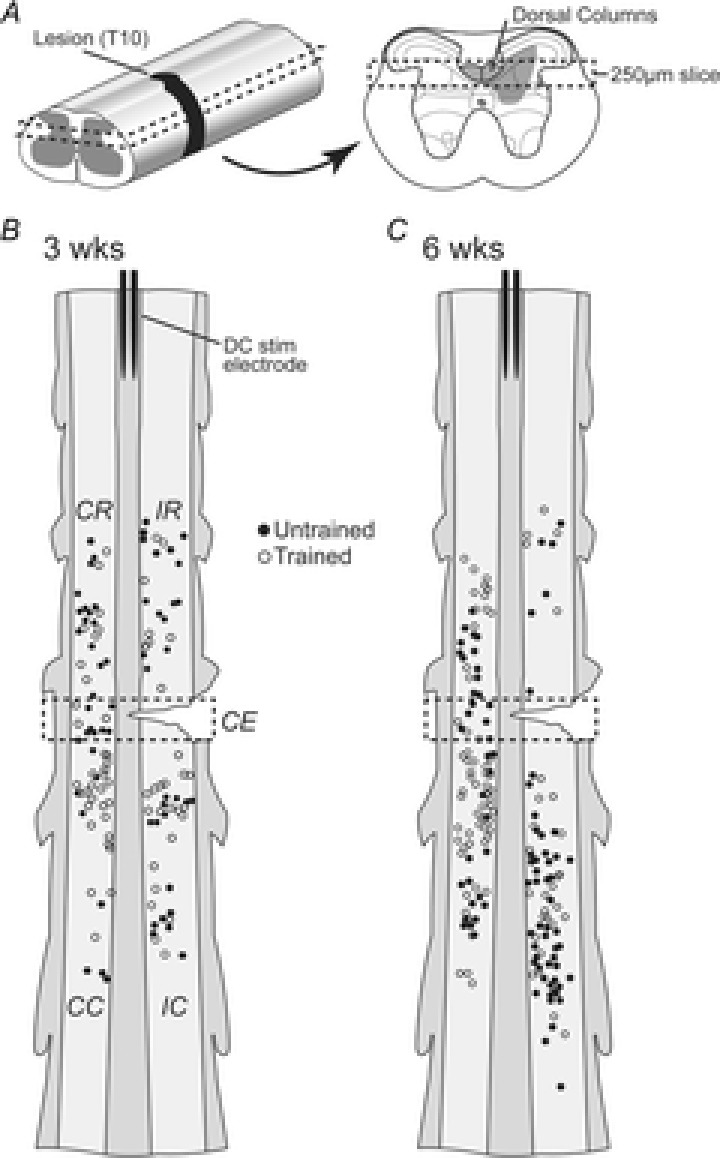
A, left panel: schematic diagrams showing spinal cord isolated for whole-cell patch-clamp experiments. A single horizontal slice containing the DCs was used for recordings (dashed rectangle). A, right panel: transverse spinal cord schematic showing the DCs and projection pattern of DC terminals in the deep dorsal horn (grey shading). Dashed rectangle in right panel shows the structures contained within the horizontal slice used for recordings. B, schematic showing the location of recorded neurons in the horizontal spinal cord slice in 3 weeks SCI mice. Light and dark grey shading represent grey and white matter, respectively. Neurons from untrained (filled circles) and trained (open circles) mice were recorded within two segments of the hemisection (dashed rectangle: lesion between T10 and T11 spinal nerves). Recording location relative to the lesion was designated ipsilateral–rostral (IR), ipsilateral–caudal (IC), contralateral–rostral (CR), contralateral–caudal (CC) and contralateral–epicentre (CE; inside dashed rectangle). A bipolar stimulating electrode was placed in the DCs at the rostral end of the slice for stimulating descending inputs. C, same as B, but in 6 weeks SCI mice.
Electrophysiological recordings
Recordings were made at room temperature using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Data were collected (sampled at 50 kHz, filtered between 2 and 10 kHz) and stored on a computer using AxoGraph X software (Axograph Scientific, Sydney, Australia). Neurons within two spinal segments of the lesion were targeted for recording using infrared differential interference contrast optics. Patch pipettes were pulled from borosilicate glass (1.5 mm o.d. × 1.16 mm i.d.; Harvard Apparatus, Edenbridge, Kent, England, UK) to a tip resistance of 3–5 MΩ and were filled with a K+-based internal solution containing (in mm): 135 KCH3SO4, 6 NaCl, 2 MgCl2, 10 Hepes, 0.1 EGTA, 2 MgATP and 0.3 NaGTP. Reported membrane potentials were corrected for a 10 mV junction potential (Barry & Lynch, 1991). At the completion of each recording session, the location of the recorded interneuron was mapped as described previously (Flynn et al. 2013). Briefly, images of the spinal cord slice were captured using a digital camera while the recording electrode remained attached to the recorded interneuron. The image was imported into Adobe Illustrator and the location of the recorded neuron plotted on a standardised template of the horizontal slice (Fig. 1).
The whole-cell recording configuration was initially established in voltage clamp (holding potential −60 mV; series resistance <20 MΩ). A 5 mV square hyperpolarising step (10 ms duration, 10 repetitions) was used to monitor series and input resistance. Action potential (AP) properties and discharge characteristics were examined by injecting square current pulses (20 pA increments, 800 ms duration). Individual action potentials were captured using a derivative threshold method, with the threshold set at a dV/dt value between 10 and 15 V s−1. Rheobase current was defined as the smallest step current that would elicit at least one AP. AP threshold, peak amplitude (measured from point of inflection to peak), afterhyperpolarisation (AHP) peak (measured from point of AHP inflection to negative peak) and latency were quantified from rheobase APs.
Subthreshold currents were activated and identified by applying a voltage-clamp protocol from a holding potential of −60 mV (Graham et al. 2007). A hyperpolarising pulse to −90 mV (1 s duration) was delivered followed by a depolarising step to −40 mV (200 ms duration) (Fig. 5A, lower left). The automated P/N leak subtraction method, within the Axograph X software, was used to remove capacitive and leakage currents. This protocol specifically identified three subthreshold ionic currents: the rapid and slow outward potassium currents (IAr and IAs), and the inward T-type calcium current (ICa) (Grudt & Perl, 2002; Graham et al. 2007; Tadros et al. 2012).
Figure 5. Incidence of voltage-activated subthreshold currents in control mice and untrained and exercise trained SCI mice at 3 and 6 weeks.
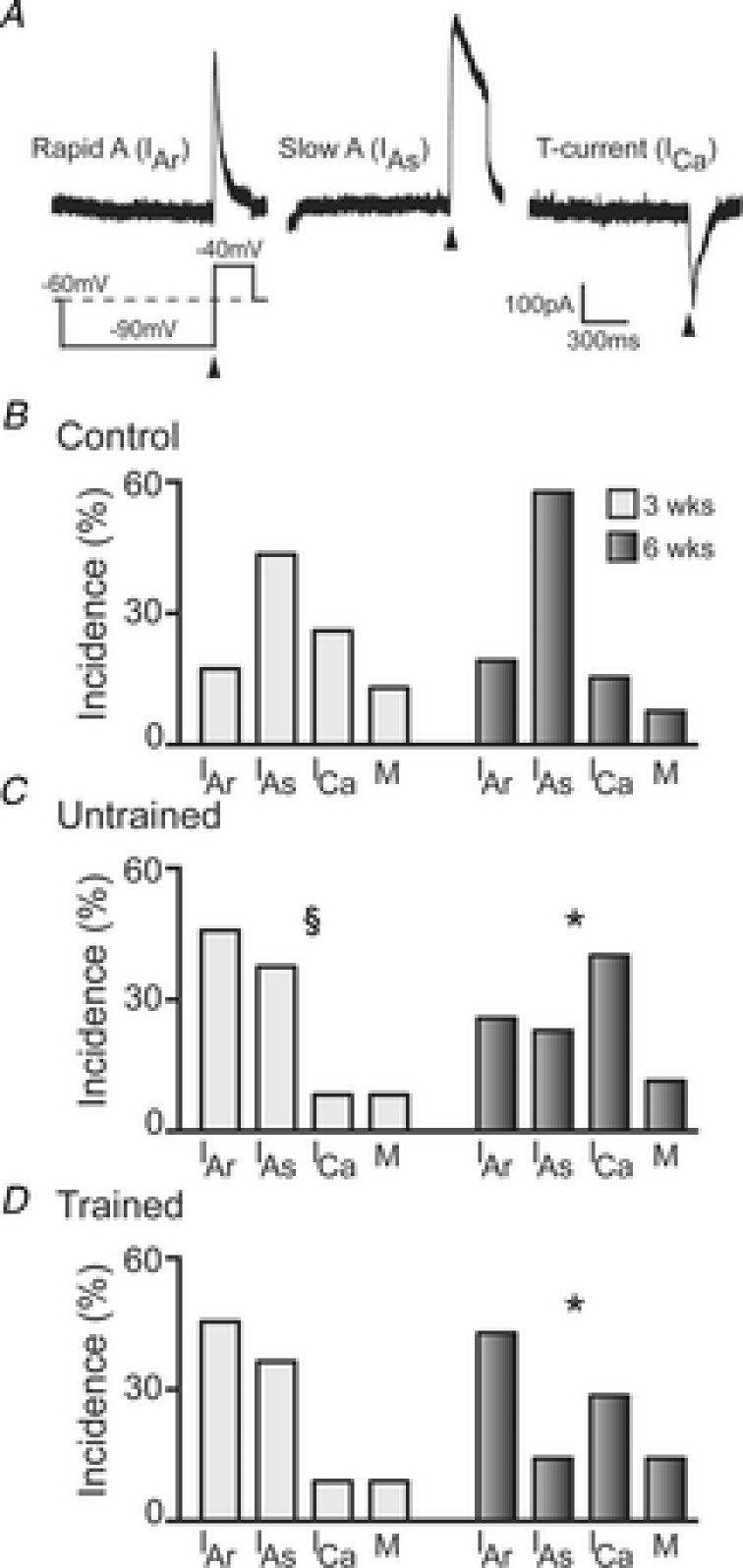
A, representative traces of the three ‘dominant’ voltage-activated subthreshold currents present in DDH interneurons. Voltage-step protocol used to elicit subthreshold currents is shown below current trace for Rapid A (IAr). B, group data showing the incidence of each subthreshold current in control animals for the 3 weeks (light grey bars, n = 23 neurons) and 6 weeks (dark grey bars, n = 26) time points. C, incidence of subthreshold currents in untrained SCI animals at 3 weeks (light grey bars, n = 24) and 6 weeks (dark grey bars, n = 35) time points. D, incidence of subthreshold currents in exercise trained SCI animals at 3 weeks (light grey bars, n = 11) and 6 weeks (dark grey bars, n = 21) time points. §P < 0.05 for 3 weeks untrained group compared to 6 weeks untrained group; *P < 0.05 for 6 weeks untrained and 6 weeks trained group compared to 6 weeks control group. Significance obtained using χ2 test.
Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded for a minimum of 3 min (holding potential −60 mV). sEPSCs were captured and analyzed using Axograph software. Captured sEPSCs were averaged and rise-time (calculated over 10–90% of peak amplitude), peak amplitude (baseline to peak negative current), and decay time constant (calculated over 20–80% of the decay phase) were obtained from the averaged event. sEPSC frequency was calculated from at least 150 s of data. Synaptic responses evoked by DC stimulation were recorded in voltage clamp (holding potential −60 mV). The DCs were stimulated, using a bipolar tungsten electrode, at 1.2 × threshold with a 0.1 ms current pulse at 0.2 Hz. Peak amplitude (baseline to peak negative current) was obtained from an averaged response (15 trials).
Statistical analysis
All comparisons of intrinsic, synaptic and DC stimulation-evoked properties based on cell location were made using a one-way ANOVA with a Tukey post hoc test. A Kruskal–Wallis non-parametric one-way analysis of variance with a Dunn post hoc test was used for data that did not meet the assumptions for ANOVA. Additional analyses were made to compare DC stimulation threshold and amplitude of DC evoked EPSCs for cells above versus below the hemisection using a Mann–Whitney U test. Comparisons of intrinsic and synaptic properties as well as DC evoked responses from control, untrained and trained groups at 3 and 6 week time points were made using a multiple comparison two-way ANOVA with a Tukey post hoc test. The incidence of AP discharge patterns, or subthreshold currents were compared using a χ2 test with a Williams correction. To avoid clutter on figures, indications of significance are only provided for results obtained using either two-way ANOVA (main effects only, not interactions) or χ2 tests. Effect sizes (described below) are not shown on figures.
Following two-way ANOVA comparison, post hoc tests were often unable to reveal significant differences between individual groups, as a large number of comparisons were required to identify the location of specific differences between groups. To provide the necessary statistical power for such two-way ANOVA and Tukey's post hoc tests to reveal significance in these data, a very large n for each group would be required. Sample size calculations revealed that a minimum of a 50% increase in the number of neurons included in each group would be required to reveal statistical significance using this approach. Due to ethical limitations on excessive animal use we therefore chose to employ a statistic that allows a better understanding of the magnitude of differences in means between response categories when using smaller numbers. This statistic is increasingly common in human intervention studies and particularly valuable where the number of patients or participants is limited such as in rare conditions. To this end, we calculated the standardised difference (Cohen's d), termed effect size (ES), for all possible pairwise comparisons between controls and untrained and trained groups at 3 and 6 week time points for intrinsic, synaptic and DC evoked properties.
Cohen's d is calculated as the difference between two means divided by a standard deviation for the data and describes the magnitude of differences between the means of two groups in a consistent and standardised way. The magnitude of differences between groups is what is important, with larger effect sizes more likely indicating biologically relevant changes. Effect size calculations have several advantages over traditional statistical methods. Most notably, the effect size statistic is less susceptible to the influence of sample size, meaning that biologically relevant effects can be detected without requiring large sample sizes. The effect size statistic also accommodates complex designs and allows multiple comparisons without sacrificing statistical power. We designated values of d < 0.3 to indicate trivial effect size, d of 0.3–0.5 a small effect size, d of 0.5–0.8 a moderate effect, and d > 0.8 to indicate a large effect.
For all comparisons, normality of the data was first assessed using a Kolmogorov–Smirnov test. Significance for these statistical tests was set at P < 0.05. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Data from SCI and age-matched control mice in the ‘3 weeks’ group were obtained from 198 interneurons (67 control, 65 untrained, 66 trained) from 45 adult mice (19 control, 14 untrained, 12 trained). Data from the ‘6 weeks’ group were obtained from 231 interneurons (57 control, 88 untrained, 86 trained) from 40 adult mice (13 control, 13 untrained, 14 trained). The average yield (number of neurons recorded per animal) was not different (P = 0.23) for control mice at 3 weeks (3.5 ± 0.4) and 6 weeks (4.4 ± 0.5). The average yield for SCI animals was lower in the 3 weeks compared to 6 weeks group (5.1 ± 0.4 vs. 6.6 ± 0.6 neurons per animal; P = 0.03), with no differences in yield between untrained and trained groups at either 3 or 6 weeks.
Effect of neuron location relative to spinal cord lesion
All recorded neurons from SCI animals were located within two spinal segments of the T10 hemisection (Fig. 1B and C). As the axon trajectory of interneuron populations can vary and thus play differing roles in reforming intraspinal circuits near a SCI (Fenrich et al. 2007; Fenrich & Rose, 2009, 2011) we first determined whether neurons responded differently according to their location relative to the hemisection (Fig. 1B and C). The five locations relative to the lesion were: contralateral–rostral (CR), ipsilateral–rostral (IR), ipsilateral–caudal (IC), contralateral–caudal (CC) and contralateral–epicentre (CE area immediately contralateral to the lesion).
In both untrained and trained SCI animals there were no differences in passive or active membrane properties (input resistance, rheobase current, resting membrane potential, AP threshold, AP amplitude, AHP peak and AHP latency) for cells within any given group (i.e. 3 weeks untrained) according to neuron location. This was true for both 3 and 6 weeks SCI groups. Similarly sEPSC properties (rise time, decay time constant, amplitude, and frequency) and DC evoked responses (threshold and amplitude) did not differ according to recording location. These data suggest that all neurons within close proximity (<2 spinal segments) of the spinal cord lesion were affected similarly in our model. We therefore pooled data across recording location and hereafter results are presented as neurons in the vicinity of a SCI in untrained and trained mice. For control animals, recordings were made from neurons in the same spinal cord segments (i.e. T8–T12).
Passive membrane properties
For each passive intrinsic membrane property (input resistance, rheobase current and RMP) a two-way ANOVA identified significant interactions (P < 0.05) between time (3 or 6 weeks) and group (control, untrained or trained) (Fig. 2). Pairwise comparisons using Cohen's d as the indicator of the magnitude of differences between means (i.e. effect size, ES) showed the following.
Figure 2. Passive intrinsic membrane properties in uninjured age-matched controls and untrained and trained SCI mice.
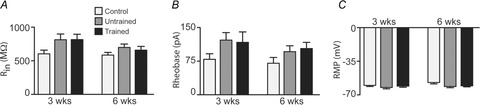
Bar plots compare mean ± SEM values for passive membrane properties for controls and (light grey bars), untrained (dark grey bars) and trained (black bars) SCI mice. A, input resistance (Rin) for 3 weeks control (n = 67 neurons), untrained (n = 65) and trained (n = 66) as well as 6 weeks control (n = 57), untrained (n = 88) and trained (n = 86) mice. B, rheobase in 3 weeks control (n = 44), untrained (n = 45) and trained (n = 44) and 6 weeks control (n = 39), untrained (n = 65) and trained (n = 61) mice. C, resting membrane potential (RMP) for neurons in 3 weeks control (n = 55), untrained (n = 61) and trained (n = 61) as well as 6 weeks control (n = 47), untrained (n = 67) and trained (n = 73) mice.
Input resistance was higher in both untrained (ES small, d = 0.37) and trained (ES small, d = 0.39) SCI groups compared to control mice at 3 weeks. This difference was not present at 6 weeks (d = 0.30). Rheobase current was higher in both untrained (ES small, d = 0.44) and trained (ES small, d = 0.32) SCI groups compared to controls at 3 weeks. At 6 weeks, small differences in rheobase current were still evident in trained (ES small, d = 0.35) but not untrained (d = 0.28) SCI groups compared to the controls. For RMP there were no differences among controls and both SCI groups at 3 weeks. By 6 weeks, there were small differences between controls and both untrained (ES small, d = 0.42) and trained (ES small, d = 0.37) SCI groups, with RMP being more hyperpolarised in both SCI groups. There was a small change (ES small, d = 0.32) in RMP in control mice when comparing data from 3 and 6 weeks SCI groups. In summary, there were a number of small differences between the controls and both SCI groups for all three variables at 3 and 6 weeks. There were, however, no differences between the SCI groups, suggesting that exercise training did not affect passive membrane properties at either time point.
Active membrane properties
Selected active membrane properties (AP threshold, AP amplitude, AHP peak and AHP latency) are presented in Fig. 3. A two-way ANOVA revealed interaction effects as follows: AP threshold (P = 0.05), AP amplitude (P = 0.18), AHP peak (P = 0.04) and AHP latency (P = 0.07). There was a significant main effect for only one active membrane property: AP threshold was more hyperpolarised in both untrained (P = 0.006) and trained (P < 0.0001) SCI groups when compared to controls at the 6 week time point. This significance is further supported by the presence of moderate and large effect sizes as described below.
Figure 3. Active intrinsic membrane properties in uninjured age-matched controls and untrained and trained SCI mice.
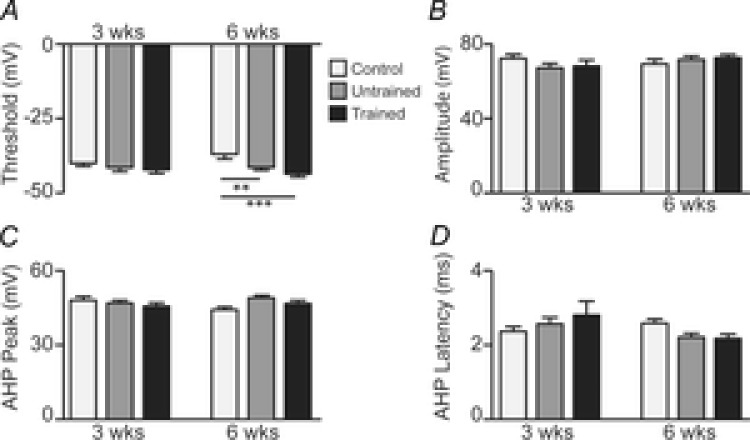
A–D, bar plots comparing mean ± SEM values for four active intrinsic membrane properties (A, threshold; B, peak amplitude; C, AHP peak; D, AHP latency) in 3 weeks controls and (light grey bars; n = 44 neurons), untrained (dark grey bars; n = 45) and trained (black bars; n = 44) SCI mice and in 6 weeks controls (n = 39), and untrained (n = 66) and trained (n = 61) SCI mice. Significance obtained from two-way ANOVA comparison: **P < 0.01, ***P < 0.0001.
For AP threshold (Fig. 3A), a small difference (d = 0.38) was observed between the controls and trained SCI groups at 3 weeks. By 6 weeks, AP threshold was more hyperpolarised in the untrained (ES moderate, d = 0.69) and trained (ES large, d = 0.96) groups compared to controls; there was also a small difference (d = 0.32) between the untrained and trained SCI groups. In the control group, AP threshold was more depolarised at 6 weeks compared to 3 weeks (ES moderate, d = 0.50) but there were no changes in the SCI groups over this time. For AP amplitude (Fig. 3B), there were no meaningful differences within the 3 or 6 weeks groups, nor changes between 3 and 6 weeks. AHP peak (Fig. 3C) did not differ among the groups at 3 weeks. By 6 weeks, AHP peak amplitude was higher in both untrained (ES moderate, d = 0.63) and trained (ES small, d = 0.31) SCI groups compared to controls. From 3 to 6 weeks, AHP peak amplitude decreased in the control mice (ES small, d = 0.43) whereas there were no changes in either SCI group. AHP latency (Fig. 3D) did not differ among the groups at 3 weeks, but by 6 weeks, AHP latency was shorter in both the untrained (ES small, d = 0.49) and trained (ES moderate; d = 0.50) SCI groups compared to controls. From 3 to 6 weeks, there were small decreases in AHP latency in the untrained (ES small, d = 0.37) and trained (ES small, d = 0.36) SCI groups.
In summary, there were mainly only small differences among the three groups for all four variables at 3 weeks. However, at 6 weeks AP threshold was more hyperpolarised, AHP peak amplitude was higher, and AHP latency was lower in the SCI groups compared to controls. With the exception of small effect on AP threshold, there was no effect of exercise training on any other active membrane properties.
Discharge patterns
Injection of a square step depolarising current (800 ms long) resulted in one of four AP discharge patterns: tonic firing (TF), initial bursting (IB), delayed firing (DF) and single spiking (SS) (Fig. 4A). We documented the incidence of discharge patterns across each group because these properties reflect specific ion channel expression patterns and are known to change in spinal neurons during development (Tadros et al. 2012), and in response to various perturbations such as injury and ion channel mutations (Graham et al. 2007; Lu et al. 2009; Harris et al. 2014).
Figure 4. Incidence of AP discharge patterns in controls and untrained and trained SCI mice.
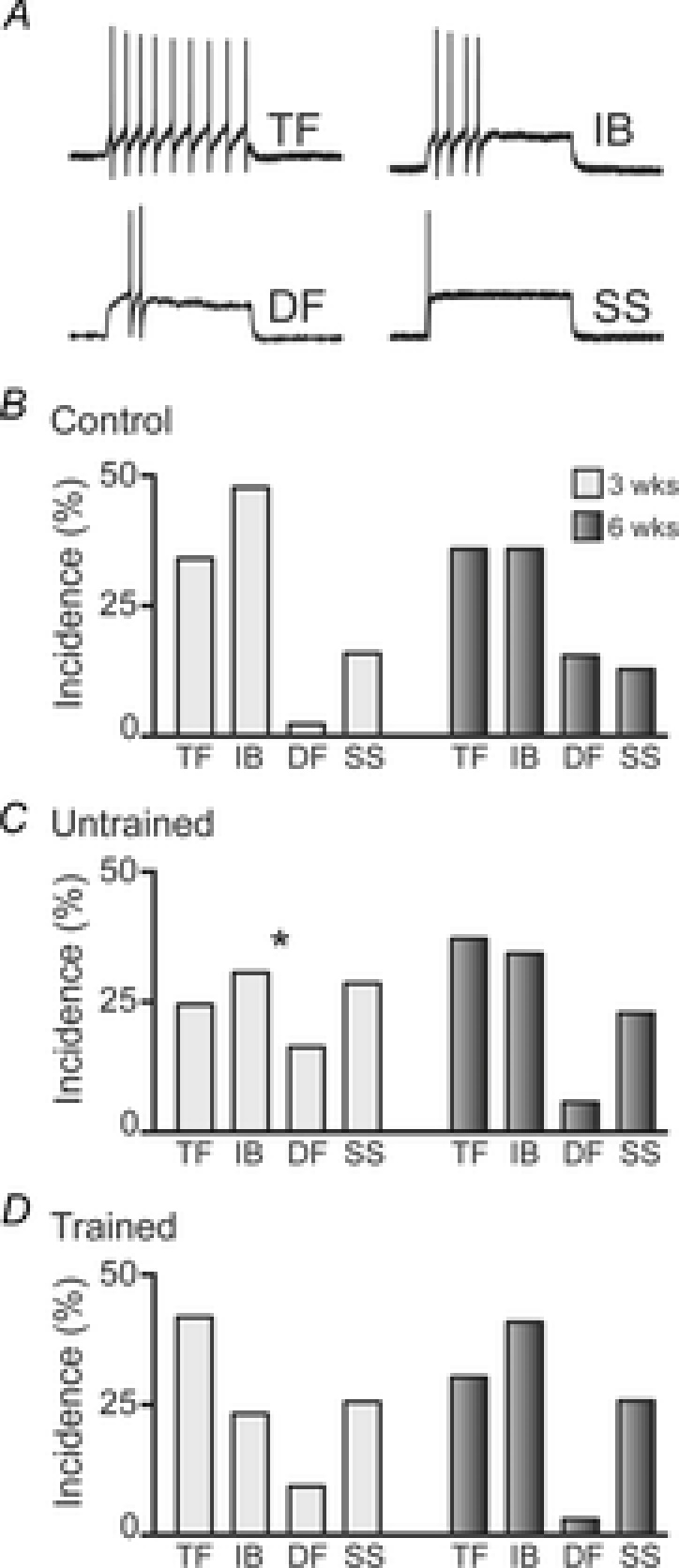
A, representative traces showing each of the four distinct AP discharge patterns exhibited by DDH interneurons in response to a 800 ms square step depolarising current injection: tonic firing (TF), initial bursting (IB), delayed firing (DF) and single spiking (SS). B, group data showing the incidence of each of the four AP discharge patterns in age-matched uninjured control animals for the 3 weeks (light grey bars, n = 44 neurons) and 6 weeks (dark grey bars, n = 39) time points. C, incidence of AP discharge patterns in untrained SCI animals at 3 weeks (light grey bars, n = 49) and 6 weeks (dark grey bars, n = 70) time points. D, incidence of AP discharge patterns in trained SCI animals at 3 weeks (light grey bars, n = 43) and 6 weeks (dark grey bars, n = 66). *P < 0.05 for 3 week untrained group compared to 3 week control group. Significance obtained using χ2 test.
At 3 weeks, the incidence of the four discharge patterns was significantly different in untrained SCI animals compared to controls (χ2 test, P = 0.03). This was due to a reduction in the incidence of TF and IB neurons and increased incidence of DF and SS neurons in the 3 weeks untrained SCI animals (Fig. 4B and C). The incidence of each discharge pattern in 3 weeks trained animals was not significantly different to controls. At 6 weeks, there were no significant differences in the incidence of the four discharge patterns between controls and SCI groups (Fig. 4B–D). Together, these data suggest there is reorganisation of AP discharge properties at 3 weeks after SCI, and exercise training limits this reorganisation. However, by 6 weeks AP discharge properties are comparable to controls and there is no effect of exercise training.
Voltage-gated subthreshold currents
The expression of several voltage-gated ion channels is known to underlie the different AP discharge patterns that exist in dorsal horn interneurons (Yoshimura & Jessell, 1989; Ruscheweyh et al. 2004; Graham et al. 2007). We used a voltage-clamp protocol that allows the evaluation of three voltage-gated, subthreshold currents that are known to shape AP discharge in dorsal horn neurons. Subthreshold currents were activated and identified using a protocol that first hyperpolarises the neuron to −90 mV (1 s duration), from a holding potential of −60 mV. This is followed by a depolarising step to −40 mV (200 ms duration) (Fig. 5A lower left).
Using this protocol, three major subthreshold currents were identified that are consistent with those previously described in detail for dorsal horn neurons (Yoshimura & Jessell, 1989; Grudt & Perl, 2002; Graham et al. 2007; Walsh et al. 2009). Two outward K+ currents were identified: one with rapid activation and inactivation kinetics consistent with rapid A-current (IAr; Fig. 5A, left trace); and the other with slower activation and inactivation kinetics, consistent with a slow A-current (IAs; Fig. 5A, middle trace). One inward current was also identified, with rapid activation and inactivation kinetics, consistent with low-threshold activated T-type Ca2+ current (ICa; Fig. 5A, right trace). In a minority of neurons (8–14% across groups) more than one ‘dominant’ subthreshold current existed. The incidence of these ‘mixed currents’ (M), was included in the incidence calculations for each group.
Using the above protocol (Fig. 5A, lower left trace), subthreshold currents were clearly resolved in 23/35 (66%) neurons at 3 weeks and 26/37 (70%) neurons at 6 weeks for control animals. In SCI animals, subthreshold currents were resolved in 24/34 (71%) of neurons from untrained and 11/31 (35%) from trained animals at 3 weeks and 35/55 (64%) from untrained and 21/53 (40%) from trained animals at 6 weeks.
The incidence of each type of subthreshold current in controls and SCI groups at 3 and 6 weeks is shown in Fig. 5B–D. At 3 weeks, there were no significant differences in the incidence of each subthreshold current among the groups. At 6 weeks both the untrained and trained SCI animals exhibited significant differences compared to controls in the incidence of each subthreshold current (χ2 test, controls vs. untrained P = 0.02; controls vs. trained P = 0.01). Specifically, expression of IAr and ICa increased, and IAs decreased between controls and both SCI groups. Between 3 and 6 weeks, the proportion of these subthreshold currents significantly changed in the untrained SCI group (P = 0.02), with decreased expression of IAr and IAs and increased expression of ICa; similar trends were observed in the trained group. In summary, there is substantial reorganisation of the major subthreshold currents, primarily at 6 weeks in SCI animals, though an effect of exercise training is not apparent.
Spontaneous excitatory synaptic current properties (sEPSCs)
To assess any differences in synaptic properties after SCI, we recorded sEPSCs from neurons at –60 mV holding potential. Under these conditions, sEPSCs manifest as inward currents (Fig. 6A) and are mediated by the activation of AMPA-type glutamate receptors because they are abolished by 10 μm CNQX (Flynn et al. 2011a). Using a two-way ANOVA to evaluate differences, sEPSC rise time did not differ (P > 0.05) among groups at 3 weeks (mean rise times: controls 0.81 ± 0.04, untrained 0.71 ± 0.04, trained 0.74 ± 0.04 ms) or 6 weeks (controls 0.86 ± 0.05, untrained 0.84 ± 0.05, trained 0.95 ± 0.18 ms) nor were there changes from 3 to 6 weeks. Decay time constants also did not differ within groups at 3 weeks (mean decay time: controls 2.96 ± 0.18, untrained 2.64 ± 0.22, trained 2.92 ± 0.18 ms) or 6 weeks (controls: 3.07 ± 0.20, untrained 2.93 ± 0.17, trained 3.10 ± 0.37 ms), nor were there changes from 3 to 6 weeks. All comparisons of effect sizes for both rise time and decay time constants were trivial (d < 0.3). These results show the kinetics of fast, glutamatergic neurotransmission is not affected by SCI or exercise training (Fig. 6B and C).
Figure 6. Properties of spontaneous excitatory postsynaptic currents (sEPSCs).
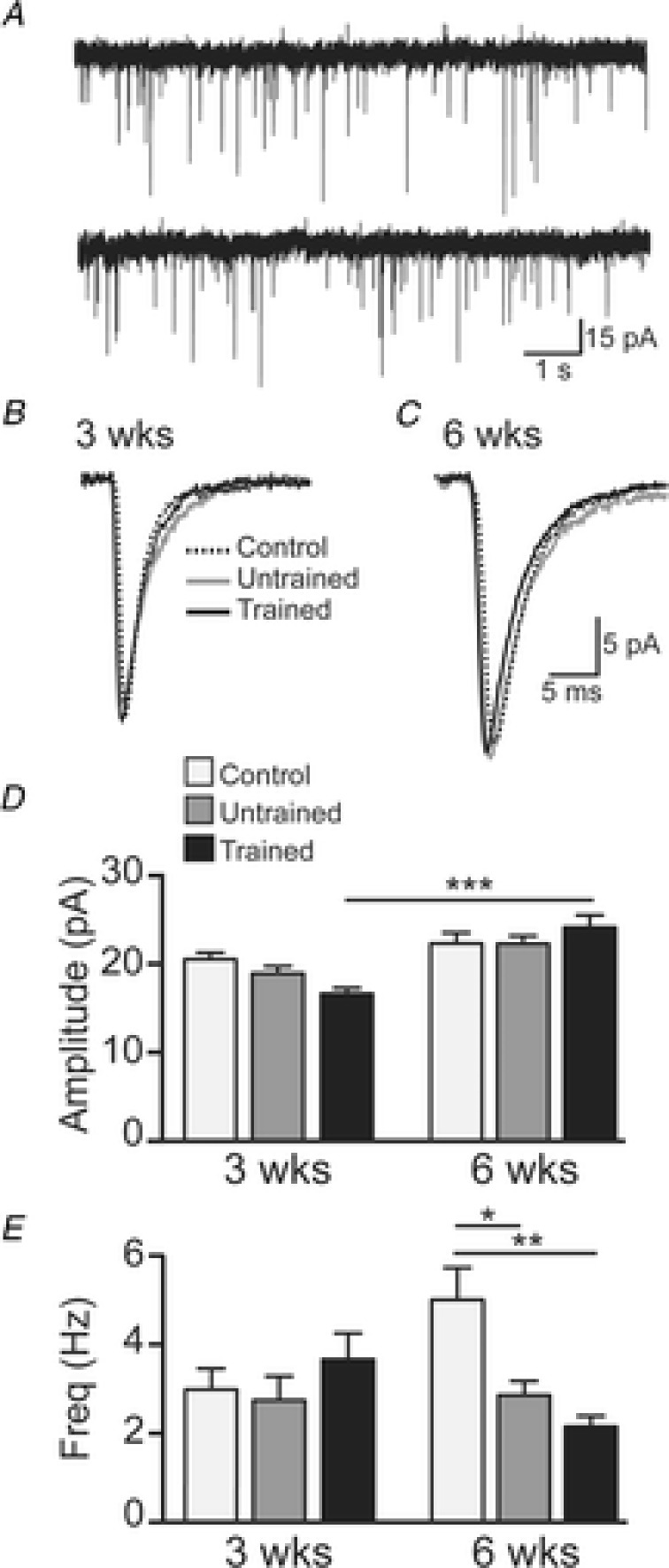
A, representative trace showing sEPSCs recorded in a 6 weeks trained SCI mouse (holding potential −60 mV). Traces shown are two 10 s epochs of continuous data. B and C, example sEPSCs (averaged from all events in 180 s of data) from 3 weeks and 6 weeks controls (black dotted line), and untrained (grey continuous line) and trained (black continuous line) SCI mice. D, group data (mean ± SEM) of sEPSC amplitude for 3 weeks controls (n = 52 neurons), and untrained (n = 46) and trained (n = 49) SCI mice and in 6 weeks controls (n = 45), and untrained (n = 63) and trained (n = 70) SCI mice. E, group data (mean ± SEM) of sEPSC frequency for 3 weeks controls (light grey bars; n = 52), and untrained (dark grey bars; n = 43) and trained (black bars; n = 54) SCI mice and in 6 weeks controls (n = 46), and untrained (n = 64) and trained (n = 72) SCI mice. Significance obtained from two-way ANOVA comparison: *P < 0.05, **P < 0.001, ***P < 0.0001.
Analysis of sEPSC amplitude (Fig. 6D) using a two-way ANOVA showed a significant increase in amplitude from 3 to 6 weeks with exercise training (P < 0.0001). Additional analysis of effect sizes revealed the following. sEPSC amplitude was lower in the trained SCI group compared to controls (ES moderate, d = 0.80) at 3 weeks but this difference was no longer present at 6 weeks. From 3 to 6 weeks, sEPSC amplitude was increased in untrained (ES moderate, d = 0.53) and in trained SCI animals (ES large, d = 0.95). These results suggest excitatory synaptic drive is increased over time after SCI and is enhanced by 6 weeks of exercise training.
Analysis of sEPSC frequency (Fig. 6E) using a two-way ANOVA showed several significant differences. Firstly, sEPSC frequency did not differ among the groups at 3 weeks; however, at 6 weeks sEPSC frequency was lower in both untrained (P = 0.02) and trained (P = 0.0002) SCI groups compared to controls. These results are supported by comparison of effect sizes which additionally demonstrate that at 6 weeks, sEPSC frequency was lower in both untrained (ES moderate, d = 0.58) and trained (ES large, d = 0.83) SCI groups compared to controls. Importantly, there was a small difference (ES small, d = 0.31) between the trained and untrained SCI groups. From 3 to 6 weeks sEPSC frequency was increased in the control group (ES moderate, d = 0.49) and decreased in the trained SCI group (ES small, d = 0.47). These results demonstrate that synaptic strength, as indicated by sEPSC frequency, is decreased with 6 weeks of exercise training.
DC evoked EPSCs
Responses evoked by stimulation of the DCs reflect the connectivity of descending spinal pathways. Importantly, the most ventral region of the DCs in rodents contains the corticospinal tract that projects to interneurons in the DDH (lamina IV and V, Fig. 1A; Tracey, 2004), where our recordings are made (Fig. 1A and B). The DC evoked EPSCs we recorded are clearly polysynaptic in nature (Fig. 7A), suggesting the activation of multiple synapses between the stimulation site and the recorded neuron.
Figure 7. Properties of DC evoked EPSCs in controls and untrained and trained SCI mice at 3 week and 6 week time points.
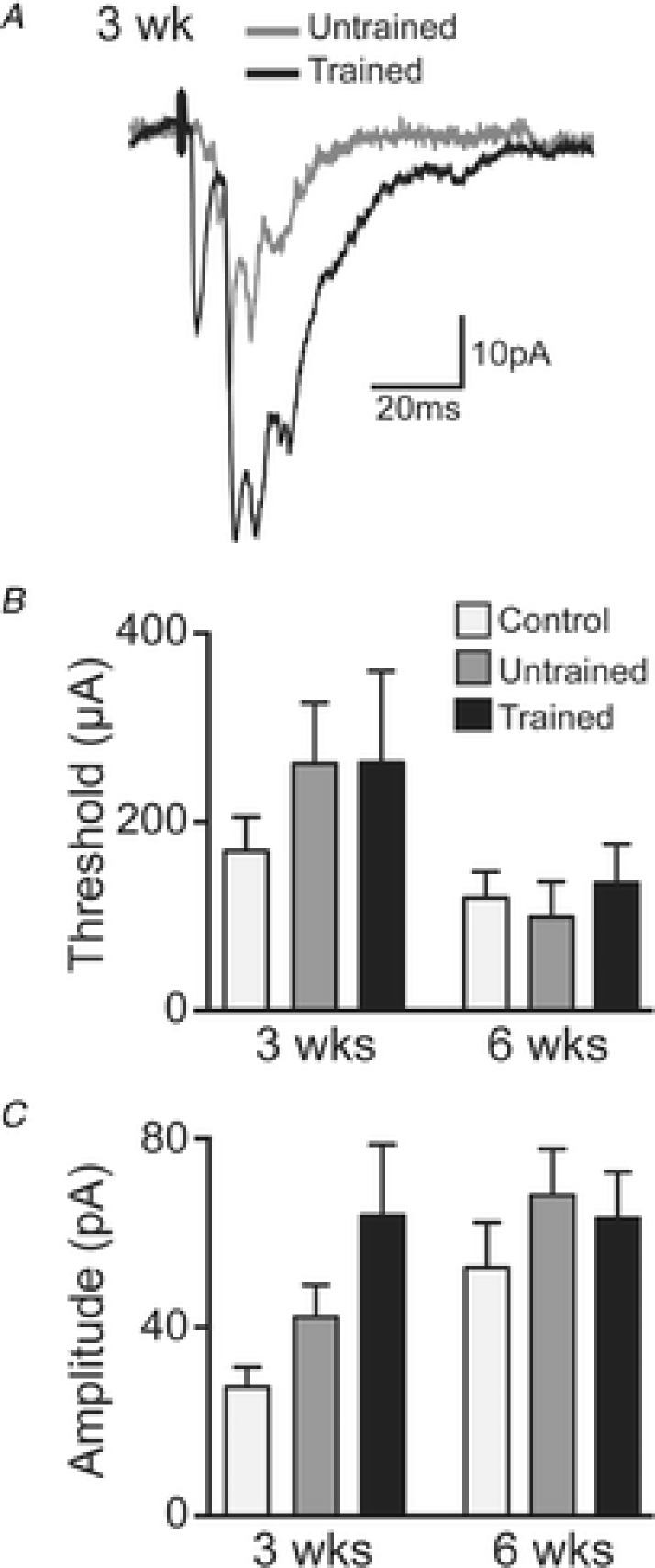
A, representative traces of averaged (15 trials) DC evoked EPSCs from 3 weeks untrained (grey trace) and trained (black trace) SCI mice. B, group data (mean ± SEM) for stimulus threshold for 3 weeks controls (light grey bars; n = 10 neurons), and untrained (dark grey bars; n = 21) and trained (black bars; n = 12) SCI mice and in 6 weeks controls (n = 13), and untrained (n = 27) and trained (n = 30) SCI mice. C, group data (mean ± SEM) of DC evoked EPSC amplitude for 3 weeks controls (n = 8), and untrained (n = 21) and trained (n = 11) SCI mice and in 6 weeks controls (n = 13), and untrained (n = 26) and trained (n = 25) SCI mice.
Interestingly, we demonstrate that the effects of DC stimulation are equivalent for interneurons rostral as well as caudal to the lesion (where half of the DCs have presumably been damaged). A Mann–Whitney U test was used to compare DC stimulation threshold and DC evoked EPSC amplitude for neurons rostral and caudal to the lesion. In 3 weeks and 6 weeks untrained and trained SCI animals there were no differences in DC stimulation threshold for neurons rostral to the lesion compared to those caudal to the lesion (average P = 0.51 ± 0.32). Likewise, there were no differences in DC evoked EPSC amplitude recorded from neurons rostral to the lesion compared to those caudal to the lesion (average P = 0.77 ± 0.14).
Analysis of stimulus threshold and amplitude of DC evoked EPSCs with a two-way ANOVA identified no significant effect of time (3 or 6 weeks) or group (control, untrained or trained), nor any significant interactions (P > 0.05) between time and group. Comparison of effect sizes, however, revealed the following. The stimulus threshold (Fig. 7B), defined as the level of the applied stimulus required to consistently evoke a response from the recorded interneuron, was higher in both the untrained (ES small, d = 0.45) and trained (ES small, d = 0.42) SCI groups compared to controls at 3 weeks. By 6 weeks, however, there were no differences among the groups. From 3 to 6 weeks there were decreases in the stimulus threshold in all groups (control: ES small d = 0.46; untrained: ES moderate, d = 0.67; trained: ES small, d = 0.45). These data suggest that stimulus threshold returns to pre-injury values over time after SCI, and is unaffected by exercise training in SCI mice.
The peak amplitude of DC evoked EPSCs (Fig. 7C), evoked with 1.2 × threshold, 0.1 ms stimulus pulses at 0.2 Hz, differed substantially between controls and both SCI groups (untrained: ES moderate, d = 0.69; trained ES large, d = 1.18), and between the untrained and trained SCI groups (ES moderate, d = 0.53) at 3 weeks. At 6 weeks, only the difference between controls and trained (ES moderate, d = 0.58) SCI groups was still observed. Although the mean peak amplitude in the untrained SCI group at 6 weeks is larger than that of trained animals (43.1 pA untrained vs. 35.8 pA trained), a higher SD in the untrained group (34.0 untrained vs. 23.3 trained) precludes a larger effect size at this time point. From 3 to 6 weeks, there were increases in amplitude in the control (ES large, d = 1.08) and trained SCI (ES moderate, d = 0.77) groups. This suggests that although by 6 weeks post injury the amplitude of DC evoked EPSCs is increased, independent of exercise training, just 3 weeks of exercise training can produce a similar increase in response amplitude. It is important to note that differences in stimulus intensity did not mask changes in DC evoked EPSC amplitude. For example, increases in DC evoked EPSCs in 6 weeks untrained and trained SCI groups occur with a concomitant reduction in the threshold of DC stimulus intensity compared to 3 weeks untrained and trained SCI groups. Again, this may reflect a recovery in the properties of descending DC axons over time after SCI.
Discussion
This study expands on previous work where we demonstrated that it was possible to use a horizontal spinal cord slice preparation to make whole-cell patch-clamp recordings from adult mice with a SCI and examine the effects of exercise training (Flynn et al. 2013). The previous study employed only 3 weeks of training after SCI. Here, we have extended treadmill exercise training to 6 weeks, and considerably expanded the number of functional measurements made on DDH interneurons. Our main aim was to compare the effects of different durations of exercise training on the functional properties of interneurons in the vicinity of a SCI. We have also included recordings from neurons in age-matched uninjured mice (i.e. controls) to assess the effects of SCI on the functional properties of interneurons. We hypothesised that 6 weeks of treadmill training would selectively enhance synaptic properties of local and descending circuits. We also predicted that both intrinsic and synaptic properties of interneurons from SCI mice would differ considerably from those recorded in age-matched uninjured control mice.
Our study had three main findings. Firstly, a lesion to the spinal cord alters some intrinsic membrane properties, but has more dramatic effects on the synaptic properties of interneurons in the vicinity of the lesion. All neurons within two spinal segments of the lesion were affected similarly in our model. Secondly, the 3 week time point in SCI animals is chiefly characterised by some plasticity in intrinsic membrane properties, whereas at 6 weeks, changes to synaptic drive and an increase in the strength of synaptic connections appear to be more prominent. Lastly, the changes we observe in the intrinsic membrane properties of DDH interneurons in the vicinity of a lesion are not affected by the exercise training protocol we employed. In contrast, considerable plasticity in excitatory synaptic drive and DC mediated synaptic activity accompanies exercise training. Importantly, both intrinsic and synaptic properties of neurons in the control group differ markedly from those in either untrained or trained SCI groups.
Properties of uninjured neurons differ markedly from SCI neurons
Our study included an uninjured age-matched control group. This allowed us to compare the intrinsic and synaptic properties of interneurons after SCI and exercise training with those recorded in undamaged spinal cord tissue. Studies on the effects of interventions, including exercise, after SCI rarely include an uninjured control group even though this is imperative to contextualise the efficacy of any intervention/treatment (Battistuzzo et al. 2012). We demonstrate that large differences in passive and active membrane properties are evident between controls and SCI mice at both the 3 and 6 weeks training-related time points, with only minor improvements being attributable to exercise training within the SCI groups (Figs 2 and 3). Likewise, synaptic properties such as sEPSC frequency and the amplitude of DC evoked EPSCs (Figs 6B and 7C) continued to differ in SCI interneurons compared to those from uninjured control mice. Our findings also confirm that the injury and associated ‘metabolic chaos’ that occurs in the vicinity of a SCI (Dusart & Schwab, 1994; Fitch et al. 1999; Schnell et al. 1999) have important implications for the functional properties of interneurons in an area much larger than the injury site. Moreover, these SCI effects persist irrespective of time since injury or exercise training.
Our study demonstrates that a SCI has similar profound effects on the neuronal membranes and synaptic networks of all DDH interneurons in the vicinity of a lesion (two spinal segments above and below the injury). When we compared the responses of neurons based on their location relative the lesion (Fig. 1B and C), there was no evidence that neurons within any given SCI group responded differently according to their location relative to the hemisection. This carries the important implication that the primary injury and secondary events following SCI (Dusart & Schwab, 1994; Fitch et al. 1999; Schnell et al. 1999) have a similar effect on interneurons that lie within two segments of the lesion, be they spared or directly injured by the SCI. Similar observations regarding functional changes such as diminished conduction velocity and AP amplitude in intact axons following compression or hemisection SCI in rodents have also been reported (Nashmi & Fehlings, 2001; Hains et al. 2004; Arvanian et al. 2009). In some cases, these functional changes have been directly attributed to secondary inflammatory and demyelination damage (Park et al. 2004; Totoiu & Keirstead, 2005; Hunanyan et al. 2011). In future studies, it will be interesting to determine how far these effects extend from the initial injury site.
Importantly, we demonstrate that the effects of DC stimulation within each SCI group are equivalent for interneurons rostral to the lesion, for which the DCs are intact, and those caudal to the lesion, for which half of the DCs have been damaged. The similarity in responses of these neurons may result from the profound primary and secondary effects following SCI, as discussed above. However, the failure to find significant differences between the rostral and caudal populations of interneurons may not necessarily indicate that these populations of interneurons are identical; rather we simply have no positive evidence that they differ. Further exploration of this phenomenon is warranted.
Passive membrane properties show increased instability in acute injury
Our study demonstrates that in the comparatively acute stages of SCI (i.e. the 3 week time point) passive membrane properties of DDH interneurons show larger differences compared to controls than in later stages after SCI (i.e. 6 week time point). Specifically, input resistance and rheobase current are increased to a greater extent compared to controls in SCI mice at 3 weeks vs. at 6 weeks (Fig. 2A and B). Changes in both these properties reflect plasticity in membrane excitability. At the 6 week time point, intrinsic membrane properties differ less substantially compared to controls. Together, our 3 and 6 weeks data suggest that the cellular membrane of interneurons is in a greater state of flux at the 3 week time point and that this stabilises over time following injury. Anatomical and structural changes after SCI such as sprouting of collateral fibres (Fouad et al. 2001; Raineteau et al. 2002; Bareyre et al. 2004; Ballermann & Fouad, 2006; Martinez et al. 2011) and demyelination (Hunanyan et al. 2011) usually require a minimum of 3 weeks before they stabilise. Additionally, increased rheobase values, such as those we observe in 3 weeks vs. 6 weeks SCI animals, have been reported to result from changes in the distribution of Nav1.6 sodium channels on damaged and demyelinated neurons after a hemisection injury (Hunanyan et al. 2011). The resulting reduction in conduction velocity persists past 4 weeks post injury (Hubscher & Johnson, 2002). The variability and instability that we observe in passive membrane properties in neurons from animals 3 weeks post SCI are therefore consistent with the known anatomical and structural plasticity present in the acute stages of injury.
Synaptic properties are preferentially altered by exercise training
Our data show that both passive and active intrinsic membrane properties of interneurons in the vicinity of a spinal cord hemisection are not affected by exercise training, but rather are changed as a function of the SCI itself. In contrast, both excitatory synaptic drive and descending drive are preferentially affected by exercise training. Specifically, both spontaneous and DC evoked EPSCs are enhanced in SCI animals after 3 or 6 weeks of treadmill exercise training (Figs 6D and 7C). In the case of DC evoked EPSCs, a marked increase in the amplitude of responses occurred after as little as 3 weeks of training and these changes were increased further by 6 weeks of exercise. Furthermore, an increase in the amplitude of DC evoked EPSCs was also evident in 6 weeks untrained mice, indicating that although 3 weeks of exercise training is sufficient to increase the amplitude of DC evoked EPSCs, by 6 weeks post injury the untrained group has ‘caught up’. Overall our data demonstrate that exercise seems to preferentially promote synaptic over intrinsic plasticity in our model of SCI. This finding is consistent with anatomical studies, which demonstrate significant axonal sprouting in the proximity of a SCI, where these de novo and reorganised synapses are enhanced by exercise training following SCI and can contribute to functional recovery (Goldstein et al. 1997; Fouad et al. 2001; Bareyre et al. 2004; Engesser-Cesar et al. 2007; Goldshmit et al. 2008; Onifer et al. 2011). How the synaptic changes we report here lead to functional recovery is not known. However, these changes in excitatory synaptic connections do suggest that the ‘new’ synaptic contacts observed in anatomical studies are functional and are enhanced by exercise training. This would lead to an increased overall level of excitability within the DDH interneuron synaptic network, such as the increased descending synaptic drive we observed.
Strengths and limitations of this study
Our study provides an extensive and systematic electrophysiological characterisation of the intrinsic and synaptic properties of neurons in the vicinity of a SCI in response to the injury itself, and to varying durations of treadmill exercise training. We used randomisation when allocating animals to untrained or trained groups and blinding of the investigators who collected the electrophysiological data. These quality control steps are often lacking in SCI studies on animals (Battistuzzo et al. 2012). Additionally, we included an age-matched uninjured control group to provide important comparative data. Inclusion of such control data is often overlooked in SCI studies in animals (Battistuzzo et al. 2012). Our study design included different durations of treadmill exercise training (3 and 6 weeks), thus providing a longitudinal view of changes occurring over time after injury and with longer interventions. We believe these features add to the strength of our study.
After a SCI, it is possible that certain neuronal subtypes may be more susceptible to secondary cell death and damaging cellular reactions such as invasion of inflammatory cells and increased astrocyte activity (Dusart & Schwab, 1994; Fitch et al. 1999; Schnell et al. 1999). We included an uninjured control group in our study to gain further insights into these processes. Many of the largest differences in cellular and synaptic properties we report are between uninjured controls and SCI groups (both untrained and trained). While our data cannot confirm this, it is possible that the loss of a discrete subtype of neuron after the SCI contributes to the magnitude of differences we observed. Further studies are necessary to investigate this possibility.
Conclusions
In conclusion, our study provides the first systematic characterisation of the intrinsic and synaptic changes that occur in DDH interneurons after SCI and in response to different durations of treadmill exercise training. By including uninjured age-matched controls we show that the changes to intrinsic and synaptic properties of DDH interneurons from SCI alone are considerable. We have also demonstrated that neurons within two spinal segments of a hemisection injury are similarly affected by a spinal cord hemisection. Each of these findings emphasises the profound anatomical and functional changes caused by an injury to the spinal cord. We also show that the intrinsic properties of DDH interneurons show greater plasticity in the acute (3 weeks) versus chronic (6 weeks) phases of SCI. Synaptic activity, both local and descending, are preferentially enhanced by exercise training at both 3 and 6 weeks suggesting that exercise training exerts a positive and maintained effect on synaptic activity in DDH interneurons in the vicinity of a SCI.
Acknowledgments
We thank Lynda R. Dunn for her technical assistance and hard work in establishing post-surgical animal husbandry protocols.
Glossary
- AHP
afterhyperpolarisation
- AP
action potential
- ACSF
artificial cerebrospinal fluid
- CC
contralateral caudal
- CE
contralateral epicentre
- CR
contralateral rostral
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DC
dorsal column
- DDH
deep dorsal horn
- DF
delayed firing
- ES
effect size
- IAr
rapid A-current
- IAs
slow A-current
- IB
initial bursting
- IC
ipsilateral caudal
- ICa
T-type calcium current
- IR
ipsilateral rostral
- Rin
input resistance
- RMP
resting membrane potential
- SCI
spinal cord injury
- sEPSC
spontaneous excitatory postsynaptic potential
- SS
single spiking
- TF
tonic firing
Additional information
Competing interests
The authors have no competing interests.
Author contributions
All experiments were performed in the School of Biomedical Sciences and Pharmacy, University of Newcastle, Callaghan, NSW, Australia. Study concept and design: M.P.G., R.J.C., R.C., C.R.B., M.M.R. and J.R.F. Acquisition of data: M.M.R. and J.R.F. Analysis and interpretation of data: M.M.R., J.R.F., M.P.G., R.C. and R.J.C. Drafting of manuscript: M.M.R., J.R.F., R.C. and R.J.C. All authors approved the final version of the manuscript.
Funding
This research was supported by the National Health and Medical Research Council (Australia) grant ID 628765.
References
- Arvanian VL, Schnell L, Lou L, Golshani R, Hunanyan A, Ghosh A, Pearse DD, Robinson JK, Schwab ME, Fawcett JW. Mendell LM. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Exp Neurol. 2009;216:471–480. doi: 10.1016/j.expneurol.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML. Fitzgerald M. Intrinsic firing properties of developing rat superficial dorsal horn neurons. Neuroreport. 2005;16:1325–1328. doi: 10.1097/01.wnr.0000175612.08560.10. [DOI] [PubMed] [Google Scholar]
- Ballermann M. Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O. Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barry PH. Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Battistuzzo CR, Callister RJ, Callister R. Galea MP. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma. 2012;29:1600–1613. doi: 10.1089/neu.2011.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E. Gardiner P. Effects of daily spontaneous running on the electrophysiological properties of hindlimb motoneurones in rats. J Physiol. 2002;540:129–138. doi: 10.1113/jphysiol.2001.013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E. Gardiner PF. Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve. 2003;27:228–236. doi: 10.1002/mus.10308. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Kaloustian S, Rousseau G. Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci Res. 2008;62:147–154. doi: 10.1016/j.neures.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Bowden MG. Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86:1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR. Edgerton VR. Plasticity of functional connectivity in the adult spinal cord. Philos Trans R Soc Lond B Biol Sci. 2006;361:1635–1646. doi: 10.1098/rstb.2006.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP. Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci. 2004;24:11317–11327. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A. Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR. Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR. Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Dunlop SA. Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci. 2008;31:410–418. doi: 10.1016/j.tins.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Dusart I. Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD. Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW. Anderson AJ. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur J Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- Fenrich KK. Rose PK. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J Neurosci. 2009;29:12145–12158. doi: 10.1523/JNEUROSCI.0897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich KK. Rose PK. Axons with highly branched terminal regions successfully regenerate across spinal midline transections in the adult cat. J Comp Neurol. 2011;519:3240–3258. doi: 10.1002/cne.22686. [DOI] [PubMed] [Google Scholar]
- Fenrich KK, Skelton N, MacDermid VE, Meehan CF, Armstrong S, Neuber-Hess MS. Rose PK. Axonal regeneration and development of de novo axons from distal dendrites of adult feline commissural interneurons after a proximal axotomy. J Comp Neurol. 2007;502:1079–1097. doi: 10.1002/cne.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE. Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR, Brichta AM, Galea MP, Callister RJ. Graham BA. A horizontal slice preparation for examining the functional connectivity of dorsal column fibres in mouse spinal cord. J Neurosci Methods. 2011a;200:113–120. doi: 10.1016/j.jneumeth.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Flynn JR, Dunn LR, Galea MP, Callister R, Callister RJ. Rank MM. Exercise training after spinal cord injury selectively alters synaptic properties in neurons in adult mouse spinal cord. J Neurotrauma. 2013;30:891–896. doi: 10.1089/neu.2012.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR, Graham BA, Galea MP. Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011b;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pedersen V, Schwab ME. Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Fouad K. Tetzlaff W. Rehabilitative training and plasticity following spinal cord injury. Exp Neurol. 2012;235:91–99. doi: 10.1016/j.expneurol.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Galea MP. Spinal cord injury and physical activity: preservation of the body. Spinal Cord. 2012;50:344–351. doi: 10.1038/sc.2011.149. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Lythgo N, Galea MP. Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;25:449–465. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Little JW. Harris RM. Axonal sprouting following incomplete spinal cord injury: an experimental model. J Spinal Cord Med. 1997;20:200–206. doi: 10.1080/10790268.1997.11719469. [DOI] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Schofield PR. Callister RJ. Altered potassium channel function in the superficial dorsal horn of the spastic mouse. J Physiol. 2007;584:121–136. doi: 10.1113/jphysiol.2007.138198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ. Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Lo AC. Waxman SG. Sodium channel blockade with phenytoin protects spinal cord axons, enhances axonal conduction, and improves functional motor recovery after contusion SCI. Exp Neurol. 2004;188:365–377. doi: 10.1016/j.expneurol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Harris BM, Hughes DI, Bolton PS, Tadros MA, Callister RJ. Graham BA. Contrasting alterations to synaptic and intrinsic properties in upper-cervical superficial dorsal horn neurons following acute neck muscle inflammation. Mol Pain. 2014;10:25. doi: 10.1186/1744-8069-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher CH. Johnson RD. Differential effects of chronic spinal hemisection on somatic and visceral inputs to caudal brainstem. Brain Res. 2002;947:234–242. doi: 10.1016/s0006-8993(02)02930-x. [DOI] [PubMed] [Google Scholar]
- Hunanyan AS, Alessi V, Patel S, Pearse DD, Matthews G. Arvanian VL. Alterations of action potentials and the localization of Nav1.6 sodium channels in spared axons after hemisection injury of the spinal cord in adult rats. J Neurophysiol. 2011;105:1033–1044. doi: 10.1152/jn.00810.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husch A, Van Patten GN, Hong DN, Scaperotti MM, Cramer N. Harris-Warrick RM. Spinal cord injury induces serotonin supersensitivity without increasing intrinsic excitability of mouse V2a interneurons. J Neurosci. 2012;32:13145–13154. doi: 10.1523/JNEUROSCI.2995-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR. Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, Colmers WF, Dawbarn D, Ballanyi K. Smith PA. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587:1013–1032. doi: 10.1113/jphysiol.2008.166306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Delivet-Mongrain H, Leblond H. Rossignol S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J Neurophysiol. 2011;106:1969–1984. doi: 10.1152/jn.00368.2011. [DOI] [PubMed] [Google Scholar]
- Nashmi R. Fehlings MG. Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience. 2001;104:235–251. doi: 10.1016/s0306-4522(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Smith GM. Fouad K. Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics. 2011;8:283–293. doi: 10.1007/s13311-011-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Velumian AA. Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, Edgerton VR. Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Bareyre FM. Schwab ME. Reorganization of descending motor tracts in the rat spinal cord. Eur J Neurosci. 2002;16:1761–1771. doi: 10.1046/j.1460-9568.2002.02243.x. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Ikeda H, Heinke B. Sandkuhler J. Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. J Physiol. 2004;555:527–543. doi: 10.1113/jphysiol.2003.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Klassen H, Schwab ME. Perry VH. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999;11:3648–3658. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- Tadros MA, Harris BM, Anderson WB, Brichta AM, Graham BA. Callister RJ. Are all spinal segments equal: intrinsic membrane properties of superficial dorsal horn neurons in the developing and mature mouse spinal cord. J Physiol. 2012;590:2409–2425. doi: 10.1113/jphysiol.2012.227389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totoiu MO. Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Tracey DJ. Ascending and descending pathways in the spinal cord. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academic Press; 2004. pp. 149–164. [Google Scholar]
- Walsh MA, Graham BA, Brichta AM. Callister RJ. Evidence for a critical period in the development of excitability and potassium currents in mouse lumbar superficial dorsal horn neurons. J Neurophysiol. 2009;101:1800–1812. doi: 10.1152/jn.90755.2008. [DOI] [PubMed] [Google Scholar]
- Yoshimura M. Jessell TM. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]


