Abstract
The treatment of aplastic anemia is currently with immunosuppressive therapy (IST) with antithymocyte globulin and cyclosporine, to which two thirds of patients respond. However, a significant proportion of these responders relapse and many have persistent cytopenias. The management of these patients is challenging. Modifications to this standard approach using alternative immunosuppressive agents or adding hematopoietic cytokines such as G-CSF and erythropoietin have not improved outcome. A recent trial has shown that eltrombopag, a thrombopoeitin mimetic, is efficacious in the treatment of patients with severe aplastic anemia (SAA) refractory to IST. There is evidence that this drug works by directly stimulating marrow stem and progenitor cells thereby promoting hematopoietic recovery in patients with bone marrow failure. Several trials are ongoing in our institution using this very promising drug in combination therapy in the upfront treatment of SAA, in IST refractory SAA and in moderate disease.
INTRODUCTION
Aplastic anemia is a rare haematological disorder characterized by pancytopenia and a hypocellular marrow. Patients are usually symptomatic on presentation but some are detected incidentally when unexpected cytopenias are found on a routine blood count. The diagnosis of severe aplastic anemia is made in the setting of a hypocellular bone marrow when 2 of 3 blood counts are met: absolute neutrophil count < 500/μL, absolute reticulocyte count < 60 000/μL, and platelet count < 20 000/μL and myelodysplastic syndrome is outruled1. The principle mechanism leading to bone marrow failure in most cases is immune-mediated destruction of hematopoietic stem and progenitor cells2. There is evidence that effector T-cells such as activated cytotoxic T-cells are elevated in the bone marrow of patients with aplastic anemia.3–5 The effects exerted by cytotoxic T-lymphocytes are mediated in part due to Fas ligand-induced apoptosis of hematopoietic progenitor cells. T cell receptor gene rearrangement studies have demonstrated clonal expansion of these cells in aplastic bone marrows.6 Immune-mediated marrow destruction with many similarities to the pathophysiology of human aplastic anemia can be modeled in the mouse.7 The result of this immune attack is profound hematopoietic stem cell (HSC) depletion.8
The clinical consequences are anemia, usually with a requirement for frequent red blood cell transfusions, life-threatening bleeding from thrombocytopenia, and infection as a result of neutropenia. Bacterial and fungal infections are most common and are a significant cause of morbidity and mortality.
Treatment should be instituted as soon as the diagnosis is established. Definitive and potentially curative therapy with allogeneic bone marrow transplantation is generally only available for younger patients with histocompatible donors9. Survival rates with allogeneic hematopoietic stem cell transplantation from a histocompatible sibling are excellent and have been reported to be as high as 90% from single institution studies, and approximately 70% in registry data.10,11 Graft-versus-host disease, however, continues to be a major toxicity and affects long-term quality of life.12 Initial treatment with immunosuppressive therapy (IST) in patients without transplant options ameliorates disease in about two thirds of cases. Horse ATG and cyclosporine is now considered the standard of care for newly diagnosed patients13. Response is associated with a favourable long term outlook. However, of these responders, 30–40% relapse13,14. Retreatment of relapse with IST, usually only reinstitution of cyclosporine but occasionally repeating lymphocyte depletion with rabbit antithymocyte globulin or alemtuzumab, can be successful; patients who relapse are maintained on cyclosporine for years, often with clear dependence of blood counts on drug dose. A significant minority of patients do not respond to initial immunosuppression, and cytopenias persist in these “refractory” cases, with long-term transfusion dependence. It is unknown whether cytopenias in individual refractory patients represent a failure of immunosuppression to completely ablate autoreactive immune cells (30–40% of patients who fail treatment with horse ATG respond to alternative IST), or a persistent stem cell deficiency in the absence of active immune cell attack. The management of persistently refractory patients in challenging. Eltrombopag has recently been shown to be efficacious in the setting of refractory severe aplastic anemia (SAA) with trilineage responses in some patients and many achieving transfusion independence15,16.
THROMBOPOIETIN AND HEMATOPOIESIS
Thrombopoietin (TPO) is a glycoprotein class 1 hematopoietic cytokine, produced primarily in the liver17. It binds to the receptor c-mpl and is the primary regulator of megakaryopoiesis. After 3 decades of failed attempts to purify TPO, the discovery of the proto-oncogene Mpl aided cloning of TPO in 1994 and its many functional properties became evident thereafter through in vitro studies18.
TPO’s role in hematopoiesis was elucidated initially in cell culture experiments. The c-mpl receptor was shown to be expressed and functional on primitive hematopoietic stem and progenitor cells19 and these cells could proliferate in the presence of TPO when other cytokines were added20,21. The generation of transgenic mice lacking the TPO or c-mpl gene allowed direct in vivo investigation of the function of TPO. In transplantation studies, “knockout” mice had decreased HSC expansion compared to wild type controls22 and reduced numbers of hematopoietic progenitors12. In humans, loss of function mutations in c-MPL result in congenital amegakaryocytic thrombocytopenia, with affected children presenting initially with isolated thrombocytopenia and progressive pancytopenia due to a reduction in bone marrow HSCs23. Recently, a mutation in the gene encoding TPO (THPO) was demonstrated in a family with autosomal recessive aplastic anemia24.
RATIONALE FOR THROMBOPOIETIN STIMULATION IN APLASTIC ANEMIA
The control of TPO levels and TPO production is complex and involves sensing of c-mpl receptor occupancy, with TPO levels inversely proportional to megakaryocyte mass rather than peripheral platelet counts. TPO levels are high in bone marrow failure syndromes such as myelodysplastic syndromes and SAA and low to normal in chronic ITP25–27.
Hematopoietic cytokines such as erythropoietin and granulocyte stimulating growth factor (G-CSF) have historically had a very limited role in the treatment of patients with SAA28,29. A large, randomized study comparing standard IST with anti-thymocyte globulin and cyclosporine versus the same IST with the addition of G-CSF showed no difference in hematological response30. Erythropoietin (Epo) levels in SAA are also very high, and not surprisingly, the addition of Epo to IST, often in addition to G-CSF, has also not shown benefit in terms of increasing response in red cell or other lineages31. As noted above, TPO levels are increased in SAA patients. Why then should treatment with TPO receptor agonists be beneficial? Other hematopoietic growth factors act only on more committed myeloid or erythroid progenitors, which are largely lacking in SAA since the defect is at a much early hematopoietic stem cell stage 8. TPO acts on HSCs as well as megakaryocytes, and thus despite the high endogenous levels of TPO in SAA, studying the clinical activity of TPO mimetics in refractory bone marrow failure syndromes was an attractive concept.
Eltrombopag is a synthetic, non-peptide TPO mimetic, which can be administered orally. It was originally developed for the treatment of chronic immune thrombocytopenic purpura32. Eltrombopag selectively binds to c-mpl at the transmembrane and juxtamembrane domains of the thrombopoietin receptor, at sites distinct from the binding site of TPO. Eltrombopag therefore does not compete for binding with the native molecule17.
Unfortunately, in vivo animal studies investigating the impact and potential mechanism of action of eltrombopag are not possible, since eltrombopag binds only to the human or chimpanzee TPO receptors. The NOD/SCID mouse xenotransplant model was utilized in one report to show that eltrombopag could selectively in vivo expand engrafting human cord blood hematopoietic stem/progenitor cells 33.
TPO is primarily synthesized by the liver, but also by bone marrow stromal cells, and eltrombopag may influence the microenvironment directly in a paracrine fashion. As previous mentioned, high serum levels of TPO are seen in aplastic anemia. The concentration of TPO in the bone marrow niche may be more important for stimulating stem cells and eltrombopag, with small molecule kinetics, may be capable of entering the niche more effectively than TPO. Alternatively, eltrombopag and endogenous TPO may be capable of inducing alternative signal transduction cascades given the potential for dual stimulation of the TPO receptor by both eltrombopag and endogenous TPO. TPO clearly has a pleiotropic role in regulating hematopoiesis beyond megakaryopoiesis and platelet production. Recent in vitro studies also implicate roles for TPO in DNA repair, and some of the effects of eltrombopag may be independent of TPO receptor stimulation34,35.
ELTROMBOPAG IN REFRACTORY APLASTIC ANEMIA
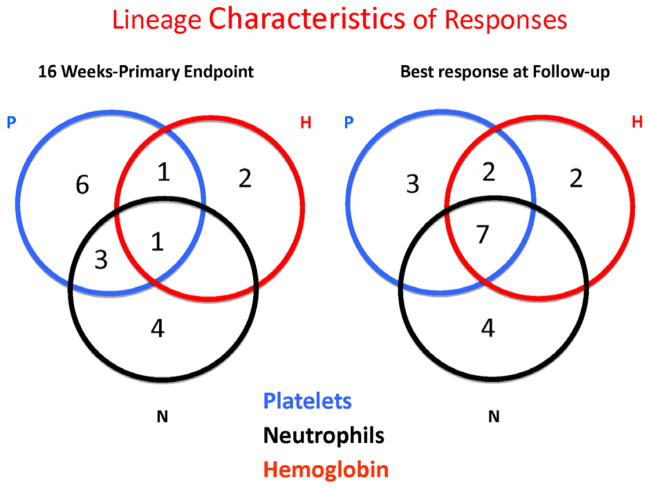
Recently, we published the results of a non-randomized, phase 2 study from the National Institutes of Health using eltrombopag in SAA16. 43 patients with SAA refractory to at least one course of IST initiated at least 6 months previously and with platelet counts less than 30x103/ul were enrolled. Subjects were treated using a dose escalation schedule starting at 50 mg and increasing every 2 weeks by 25 mg to a maximum of 150 mg. The primary endpoint was hematological response at 3–4 months. If patients responded, they were offered entry to the extension phase of the study, and they could remain on drug indefinitely or until a decision was made to discontinue. The patients enrolled were heavily pre-treated, having received a median of 2 prior courses of IST. Almost all were both red cell and platelet transfusion dependent, and 6 patients met criteria for very severe aplastic anemia defined as SAA and a neutrophil count of <200/ul. Seventeen of 43 patients (40%) had a hematological response to eltrombopag. Several multilineage responses were seen (Figure 1) at response assessment. The majority of patients who remained on eltrombopag showed continued improvement of blood counts, and several of the unilineage responders improved other lineages. Seven patients eventually showed trilineage responses. Only baseline reticulocyte count was a pretreatment predictor for response, perhaps reflecting residual HSC numbers, as has previously been demonstrated in IST36. Eltrombopag was well tolerated with no dose limiting toxicities, apart from infrequent reversible transaminitis, similar to the larger safety and efficacy studies in ITP37. All patients had bone marrow biopsies at baseline and while on drug and none showed an increase in reticulin staining, which was a concern in a recent study of ITP38. An increase in bone marrow cellularity was seen in several responders (figure 2).
Figure 1.
Responses to eltrombopag by lineage in patients with SAA refractory to IST. These Venn diagrams show the numbers of patients with uni- and multilineage responses at response assessment (A) and best response at follow-up (B). Reprinted with permission16.
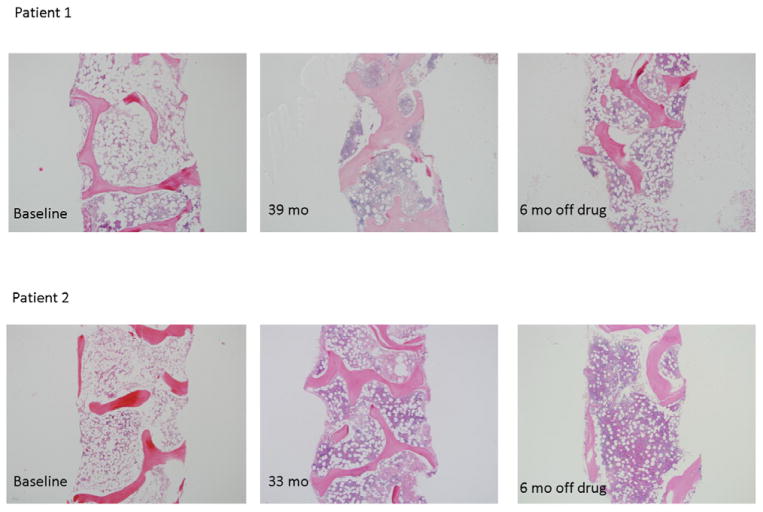
Figure 2.
Bone marrow cellularity in two patients responding to eltrombopag. The left panels show cellularity in these robust responders at baseline. The middle panels show cellularity just prior to discontinuing eltrombopag. The right panels demonstrate that, for Patients 1 and 2, the marrows remain cellular. The images were taken on an Olympus BX41 microscope with an Olympus DP72 camera, using a 4× UPlanFL N Olympus objective (original magnification ×40). Reprinted with permission16.
CAN ELTROMBOPAG BE DISCONTINUED IN REFRACTORY SAA WITHOUT RISK OF RELAPSE?
One patient in the National Institutes of Health study had eltrombopag withdrawn at 10 weeks because of a cataract misdiagnosis. Despite stopping study drug, he went on to have a trilineage response and remains transfusion independent. The protocol was subsequently amended to allow discontinuation of drug when blood counts in all three lineages reached acceptable levels and transfusions were no longer necessary. Six patients had drug withdrawn and all continue to maintain stable counts with normocellular bone marrow biopsies at a median of 24 months off drug (Figure 2). It is possible to conclude that when a critical mass of HSCs is generated, functional hematopoiesis can sustain blood counts in the near normal range without need for continued or continual exposure to eltrombopag. Whether the primitive progenitor cells stimulated by this agent have more favorable repopulating capabilities is unknown, but is an area currently under investigation. While the follow up time is relatively short, we believe there is evidence that transient exposure to eltrombopag may be sufficient, at least in a subset of patients, for hematopoietic recovery.
CLONAL EVOLUTION
Clonal evolution was a concern in this study. Eight of 43 patients developed new cytogenetic abnormalities. Most were non-responders at response assessment but 2 had previously responded. All patients who clonally progressed had eltrombopag withdrawn. Since publication one patient who was a non-responder and developed trisomy 21 at 3 months went on to develop monosomy 7 at 6 months with disappearance of the previous cytogenetic abnormality. However, on follow up marrow examination, there was normal karyotype; at least in this case, abnormal clones appeared transiently and disappeared with discontinuation of drug. In this particular patient, the platelet count further increased after study drug had been discontinued. Only two of the eight patients with newly detected cytogenetic abnormalities had dysplastic changes on the marrow examined at the time the abnormal cytogenetic clone was detected. No patient has developed AML, although 5 patients went on to have allogeneic stem cell transplant. The commonest chromosomal changes were chromosome 7 abnormalities, which developed in 5 of the 8 evolvers. This clonal transformation is associated with a poor outcome1, but all patients who developed chromosome 7 changes in the current series were successfully transplanted. Two eltrombopag responders evolved at 9 and 13 months with 13q deletion, which is considered a good prognostic cytogenetic change in patients with MDS transformed from SAA39,40. One of these patients has maintained his response off drug.
Dormant clones, undetectable to conventional cytogenetic analysis at baseline, may have been stimulated by eltrombopag. We performed CGH-SNP arrays on pretreatment marrow samples to investigate whether clones were detectable by alternative methods sensitive to rearrangement and loss of smaller amounts of chromatin, with negative results. Another hypothesis is that growth factor stimulation caused destabilization of the genome by driving proliferation of HSCs, leading to accelerated telomere attrition and subsequent emergence of clonal hematopoiesis. Patients with SAA have a baseline risk of developing clonal marrow dysfunction: 15% will show clonal progression by 10 years from diagnosis41. Concern for higher rates of transformation to AML in the treatment arm of a phase 3 study using the other available TPO receptor agonist Romiplostim in MDS caused this trial to be terminated early. However, subsequent follow up of these data (published in abstract form) found no difference in rates of progression between placebo and treatment arms42. Refractoriness to therapy is a particular risk factor for progression but true rates for evolution in this group are unknown43. While direct comparison with historical series is difficult, the association of clonal evolution with eltrombopag is possible and the drug should currently be used in aplastic anemia in the setting of a clinical research trial or with very close monitoring for transformation in refractory disease.
FUTURE DIRECTIONS
Clinical trials are ongoing in the NIH in other settings of bone marrow failure. We are conducting a pilot, non-randomized phase II trial at our institution using eltrombopag in addition to horse ATG and cyclosporine for treatment naïve SAA. The aim is to augment the quality of the hematologic response to immunosuppression by stimulating stem cells during recovery. This may prevent both short-term complications, namely infections and bleeding, but could also prevent clonal evolution. Robust hematologic responses with IST are associated with better long-term survival and a decreased risk of clonal evolution1. A larger, more heterogeneous stem cell reserve may ensure less proliferative stress on an individual clone, thereby limiting oncogenesis. To date, the overall response rate appears higher and time to transfusion independence is shortened. However, these are early results and it is unclear whether the risk of clonal evolution will be decreased with the use of eltrombopag in the upfront setting, but the risk of relapse (likely mediated by residual pathophysiologic T cells) is unlikely to be altered. Larger randomized trials comparing the use of IST with and without eltrombopag are ultimately required to determine eltrombopag’s influence on clonal evolution. Ongoing and future trials aim to abbreviate exposure and institute urgent treatment at diagnosis. These trials will be critical for informing safe prescribing practices given the existing temptation for physicians to use the drug off-label. The well-tolerated combination of eltrombopag and cyclosporine is also evident from these trials and may prove efficacious as therapy for SAA in developing countries where ATG is unavailable or simply not feasible.
Based on the initial results achieved in refractory SAA15 we initiated a new trial in refractory aplastic anemia, using a fixed dose of 150mg of eltrombopag and assessing patients after six months of therapy. The treatment period was extended because of indications that some subjects in the initial study had signs of response at the 3–4 month response assessment but were unable to continue therapy because they could not be deemed responders by protocol definition. We are, also, treating patients with moderate aplastic anemia (MAA) and unilineage cytopenic syndromes with higher doses of eltrombopag with a maximum of 300 mg allowed in this study. The treatment period in this single arm trial is 16 weeks. Most patients have not previously been treated with IST suggesting that this group may not have a strong immunological cause for their disease. Lower rates of response to IST have been suggested in patients with moderate compared to SAA44. We have observed robust hematological responses in this cohort and have seen no instances of clonal evolution despite higher doses being utilized. A simple and safe oral treatment for these patients with currently no licensed drugs available makes this a very exciting therapeutic approach.
Eltrombopag represents an important new strategy to treat aplastic anemia. To date, suppression of the aberrant immune cells was the only available approach. The addition of alternative immunosuppressive agents to ATG and cyclosporine did not improve response rates in large randomized trials9. Based on the results of our study there is strong evidence that eltrombopag stimulates HSCs and can reconstitute hematopoiesis in patients with aplastic marrows. Ongoing research into the use of this agent in other acquired bone marrow failure syndromes such as MDS are underway in our institute and others and is described elsewhere in this journal. Recent reports of the successful use of eltrombopag in inherited thrombocytopenia derived from MYH9 mutations have been published45. One patient on our MAA study with a congenital red cell aplasia responded to eltrombopag, suggesting that there may be some utility in the treatment of other inherited disorders of hematopoiesis. A phase 3 randomized study in upfront SAA is planned which will elucidate the risk of clonal evolution in patients who are IST naïve.
IST refractory SAA is a very challenging disease to treat. GlaxoSmithKline, the manufacturers of eltrombopag have recently made a submission to the Federal Food and Drug Agency for a licensed indication in refractory SAA and received breakthrough therapy designation allowing for an expedited review process.
Footnotes
The authors declare that they have no conflicts of interest or competing financial or personal relationships that could inappropriately influence the content of this article
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA : the journal of the American Medical Association. 2003 Mar 5;289(9):1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 2.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006 Oct 15;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoumbos NC, Gascon P, Djeu JY, Trost SR, Young NS. Circulating activated suppressor T lymphocytes in aplastic anemia. N Engl J Med. 1985 Jan 31;312(5):257–265. doi: 10.1056/NEJM198501313120501. [DOI] [PubMed] [Google Scholar]
- 4.Young NS, Leonard E, Platanias L. Lymphocytes and lymphokines in aplastic anemia: pathogenic role and implications for pathogenesis. Blood Cells. 1987;13(1–2):87–100. [PubMed] [Google Scholar]
- 5.Maciejewski JP, Hibbs JR, Anderson S, Katevas P, Young NS. Bone marrow and peripheral blood lymphocyte phenotype in patients with bone marrow failure. Exp Hematol. 1994 Oct;22(11):1102–1110. [PubMed] [Google Scholar]
- 6.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004 Jul 24–30;364(9431):355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- 7.Chen J. Animal models for acquired bone marrow failure syndromes. Clin Med Res. 2005 May;3(2):102–108. doi: 10.3121/cmr.3.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. A severe and consistent deficit in marrow and circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood. 1996 Sep 15;88(6):1983–1991. [PubMed] [Google Scholar]
- 9.Scheinberg P. Aplastic anemia: therapeutic updates in immunosuppression and transplantation. Hematology Am Soc Hematol Educ Program. 2012;2012:292–300. doi: 10.1182/asheducation-2012.1.292. [DOI] [PubMed] [Google Scholar]
- 10.Storb R, Etzioni R, Anasetti C, et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood. 1994 Aug 1;84(3):941–949. [PubMed] [Google Scholar]
- 11.Deeg HJ, Leisenring W, Storb R, et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998 May 15;91(10):3637–3645. [PubMed] [Google Scholar]
- 12.Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010 Sep 17; doi: 10.3324/haematol.2010.026682. 2010:haematol.2010.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheinberg P, Nunez O, Weinstein B, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011 Aug 4;365(5):430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012 Aug 9;120(6):1185–1196. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. The New England journal of medicine. 2012 Jul 5;367(1):11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014 Mar 20;123(12):1818–1825. doi: 10.1182/blood-2013-10-534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garnock-Jones KP, Keam SJ. Eltrombopag. Drugs. 2009;69(5):567–576. doi: 10.2165/00003495-200969050-00005. [DOI] [PubMed] [Google Scholar]
- 18.Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol. 2014 Apr;165(2):259–268. doi: 10.1111/bjh.12772. [DOI] [PubMed] [Google Scholar]
- 19.Zeigler FC, Desauvage F, Widmer HR, et al. In-Vitro Megakaryocytopoietic and Thrombopoietic Activity of C-Mpl Ligand (Tpo) on Purified Murine Hematopoietic Stem-Cells. Blood. 1994 Dec 15;84(12):4045–4052. [PubMed] [Google Scholar]
- 20.Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996 Jun 1;87(11):4544–4551. [PubMed] [Google Scholar]
- 21.Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996 Jun 15;87(12):4998–5005. [PubMed] [Google Scholar]
- 22.Fox N, Priestley G, Papayannopoulou T, Kaushansky K. Thrombopoietin expands hematopoietic stem cells after transplantation. The Journal of clinical investigation. 2002 Aug;110(3):389–394. doi: 10.1172/JCI15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ihara K, Ishii E, Eguchi M, et al. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci U S A. 1999 Mar 16;96(6):3132–3136. doi: 10.1073/pnas.96.6.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasouki MJ, Rafi SK, Olm-Shipman AJ, et al. Exome sequencing reveals a thrombopoietin ligand mutation in a Micronesian family with autosomal recessive aplastic anemia. Blood. 2013 Nov 14;122(20):3440–3449. doi: 10.1182/blood-2012-12-473538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emmons RV, Reid DM, Cohen RL, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996 May 15;87(10):4068–4071. [PubMed] [Google Scholar]
- 26.Wang W, Matsuo T, Yoshida S, et al. Colony-forming unit-megakaryocyte (CFR-meg) numbers and serum thrombopoietin concentrations in thrombocytopenic disorders: an inverse correlation in myelodysplastic syndromes. Leukemia. 2000 Oct;14(10):1751–1756. doi: 10.1038/sj.leu.2401898. [DOI] [PubMed] [Google Scholar]
- 27.Feng X, Scheinberg P, Wu CO, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011 Apr;96(4):602–606. doi: 10.3324/haematol.2010.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh JC, Ganser A, Stadler M. Hematopoietic growth factors in the treatment of acquired bone marrow failure states. Semin Hematol. 2007 Jul;44(3):138–147. doi: 10.1053/j.seminhematol.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Shao Z, Chu Y, Zhang Y, Chen G, Zheng Y. Treatment of severe aplastic anemia with an immunosuppressive agent plus recombinant human granulocyte-macrophage colony-stimulating factor and erythropoietin. Am J Hematol. 1998 Nov;59(3):185–191. doi: 10.1002/(sici)1096-8652(199811)59:3<185::aid-ajh2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Tichelli A, Schrezenmeier H, Socie G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011 Apr 28;117(17):4434–4441. doi: 10.1182/blood-2010-08-304071. [DOI] [PubMed] [Google Scholar]
- 31.Gurion R, Gafter-Gvili A, Paul M, et al. Hematopoietic growth factors in aplastic anemia patients treated with immunosuppressive therapy-systematic review and meta-analysis. Haematologica. 2009 May;94(5):712–719. doi: 10.3324/haematol.2008.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007 Nov 29;357(22):2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 33.Sun H, Tsai Y, Nowak I, Liesveld J, Chen Y. Eltrombopag, a thrombopoietin receptor agonist, enhances human umbilical cord blood hematopoietic stem/primitive progenitor cell expansion and promotes multi-lineage hematopoiesis. Stem Cell Res. 2012 Sep;9(2):77–86. doi: 10.1016/j.scr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012 Jul 12;120(2):386–394. doi: 10.1182/blood-2011-12-399667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugita M, Kalota A, Gewirtz AM, Carroll M. Eltrombopag inhibition of acute myeloid leukemia cell survival does not depend on c-Mpl expression. Leukemia. 2013 Apr;27(5):1207–1210. doi: 10.1038/leu.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009 Jan;144(2):206–216. doi: 10.1111/j.1365-2141.2008.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleh MN, Bussel JB, Cheng G, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013 Jan 17;121(3):537–545. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 38.Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009 Oct 29;114(18):3748–3756. doi: 10.1182/blood-2009-05-224766. [DOI] [PubMed] [Google Scholar]
- 39.Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002 May 1;99(9):3129–3135. doi: 10.1182/blood.v99.9.3129. [DOI] [PubMed] [Google Scholar]
- 40.Hosokawa K, Katagiri T, Sugimori N, et al. Favorable outcome of patients who have 13q deletion: a suggestion for revision of the WHO ‘MDS-U’ designation. Haematologica. 2012 Dec;97(12):1845–1849. doi: 10.3324/haematol.2011.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maciejewski JP, Selleri C. Evolution of clonal cytogenetic abnormalities in aplastic anemia. Leuk Lymphoma. 2004 Mar;45(3):433–440. doi: 10.1080/10428190310001602363. [DOI] [PubMed] [Google Scholar]
- 42.Kantarjian HM, Mufti GJ, Fenaux P, et al. Treatment with the Thrombopoietin (TPO)-Receptor Agonist Romiplostim in Thrombocytopenic Patients (Pts) with Low or Intermediate-1 (int-1) Risk Myelodysplastic Syndrome (MDS): Follow-up AML and Survival Results of a Randomized, Double-Blind, Placebo (PBO)-Controlled Study. Blood. 2012 Nov 16;120(21) [Google Scholar]
- 43.Kojima S, Ohara A, Tsuchida M, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002 Aug 1;100(3):786–790. doi: 10.1182/blood.v100.3.786. [DOI] [PubMed] [Google Scholar]
- 44.Young N, Griffith P, Brittain E, et al. A multicenter trial of antithymocyte globulin in aplastic anemia and related diseases. Blood. 1988 Dec;72(6):1861–1869. [PubMed] [Google Scholar]
- 45.Favier R, Feriel J, Favier M, Denoyelle F, Martignetti JA. First successful use of eltrombopag before surgery in a child with MYH9-related thrombocytopenia. Pediatrics. 2013 Sep;132(3):e793–795. doi: 10.1542/peds.2012-3807. [DOI] [PubMed] [Google Scholar]