Abstract
Objective
Delineate prenatal features of Costello syndrome (caused by HRAS mutations) which consists of mental retardation, facial, cardiovascular, skin, and musculoskeletal anomalies, and tumor predisposition.
Methods
Literature and new cases classified as Group I (pre-HRAS), Group II (HRAS confirmed), and Group III (HRAS confirmed in natural history study, plus three contributed cases).
Results
Polyhydramnios occurred in most (mean 79%) pregnancies of cases in Groups I (98), II (107), and III (17), advanced paternal age and prematurity were noted in approximately half. Less frequent were nuchal thickening, ascites, shortened long bones, abnormal hand posture, ventriculomegaly, macrosomia, and macrocephaly. Fetal arrhythmia occurred in 9 cases (6 supraventricular or unspecified tachycardia, 1 unspecified arrhythmia, 2 premature atrial contractions, PACs); excluding 3 new cases and 2 with PACs, the estimated prenatal frequency is 4/222 (2%).
Conclusion
Costello syndrome can be suspected prenatally when polyhydramnios is accompanied by nuchal thickening, hydrops, shortened long bones, abnormal hand posture, ventriculomegaly, large size, and macrocephaly, and especially fetal atrial tachycardia. Consideration should be given for timely prenatal diagnostic studies for confirmative HRAS gene mutations, and for maternal treatment of serious fetal arrhythmia.
Keywords: chaotic atrial rhythm, chaotic atrial tachycardia, Costello syndrome, fetal arrhythmia, HRAS gene, polyhydramnios, prenatal ultrasound, supraventricular tachycardia
INTRODUCTION
Costello syndrome typically presents with polyhydramnios, hypotonia and edema, accompanied by failure to thrive, short stature, neurodevelopmental and musculoskeletal problems and an approximate 15% risk for malignant tumors (Gripp, 2005; Gripp and Lin, 2006). There is relative macrocephaly, epicanthal folds, a wide nasal bridge, short nose, full lips, large mouth, nasal papillomata, wrist deviation, deep palmar and plantar creases, and loose skin. Cardiac manifestations include hypertrophic cardiomyopathy, pulmonic stenosis, and arrhythmias (Lin et al., 2002; Gripp et al, 2006). Costello syndrome overlaps with other syndromes of the Ras/MAPK pathway, especially Noonan syndrome (Quezada and Gripp, 2007). HRAS is considered the single etiologic gene for Costello syndrome (Aoki et al, 2005; Kerr et al. 2008). Somatic mosaicism has been reported (Gripp et al., 2007; Sol-Church et al., 2009). The purpose of our study was to better characterize the prenatal features of Costello syndrome.
PATIENTS
CASE 1
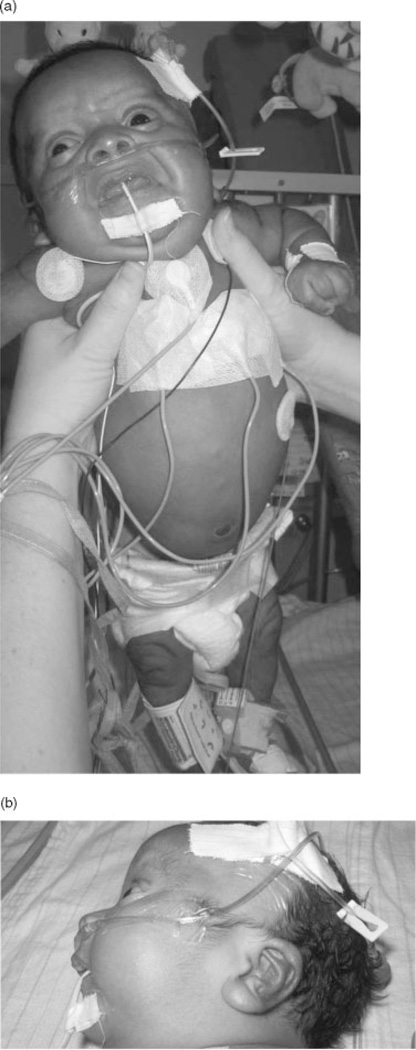
This male, born to a 29-year-old G5 mother and 40-year-old father, had a 28 week prenatal ultrasound showing macrocephaly, significant ascites, and supraventricular tachycardia (SVT) (fetal heart rate 200–230 beats per minute, bpm). Maternal treatment with intravenous digoxin and flecainide suppressed the fetal SVT and reduced the ascites. At 34 weeks, the fetal heart rate was 140–150 bpm with frequent premature atrial contractions (PACs). Ultrasound at 35 weeks showed normal sinus rhythm, shortened femurs (4 weeks < gestational age), macrocephaly (5 weeks > gestational age) with appropriate ventricular and cerebellum size. At 36 weeks, this hypotonic male was delivered by spontaneous vaginal delivery in moderate respiratory distress. Apgar scores were 7 and 8 at 1 and 5 minutes, respectively. His weight was 3.3 kg (>90th centile), length was 49 cm (80th centile) and OFC was 36 cm (>> 90th centile). He had (Figure 1 A, B) macrocephaly, hypertelorism, prominent eyes, pinpoint cataracts, low-set ears, overfolded helices, short neck, deep palmar creases, hyperextensible digits, tightly clenched fists with overlapping first and third fingers, spoon-shaped nails, extra folds of skin on the forearm, shoulder and back, hyperextensible elbows, large feet with overlapping toes, diffuse hypotonia and opisthotonic positioning.
Figure 1.
Case 1. Typical features of a newborn with Costello syndrome showing (A) hypertelorism, wrinkled skin, short nose, full nasal tip, wide mouth, full, prominent lips and clenched hand with overlapping fingers (reminiscent of, but distinct from, trisomy 18 syndrome); (B) and in lateral view the low-set ears with fleshy lobule.
Chromosome analysis was 46,XY. Costello syndrome was confirmed by molecular testing which revealed the typical missense mutation in the HRAS gene, p.Gly12Ser. Two-dimensional echocardiogram showed mild pulmonic valve stenosis, without cardiac hypertrophy. Nearly continuous atrial tachyarrhythmias (Figure 2 A, B) were interpreted at various times as SVT, chaotic atrial rhythm, multifocal atrial tachycardia, ectopic atrial tachycardia with variable AV block, and later, atrial flutter depending on P wave morphology and rhythm regularity. The maximal rate of 375 bpm was resistant to digoxin, propranolol, amiodarone, and flecanide. At age 3 months, and after 2 weeks of sinus rhythm, hemodynamically significant atrial tachycardia returned, resisting cardioversion. A tracheostomy treated epiglottic and vocal cord areas of soft tissue obstruction. The patient died at age 4 months following an episode of severe tachycardia. Parents declined autopsy.
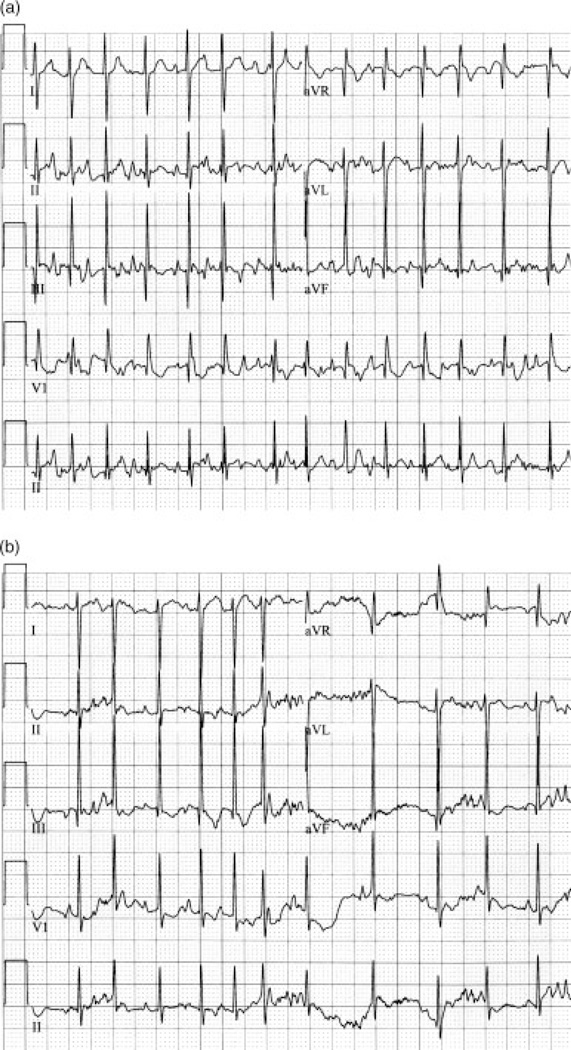
Figure 2.
Case 1. Postnatal electrocardiograms illustrate chaotic atrial rhythm. (A) Day of life 3: Bouts of CAR start and stop, with evidence of multiple P wave morphologies and faster AV conduction (mean HR 200). (B) At 7 weeks of age, the tachycardia appears less chaotic, but multiple P waves are again noted.
CASE 2
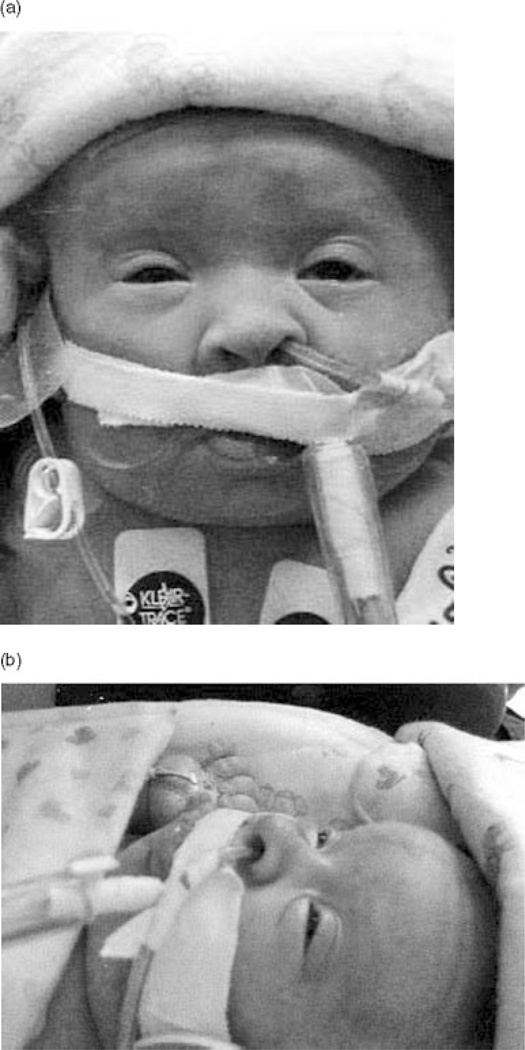
This female was born to a 33-year-old G1 mother and 31-year-old father. The pregnancy was complicated by well-controlled insulin-dependent gestational diabetes. Multiple marker screening suggested an increased risk for Down syndrome (1:237). Polyhydramnios noted at 24 weeks gestation became severe at 27 weeks necessitating amnioreduction (AFI 52 to 28 cm). Fetal MRI at 27 weeks showed ventricles at the upper limits of normal size. At 30 weeks, fetal edema and bilateral pleural effusions developed, and a second amnioreduction was performed. Fetal echocardiogram at 30 weeks gestation saw no structural anomalies or pericardial effusion, only PACs. Worsening fetal edema and bilateral pleural effusions led to scheduled Caesarean delivery at 32 weeks. Placental enlargement was present with a weight of 793 g (normal range 250–450 g). Birth weight was 2.7 kg (> 90th centile); estimated dry weight was 2 kg (75th centile). Length was 40 cm (25th centile). OFC 34.5 cm (>90th centile), but affected by severe edema. Apgar scores were 2 and 7 at 1 and 5 minutes, respectively. A protruding tongue (Figure 3), widely spaced nipples and hyperconvex nails were seen. Echocardiogram at age 2 days was normal. At age 11 and 18 days, there was sinus tachycardia (189–192 bpm) with multifocal PACs. Electrocardiogram tracings at 18 and 27 days showed a wide QRS tachycardia (198–203 bpm) consistent with ventricular tachycardia although atrial tachycardia with aberrant conduction could not be ruled out. Recurring pleural effusions and chylothorax necessitated chest tubes, and multi-organ failure ensued with death at age 40 days.
Figure 3.
Case 2, frontal and lateral. The facial features of Costello syndrome are illustrated in this premature infant, though obscured by the endotracheal and feeding tubes, and adhesive tape. There is a full nasal tip, periorbital puffiness and protruding tongue.
On autopsy, lung hypoplasia, nephromegaly and a liver hemangioma were noted. Multiple chromosome studies performed on amniocytes and peripheral blood cells had been normal, as was molecular testing for PTPN11, SOS1, KRAS and RAF1 mutations. However, postmortem molecular testing detected an HRAS mutation resulting in a predicted p.Gly12Cys amino acid change, consistent with Costello syndrome.
CASE 3
This female, born to a 39-year-old G2 mother and unrelated 41-year-old father, developed SVT at 24 weeks gestation treated successfully with maternal digoxin and sotalol. Prenatal ultrasound showed polyhydramnios, hepatomegaly, thickened nuchal fold, macroglossia and shortened limbs. Labor was induced at 36 weeks gestation. The birth weight was 2.8 kg (50th centile), length 43 cm (5th centile) and OFC was 34 cm (75th centile). She had coarse facial features with a prominent forehead, periorbital fullness, epicanthal folds, small midface with an upturned nose, dysplastic and thickened ears, short neck with a wide chest, widely spaced nipples, and short thickened hands. At age 4 months, deep palmar creases and excess skin over the dorsal aspects of her hands and feet were noted. Tracheostomy and gastrostomy tubes were required by age 3 months (Figure 4).
Figure 4.
Case 3. This child also has the distinctive clenched hands with overlapping fingers, short nose, full nasal tip, thick earlobe and periorbital fullness.
Serial echocardiograms showed biventricular hypertrophy, and mildly thickened atrioventricular valve leaflets. Refractory tachyarrhythmias showed variable P wave morphology described at various times as SVT, atrial fibrillation, atrial flutter, and occasional wide QRS tachycardia, though ultimately diagnosed as chaotic atrial rhythm. While reasonably well-controlled with digoxin, sotalol, and flecainide, she became markedly bradycardic, with sinus pauses up to 8 seconds, ultimately leading to pacemaker insertion at age 3 1/2 months. At age 9 months, she began having prolonged episodes of wide QRS tachycardia (greater than 40 minutes), for which sotalol and digoxin were discontinued and amiodarone was initiated. Systemic hypertension, with blood pressure ranging from 130–140/95, developed at age 10 months. While hospitalized at age 13 months, she had cardiovascular collapse with a terminal rhythm reported as ventricular tachycardia and fibrillation.
Postmortem examination of the heart confirmed the absence of structural heart defects, and normal semi-lunar and mitral valves, with slight redundancy of the tricuspid valve leaflets. There was hypertrophy of the left ventricle (left ventricle free wall 9 mm) and interventricular septum (15 mm), with mild subaortic stenosis. Microscopic examination showed mild disorganization of the myofibers, mainly in the central region. The coronary arteries and intra-myocardial vessels, pulmonary artery and aorta were normal. Neuropathological examination was consistent with old hypoxic-ischemic injuries. Storage disease, especially mucopolysaccharidosis, had been considered in the first months of life. Costello syndrome was suspected at age 3 months and confirmed by HRAS mutation testing two years postmortem showing the most common p.Gly12Ser mutation.
Literature Review and Natural History Study
Cases from the English literature from 1971 to 2008 were assigned to Group I (pre-HRAS) or Group II (HRAS confirmed). Group I cases required adequate clinical description and/or photograph. We excluded reviews with limited prenatal history (Lin et al., 2002; Axelrad et al., 2004; Gripp et al., 2005; Aoki et al., 2005; Della Marca et al., 2006; Axelrad et al., 2007; Schulz et al., 2008), and mosaicism (Gripp, et al. 2006; Sol-Church et al., 2009). We mailed a data collection form to the parents of HRAS mutation positive patients who had been enrolled in an ongoing natural history study of Costello Syndrome (Group III), and also included 3 additional cases cared for by authors.. Informed consent was obtained based on a protocol at the A. I. duPont Hospital for Children (IRB #2003-006 and #2005-051). Mutation analysis and clinical methods were performed as described previously (Gripp et al., 2006). Although chaotic atrial rhythm, chaotic atrial tachycardia and multifocal atrial tachycardia are equivalent terms (Walsh and Triedman, 2001; Walsh et al; 2006), but we used the former in this paper.
RESULTS
There were 222 Costello syndrome cases in Groups I (98), II (107) and III (17) (Table I). Groups II and III had molecular confirmation, but differ because of ascertainment. Though the smallest, Group III provided the most information since all parents completed the questionnaire, and most of medical information was validated from medical records.
Table 1.
Prenatal features of patients with Costello syndrome: 98 literature cases identified before the period of HRAS gene diagnosis (Group I), 107 literature cases with HRAS confirmation (Group II), and 17 new cases (14 from natural history study, 3 contributed cases) (Group III).
| Feature | Group I1 | Group II2,3 | Group III |
|---|---|---|---|
| n=98 | n= 107 | n=17 | |
| Denominator reflects number of informative cases | |||
| Male | 52/97 (54%) | 30/66(45%) | 9/17 (53%) |
| Female | 45/97 (46%) | 36/66 (54%) | 8/17 (47%) |
| Advanced maternal age (>35 years) | 10/66 (15%) | 5/12 (42%) | 3/16 (19%) |
| Mean 30.5 | Mean 33.1 | Mean 31.6 | |
| Advanced paternal age (>35 years) | 29/68 (43%) | 13/21 (62%) | 7/16 (43%) |
| Mean 34.4 | Mean 37.2 | Mean 37.1 | |
| Preterm delivery (<37 weeks) | 34/85 (40%) | 11/22 (50%) | 9/17 (53%) |
| Ultrasound findings; | |||
| Polyhydramnios4 | 48/71 (68%) | 55/60 (92%) | 13/17 (76%) |
| Severe | 2/48 (4%) | 6/55 (11%) | 3/13 (23%) |
| Nuchal thickening | 1/48 (2%) | 3/55 (5%) | 2/17 (12%) |
| Ascites, effusions, and/or hydrops | 0 | 3/55 (5%) | 2/17 (12%) |
| Shortened long bones | 0 | 2/55 (4%) | 5/17 (29%) |
| Abnormal hand posturing | 0 | 2/55 (4%) | 0 |
| Ventriculomegaly | 0 | 0 | 3/17 (18%) |
| Macrocephaly | 12/48 (25%) | 9/18(50%) | 3/17 (18%) |
| Prenatal cardiac abnormalities | 4/71 (6%) | 1/58 (2%) | 5/17 (29%) |
| 1 Arrhythmia nos | 1 Tachycardia nos | 3 SVT | |
| 1 VSD nos | 2 PACs | ||
| 1 Tachycardia nos | |||
| 1 PAT | |||
Costello et al., 1977 and Costello et al., 1996; Der Kaloustian et al., 1991; Martin and Jones, 1991; Borochowitz et al., 1992; Teebi et al., 1993; DiRocco et al., 1993; Izumikawa et al.,1993; Kondo et al.,1993; Patton et al., 1993; Phillip and Mancini, 1993; Zampino et al., 1993; Say et al., 1993; Yoshida et al., 1993; Davies et al., 1994; Okamoto et al., 1994; Fryns et al., 1994; Torres et al., 1994; Umans et al., 1994; Torrelo et al., 1995; Mori et al., 1996; Fukao et al., 1996; Johnson et al., 1998; Pratesi et al., 1998; Tomita et al., 1998; Siwik et al., 1998; Franceshini et al., 1999; Szalai et al, 1999; van Eeghen et al., 1999; Bisogno et al., 1999; Innes and Chudley, 2000; Moroni et al., 2000; Sigaudy et al., 2000; Hatamochi et al., 2000; Boente et al., 2001; Gripp et al., 2002; Kaji et al., 2002; Kamoda et al., 2002; van den Bosch et al., 2002; Urakami et al., 2002; Kawame et al., 2003; Delrue et al., 2003; Mancini et al., 2003; DiRocco et al., 2003; Nasca et al., 2003; Cakir et al., 2004; Waldburg et al., 2004; Dickson et al., 2004; Gregersen et al., 2004; Stein et al., 04; Hinek et al., 2005; Alexander et al., 2005; White et al., 2005.
Lin et al., 2008 represented the compilation of Gripp et al., 2006 and Estep et al., 2006; Kerr et al., 2006; Steens et al., 2006; Zampino et al., 2006; Digilio et al., 2007; Limongelli et al., 2007; Denayer et al., 2007; Lo et al., 2008; Hou et al., 2008; Gripp et al., 2008.
Case reports in the literature who were later included in extensive reviews were generally reported only in Group II, e.g. patients 1 and 3 in Zampino et al., 1993 were also patients CS-01 and CS-04 in Zampino et al., 2006).
Polyhydramnios was recorded if reported by ultrasonographic examination, and in one additional patient observed clinically at delivery.
CAR, chaotic atrial rhythm; NOS, not otherwise specified; NS, not stated; PACs, premature atrial contractions; PAT, paroxysmal atrial tachycardia; VSD. Ventricular septal defect; SVT, supraventricular tachycardia
Polyhydramnios occurred in at least two-thirds (mean 79%) of cases in all groups, often reported as “severe”, “marked”, or requiring serial amniotic fluid reduction. Advanced paternal (> 35 years) and preterm delivery (< 37 gestational weeks) were reported approximately half of the cases. A detailed analysis of the causes of prematurity was not possible, but polyhydramnios occurred in 64% and 78% in Groups II and III, which did not differ from overall frequency. In Group III, at least one other abnormality was identified in addition to polyhydramnios in 9 of 17 (53%). Infrequent findings included hydrops (ascites, pleural and/or pericardial effusions), nuchal thickening, shortened long bones and abnormal hand posture.
Any type of cardiac abnormality was reported postnatally in 67%, 76% and 82%, with prenatal detection in 6%, 2%, 29% of cases in Groups I, II and III, respectively (Table 1). The absence of prenatal hypertrophic cardiomyopathy was striking since this was reported postnatally in 45%, 55% and 41% of cases in Groups I, II and III, respectively. A congenital heart defect was reported in one fetus from Group I (small ventricular septal defect in Siwik et al., 1998), and postnatally in 19%, 17% and 47% of Group I, II and III, respectively (Table 2).
Table 2.
Prenatal and subsequent postnatal features of cardiac abnormalities in Costello syndrome.
| Group I n=98 |
Group II n= 107 |
Group III n=17 |
Outcome of respective patients |
|---|---|---|---|
| Prenatal findings | Subsequent postnatal | ||
|
Siwik et al., 1998 35 weeks, male VSD, small (nos) |
VSD, perimem Severe HCM, BVH Frequent PACs |
||
|
Gripp et al., 2002 Case 2 33 weeks, female Tachycardia |
EAT | ||
|
Gripp et al., 2002 Case 3 37 weeks, female PAT |
SubPS HCM Atrial fibrillation |
||
|
Dickson, et al., 2004 35 weeks, male Arrhythmia (nos) |
Coarctation Abnormal aortic valve HCM “Multiple SVTs”, CAR1 |
||
|
Lin et al., 2008 Case 42 (Estep et al., 2006) Tachycardia nos |
HCM | ||
| (Previously reported in Berberich et al., 1990; Johnson et al, 1998 Case 4) |
Natural history study case 11 Term, male SVT |
PS, BAV HCM SVT |
|
| (Previously reported in Gripp et al., 2006; Lin et al., 2008) |
Natural history study case 12 34 weeks male PACs |
EAT, PAT, MAT, SVT, CAR |
|
| New case 1 36 weeks male SVT, PACs |
PSV SVT, EAT, MAT, CAR |
||
| New case 2 32 weeks, female PACs |
Sinus tachycardia with PACs and VT (or atrial tachy with aberrant conduction) |
||
| New case 3 36 weeks, female SVT |
SVT, wandering atrial pacemaker, CAT, |
||
The postnatal arrhythmias were re-evaluated by the authors of the original paper and A.E.L. and C.B. who reviewed the original electrocardiograph tracings (Dickson et al., 2001, personal correspondence). Chaotic atrial rhythm was present.
BAV, bicuspid aortic valve; BVH, biventricular hypertrophy; CAR, chaotic atrial rhythm; CAT, chaotic atrial tachycardia; EAT, ectopic atrial tachycardia; HCM, hypertrophic cardiomyopathy; NOS, not otherwise specified; NS, not stated; PACs, premature atrial contractions; PAT, paroxysmal atrial tachycardia; PS, pulmonic stenosis; SVT, supraventricular tachycardia; VSD, perimem, Ventricular septal defect, perimembranous; VT, ventricular tachycardia
Arrhythmia occurred prenatally in 6%, 2%, and 29%, and postnatally in 24%, 35% and 82% in Groups I, II and III. Case 1 and Case 3 had prenatal SVT and later developed postnatal atrial tachycardia with multiple P wave morphology, interpreted as multifocal or chaotic atrial rhythm/tachycardia (Walsh et al., 2006). The frequency of a fetal arrhythmia in Costello syndrome can be estimated as 9/222 (4%) total cases, 7/222 (3%) excluding two cases with premature atrial contractions, or 4/222 (2%) excluding the 3 new cases.
DISCUSSION
The severe neonatal (Hinek et al., 2005; Digilio et al., 2007; Lo et al., 2007) and prenatal characteristics of Costello syndrome have been described in case reports and table summaries listing polyhydramnios (Philip and Sigaudy, 1998; Zampino et al., 2006; Digilio et al., 2007), increased nuchal thickness on early screening ultrasound (Kerr et al., 1998) and large size for gestational age (Fryns et al., 1996; Van den Bosch et al., 2002). Moroni et al. (2000) provided the first prenatal images of one case noting that ultrasound examinations were normal until the third trimester when macrosomia and polyhydramnios developed. Although prenatal cardiac abnormalities were not reported, hypertrophic cardiomyopathy was observed at age five months. Costello syndrome has been associated with advanced paternal age (Lurie, 1994; Zampino et al., 2007), reflecting the paternal origin of most, but not all, Costello syndrome causing mutations (Sol-Church et al, 2006).
We confirm the high frequency of polyhydramnios which can be severe. Three skeletal markers are nonspecific, observed with skeletal dysplasia or chromosome abnormality syndromes, i.e. shortened long bones, macrocephaly and abnormal hand posturing. Likewise, the detection of hydrops, ascites, pleural and/or pericardial effusions, large for gestational age, nuchal thickening, and ventriculomegaly are not diagnostic for Costello syndrome. Polyhydramnios and other ultrasonographic markers were present in over half of the well-studied cases in Group III. In addition to polyhydramnios, the 3 new cases show some of the less common findings of the severe neonatal phenotype (Lo et al., 2007), such as pleural effusions, need for tracheostomy and early death. In our study, there was no correlation between the presence of a fetal arrhythmia and polyhydramnios, hydrops, ascites or effusion (data calculated, but not shown on Table 1).
Fetal atrial tachycardia
This study highlights the importance of fetal arrhythmia in Costello syndrome, which is usually diagnosed in utero as SVT and followed postnatally by atrial tachycardia, especially chaotic atrial rhythm. In contrast to ectopic atrial tachycardia in which there is a single non-sinus atrial focus, chaotic atrial rhythm reflects multiple (≥ 3 P waves) foci of enhanced automaticity (Walsh et al., 2006). Chaotic atrial rhythm is usually idiopathic in children, or may occur postoperatively with a congenital heart defect. The occurrence with Costello syndrome has been reported (Lin et al., 2002; Gripp et al., 2006), and appears to be the most common syndromic association. Thus, chaotic atrial rhythm in an infant with polyhydramnios, high birth weight, edema, unusual facial features, and joint laxity, especially when accompanied by hypertrophic cardiomyopathy, and/or pulmonic stenosis, should lead to an evaluation for Costello syndrome.
Among literature and new patients with a nonspecific fetal SVT followed by postnatal chaotic atrial rhythm (Table 2), none have had ECG tracings to document its prenatal characteristics. Costello syndrome should be considered when polyhydramnios, ascites, thick nuchal fold, large for gestational, and shortened femurs are accompanied by SVT. The postnatal electrocardiogram tracings (Figure 2) of Case 1 show representative examples of chaotic atrial rhythm, which may pose a diagnostic and management challenge to the pediatric cardiologist.
Fetal intermittent extrasystoles are usually hemodynamically insignificant, except when they initiate sustained tachycardia. Two patients (Case 12, new patient 2) had only fetal PACs and later developed serious postnatal tachycardia including chaotic atrial rhythm (Case 12) and wide QRS tachycardia (new patient 2) suggesting that their appearance in a fetus with suspected Costello syndrome may have prognostic value. Symptomatic fetal tachycardias are usually supraventricular in origin and may be associated with the development of hydrops fetalis (Kleinman and Nehgme, 2004). In our study, there was no correlation between the presence of an arrhythmia and polyhydramnios, hydrops, ascites, pleural or pericardial effusions. Patients with fetal arrhythmias may respond to anti-arrhythmic drugs, given either through the maternal or fetal route. There is insufficient data to generalize the predictive value of response to maternal therapy in Costello syndrome fetuses. The persistence of fetal atrial tachycardia in Case 1 was consistent with failure to respond to antiarrhythmic therapy postnatally.
Comparison with other Ras/MAPK syndromes
Currently, chaotic atrial rhythm appears to distinguish Costello syndrome from other syndromes in the Ras/MAPK pathway (Lin et al., 2002; Gripp et al., 2007). The Cardiofaciocutaneous (CFC) syndrome is also associated with a similar frequency of polyhydramnios and prematurity in a smaller series (Armour and Allanson, 2008). Prenatal ultrasonographic findings have been reported in two patients with CFCS and polyhydramnios, increased nuchal translucency, pyelectasis, ventriculomegaly, macrosomia and short femurs, similar to the features in the Costello syndrome patients in this report (Witters et al., 2008). Fetal atrial tachycardia was not observed.
Study limitations and strengths
In this descriptive study, statistical analysis of genotype-phenotype correlation was not possible because it is skewed in favor of the G12S mutation. The prenatal information for Group II was not collected in a systematic fashion, and there is a bias of ascertainment in Group III because the 3 cases were chosen for their antenatal cardiac presentations.
An important question for the future is whether these common or distinctive feature will enhance the diagnosis of Costello syndrome. The lack of prenatally diagnosed hypertrophic cardiomyopathy in the ~50% who develop it later in life raises interesting questions about the pathophysiology of this cardiac “overgrowth”. The absence of fetal hypertrophic cardiomyopathy may be due to a lack of sensitivity of prenatal echocardiographic detection of mild forms, or may reflect the natural history of hypertrophic cardiomyopathy which appears to increase in severity as the period of failure to thrive progresses. Similarly, the lack of prenatal pulmonic stenosis may reflect the prevalence of mild forms or natural history of the defect. Future analysis could study the timing of the ultrasound markers, and whether they are second trimester findings which would permit the diagnosis at a time to change the pregnancy management.
CONCLUSION
The fetal phenotype of Costello syndrome consists of polyhydramnios, macrocephaly, large size for gestational age, shortened long bones and ventriculomegaly, which may be nonspecific ultrasonographic findings when observed alone, but together lead to a suspicion for Costello syndrome. This suspicion would be substantially strengthened by the occurrence of a fetal atrial tachycardia. Since amniocentesis will likely be offered to exclude more common causes of polyhydramnios and short limbs, concomitant HRAS testing in affected fetuses could be considered. Costello syndrome results in mental retardation, increased malignant tumor risk and multiple anomalies, and timely prenatal diagnosis will provide important options for pregnancy management. Pregnancy outcomes could be improved by increasing the awareness for (1) potential arrhythmias, (2) preterm labor, and (3) large for gestational age fetuses or macrocephaly which may alter delivery management.
ADDENDUM.
At the time of submission, we became aware of a publication which reported the first prenatal amniocentesis diagnosis of Costello syndrome (Kuniba J, Pooh RK, Sasaki K, Shimokawa O, Harada N, Kondoh T, Egashira M, Moriuchi H, Yoshiura K, Niikawa N. 2008. Prenatal diagnosis of Costello syndrome using 3D ultrasonography amniocentesis confirmation of the rare HRAS mutation G12D. Am J Med Genet Part A. DOI 10.1002/ajmg.a.32335). Prenatally, this 31 week old male had polyhydramnios, increased fetal weight and head circumference, suggestive facial appearance, wrist deviation. There was no advanced paternal age, prenatal effusions, ascites, edema, brain anomalies, macroglossia, short limbs, or cardiac problems. The uncommon G12D mutation was detected. “Cardiac hypertrophy” was noted, but not further characterized.
ACKNOWLEDGEMENTS
Katia Sol-Church is supported by funds from the NCRR to the Biomolecular Core Laboratory (NIH P20-RR020173) and by the Nemours Foundation.
We extend our gratitude to many colleagues for their expertise in diagnosing and caring for these children, and assistance in research for this paper including Drs. Patricia Dickson, Elizabeth Goldmuntz, Prapti Kanani, Elizabeth Roeder, Steven Seidner, and Robert Stratton, and Ms. Elizabeth Hopkins and Meghan Muir. We are indebted to the parent leaders (Dawn Macready Santos, Lisa Schoyer, Colin Stone), the parents and individuals of the International Costello Syndrome Family Network.
REFERENCES
- Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, Filocamo M, Kato K, Suzuki Y, Kure S, Matsubara Y. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- Alexander S, Ramandan D, Alkhayyat H, Al-Sharkawi I, Aboo Backer KC, El-Sabban F, Hussain K. Costello syndrome and hyperinsulinemic hypoglycemia. Am J Med Genet. 2005;139A:227–230. doi: 10.1002/ajmg.a.31011. [DOI] [PubMed] [Google Scholar]
- Armour CM, Allanson JE. Further delineation of cardio-facio-cutaneous syndrome: clinical features of 38 individuals with proven mutations. J Med Genet. 2008;45:249–254. doi: 10.1136/jmg.2007.054460. [DOI] [PubMed] [Google Scholar]
- Berberich MS, Carey JC, Hall BD. Resolution of the perinatal and infantile failure to thrive in a new autosomal recessive syndrome with the phenotype of a storage disorder and furrowing of palmar creases. Proc Greenwood Genet Center. 1991;10:78. [Google Scholar]
- Bodkin NM, Mortimer ES, Demmer LA. Male-to-male transmission of Costello syndrome consistent with autosomal dominant inheritance. Am J Hum Genet. 1999;65(Suppl):A143. [Google Scholar]
- Boente MC, Carrero-Valenzuela RD, Frontini MV, Asial RA. Costello syndrome: report of a new case with choanal atresia and fatal outcome. Eur J Dermatol. 2001;11:453–457. [PubMed] [Google Scholar]
- Borochowitz Z, Pavone L, Mazor G, Rizzo R, Dar H. Facio-cutaneous-skeletal syndrome: New nosological entity or Costello syndrome? Am J Med Genet. 1993;47:173. doi: 10.1002/ajmg.1320430405. [DOI] [PubMed] [Google Scholar]
- Costello JM. Costello syndrome: update on the original cases and commentary. Am. J Med Genet. 1996;62:199–201. doi: 10.1002/ajmg.1320620203. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Hughes HE. Costello syndrome: natural history and differential diagnosis of cutis laxa. J Med Genet. 1994;31:486–489. doi: 10.1136/jmg.31.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Marca G, Vasta I, Scarano E, Rigante M, De Feo E, Mariotti P, Rubino M, Vollono C, Mennuni GF, Tonali P, Zampino G. Obstructive sleep apnea in Costello syndrome. Am J Med Genet. 2006;40A:257–262. doi: 10.1002/ajmg.a.31076. [DOI] [PubMed] [Google Scholar]
- Delrue MA, Chatail JF, Arveiler B, Lacombe D. Costello syndrome and neurologic abnormalities. Am J Med Genet A. 2003;123A:301–305. doi: 10.1002/ajmg.a.20330. [DOI] [PubMed] [Google Scholar]
- Der Kaloustian VM, Moroz B, McIntosh N, Watters AK, Blaichman S. Costello syndrome. Am J Med Genet. 1991;41:69–73. doi: 10.1002/ajmg.1320410118. [DOI] [PubMed] [Google Scholar]
- Dickson PI, Briones NY, Baylen BG, Jonas AJ, French SW, Lin HJ. Costello syndrome with pancreatic islet cell hyperplasia. Am J Med Genet. 2004;130A:402–405. doi: 10.1002/ajmg.a.30288. [DOI] [PubMed] [Google Scholar]
- Di Rocco M, Dodero P. Concerning “Five additional Costello syndrome patients with rhabdomyosarcoma: Proposal for a tumor screening protocol”. Am J Med Genet. 2003;118A:199. doi: 10.1002/ajmg.a.10879. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Sarkozy A, Capolino R, Testa MBC, Esposito G, deZorzi A, Cutrera R, Marino B, Dallapiccola B. Costello syndrome: clinical diagnosis in the first year of life. Eur J Pediatr. 2008;167:621–628. doi: 10.1007/s00431-007-0558-0. [DOI] [PubMed] [Google Scholar]
- Gregersen N, Viljoen D. Costello syndrome with growth hormone deficiency and hypoglycemia: a new report and review of the endocrine associations. Am J Med Genet. 2004;129A:171–175. doi: 10.1002/ajmg.a.30189. [DOI] [PubMed] [Google Scholar]
- Feingold M. Costello syndrome and rhabdomyosarcoma. J Med Genet. 1999;36:582–583. [PMC free article] [PubMed] [Google Scholar]
- Fransceschini P, Licata D, Di Cara G, Guala A, Bianchi M, Ingrosso G, Franceschini D. Bladder carcinoma in Costello syndrome: report on a patient born to consanguineous parents and review. Am J Med Genet. 1999;86:174–179. [PubMed] [Google Scholar]
- Fryns JP, Devlieger H, Lukusa P, Devlieger H, Gewillig M, Lukusa P, Devriendt K. Polyhydramnios and paroxysmal atrial tachycardia as the first clinical signs in Costello Syndrome. Genet Couns. 1996;7:237–239. [PubMed] [Google Scholar]
- Fukao T, Sakai S, Shimozawa N, Kuwahara T, Kano M, Goto E, Nakashima Y, Katagiri-Kawade M, Ichihashi H, Masuno M, Orii T, Kondo N. Life-threatening cardiac involvement throughout life in a case of Costello syndrome. Clin Genet. 1996;50:244–247. doi: 10.1111/j.1399-0004.1996.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Scott CI, Jr, Nicholson L, Figueroa TE. A second case of bladder carcinoma in a patient with Costello syndrome. Am J Med Genet. 2000;90:256–259. doi: 10.1002/(sici)1096-8628(20000131)90:3<256::aid-ajmg16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Scott CI, Jr, Nicholson L, McDonald-McGinn DM, Ozeran JD, Jones MC, Lin AE, Zackai EH. Five additional Costello syndrome patients with rhabdomyosarcoma: Proposal for a tumor screening protocol. Am J Med Genet. 2002;108:80–87. doi: 10.1002/ajmg.10241. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Kawame H, Viskochil DH, Nicholson L. Elevated catecholamine metabolites in patients with Costello syndrome. Am J Med Genet. 2004;128A:48–51. doi: 10.1002/ajmg.a.30100. [DOI] [PubMed] [Google Scholar]
- Gripp KW. Tumor predisposition in Costello Syndrome. Am J Med Genet, C Semin Med Genet. 2005;137:72–77. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- Gripp K, Lin A. Costello Syndrome in: GeneReviews at GeneTests: Medical Genetics Information Resource [database online] Seattle: Copyright, University of Washington; 2006. [Accessioned September 8, 2008]. 1997–2006. Available at http://www.genetests.org. [Google Scholar]
- Gripp KW, Lin AE, Stabley DL, Nicholson L, Scott CI, Doyle D, Aoiki Y, Matsubara Y, Zackai EH, Lapunizina P, Gonzalez-Meneses A, Holbrook J, Agresta CA, Gonzalez IL, Sol-Church K. HRAS mutation analysis in Costello syndrome: genotype and phenotype correlation. Am J Med Genet A. 2006;140:1–7. doi: 10.1002/ajmg.a.31047. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Lin AE, Nicholson L, Allen W, Cramer A, Jones K, Kutz W, Peck D, Wheeler PG, Wilson W, Al-Rahawan MM, Stabley DL, Sol-Church K. Further delineation of the phenotype resulting from BRAF or MEK1 germline mutations helps differentiate Cardio-facio-cutaneous syndrome from Costello syndrome. Am J Med Genet. 2007;143A:1472–1480. doi: 10.1002/ajmg.a.31815. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Stabley DL, Nicholson L, Hoffman JD, Sol-Church K. Somatic mosaicism for an HRAS mutation causes Costello Syndrome. Am J Med Genet. 2006;140A:2163–2169. doi: 10.1002/ajmg.a.31456. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Innes AM, Axelrad ME, Gillan TL, Parboosingh JS, Davies C, Leonard NJ, Lapointe M, Doyle D, Catalano S, Nicholson L, Stabley DL, Sol-Church K. Costello syndrome associated with novel germline HRAS mutations: An attenuated phenotype? Am J Med Genet. 2008;146A:683–690. doi: 10.1002/ajmg.a.32227. [DOI] [PubMed] [Google Scholar]
- Hinek A, Teitell MA, Schoyer L, Allen W, Gripp KW, Hamilton R, Weksberg R, Lin AE. Myocardial storage of chondroitin sulfate-containing moieties in Costello syndrome patients with severe hypertrophic cardiomyopathy. Am J Med Genet. 2005;133A:1–12. doi: 10.1002/ajmg.a.30495. [DOI] [PubMed] [Google Scholar]
- Ioan DM, Fryns JP. Costello syndrome in two siblings and minor manifestations in their mother. Further evidence for autosomal dominant inheritance? Genet Couns. 2002;13:353–356. [PubMed] [Google Scholar]
- Izumikawa Y, Naritomi K, Tohma T, Shiroma N, Hirayama K. The Costello syndrome: a boy with thick mitral valves and arrhythmias. Jpn J Hum Genet. 1993;38:329–334. doi: 10.1007/BF01874143. [DOI] [PubMed] [Google Scholar]
- Johnson JP, Golabi M, Norton ME, Rosenblatt RM, Feldman GM, Yang SP, Hall BD, Fries MH, Carey JC. Costello syndrome: phenotype, natural history, differential diagnosis, and possible cause. J Pediatr. 1998;133:441–448. doi: 10.1016/s0022-3476(98)70284-7. [DOI] [PubMed] [Google Scholar]
- Kaji M, Kurokawa K, Hasegawa T, Oguro K, Saito A, Fukuda T, Ito M, Sugie H. A case of Costello syndrome and glycogen storage disease type III. J Med Genet. 2002;39:E8. doi: 10.1136/jmg.39.2.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoda T, Matsui A, Ochiai N. Osteofibrous dysplasia in a Japanese boy with Costello syndrome. Clin Dysmorphol. 2003;12:211–212. doi: 10.1097/01.mcd.0000080412.95344.29. [DOI] [PubMed] [Google Scholar]
- Kawame H, Matsui M, Kurosawa K, Matsuo M, Masuno M, Ohashi H, Fueki N, Aoyama K, Miyatsuka Y, Suzuki K, Akatsuka A, Ochiai Y, Fukushima Y. Further delineation of the behavioral and neurologic features in Costello syndrome. Am J Med Genet. 2003;118A:8–14. doi: 10.1002/ajmg.a.10236. [DOI] [PubMed] [Google Scholar]
- Kerr B, Eden TOB, Dandamudi R, Shannon N, Quarrell O, Emmerson A, Ladusans E, Gerrard M, Donnai D. Costello syndrome: Two cases with embryonal rhabdomyosarcoma. J Med Genet. 1998;335:1036–1039. doi: 10.1136/jmg.35.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Einaudi MA, Clayton M, Gladman G, Eden T, Saunier P, Genevieve D, Philip N. Is growth hormone treatment beneficial or harmful in Costello syndrome? J Med Genet. 2003;40:e74. doi: 10.1136/jmg.40.6.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Delrue MA, Sigaudy S, Perveen R, Marche M, Burgelin I, Stef M, Tang B, Eden OB, O’sullivan J, De Sandre-Giovannoli A, Reardon W, Brewer C, Bennett C, Quarell O, M’cann E, Donnai D, Stewart F, Hennekam R, Cave H, Verloes A, Philip N, Lacombe D, Levy N, Arveiler B, Black G. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet. 2006;43:401–405. doi: 10.1136/jmg.2005.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Allanson J, Delrue M, Gripp K, Lacombe D, Lin A, Rauen K. The diagnosis of Costello Syndrome nomenclature in Ras/MAPK pathway disorders. Am J Med Genet. 2008;146A:1218–1220. doi: 10.1002/ajmg.a.32273. [DOI] [PubMed] [Google Scholar]
- Kleinman CS, Nehgme RA. Cardiac arrhythmias in the Human Fetus. Pediatric Cardiology. 2004;25:234–251. doi: 10.1007/s00246-003-0589-x. [DOI] [PubMed] [Google Scholar]
- Kondo I, Tamanaha K, Ashimine K. The Costello syndrome: report of a case and review of the literature. Jpn J Hum Genet. 1993;38:433–436. doi: 10.1007/BF01907991. [DOI] [PubMed] [Google Scholar]
- Lin AE, Grossfeld PD, Hamilton R, Smoot L, Proud V, Weksberg R, Gripp K, Wheeler P, Picker J, Irons M, Zackai E, Scott CI, Nicholson L. Further delineation of cardiac anomalies in Costello syndrome. Am J Med Genet. 2002;111:115–129. doi: 10.1002/ajmg.10558. [DOI] [PubMed] [Google Scholar]
- Lin AE, Rauen KA, Gripp KW, Carey JC. Clarification of previously reported Costello syndrome patients. Am J Med Genet. 2008;146A:940–943. doi: 10.1002/ajmg.a.32164. [DOI] [PubMed] [Google Scholar]
- Lo IFM, Brewer C, Shannon N, Shorto J, Tang B, Black G, Soo MT, Ng DKK, Lam STS, Kerr B. Severe neonatal manifestations of Costello syndrome. J Med Genet. 2008;45:167–171. doi: 10.1136/jmg.2007.054411. [DOI] [PubMed] [Google Scholar]
- Lurie IW. Genetics of the Costello syndrome. Am J Med Genet. 1994;52:358–359. doi: 10.1002/ajmg.1320520321. [DOI] [PubMed] [Google Scholar]
- Martin RA, Jones KL. Delineation of the Costello syndrome. Am J Med Genet. 1991;41:346–349. doi: 10.1002/ajmg.1320410316. [DOI] [PubMed] [Google Scholar]
- Moroni I, Bedeschi F, Luksch R, Casanova M, D'Incerti L, Uziel G, Selicorni A. Costello syndrome: a cancer predisposing syndrome? Clin Dysmorphol. 2000;9:265–268. doi: 10.1097/00019605-200009040-00006. [DOI] [PubMed] [Google Scholar]
- Mori M, Yamagata T, Mori Y, Nokubi M, Saito K, Fukushima Y, Momoi MY. Elastic fiber degeneration in Costello syndrome. Am J Med Genet. 1996;61:304–309. doi: 10.1002/(SICI)1096-8628(19960202)61:4<304::AID-AJMG2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Chiyo H, Imai K, Otani K, Futagi Y. A Japanese patient with the Costello syndrome. Hum Genet. 1994;93:605–606. doi: 10.1007/BF00202834. [DOI] [PubMed] [Google Scholar]
- Patton MA, Baraitser M. Cutis laxa and the Costello syndrome. J Med Genet. 1993;30:622. doi: 10.1136/jmg.30.7.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N, Mancini J. Costello syndrome and facio-cutaneous-skeletal syndrome. Am J Med Genet. 1993;47:174–175. doi: 10.1002/ajmg.1320470209. [DOI] [PubMed] [Google Scholar]
- Philip N, Sigaudy S. Costello syndrome. J Med Genet. 1998;35:238–240. doi: 10.1136/jmg.35.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratesi R, Santos M, Ferrari I. Costello syndrome in two Brazilian children. J Med Genet. 1998;35:54–57. doi: 10.1136/jmg.35.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada E, Gripp KW. Costello syndrome and related disorders. Curr Opin Pediatr. 2007;19 doi: 10.1097/MOP.0b013e3282f161dc. 636-633. [DOI] [PubMed] [Google Scholar]
- Say B, Güçsavaş M, Morgan H, York C. The Costello syndrome. Am J Med Genet. 1993;47:163–165. doi: 10.1002/ajmg.1320470203. [DOI] [PubMed] [Google Scholar]
- Schulz AL, Albrecht B, Arici C, van der Burgt I, Buske A, Gillessen-Kaesbach G, Heller R, Horn D, Hübner CA, Korenke GC, König R, Kress W, Krüger G, Meinecke P, Mücke J, Plecko B, Rossier E, Schinzel A, Schulze A, Seemanova E, Seidel H, Spranger S, Tuysuz B, Uhrig S, Wieczorek D, Kutsche K, Zenker M. Mutation and phenotypic spectrum in patients with cardio-facio-cutaneous and Costello syndrome. Clin Genet. 2008;73:62–70. doi: 10.1111/j.1399-0004.2007.00931.x. [DOI] [PubMed] [Google Scholar]
- Sigaudy S, Vittu G, David A, Vigneron J, Lacombe D, Moncla A, Flori E, Philip N. Costello syndrome: report of six patients including one with an embryonal rhabdomyosarcoma. Eur J Pediatr. 2000;159:139–142. doi: 10.1007/s004310050037. [DOI] [PubMed] [Google Scholar]
- Siwik ES, Zahka KG, Wiesner GL, Limwongse C. Cardiac disease in Costello syndrome. Pediatrics. 1998;101:706–709. doi: 10.1542/peds.101.4.706. [DOI] [PubMed] [Google Scholar]
- Sol-Church K, Stabley DL, Nicholson L, Gonzalez IL, Gripp KW. Paternal Bias in Parental Origin of HRAS Mutations in Costello Syndrome. Hum Mutat. 2006;27:736–741. doi: 10.1002/humu.20381. [DOI] [PubMed] [Google Scholar]
- Sol-Church K, Stabley DL, Demmer LA, Agbulos A, Lin AE, Smoot L, Nicholson L, Gripp KW. Male-to-male transmission of Costello syndrome: G12S HRAS germline mutation inherited from a father with somatic mosaicism. Am J Med Genet. 2009 doi: 10.1002/ajmg.a.32639. 10.1002/ajmg.a.32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RI, Legault L, Daneman D, Weksberg R, Hamilton J. Growth hormone deficiency in Costello syndrome. Am J Med Genet Part A. 2004;129A:166–170. doi: 10.1002/ajmg.a.30187. [DOI] [PubMed] [Google Scholar]
- Szalai S, Becker K, Torok E. Costello syndrome with decreased glucose tolerance. Eur J Dermatol. 1999;9:533–536. [PubMed] [Google Scholar]
- Teebi AS. Costello or facio-cutaneous-skeletal syndrome? Am J Med Genet. 1993;47:172–173. doi: 10.1002/ajmg.1320470207. [DOI] [PubMed] [Google Scholar]
- Vila Torres J, Pineda Marfa M, González Enseñat MA, Lloreta Trull J. Pathology of the elastic tissue of the skin in Costello syndrome. An image analysis study using mathematical morphology. Anal Quant Cytol Histol. 1994;16:421–429. [PubMed] [Google Scholar]
- Tomita H, Fuse S, Ikeda K, Matsuda K, Chiba S. An infant with Costello syndrome complicated with fatal hypertrophic obstructive cardiomyopathy. Acta Paediatr Jpn. 1998;40:608–611. doi: 10.1111/j.1442-200x.1998.tb02000.x. [DOI] [PubMed] [Google Scholar]
- Torrelo A, López-Avila A, Mediero IG, Zambrano A. Costello syndrome. J Am Acad Dermatol. 1995;32:904–907. doi: 10.1016/0190-9622(95)91559-1. [DOI] [PubMed] [Google Scholar]
- Umans S, Decock P, Fryns JP. Costello syndrome: the natural history of a true postnatal growth retardation syndrome. Genet Couns. 1995;6:121–125. [PubMed] [Google Scholar]
- Van den Bosch T, van Schoubroeck D, Fryns JP, Naulaers G, Inion AM, Devriendt K. Prenatal Findings in a monozygotic twin pregnancy with Costello Syndrome. Prenat Diagn. 2002;22:415–417. doi: 10.1002/pd.333. [DOI] [PubMed] [Google Scholar]
- Van Eeghen AM, van Gelderen I, Hennekam RC. Costello syndrome: report and review. Am J Med Genet. 1999;82:187–193. doi: 10.1002/(sici)1096-8628(19990115)82:2<187::aid-ajmg17>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Van Steensel MA, Vreeburg M, Peels C, van Ravenswaaij-Arts CM, Bijlsma E, Schrander-Stumpel CT, van Geel M. Recurring HRAS mutation G12S in Dutch patients with Costello syndrome. Exp Dermatol. 2006;15:731–734. doi: 10.1111/j.1600-0625.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- Waldburg N, Buehling F, Evert M, Burkhardt O, Welte T. Pulmonary infiltrates in Costello Syndrome. Eur Respir J. 2004;23:783–785. doi: 10.1183/09031936.04.00073704. [DOI] [PubMed] [Google Scholar]
- Walsh EP, Berul CI, Triedman JK. Cardiac arrhythmias. In: Keane JK, Lock JE, Fyler DC, editors. Nadas’ Pediatric Cardiology. Second Edition. Philadelphia: Saunders Elsevier; 2006. pp. 484–488. 2006. [Google Scholar]
- White SM, Graham JM, Jr, Kerr B, Gripp K, Weksberg R, Cytrynbaum C, Reeder JL, Stewart FJ, Edwards M, Wilson M, Bankier A. The adult phenotype in Costello syndrome. Am J Med Genet. 2005;136A:128–135. doi: 10.1002/ajmg.a.30747. [DOI] [PubMed] [Google Scholar]
- Witters I, Denayer E, Brems H, Fryns JP, Legius E. The cardiofaciocutaneous syndrome: prenatal findings in two patients. Prenat Diagn. 2008;28:53–55. doi: 10.1002/pd.1891. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Fukushima Y, Ohashi H, Asoh M, Fukuyama Y. The Costello syndrome: are nasal papillomata essential? Jpn J Hum Genet. 1993;38:437–444. doi: 10.1007/BF01907992. [DOI] [PubMed] [Google Scholar]
- Zampino G, Mastroiacovo P, Ricci R, Zollino M, Segni G, Martini-Neri ME, Neri G. Costello syndrome: further clinical delineation, natural history, genetic definition, and nosology. Am J Med Genet. 1993;47:176–183. doi: 10.1002/ajmg.1320470210. [DOI] [PubMed] [Google Scholar]
- Zampino G, Pantaleoni F, Carta C, Cobellis G, Vasta I, Neri C, Pogna EA, De Feo E, Delogu A, Sarkozy A, Atzeri F, Selicorni A, Rauen KA, Cytrynbaum CS, Weksberg R, Dallapiccola B, Ballabio A, Gelb BD, Neri G, Tartaglia M. Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum Mutat. 2007;28:265–272. doi: 10.1002/humu.20431. [DOI] [PubMed] [Google Scholar]