Abstract
Background
We examined the effects of moderate prenatal alcohol exposure and/or prenatal stress exposure on D1 receptor binding in a nonhuman primate model. The dopamine D1 receptor is involved in executive function, and it may play a role in cognitive behavioral deficits associated with prenatal alcohol and/or stress exposure. Little is known, however, about the effects of prenatal alcohol and/or stress exposure on the D1 receptor. We expected that prenatal insults would lead to alterations in D1 receptor binding in prefrontal cortex and striatum in adulthood.
Methods
Rhesus macaque females were randomly assigned to moderate alcohol exposure and/or mild prenatal stress as well as a control condition during pregnancy. Thirty eight offspring were raised identically and studied as adults by non-invasive in vivo neuroimaging using positron emission tomography (PET) with the D1 antagonist radiotracer [11C]SCH 23390. Radiotracer binding in prefrontal cortex and striatum was evaluated by 2 (alcohol) × 2 (stress) × 2 (sex) analysis of variance.
Results
In prefrontal cortex, a significant alcohol × sex interaction was observed with prenatal alcohol exposure leading to increased [11C]SCH 23390 binding in male monkeys. No main effect of prenatal alcohol or prenatal stress exposure was observed.
Conclusions
These results suggest that prenatal alcohol exposure results in long-term increases in prefrontal dopamine D1 receptor binding in males. This may help explain gender differences in the prevalence of neurodevelopmental disorders consequent to prenatal alcohol exposure.
Keywords: rhesus macaque, dopamine D1 receptor, prenatal alcohol, prenatal stress, sex differences
Introduction
Prenatal alcohol exposure, while one of the most preventable causes of childhood developmental disabilities, remains a leading cause of intellectual disabilities and other neurobehavioral disorders. Consequences of prenatal alcohol exposure range from growth retardation, craniofacial abnormalities, and intellectual and behavioral impairments in children with severe adverse effects, i.e. fetal alcohol syndrome (FAS), to problems with inattention, learning, emotion regulation, speed of processing, motor function, and executive function in children without full-blown FAS (Jacobson et al., 1993; Mattson et al., 2011; Streissguth et al., 2004). Prenatal alcohol-exposed individuals are also at risk for major depressive disorders of childhood (O'Connor and Kasari, 2000), conduct disorder, ADHD (Herman et al., 2008), elevated risk for suicide, and substance abuse problems (Baer et al., 2003).
To address the wide range of outcomes associated with fetal alcohol exposure, the term fetal alcohol spectrum disorder (FASD) is currently used (Sokol et al., 2003). FASD is an important issue today because of the high prevalence of prenatal alcohol exposure. Indeed, over 50% of women in the United States of childbearing age reported using alcohol in the previous month (Rasmussen et al., 2009) and many of these women consume alcohol before pregnancy is detected. Better information on vulnerable behavioral and brain systems is needed to develop improved intervention strategies for prenatal alcohol-exposed individuals.
Fetal alcohol-exposed children are often conceived within the context of a stressful environment, yet interactions between prenatal alcohol and prenatal stress are an understudied yet common condition in children. In human studies it is difficult to determine the causal links between prenatal alcohol exposure, prenatal stress, and poor developmental outcomes due to confounding variables such as nutrition, other lifestyle factors as well as selection bias. Nonhuman primates serve as excellent models for studying prenatal perturbations with experimental control because of the similarity to humans in complex cognitive and social behaviors and in brain structures and biological processes. Studies with pig-tailed macaques (Macaca nemestrina), crab-eating macaques (Macaca fascicularis), and rhesus macaques (Macaca mulatta) have found a wide range of deficits from prenatal alcohol exposure which appeared to depend upon the dose and timing of exposure (Altshuler and Shippenberg, 1981; Clarren et al; 1992). Most of these studies, however, included few subjects and relatively high doses of prenatal alcohol exposure (see Schneider et al., 2011, for more detailed review).
The rhesus monkeys in the present study are part of a longitudinal experiment that independently manipulated moderate dose prenatal alcohol and prenatal stress exposure. In this study, with a relatively large sample (38 subjects), we examined prenatal alcohol-exposed rhesus macaques whose mothers voluntarily ingested 0.6 g/kg alcohol daily throughout pregnancy. This is comparable to an average size woman consuming two drinks daily throughout pregnancy.
The primary goal of the present report was to determine whether moderate level prenatal alcohol exposure would disrupt dopamine (DA) D1 receptor (D1R) binding in adult rhesus monkeys. The D1R is a critical component of the DA system and it is amenable to study by noninvasive radiotracer imaging. In the present study, [11C]SCH 23390 was used to determine the D1R binding potential, an index of receptor density and ligand affinity, in rhesus monkeys from prenatal alcohol- and/or prenatal stress-exposed pregnancies compared to controls (Halldin et al., 1986; DeJesus et al., 1987). The positron emission tomography (PET) radiotracer [11C]SCH 23390 is well-established for use in rhesus macaques to study the response of D1R binding to a variety of factors including age (Harada et al., 2002), administration of dopaminergic drugs (Ekesbo et al., 1999), experimental lesioning (Doudet et al., 2002), and electroconvulsive therapy (Landau et al., 2011).
D1 class dopamine receptors (D1 and D5) are located postsynaptically and, when activated by DA, initiate an excitatory secondary messenger transduction cascade (Beaulieu and Gainetdinov, 2011). D1R and their role in human behavior has been studied by PET. For example, in a study of healthy humans, D1R binding in hippocampus and parts of the striatum predicted executive performance, speed, and accuracy in general information on the subtest of the WAIS-R (Karlsson et al., 2011). In another study of healthy humans, decreases in cortical D1R binding predicted improvements in working memory in response to working memory training (McNab et al., 2009). Moreover, in schizophrenia patients, D1R binding in dorsolateral prefrontal cortex was elevated compared to controls, and increased D1R binding predicted decreased working memory performance (Abi-Dargham et al., 2002). Taken together, these PET studies are consistent with conclusions from the animal literature that D1R is involved in motor function, reward mechanisms, learning, and working memory (Beaulieu and Gainetdinov, 2011).
In rodents, the effects on D1R of experimental exposure to prenatal alcohol have been studied. For example, D1 receptors were increased by 5-10% in the striatum in rats exposed prenatally to alcohol (Gillespie et al., 1997). More recently, moderate alcohol during the third trimester equivalent in rats reduced D1R-mediated facilitation of GABA transmission in basolateral amygdala (Diaz et al., 2014). Given the role of the basolateral amygdala in emotional processing, it is possible that altered D1R binding might be an important mechanism of reduced behavioral adaptation that is found in FASD. Studies with mice showed a developmentally transient increase in D1R binding as a consequence of prenatal alcohol exposure (Boggan et al., 1996) in contrast to another study with rats showing a developmentally transient decrease in D1R binding (Druse et al., 1990). Moreover, D1R agonists and antagonists altered oral and sniffing behaviors in a way that suggested increased D1R drug sensitivity as a result of prenatal alcohol exposure (Sobrian et al., 2005). Furthermore, it is interesting that male prenatal alcohol-exposed rats, but not female, showed enhanced sensitivity to the D1R agonist effects of methylphenidate, amphetamine, and apomorphine in the open field test (Means et al., 1984; Hannigan et al., 1990; Blanchard et al., 1987; Ulug and Riley, 1983). In addition, prenatal alcohol exposure, in male but not female rats, delayed the emergence of the mature cataleptic response to pharmaological doses of the D1-antagonist SCH 23390 (Hannigan 1990b). These findings of differential responses of prenatally-exposed males compared to females suggest that prenatal alcohol exposure might increase activity of DA receptors preferentially in males.
In this longitudinal experiment with rhesus monkeys, we previously reported several findings with respect to DA function. Using in vivo imaging with PET to measure receptor and transporter binding as well as enzyme activity, we found that the moderate prenatal alcohol combined with prenatal stress condition produced increased D2 receptor binding (Roberts et al., 2004). Moreover, prenatal stress resulted in an increase of 15% in DAT binding in striatum (Converse et al., 2013). Finally, alterations in DA-regulated behaviors, including reduced neonatal orienting and motor maturity (Schneider et al., 1997) and reduced executive function at 30 months of age (Schneider et al., 2001), were found in monkeys from the prenatal alcohol condition compared with non-exposed controls.
In the present report, PET was used with the radiotracer [11C]SCH 23390 to test the hypothesis that prenatal alcohol exposure and prenatal stress, alone or in combination, would alter D1R binding compared to non alcohol- and non stress-exposed monkeys. Because of their major role in the modulation of cognitive and motor functions, we examined D1R binding in the following brain regions rich in dopaminergic innervation: striatum (putamen, caudate, and nucleus accumbens), prefrontal cortex (PFC), and substantia nigra/ventral tegmental area (SN/VTA).
Methods
Maternal Alcohol and Stress Treatments
As described previously (Schneider et al., 1997), healthy adult female rhesus monkeys in the breeding colony were screened for voluntary consumption of 0.6g/kg of a 6% volume/volume alcohol solution sweetened with Nutrasweet (300 mg/100 ml) (Equal Sweetener, Merisant US Inc., Chicago, IL). Animals in the present study were derived from females that reliably consumed alcohol and were randomly assigned to the control group or one of three experimental groups prior to breeding: prenatal alcohol only, prenatal stress only, or prenatal alcohol + stress. In the alcohol conditions, mothers voluntarily consumed the alcohol solution daily at 1600 hours. The control mothers consumed a sucrose solution that was designed to be approximately equivolemic and equicaloric (8g/100 ml of water, amounting to approximately 5% of daily calories) to the alcohol solution. The stress treatment was administered five times per week at approximately 1530 hours during mid-to-late gestation (Day 90 through Day 145 post-conception). The treatment involved removing the pregnant female from the home cage, placing her in a transport cage and taking her to a darkened room where three noise bursts (1300 Hz, 115 dB intensity at 1 m) were randomly administered over a 10-min period. All females were housed identically, undisturbed except for routine animal husbandry.
Subjects
The offspring subjects in this study were 38 rhesus monkeys (Macaca mulatta) that resulted from one of the 4 pregnancy conditions described above. Twenty-two dams contributed the 38 offspring: 11 dams contributed 1 offspring each, 6 dams contributed 2 offspring each, and 5 dams contributed 3 offspring each. In no cases did dams contribute infants all in the same conditions. All dams were multiparous with a minimum of 1 previous pregnancy. There were no differences across treatment groups in pre-pregnancy weights, age at delivery, or number of previous pregnancies (see Maternal Characteristics in Table 1). Among the offspring, there were 12 controls (9F, 3M), 10 prenatal alcohol-exposed (7F, 3M), 8 prenatal-stressed (2F, 6M) and 8 prenatal alcohol + stress-exposed offspring (3F, 5M). The study initially had 40 monkeys, however, two monkeys were dropped due to clinical conditions unrelated to the study. The rearing conditions and previous testing of these subjects were described in detail elsewhere (Schneider et al., 1997). Briefly, all infant monkeys were housed with their mothers in individual cages during the first 6 months of life. They underwent brief separations weekly for neurobehavioral testing during the first month of life. When they were 6 months old they were separated permanently from their mothers and reared in mixed-sex peer groups consisting of 5-6 monkeys from similar prenatal conditions. They were maintained on a diet of Purina Monkey Chow supplemented 3 times weekly with fresh fruit. Housing conditions were 8 hours dark and 16 hours light. Temperature was controlled at 21 ± 0.5 degree C. They were approximately 14 years old at the time of this study (See Table 1 for further details). Subjects were not exposed to alcohol after birth. These studies were conducted in accordance with the Institutional Animal Care and Use Committee.
Table 1. Subject characteristics, maternal characteristics, and radiotracer parameters.
| In Utero Maternal Treatment, Mean ± sem | ||||
|---|---|---|---|---|
|
|
||||
| Control | Alcohol | Stress | Alcohol + Stress | |
| Subject Characteristics | ||||
| n | 12 | 10 | 8 | 8 |
| F (%) | 75 | 70 | 25 | 38 |
| Age (yr) | 14.42 ± 0.18 | 14.15 ± 0.26 | 13.96 ± 0.44 | 13.32 ± 0.42 |
| Weight (kg) | 10.27 ± 0.51 | 9.13 ± 0.74 | 10.75 ± 0.49 | 10.59 ± 0.56 |
| Maternal Characteristics | ||||
| Pre-pregnancy weight | 6.7 ± 0.3 | 5.9 ± 0.2 | 6.8 ± 0.4 | 6.9 ± 0.3 |
| Age at delivery | 11.2 ± 1.0 | 11.2 ± 1.2 | 14.8 ± 8 | 12.9 ± 1.5 |
| Previous pregnancies | 5.2 | 6.0 | 7.8 | 6.8 |
| Radiotracer Parameters | ||||
| ID (MBq) | 223.4 ± 4.1 | 217.9 ± 5.6 | 218.9 ± 4.3 | 224.2 ± 4.6 |
| ID/BW (kBq/g) | 22.3 ± 1.1 | 25.3 ± 2.2 | 20.7 ± 1.0 | 21.6 ± 1.1 |
| SA (MBq/nmol) | 20.2 ± 2.0 | 20.7 ± 2.1 | 17.0 ± 1.6 | 22.9 ± 1.6 |
| IM/BW (pmol/g) | 1.18 ± 0.09 | 1.29 ± 0.11 | 1.28 ± 0.11 | 0.98 ± 0.09 |
| Aref (g/mL) | 0.71 ± 0.02 | 0.69 ± 0.04 | 0.79 ± 0.03 | 0.71 ± 0.03 |
| Mref (pmol/mL) | 0.85 ± 0.08 | 0.88 ± 0.08 | 1.00 ± 0.08 | 0.68 ± 0.06 |
ID = injected dose of [11C]SCH 23390; ID/BW = injected dose per body weight; SA = specific activity of radiotracer at injection; IM/BW = injected mass per body weight; Aref = reference region radioactivity/(injected dose/body weight) 20-60 min after injection; Mref = reference region radioactivity/SA = reference region tracer mass concentration 20-60 min after injection.
PET Procedure
Radiotracer
11C was produced as [11C]CH4 on the UW Medical Physics RDS112 prototype cyclotron using the 14N(p,α)11C reaction. [11C]CH4 was converted to [11C]CH3I using the recirculating gas loop synthesis method. [11C]CH4 was trapped on Hayesep Q at -180°C, flushed with He and released into a recirculating loop, passing through an Iodine furnace (70°C) and a reaction furnace (720°C), after which the [11C]CH3I was trapped on Hayesep Q at -10°C, with the rest of the unreacted gases passing though the reaction zone again. Upon completion of the reaction the gases were vented to waste, and the [11C]CH3I was released from the Hayesep at 200°C, bubbling slowly into the precursor solution. [11C]SCH 23390 was prepared by 11C-methylation of (5R)-8-chloro-2,3,4,5-tetrahydro-5-phenyl-1H-3-Benzazepin-7-ol (SCH 24518). Initially, [11C]SCH 23390 was prepared by bubbling [11C]CH3I into a mixture of SCH 24518 and sodium bicarbonate in acetonitrile as described in literature (DeJesus et al., 1987). [11C]SCH 23390 was obtained in 45% (n=10) decay corrected yield with an average specific activity of 1163 mCi/micromole. Later, [11C]SCH 23390 was prepared in improved yields using [11C]MeOTf as the 11C-methylation agent. Thus, [11C]MeOTf, prepared by passing [11C]CH3I through a column of AgOTf/GraphPac at 210°C, was bubbled through a solution of SCH 24518 (0.4 mg) in methyl ethyl ketone (0.15 mL). After 4 minutes at room temperature the product was purified using HPLC. The [11C]SCH 23390 HPLC fraction was evaporated to near dryness in vacuo and the residue was taken up in saline (5 mL) and passed through 0.2 μ filter (Millex-LG, 13 mm). The decay corrected radiochemical yield was 80% (n=36) with an average specific activity of 1397 mCi/micromole.
PET scans
The PET scanning protocol, image reconstruction, image processing, and pharmacokinetic modeling were generally carried out as detailed elsewhere (Converse et al., 2013). Briefly, isoflurane anesthetized subjects were scanned for 60 minutes in a microPET P4 (Siemens, Knoxville) starting with radiotracer injection. Quantitative dynamic images were reconstructed, and time-activity curves for anatomically defined regions of interest were analyzed with respect to a cerebellar reference region using the Logan slope at 20-60 minutes post-injection to determine the binding potential with respect to non-displaceable radiotracer, BPND (see Table 1 for scan details).
Statistical analysis
Prenatal treatment effects on [11C]SCH 23390 binding were evaluated for the prefrontal cortex (PFC) and striatum ROI's in a 2 (Prenatal Alcohol) × 2 (Prenatal Stress) × 2 (Sex) factorial analysis of variance (ANOVA). Effects meeting p<0.05 were considered significant. The sub-ROI's of the PFC (medial, ventrolateral, dorsolateral, frontopolar, and orbitofrontal) and striatum (caudate nucleus, putamen, and nucleus accumbens) were tested contingent on significant treatment effects in the larger regions. To qualitatively confirm the results of the ROI analysis, a voxelwise statistical map of the Sex × Alcohol interaction was subsequently created (SPM8) using uptake ratio images, which indicate for each subject the radioactivity concentration 20-60 minutes post injection of tracer divided by the concentration in the cerebellar reference region. In these images, the uptake ratio is a good proxy for the binding potential (r2 = 0.92 in putamen).
Results
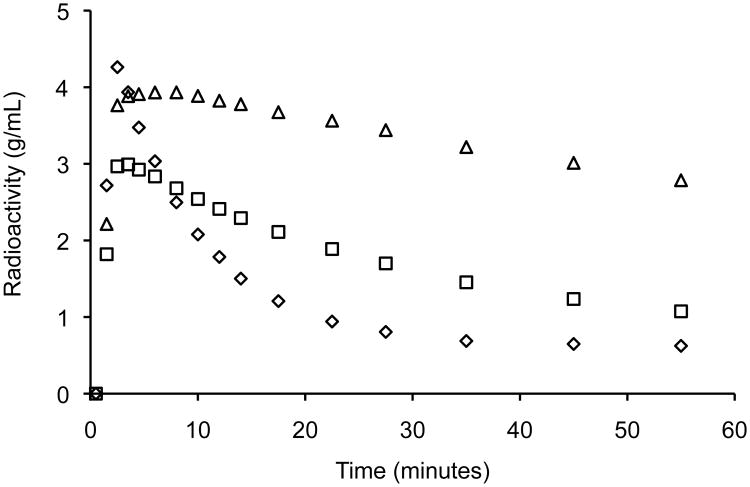
Figure 1 presents the mean radioactivity image of the control animals from 0 to 60 minutes following injection of [11C]SCH 23390. As expected, high binding is seen in the striatum reflecting dense nigrostriatal afferents; in addition, widely distributed diffuse binding is seen in the cortex, which is the target of mesocorticolimbic axons. Figure 2 shows average time-activity curves for the control animals. (See also Supplementary Figure 1 for individual results).
Figure 1.
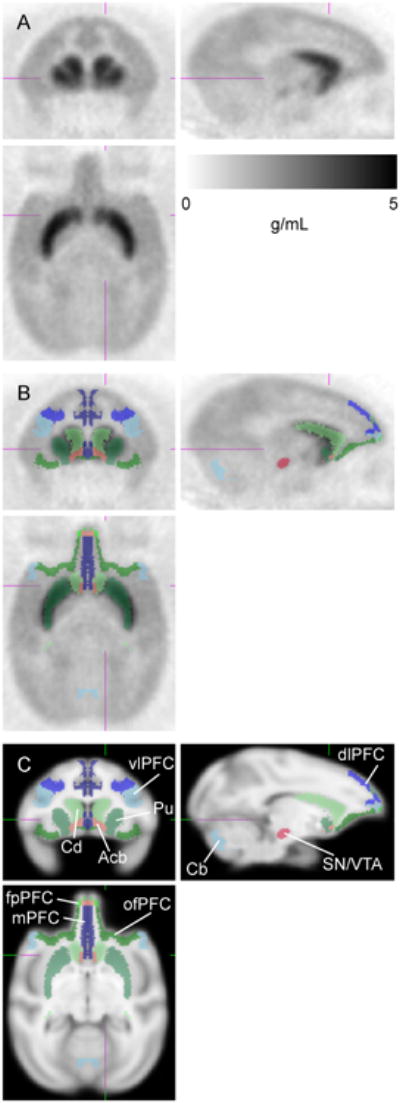
[11C]SCH 23390 image. (A) Mean radioactivity image of control subjects (n=12, 0-60 min) scaled to injected dose/body weight. (B) Regions of interest overlaid on radioactivity image. (C) Regions of interest overlaid on MRI template image. Coronal (upper left), sagittal (upper right), and axial (lower) slices shown at right striatum. Subregions of prefrontal cortex (PFC): vlPFC = ventrolateral PFC, dlPFC = dorsolateral PFC, mPFC = medial PFC, ofPFC = orbitofrontal PFC, fpPFC = frontopolar PFC. Subregions of striatum: Cd = caudate nucleus, Pu = putamen, Acb = nucleus accumbens. SN/VTA = substantia nigra/ventral tegmental area. Cb = cerebellar reference region.
Figure 2.
Time-activity curves. Mean radioactivity concentration for control subjects (n=12) illustrating relatively rapid washout from the cerebellar reference region (diamonds) along with binding in prefrontal cortex (squares) and striatum (triangles). Radioactivity concentration is scaled to injected dose/body weight.
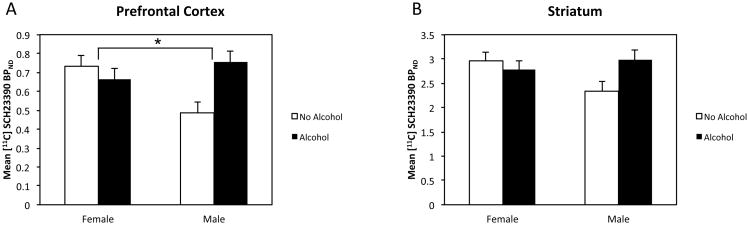
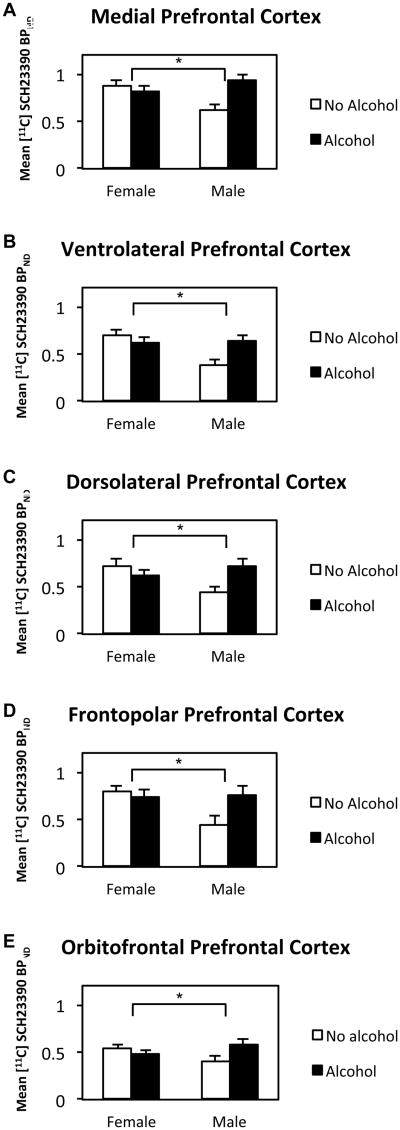
Analysis of [11C]SCH 23390 binding revealed a significant interaction of Alcohol × Sex in PFC (p = 0.012) and a trend in striatum (p = 0.072) (Table 2). There was a significant increase in D1 binding in alcohol-exposed males compared to non-alcohol-exposed males whereas females did not show this effect. All sub-ROI's of PFC also showed significant Alcohol × Sex interactions. For all sub-ROI's of the PFC in Table 2, the difference between No Alcohol and Alcohol-exposed males was significantly larger than the difference between No Alcohol and Alcohol-exposed females. The mean binding potentials in PFC and striatum are shown in Figure 3. The sub-ROI's of the PFC are shown in Figure 4 (further details are provided in Supplementary Table 1 and Supplementary Figure 2). There were no main effects or interactions with prenatal stress. A confirmatory voxelwise map of the Sex × Alcohol interaction is displayed in Figure 5 and Supplementary Movie 1.
Table 2. Effects of alcohol, sex and interaction of [11C]SCH 23390 binding potential.
| Alcohol | Sex | Alcohol × Sex | ||||
|---|---|---|---|---|---|---|
| Region of Interest | F (1,30) | p | F (1,30) | p | F (1,30) | p |
| Prefrontal Cortex | 2.43 | 0.13 | 1.59 | 0.22 | 7.24 | 0.012* |
| Lateral PFC | 1.7 | 0.203 | 3.09 | 0.09+ | 7.53 | 0.01* |
| Ventrolateral PFC | 1.77 | 0.193 | 5.14 | 0.031* | 7.11 | 0.012* |
| Dorsolateral PFC | 1.57 | 0.221 | 1.8 | 0.19 | 7.5 | 0.01* |
| Medial | 3.19 | 0.084+ | 0.81 | 0.37 | 6.8 | 0.014* |
| Frontopolar | 2.04 | 0.163 | 2.93 | 0.097+ | 4.37 | 0.045* |
| Orbitofrontal | 1.34 | 0.256 | 0.08+ | 0.784 | 4.66 | 0.039* |
| Striatum | 1.12 | 0.3 | 0.91 | 0.35 | 3.48 | 0.072+ |
| Caudate | 1.92 | 0.176 | 0.41 | 0.53 | 3.12 | 0.088+ |
| Putamen | 0.54 | 0.468 | 1.51 | 0.23 | 3.54 | 0.07+ |
| Nucleus accumbens | 4.47 | 0.043* | 0.57 | 0.46 | 3.81 | 0.0603+ |
| SN/VTA | 3.78 | 0.0614+ | 3.12 | 0.088+ | 1.38 | 0.249 |
ROI's are hierarchically structured. F's and p's are from 2(alcohol) × 2(prenatal stress) × 2(sex) ANOVA's. SN/VTA=substantia nigra/ventral tegmental area.
p<0.05
p<0.10
Figure 3.
Mean [11C]SCH 23390 BPND in prefrontal cortex (A) and striatum (B) as a function of prenatal alcohol exposure and sex of animal. In prefrontal cortex, the prenatal alcohol × sex interaction was significant F=7.24, p<0.012. In striatum, F=3.48 p<0.072. *p<0.05.
Figure 4.
Mean [11C]SCH 23390 BPND sub-ROI's of prefrontal cortex as a function of prenatal alcohol treatment and sex of animal. In all sub-regions of the prefrontal cortex, the prenatal alcohol × sex interaction was significant. *p<0.05 (see Table 2 for statistical details.)
Figure 5.
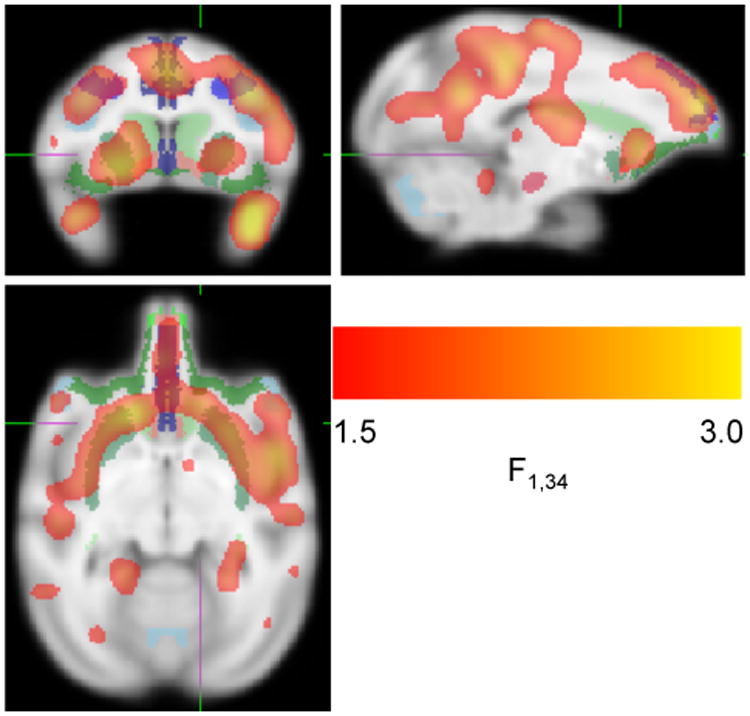
Sex × Alcohol interaction. To qualitatively confirm the results of the ROI analysis, a voxelwise map of the F test for the Sex × Alcohol interaction was calculated. Males exhibit relatively greater D1 binding in response to prenatal alcohol compared to females in prefrontal cortex and striatum. For display purposes the map has been smoothed with a σ = 2 mm Gaussian filter and arbitrarily thresholded at 1.5 < F < 3.0. The map is shown overlaid on the MRI template along with the regions of interest.
Discussion
This is the first study to our knowledge of D1R binding in nonhuman primates exposed prenatally to moderate level alcohol and/or stress. We found an Alcohol × Sex interaction such that D1R binding in males was increased more by moderate level prenatal alcohol exposure than for females. Our study was conducted when the animals were approximately 14 years old and not exposed to alcohol after birth, indicating likely persistence of adverse effects of prenatal alcohol exposure on DA system function in males. Age 14 in rhesus macaques translates approximately to middle-age in humans. The interpretation of enduring effects is consistent with human studies that have shown long-term developmental consequences of prenatal alcohol exposure (Spohr and Steinhausen, 1993). For example, Day and colleagues (2013) concluded that prenatal low-moderate alcohol exposure predicted problem behaviors, including internalizing, externalizing, and attention problems, when offspring were 22 years old. Effects resulted from exposures at each of the 3 trimesters.
DA D1R activation has been shown to have a modulatory effect on cell function in the dorsolateral prefrontal cortex (DLPFC) in macaque monkeys (Gonzalez-Burgos et al., 2002). More recently, Diaz and colleagues found that third trimester equivalent alcohol exposure in rats also decreased the DA precursor, (L-3,4-dihydroxyphenylalanine) L-DOPA as well as reduced levels of DA metabolites (homovanillic acid) in the basolateral amygdala (Diaz et al., 2014). Given this finding, it is possible that the increased D1R binding shown in male prenatal alcohol exposed monkeys in this study might represent a compensatory mechanism or adaptation to reduced DA synthesis or DA release in PFC. Moreover, because the DLPFC is involved in executive function and working memory, our finding of increased D1R binding in prenatal alcohol-exposed males in DLPFC could be related to deficits in executive function or working memory shown previously in this cohort (Schneider et al., 2001) as well as in children with FASD (Mattson et al., 2011).
There are no previous studies of prenatal-alcohol exposure and D1R binding in non-human primates. In one study of poly-drug exposed human fetal tissue, it was found that self-reported maternal alcohol use was related to reduced D1R mRNA transcription (DiNieri et al., 2011). In rodents, however, there is more extensive evidence that prenatal alcohol exposure affects D1R functioning, and there is also evidence of enhanced vulnerability of the D1R system of male animals compared to females to a range of early insults.
Prenatal alcohol exposure in rodents has increased, reduced, or had no significant effects on D1R binding in the frontal cortex and/or striatum (Boggan et al., 1996; Carneiro et al., 2005; Druse et al., 1990; Gillespie et al., 1997; Randall et al., 1999; Sobrian et al., 2005). These studies vary widely on factors such as the route and pattern of alcohol administration, dose and timing of exposure, species, age of assessment, and methods for measuring D1R binding. An increase in D1R binding in the striatum and no changes in frontal cortex was found by Gillespie et al., (1997) in PN19 rats. In mice, Boggan et al., (1996) found an increase in D1R binding in striatum but it was developmentally transient. In contrast to these studies showing increases in D1R binding, Carneiro et al., (2005) found reduced D1R density in striatum and hippocampus in rats. Developmentally transient decreases of D1 binding in frontal cortex and striatum were reported by Druse et al., (1990). Finally, Randall and Hannigan (1999) reported no effects on D1R binding sites in frontal cortex, striatum, ventral tegmental area and substantia nigra.
In spite of the mixed findings in direct assessments of D1R binding sites in rodents, pharmacological studies have shown altered sensitivity to DA releasing drugs in prenatal alcohol-exposed rats. For example, rats exposed to alcohol prenatally showed increased responsiveness to amphetamine and methylphenidate (Blanchard et al., 1987; Ulug and Riley, 1983). In a post-weaning methylphenidate challenge, Randall and Hannigan (1999) found behavioral sensitization and increased locomotor behavior in prenatally alcohol-exposed rats that they interpreted to be mediated by altered DA function. Reduced sensitivity to DA antagonists is consistent with increased D1R binding. Moreover, Hannigan (1990b) found that prenatally alcohol-exposed rats showed decreased sensitivity to the cataleptogenic effects of pharmacological doses of the D1 receptor antagonist SCH 23390. Decreased sensitivity to DA blocking drugs is considered to be due to either increased sensitivity or higher concentration of post-synaptic D1 receptors. Our findings of increased D1R binding in prenatal alcohol-exposed male monkeys are consistent with these pharmacological studies.
Sex differences similar to those of the present study have been found in rodent research in the vulnerability of the D1 system to early life perturbations. Our study found increased D1 receptor binding in PFC (and marginally in striatum) in male rhesus monkeys exposed to alcohol prenatally compared with non-exposed males, whereas this did not occur in females. Our finding is consistent with a body of evidence that sex of the animal influences the effects of prenatal alcohol exposure on the DA system. For example, Boggan et al., (1996) found that prenatal alcohol exposure resulted in lower DA concentrations and higher DOPAC/DA ratios in young male compared to female mice, although no sex × alcohol interaction was reported in D1 binding. Hannigan et al. (1990b) found that the mature response to the D1 receptor antagonist SCH 23390 was delayed in male but not female prenatal alcohol-exposed rats. In addition, the dose response curve to apomorphine (a DA agonist) for motor activity responsiveness was shifted higher in prenatally alcohol-exposed males but not females (Hannigan et al., 1990b). Finally, prenatal alcohol-exposed male rats showed greater sensitivity than females to acute amphetamine challenge (Blanchard et al., 1987). Sobrian et al., (2005), on the other hand, did not find sex differences in DA-related drug response for prenatal alcohol-exposed rats. Overall, males appear to be more vulnerable to the adverse effects on the DA system from prenatal alcohol exposure, consistent with human clinical studies showing that FASD boys were more likely to have the DA-related diagnosis of ADHD than FASD girls (Herman et al., 2008).
Other types of developmental perturbations have induced heightened vulnerabilities in males. For example, perinatal anoxia altered DA and DA metabolite levels in the PFC and nucleus accumbens in male rats compared to females (Laplante et al., 2012). Moreover, lead-exposed male rats showed behavioral impairments (hyperactivity in novel environments and spatial memory impairments) compared with females (Mansouri et al., 2012). Acute cocaine administration reduced D1R binding in caudate/putamen in male rats but not females (Festa et al., 2006). Furthermore, male rats compared with females exhibited prenatal cocaine-induced impairments in striatal DA release (Glatt et al., 2004). Similarly, prenatal cocaine-exposed male rats as opposed to females showed D1R density changes in PFC and prelimbic regions, which endured into adulthood (Ferris et al., 2007). Interestingly, in mice, Boggan and colleagues (1996) reported that D1R binding was about 40% greater in females compared with male mice. There were also lower DA concentrations and higher DOPAC/DA ratios in males compared with female mice suggesting more rapid DA turnover or less rapid development of DA system in males. Taken together, these studies suggest that the DA system in males and females is differentially sensitive to early life perturbations. Overall this literature on sex differences in animals suggests that D1 receptors may be one important part of the neural substrate for sex differences in the effects of prenatal alcohol in humans.
The mechanisms behind such sex dependent findings need to be elucidated. One possibility is that they are partly due to the maturational delay in DA metabolism and innervation in male rats compared with females (Ovtscharoff et al., 1992). If this sex difference in the timing of the dopamine system maturation holds across mammalian species, then the male lag in DA maturation may enhance neuronal vulnerability to a variety of developmental toxicants including prenatal alcohol, depending on the timing of exposure. Two particular processes that may be affected by sex differences in DA maturation are long-term potentiation (LTP) and long-term depression (LTD), two forms of synaptic plasticity in the striatum (Centonze et al., 2001). Conversion of LTP to LTD takes place around the postnatal third week in the rat (Partridge et al., 2000) with the DA system playing a central role in this change (Tang et al., 2002). LTP is a principal form of plasticity that emerges when synapses are beginning to function in striatum (Partridge et al., 2000). LTD emerges later in order to better calibrate synapses for skilled movement and sequencing of behavior (Di Filippo et al., 2009). Zhou et al. (2012) argued that D1 receptor up-regulation is one of the possible mechanisms underlying abnormalities in synaptic plasticity. Altered synaptic plasticity, in turn, could underlie neurobehavioral difficulties observed in prenatal alcohol-exposed offspring. Zhou and colleagues (2012) found that a high dose of prenatal alcohol exposure in rats (6 g/kg/day, gestational days 7-20, by intragastric intubation) led to the emergence of abnormal LTP instead of LTD at PN30 via altering D1 and D2 functions in the dorsolateral striatum of young male offspring.
A major strength of this study is the use of an experimental design enabling direct testing of the timing and dose of prenatal alcohol exposure on D1R binding, a degree of experimental control impossible to achieve in human studies. Also, the relatively long life span of rhesus macaques and the close similarity of their brain morphology and function to humans facilitate comparison to humans. Finally, the use of high resolution PET permits analysis of well-defined anatomical regions. The present study is limited by the need for caution in generalizing from monkey studies to humans, as always. Furthermore, approximately one quarter of [11C]SCH 23390 binding in cortex has been estimated to be to 5-HT2A receptors (Ekelund et al., 2007), although given the magnitude of the effects in the present study, they likely reflect differences in D1 binding. Further studies are underway of other neurotransmitter systems that represent important aspects of functional regulation in the frontal-striatal circuitry. An additional question is whether D1R binding changes resulting from prenatal alcohol exposure alter levels of voluntary alcohol drinking and brain neuroadaptation after chronic alcohol drinking in this cohort. Such studies are currently ongoing.
In summary, we found that prenatal alcohol-exposed male adult rhesus monkeys had increased D1R binding in PFC. Our findings are consistent with rodent studies showing that prenatal alcohol-exposed offspring, in particular males, were more drug sensitive than controls, indicating preferential targeting of the D1R by prenatal alcohol exposure in males (Blanchard et al., 1987; Ulug and Riley 1983). The fact that the development of the DA system varies for male and female animals may lead to differential vulnerabilities to prenatal alcohol exposure. Our findings are also consistent with human studies showing that boys with FASD were more likely than girls to have ADHD, a condition associated with dopaminergic dysfunction (Herman et al., 2008). Given the high proportion of women of child-bearing age consuming alcohol, this might contribute to the disproportionate number of males with ADHD. Future epidemiological studies should therefore examine prenatal alcohol by sex interactions.
Finally, our findings with moderate dose prenatal alcohol exposure in primates fill a research gap between rodent studies and human correlational results. Given the higher prevalence of neurodevelopmental disorders in boys than in girls, our findings point to the importance of further research on sex differences in fetal alcohol effects. As the scientific evidence accumulates, it will be important to enhance public education of women of childbearing age and training of healthcare professionals on the increased vulnerability of males to prenatal alcohol.
Supplementary Material
Acknowledgments
This study was supported by grants R01AA10079(MLS) and R01AA12277(MLS) from the National Institute of Alcohol Abuse and Alcoholism and grant P30HD03352 from the National Institute of Child Health and Human Development in support of the Waisman Brain Imaging Core.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler HL, Shippenberg TS. A subhuman primate model for fetal alcohol syndrome research. Neurobehav Toxicol Teratol. 1981;3:121–126. [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2001;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Hannigan JH, Riley EP. Amphetamine-induced activity after fetal alcohol exposure and undernutrition in rats. Neurotoxicol Teratol. 1987;9:113–119. doi: 10.1016/0892-0362(87)90087-0. [DOI] [PubMed] [Google Scholar]
- Boggan WO, Xu W, Shepherd CL, Middaugh LD. Effects of prenatal ethanol exposure on dopamine systems in C57BL/6J mice. Neurotoxicol Teratol. 1996;18:41–48. doi: 10.1016/0892-0362(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Carneiro LMV, Diogenes JPL, Vasconcelos SMM, Aragao GF, Noronha EC, Gomes PB, Viana GSB. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Centonze D, Saulle E, Bernardi G, Calabresi P. Receptor and post-receptor mechanisms of ischemic long-term potentiation in the striatum. Funct Neurol. 2001;16:149–152. [PubMed] [Google Scholar]
- Clarren SK, Astley SJ, Gunderson VM, Spellman D. Cognitive and behavioral deficits in nonhuman primates associated with very early embryonic binge exposures to ethanol. Pediatrics. 1992;121:789–796. doi: 10.1016/s0022-3476(05)81917-1. [DOI] [PubMed] [Google Scholar]
- Converse AK, Moore CF, Moirano JM, Ahlers EO, Larson JA, Engle JW, Barnhart TE, Murali D, Christian BT, DeJesus OT, Holden JE, Nickles RJ, Schneider ML. Prenatal stress induces increased striatal dopamine transporter binding in adult nonhuman primates. Biol Psychiatry. 2013;74:502–510. doi: 10.1016/j.biopsych.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Helsel A, Sonon K, Goldschmidt L. The association between prenatal alcohol exposure and behavior at 22 years of age. Alcohol Clin Exp Res. 2013;37:1171–8. doi: 10.1111/acer.12073. [DOI] [PubMed] [Google Scholar]
- DeJesus OT, Van Moffaert GJ, Friedman AM. Synthesis of [11C]SCH 23390 for dopamine D1 receptor studies. Int J Rad Appl Instrum [A] 1987;38:345–348. doi: 10.1016/0883-2889(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Picconi B, Tantucci M, Ghiglieri V, Bagetta V, Sgobio C, Tozzi A, Parnetti L, Calabresi P. Short-term and long-term plasticity at corticostriatal synapses: Implications for learning and memory. Behav Brain Res. 2009;199:108–118. doi: 10.1016/j.bbr.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Jotty K, Locke JL, Jones SR, Valenzuela CF. Moderate Alcohol Exposure during the Rat Equivalent to the Third Trimester of Human Pregnancy Alters Regulation of GABAA Receptor-Mediated Synaptic Transmission by Dopamine in the Basolateral Amygdala. Frontiers in Pediatrics. 2014;2 doi: 10.3389/fped.2014.00046. article 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudet DJ, Jivan S, Ruth TJ, Wyatt RJ. In vivo PET studies of the dopamine D1 receptors in rhesus monkeys with long-term MPTP-induced Parkinsonism. Synapse. 2002;44:111–5. doi: 10.1002/syn.10057. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin N, Kuo AP, Connerty M. Effects of in utero ethanol exposure on the developing dopaminergic system in rats. J Neurosci Res. 1990;27:233–240. doi: 10.1002/jnr.490270214. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN, Hwang Y, Hwang DR, Abi-Dargham A, Laruelle M. In Vivo DA D1 Receptor Selectivity of NNC 112 and SCH 23390. Mol Imaging Biol. 2007;9:117–125. doi: 10.1007/s11307-007-0077-4. [DOI] [PubMed] [Google Scholar]
- Ekesbo A, Torstenson R, Hartvig P, Carlsson A, Sonesson C, Waters N, Tedroff J, Langstrom B. Effects of the substituted (S)-3-phenylpiperidine (-)-OSU6162 on PET measurements of [11C]SCH23390 and [11C]raclopride binding in primate brains. Neuropharmacology. 1999;38:331–338. doi: 10.1016/s0028-3908(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Silvers JM, Hasselrot U, Beaudin SA, Strupp BJ, Booze RM. Sex mediates dopamine and adrenergic receptor expression in adult rats exposed prenatally to cocaine. Int J Dev Neurosci. 2007;25:445–454. doi: 10.1016/j.ijdevneu.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Jenab S, Weiner J, Nazarian A, Niyomchai T, Russo SJ, Kemen LM, Akhavan A, Wu HBK, Quinones-Jenab V. Cocaine-induced sex differences in D1 receptor activation and binding levels after acute cocaine administration. Brain Res Bull. 2006;68:277–284. doi: 10.1016/j.brainresbull.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Gillespie RA, Eriksen J, Hao HL, Druse MJ. Effects of maternal ethanol consumption and buspirone treatment on dopamine and norepinephrine reuptake sites and D1 receptors in offspring. Alcohol Clin Exp Res. 1997;21:452–459. doi: 10.1111/j.1530-0277.1997.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Trksak GH, Cohen OS, Simeone BP, Jackson D. Prenatal cocaine exposure decreases nigrostriatal dopamine release in vitro: Effects of age and sex. Synapse. 2004;53:74–89. doi: 10.1002/syn.20036. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Kröner S, Krimer LS, Seamans JK, Urban NN, Henze DA, Barrionuevo G. Dopamine modulation of neuronal function in the monkey prefrontal cortex. Physiol Behav. 2002;77:537–43. doi: 10.1016/s0031-9384(02)00940-x. [DOI] [PubMed] [Google Scholar]
- Halldin C, Stone-Elander S, Farde L, Ehrin E, Fasth KJ, Langstrom B, Sedvall G. Preparation of 11C-labelled SCH 23390 for the in vivo study of dopamine D-1 receptors using positron emission tomography. Int J Rad Appl Instrum [A] 1986;37:1039–43. doi: 10.1016/0883-2889(86)90044-4. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Blanchard BA, Horner MP, Riley EP, Pilati ML. Apomorphine-induced motor behavior in rats exposed prenatally to alcohol. Neurotoxicol Teratol. 1990;12:79–84. doi: 10.1016/0892-0362(90)90116-t. [DOI] [PubMed] [Google Scholar]
- Hannigan JH. The ontogeny of SCH 23390-induced catalepsy in male and female rats exposed to ethanol in utero. Alcohol. 1990b;7:11–6. doi: 10.1016/0741-8329(90)90053-f. [DOI] [PubMed] [Google Scholar]
- Harada N, Nishiyama S, Satoh K, Fukumoto D, Kakiuchi T, Tsukada H. Age-related changes in the striatal dopaminergic system in the living brain: A multiparametric PET study in conscious monkeys. Synapse. 2002;45:38–45. doi: 10.1002/syn.10082. [DOI] [PubMed] [Google Scholar]
- Herman LE, Acosta MC, Chang PN. Gender and attention deficits in children diagnosed with a Fetal Alcohol Spectrum Disorder. Can J Clin Pharmacol. 2008;15:411–419. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Dev. 1993;64:1706–1721. [PubMed] [Google Scholar]
- Karlsson S, Rieckmann A, Karlsson P, Farde L, Nyberg L, Backman L. Relationship of dopamine D1 receptor binding in striatal and extrastriatal regions to cognitive functioning in healthy humans. NeuroImage. 2011;57:346–351. doi: 10.1016/j.neuroimage.2011.04.047. [DOI] [PubMed] [Google Scholar]
- Landau AM, Chakravarty MM, Clark CM, Zis AP, Doudet DJ. Electroconvulsive therapy alters dopamine signaling in the striatum of non-human primates. Neuropsychopharmacology. 2011;36:511–518. doi: 10.1038/npp.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante F, Brake WG, Chehab SL, Sullivan RM. Sex differences in the effects of perinatal anoxia on dopamine function in rats. Neurosci Lett. 2012;506:89–93. doi: 10.1016/j.neulet.2011.10.055. [DOI] [PubMed] [Google Scholar]
- Mansouri MT, Naghizadeh B, Lopez-Larrubia P, Cauli O. Gender-dependent behavioural impairment and brain metabolites in young adult rats after short term exposure to lead acetate. Toxicology Letters. 2012;210:15–23. doi: 10.1016/j.toxlet.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Mattson S, Crocker N, Nguyen T. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Means LW, Medlin CW, Hughes VD, Gray SL. Hyperresponsiveness to methylphenidate in rats following prenatal ethanol exposure. Neurobehav Toxicol Teratol. 1984;6:187–192. [PubMed] [Google Scholar]
- O'Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcohol Clin Exp Res. 2000;24:1084–1092. [PubMed] [Google Scholar]
- Ovtscharoff W, Eusterschulte B, Zienecker R, Reisert I, Pilgrim C. Sex differences in densities of dopaminergic fibers and GABAergic neurons in the prenatal rat striatum. J Comp Neurol. 1992;323:299–304. doi: 10.1002/cne.903230212. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Randall S, Hannigan JH. In utero alcohol and postnatal methylphenidate: Locomotion and dopamine receptors. Neurotoxicol Teratol. 1999;21:587–593. doi: 10.1016/s0892-0362(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Erickson JD, Reef SE, Ross DS. Teratology: from science to birth defects prevention. Birth Defects Res A Clin Mol Teratol. 2009;85:82–92. doi: 10.1002/bdra.20506. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Moore CF, DeJesus OT, Barnhart TE, Larson JA, Mukherjee J, Nickles RJ, Schueller MJ, Shelton SE, Schneider ML. Prenatal stress, moderate fetal alcohol, and dopamine system function in rhesus monkeys. Neurotoxicol Teratol. 2004;26:169–78. doi: 10.1016/j.ntt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induces attention and neuromotor impairments in primate infants. Child Dev. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: Learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25:1383–92. [PubMed] [Google Scholar]
- Schneider M, Moore C, Adkins M. The Effects of Prenatal Alcohol Exposure on Behavior: Rodent and Primate Studies. Neuropsychol Rev. 2011:1–18. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrian SK, Jones BL, James H, Kamara FN, Holson RR. Prenatal ethanol preferentially enhances reactivity of the dopamine D1 but not D2 or D3 receptors in offspring. Neurotoxicol Teratol. 2005;27:73–93. doi: 10.1016/j.ntt.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC. Prenatal alcohol exposure and long-term developmental consequences. The Lancet. 1993;341:907–910. doi: 10.1016/0140-6736(93)91207-3. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–38. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulug S, Riley EP. The effect of methylphenidate on over-activity in rats prenatally exposed to alcohol. Neurobehav Toxicol Teratol. 1983;5:35–39. [PubMed] [Google Scholar]
- Zhou R, Wang S, Zhu X. Prenatal ethanol exposure alters synaptic plasticity in the dorsolateral striatum of rat offspring via changing the reactivity of dopamine receptor. PLoS ONE. 2012;7:1–9. doi: 10.1371/journal.pone.0042443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.