Abstract
Rationale
Positively reinforcing properties of alcohol are in part mediated by activation of the ventral striatum (VS). Alcohol-induced release of endogenous opioids is thought to contribute to this response. Preclinical studies show that the opioid antagonist naltrexone (NTX) can block this cascade, but its ability to do so in treatment seeking alcoholics has not been examined.
Objectives
To study the effects of NTX on alcohol-induced VS activation and on amygdala response to affective stimuli in treatment seeking alcohol dependent inpatients.
Methods
Sixty-three treatment seeking alcoholics were randomized to receive NTX (50 mg) or placebo (PLC) daily. On day 7, participants underwent an alcohol cue reactivity session, and craving was measured using the Penn Alcohol Craving Scale. On day 9, participants received a saline infusion followed by an alcohol infusion and also viewed affective stimuli in an MR scanner.
Results
Irrespective of medication treatment condition, the alcohol infusion did not activate the VS in the alcohol dependent patients. Unexpectedly, VS activation was greater in NTX treated patients than in the PLC group. NTX treated patients also reported increased craving in response to alcohol cue exposure, and increased subjective response to alcohol (‘high’ and ‘intoxicated’) compared to PLC subjects. No significant effects of alcohol infusion on brain response to affective stimuli were in the NTX or placebo groups.
Conclusions
Unlike previous findings in social drinkers, a moderate level of intoxication did not activate the VS in treatment seeking alcoholics. This is likely to reflect tolerance to the positively reinforcing properties of alcohol in this clinical population. Our findings may help explain the efficacy of NTX to reduce heavy drinking, but not to maintain abstinence.
Keywords: Alcoholism, Dopamine, Ventral Striatum, Naltrexone, Opioid System
INTRODUCTION
Mesolimbic dopamine (DA) projections from the ventral tegmental area (VTA) to the nucleus accumbens / ventral striatum (NcAcc / VS) are thought to play an important role in positively reinforcing properties of alcohol. For instance, extracellular DA levels in the rat NcAcc are increased both by alcohol injections (Di Chiara and Imperato 1988) and by oral alcohol self-administration (Weiss et al. 1993). Dopamine levels in this region are also correlated with measures of alcohol preference in genetically selected rat lines (Katner and Weiss 2001). Alcohol induced DA release in the NcAcc / VS appears to be mediated by activation of DA neurons in the VTA, since alcohol increases their firing rate both in vivo (Gessa et al. 1985) and in vitro ( Koyama et al. 2007). Of note, neurochemical lesions show that mesolimbic DA activity, while contributing to alcohol reinforcement, is not essential to maintain alcohol intake (Rassnick et al. 1993).
Rodent studies have also shown that acute alcohol administration induces a central release of endogenous opioids [for review, see (Herz 1997)], a finding recently confirmed in humans (Mitchell et al. 2012). The µ-opioid receptor appears to be particularly important for positive reinforcement from alcohol, as measured by alcohol self-administration, as well as alcohol “reward”, as measured by conditioned place preference (CPP). Both these motivational measures are suppressed by genetic deletion of µ-opioid receptors in mice (Roberts et al. 2000; Hall et al. 2001), and similar effects are obtained with several µ-opioid antagonists [for review, see e.g. (Heilig and Egli 2006)]. Accordingly, the mu-preferring opioid antagonist naltrexone (NTX) is an FDA-approved pharmacotherapy for alcoholism, and its efficacy is supported by meta-analysis of several randomized controlled trials (Rosner et al. 2010).
Positively reinforcing properties of alcohol may in part arise from an interaction between alcohol, endogenous opioids and DA. Suppression of alcohol reinforcement by NTX in rats is associated with attenuation of alcohol-induced DA release in the NcAcc (Gonzales and Weiss 1998). This interaction may be mediated at the level of the VTA, the NcAcc / VS, or both. Thus, activation of µ-opioid receptors in the VTA results in an activation of DA neurons in this structure, through removal of inhibitory GABA-ergic tone (Spanagel et al. 1992). This mechanism has been suggested to mediate DA activation by several non-stimulant drugs, including ethanol (Tanda and Di Chiara 1998). However, a recent mouse study did not find support for VTA mediation of alcohol - opioid – DA interactions (Ramachandra et al. 2011). Direct effects of µ-opioid receptors in the NcAcc / VS may instead contribute to alcohol seeking (Heinz et al. 2005; Mitchell et al. 2012).
Accumulating evidence from fMRI studies suggests that the BOLD signal originating from the VS reflects consequences of DA release, through activation of postsynaptic DA-D1 receptors, ultimately leading to changes in postsynaptic membrane potentials [reviewed in (Knutson and Gibbs 2007)]. This influence can change on a second-to-second basis, making fMRI a useful probe into circuitry mediating drug reinforcement. Accordingly, we have previously reported that pharmacokinetically controlled IV alcohol administration to social drinkers resulted in a robust activation of the VS, as measured by fMRI BOLD (Gilman et al. 2008). Self-ratings of intoxication in that study were highly correlated with VS activation, supporting the notion that alcohol-induced activation of this structure is a neural substrate of alcohol reinforcement . A subsequent study replicated these findings in young adult social drinkers, but found attenuated alcohol-induced VS activation in non-treatment seeking heavy drinkers (Gilman et al. 2012). This raised the question whether blockade of a reinforcing alcohol – opioid – DA cascade is a plausible therapeutic mechanism for NTX in a clinical population of treatment seeking alcoholic patients.
Here, we addressed this question in an experimental inpatient study under well-controlled conditions. We randomized treatment-seeking alcoholics in early abstinence to NTX or PLC, and examined their responses to alcohol cues during an alcohol cue-reactivity experiment, as well as their fMRI BOLD brain responses to a pharmacokinetically controlled IV alcohol challenge. Because a prior study had shown that alcohol modulates brain activity to fearful faces in the amygdala and other frontal and temporal brain regions in social drinkers but not in heavy drinkers (Gilman et al, 2008;) , we also evaluated brain responses to fearful and neutral faces during alcohol and saline infusion. in a sample of treatment-seeking alcoholics
METHODS
Participants
Participants were recruited through advertisements in local media. Following phone screening, subjects were admitted to the NIH Clinical Center in Bethesda, MD, and underwent medically managed withdrawal if needed. Once they had an undetectable breath alcohol concentration and did not require benzodiazepines for withdrawal, they were evaluated for eligibility; eligibility determination typically took between five and seven days to complete. Detailed eligibility criteria are at http://www.clinicaltrials.gov/ct2/show/NCT00896038. In brief, subjects were 63 treatment-seeking alcohol-dependent individuals (63.5% males) between 21 – 50 years old, diagnosed with alcohol dependence according to the Structured Clinical Interview for DSM-IV [SCID; (First et al., 1995)], and in good physical health. Alcoholism severity was assessed using the Alcohol Dependence Scale [ADS; (Skinner and Allen 1982)] and alcohol consumption during the preceding 90 days was determined using the time-line follow-back questionnaire (Sobell et al. 1986). Subjects were excluded if they presented with complicated medical or psychiatric problems or were unable to participate in all study procedures or provide informed consent. Written informed consent was obtained as approved by the NIH Institutional Review Board. Because it has been reported that individuals with a family history of alcoholism show a greater NTX-induced attenuation of the stimulatory effects of alcohol (King et al. 1997), we included only family history positive subjects.
Subjects remained hospitalized throughout the study, and immediately following the infusion session (Day 9), they participated in standard-of-care behavioral alcoholism treatment, mainly consisting of 12-step facilitation sessions,
Pharmacologic Intervention
On Day 1, participants were randomized to receive NTX 50 mg/day or matching PLC for nine days. Both participants and study staff were blind to treatment condition. Blinding was achieved by encapsulating commercially obtained NTX and manufacturing matching PLC capsules. Randomization was carried out by the NIH Clinical Center Pharmacy.
Genotyping
Effects of NTX on clinical outcomes [for meta-analysis, see (Chamorro et al. 2012)] and on laboratory measures [see e.g. (Ray and Hutchison 2004, 2009)] differ as a function of genotype at an A118G non-synonymous single nucleotide polymorphism (SNP) in the OPRM1 gene that encodes the mu-opioid receptor. In order to control for possible confound of genetic variation at this locus, blood was collected and subjects were genotyped as described previously (Ramchandani et al. 2011). For all outcomes, sensitivity analyses were carried out, in which 118G carriers (NTX: n=3; PLC: n=2) were removed; in all analyses, results remained unchanged.
Experimental Procedures
IV alcohol infusion and fMRI
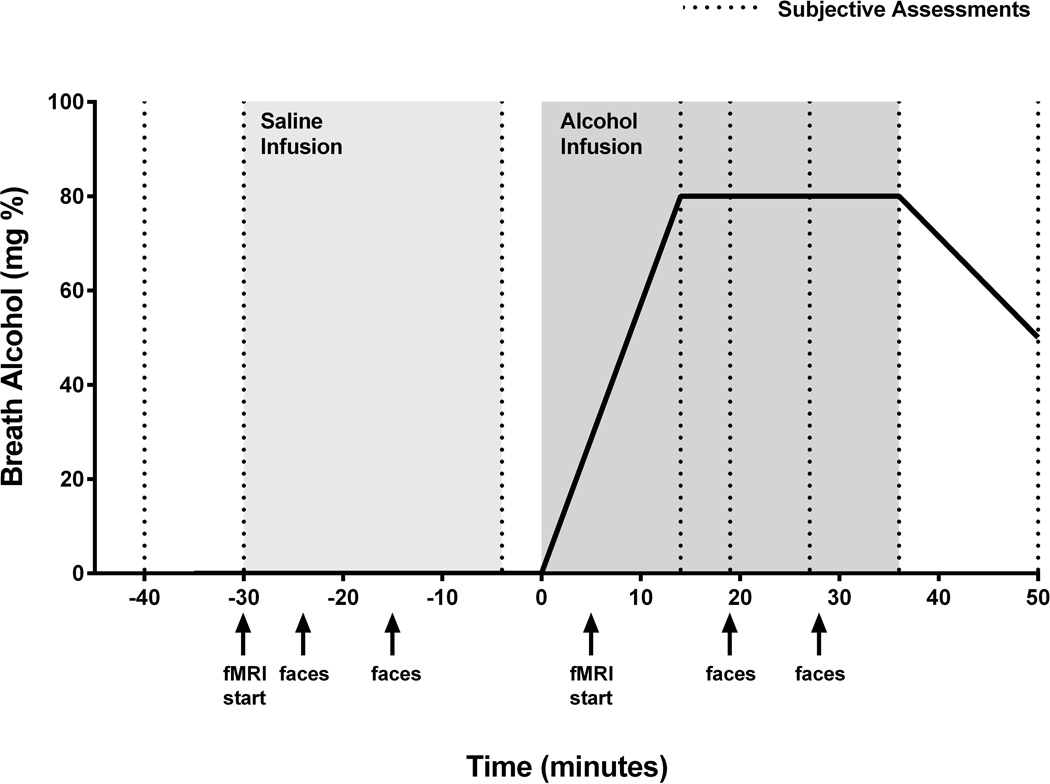
On Day 9, participants had IV catheters inserted in antecubital or arm veins, placed in the scanner, and infused with saline for 30 minutes, followed by alcohol (Fig. 1). During the alcohol infusion procedure alcohol was infused as a 6% v/v solution in saline to achieve a target BAC of 0.08 g% (+/− 0.005 g%) in 15 min and maintain that concentration for 20 min (for further details see Gilman et al. 2008). Subjective responses to alcohol were measured using the modified Drug Effects Questionnaire [DEQ; (de Wit and McCracken 1990)], at baseline (−30 min), at the end of the saline phase (−4 min) and serially during the infusion at 14, 19, 28, 36 and 50 min following the start of the alcohol infusion. Breathalyzer measurements were obtained at the end of the infusion and every 30 min thereafter until BrAC returned to zero. Safeguards used to address the risk of relapse or other adverse consequences related to alcohol exposure are specified in the following section. Structural scans were acquired as the saline infusion began, and standardized emotional facial expression (EFE) images (Matsumoto, 1988) were presented starting at the 20 min timepoint as functional scans were acquired. Participants were instructed to focus on the images and were asked to press a button every time they saw a white square on the screen in order to ensure their attention during the presentation of the stimuli.
Fig. 1.
Experimental Timeline of the IV saline/alcohol infusion and fMRI procedures.
Safeguards developed and implemented for administering ethanol to treatment-seeking alcoholics
We established a list of safeguards using the guidelines established by the National Advisory Council on Alcohol Abuse and Alcoholism and input by the NIH Combined NeuroScience Institutional Review Board. The following safeguards were applied during this study:
-
▪
All participants were medically and mentally stable.
-
▪
They were recently detoxified, therefore, not long-term abstinent.
-
▪
The amount of ethanol given was equivalent to approximately 3–4 standard drinks (1 standard drink = 12 oz. can of beer or 5 oz. glass of wine).
-
▪
A nurse was present and a physician was immediately accessible during the ethanol infusion.
-
▪
Following the ethanol infusion, subjects were debriefed by a trained nurse and/or a physician, and standard CBT techniques for coping with cravings were used to support patients. Subjects remained in the hospital for at least three weeks after the alcohol infusion session to complete the alcoholism treatment program and to monitor their craving for alcohol. If a participant expressed a desire to leave the hospital under the influence of alcohol, every effort was made to encourage them to stay in treatment and complete the study.
-
▪
Following the fMRI session, all patients were offered to receive NTX (50 mg/day) as a standard FDA-approved medication for alcoholism.
-
▪
If participants had a car on campus, their car keys were held by the unit staff during the study. This prevented participants from impulsively leaving the hospital and driving under the influence of alcohol.
-
▪
Family members or friends were strongly discouraged from providing transportation that would enable the patient to leave the program early.
fMRI Stimuli
Visual images from a series of Emotional Facial Expression (EFE) images were used in this study. In all, 45 neutral and 45 fearful faces, as well as a non-emotional control cross-hair condition that served as the inter-stimulus interval, were presented in an event-related design that lasted 8 min 30 s. The stimuli were each presented for 2 s, and the Inter-Stimulus Interval ranged from 0 to 8 s. All stimuli were projected onto a screen placed at the foot of the MRI scanner bed and were viewed using a mirror mounted on the head coil. The images were presented at 6 min and 15 min (saline infusion), and at 49 and 58 min during the alcohol infusion, as functional scans were acquired. Participants were instructed to focus on the images, but no response was required.
fMRI Acquisition
Imaging was performed using a 3 T General Electric MRI scanner with a 16-channel head coil. In all, 30 contiguous axial slices were acquired (in-plane resolution 3.75 3.75 mm), providing whole-brain coverage. High-resolution structural scans were collected using a T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) pulse sequence, which facilitated localization and co-registration of functional data (matrix 256 × 256 x 124, repetition time (TR)=100 ms, echo time (TE)=12 ms, field of view (FOV)=24 cm). Functional scans were acquired using a T2*-EPIRT sequence that measure changes in blood oxygen level dependent (BOLD) contrast (B210 volumes, TR=2 s, TE¼30 ms, flip angle=901, matrix 6464, in-plane matrix=128, FOV¼24 cm, slice thickness =3.75 mm, 1mm gap, yielding 3.75mm cubic voxels).
Cue Reactivity test
On Day 7, a cue-reactivity test was performed similarly to that of previously published studies (details in: Monti et al., 1987, Monti et al., 1999 and McGeary et al., 2006). The procedure was performed in the early afternoon and participants were exposed to visual, tactile, olfactory, and proprioceptive stimuli associated with the beverage during three 3-minute CR trials. The first consisted on the exposure to neutral cues (i.e. a bottle and glass of water), followed by three minutes of rest. The second trial consisted on the exposure to alcoholic cues (commercially-labeled preferred alcohol beverage); at each trial, an audiotape instructed the participant to sniff the glass of water or alcohol according to high/low tones. The trials were presented in the same order for all participants because of known carryover effects (Monti et al., 1987; Monti et al., 1999; Rohsenow et al., 2000). Craving was assessed using the Penn Alcohol Craving Scale (PACS) administered at baseline and after the cue reactivity test was completed. Also mean arterial pressure (MAP) and HR were continuously monitored.
Participants were monitored by clinical research staff via a one-way mirror to ensure compliance to the study procedures.
Analysis of fMRI data
Functional image analyses were performed using Analysis of Functional Neural Images (AFNI) software (Cox, 1996). Echoplanar image volumes were preprocessed as follows: (1) voxel time series were interpolated to correct for non-simultaneous slice acquisition within each volume (using sinc interpolation and the most inferior slice as a reference). (2) Volumes were corrected for motion in three dimensional space and were spatially smoothed using a 6-mm full-width half-maximum smoothing kernel. (3) A mask was created so that all of the background values outside of the brain were set to zero. Statistical maps were generated for each individual. The group comparisons for each contrast of interest were performed between two groups using the Mixed Effect Meta Analysis (MEMA) of AFNI. For each individual reprocessed time series data time-locked to single impulse response functions at the onset of various events were then analyzed by applying a general linear model (GLM). Motion correction parameters were used as regressors of no interest. The model was convolved with the canonical hemodynamic response function. In this full, factorial ANOVA, infusion (alcohol or saline) were within subject factors, group (NTX or PLC) was a between-subject factor, and ‘subject’ was a random factor.
The whole brain images were non-linearly registered to a Talairach space and the VOI volumes were extracted from these images using average parameter estimates within VOIs. VOI analyses were performed using atlas generated masks of NcAcc/VS and amygdala in a standard (i.e. AFNI Talairach) space, in order to avoid any between-subjects biases in the position of VOIs and to eliminate a potential confound of manually generating the VOI masks.
Potential covariates such as ADS and subjective responses to alcohol as measured by DEQ, were evaluated for inclusion on a model-by-model basis and did not show any significant effects, therefore they were not retained in the final analysis.
Analysis of Behavioral Measures
Data were examined for homogeneity of variance and distribution, and analyzed using general linear models (GLM, Statistica 6.0, StatSoft, Tulsa, OK). One-way analysis of variance (ANOVA) was used to compare baseline characteristics between the randomized treatment groups. Repeated measures analysis of covariance (RM-ANCOVA) was used to analyze primary and secondary outcomes associated with alcohol and saline challenge. The latter analyses included pharmacological treatment (NTX vs. PLC) as a between-subjects factor, and both challenge treatment (alcohol vs. saline) and time point (number varied according to outcome) as within-subjects factors. Potential covariates, including baseline behavioral measures and subject characteristics were evaluated for each outcome and included in the model if they were significant. The alpha value was set at 0.05 for all analyses.
RESULTS
Participant characteristics are described in Table 1. The groups did not differ significantly on baseline variables, including alcoholism severity and number of drinks per day in the 90 day interval prior to admission to the inpatient unit.
Table 1.
Demographic characteristics of participants
| Naltrexone (n = 31) |
Placebo (n = 32) |
p-value1 | |
|---|---|---|---|
| Female/Male | 13/18 | 10/22 | > 0.05 |
| Caucasian/Black/Hispanic | 18/13/0 | 20/10/2 | > 0.05 |
| Age (years) | 38.2 ± 1.8 | 39.1 ± 1.5 | > 0.05 |
| Education (years)2 | 13.3 ± 0.3 | 13.7 ± 0.4 | > 0.05 |
| Alcohol Dependence Severity (ADS) | 21.2 ± 1.3 | 19.4 ± 1.5 | > 0.05 |
| Family History Density of Alcoholism | 0.18 ± 0.04 | 0.22 ± 0.03 | > 0.05 |
| Lifetime Alcohol Use (years)2 | 20.0 ± 1.9 | 20.6 ± 1.9 | > 0.05 |
| Lifetime Treatments for Alcohol Abuse2 | 3.4 ± 0.6 | 3.9 ± 0.9 | > 0.05 |
| Time Line Follow Back (TLFB; 90 days) | |||
| Total Drinks | 947.5 ± 152.8 | 1072.0 ± 134.3 | > 0.05 |
| Drinking Days (nr) | 69.6 ± 4.0 | 70.8 ± 4.2 | > 0.05 |
| Avg Number Drinks/Day | 12.5 ± 1.7 | 14.6 ± 1.4 | > 0.05 |
| Heavy Drink Days (nr) | 59.7 ± 5.5 | 67.6 ± 4.5 | > 0.05 |
| Days Abstinent Prior to Admission | 0.7 ± 0.4 | 0.2 ± 0.1 | > 0.05 |
| Lifetime Heroin Use (years)2 | 0.3 ± 0.2 | 0.2 ± 0.1 | > 0.05 |
| Lifetime Barbituate Use (years)2 | 0.2 ± 0.1 | 0.1 ± 0.1 | > 0.05 |
| Lifetime Sedative/Hypnotic Use (years)2 | 1.5 ± 1.0 | 0.7 ± 0.4 | > 0.05 |
| Lifetime Amphetamine Use (years)2 | 0.7 ± 0.3 | 1.0 ± 0.4 | > 0.05 |
| Lifetime Cocaine Use (years)2 | 7.6 ± 2.0 | 5.5 ± 1.0 | > 0.05 |
| Lifetime Cannabis Use (years)2 | 13.7 ± 2.4 | 9.1 ± 1.8 | > 0.05 |
| Lifetime Hallucinogen Use (years)2 | 2.9 ± 1.1 | 1.3 ± 0.5 | > 0.05 |
chi-square tests for gender, t-tests for continuous variables, all p > 0.05
Measured using the Addiction Severity Index (ASI)
VS of treatment seeking alcoholics is not activated by alcohol, but is increased by NTX
To test the hypothesis that VS responses would be increased by alcohol and decreased by medication treatment, we evaluated data using a volume of interest (VOI) approach, and a mixed model two way ANOVA with treatment (NTX vs. PLC) as a between subjects factor, and challenge condition (alcohol vs. saline) as a within-subjects factor. Since we did not observe any difference in the activation of the VS or other brain regions in response to fearful vs. neutral stimuli, we collapsed our results across all affective stimuli.
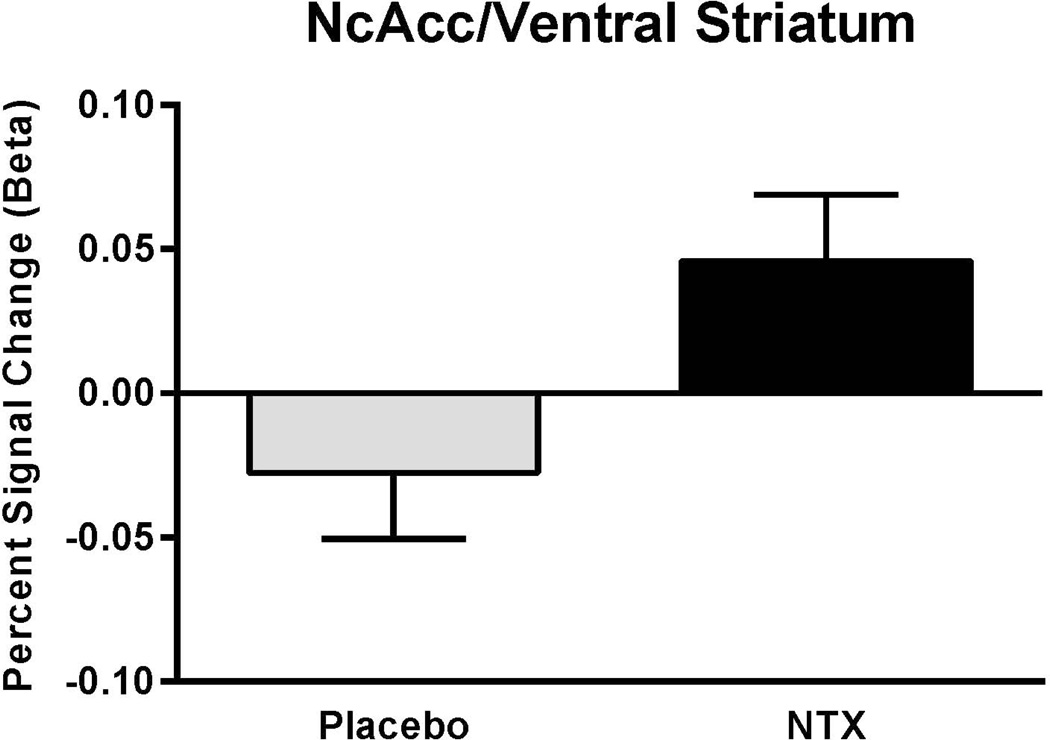
There was no main effect for alcohol to activate the VS versus the saline condition (F[1,52]=0.19, p=0.67); i.e. across all other conditions, the ALC response was not different from that to SAL. There was a main effect of medication condition (NTX vs. PLC; F[1,52]=5.1, p=0.03); the NTX response was greater than the PLC response (Fig.2).
Fig. 2.
Volume of Interest Analysis of the Ventral Striatum. There was a main effect of NTX to affect the VS BOLD signal change vs. PLC (F[1,52]=5.1, p=0.03), the NTX response was greater than the PLC response.
Amygdala responses to fearful faces were not influenced by alcohol or NTX
Similar to the VS, amygdala activity was not influenced by alcohol (main effect: F[1,52]=0.17, p=0.68). In contrast to the VS, however, amygdala activity was also unaffected by NTX treatment (main effect: F[1,52]=2.1, p=0.15). Finally, there was no main effect of the emotional condition to increase amygdala activity (F[1,52]=0.47, p=0.49). None of the interactions reached significance, although there was a trend for an interaction between NTX treatment and emotional condition (F[1,52]=3.33, p=0.074); this was driven by a higher response to fearful faces among NTX treated subjects.
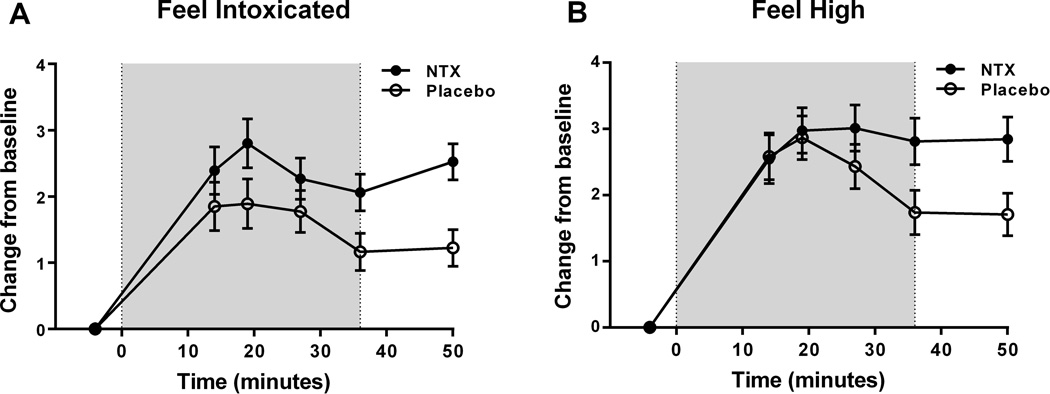
NTX increases the subjective response to alcohol
There was a main medication effect on responses to the DEQ item ‘Do you feel intoxicated?’ (F [1,43]=4.8, p<0.03). Across the session, NTX subjects experienced higher levels of subjective intoxication than subjects receiving placebo (Fig. 3a). Baseline alcohol dependence severity (ADS scores; F[1,43]=11.1, p=0.002) and race (African American vs. non-African American; F[1,43]=10.5, p=0.002) were significant covariates in this analysis, and were retained in the final model.
Fig. 3.
Subjective response to alcohol measured with the DEQ during the alcohol infusion session. (a) Ratings of ‘Feel Intoxicated’ during the alcohol infusion (starting at minute 40 of the infusion) following the saline infusion session. On average, subjects receiving NTX reported higher levels of intoxication than subjects receiving placebo; (b) Ratings of ‘Feel High’ during the alcohol infusion session. NTX subjects reported significantly higher ratings than placebo subjects during the later time points (p≤0.05).
The shaded areas on the graphs in Fig 3a and 3b indicate the timing of the alcohol infusion.
Although there was no main treatment effect for responses to the item ‘Do you feel high?’ (F[1,45]=1.9, p=0.17) there was a significant time x treatment interaction for this item (F[4,45]=2.7, p<0.03). This reflected a differential time course between the treatment groups over the course of the session. PLC subjects decreased their reported “high” toward the later time points, while NTX subjects maintained their “high” throughout the infusion (Fig. 3b). In this analysis, ADS score was a significant covariate, and was retained in the final model (F [1, 61] = 9.48, p<0.003).
NTX and PLC subjects did not show a significant difference in responses to the questions on ‘Feel Drug’, ‘Like Drug’, and ‘Want More Drug’ (data not shown).
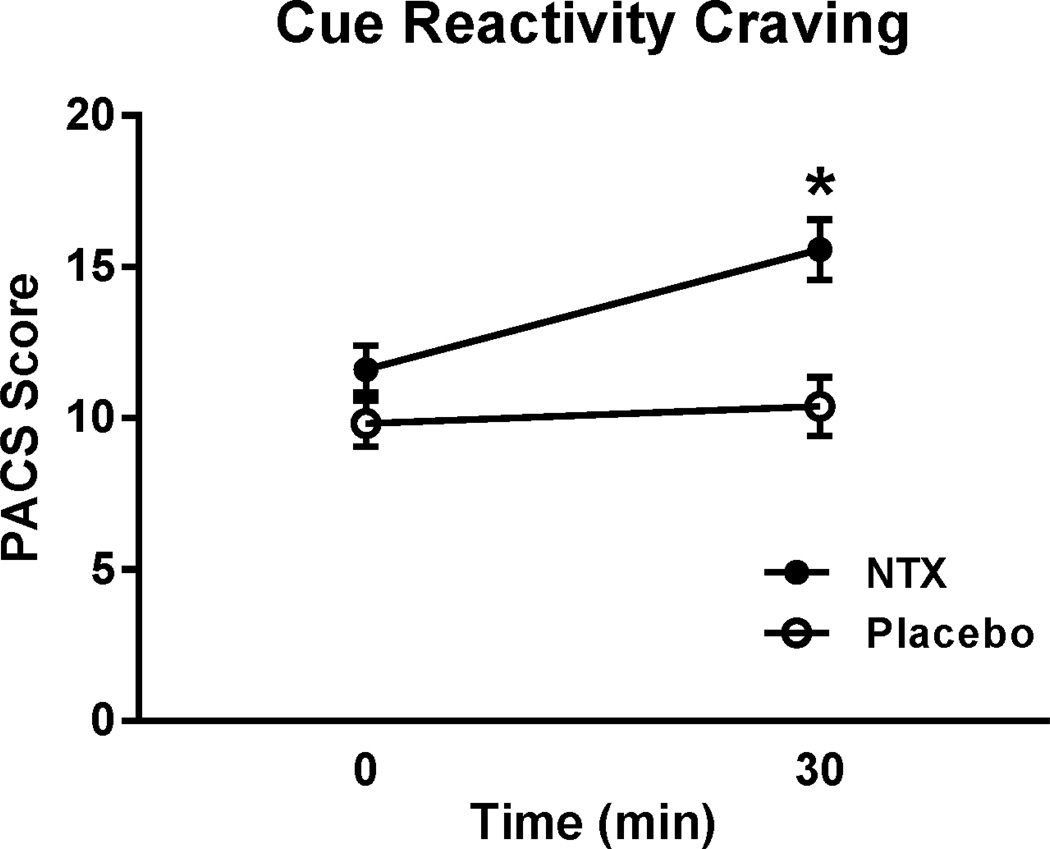
NTX increases alcohol craving in responses to alcohol cues
PACS scores following alcohol cue exposure showed a robust main effect of treatment (F[1,58]= 11.1, p=0.001) and a time x treatment interaction (F [1,58]= 6.0; p=0.001). Newman-Keuls post-hoc test showed that subjects receiving NTX reported higher craving on the PACS following the cue exposure compared both to their own pre-exposure baseline, and to the PLC treated group (Fig. 4).
Fig. 4.
Craving following the cue reactivity test, as measured by PACS. Subjects on NTX showed increased craving in response to cue exposure. For detailed statistics, see Results. *p<0.05
DISCUSSION
To our knowledge, the present study is the first to examine brain responses to alcohol in treatment seeking, alcohol dependent patients and their potential modulation by NTX. In this population, a moderate level of intoxication, 80 mg/dl, was insufficient to activate the VS as assessed by BOLD fMRI. These data complete a series of studies from our laboratory (Gilman et al. 2008; Gilman et al. 2012), which have previously shown that IV alcohol administration to this level of intoxication induces a robust activation of the VS in healthy social drinkers, but only a lesser degree of activation in heavy drinking, non-treatment seeking subjects. Taken together, this series of studies suggest that with increasing severity of alcohol use, there is increasing tolerance to the ability of alcohol to activate brain reward circuitry. This tolerance persists beyond acute withdrawal, since our data were obtained more than a week after admission, and systematic assessment confirmed that significant withdrawal symptoms were absent. We also controlled for the possibility that our results could be confounded by genetic variation at the OPRM1 A118G locus previously shown to moderate brain alcohol responses (Ramchandani et al. 2011). Of note, our data do not establish an absolute deficit in the ability of alcohol to activate the VS in our clinical population. Because ethical considerations limit the level of brain alcohol exposure that can be assessed in treatment seeking subjects, the deficit was detected at blood alcohol levels lower than those that typically result from heavy drinking. It is therefore possible that a VS response to alcohol would occur in this population at higher levels of intoxication.
We believe that our findings have important implications for understanding the motivational properties of alcohol in dependent patients. Chronic brain alcohol exposure produces profound changes in neural mechanisms that mediate positive reinforcement (Koob and Le Moal 2005). Acutely, both experimenter- and self-administered alcohol consistently increase extracellular DA levels in the NcAcc of non-dependent rats [e.g. (Di Chiara and Imperato 1988; Gonzales and Weiss 1998; Tanda and Di Chiara 1998)]. Following the development of dependence, however, this effect shows tolerance (Weiss et al. 1996). These dependence-induced deficits in NcAcc DA are associated with impaired brain reward function, as indicated by elevated intracranial self-stimulation thresholds (Schulteis et al. 1995). Human brain imaging studies have also suggested that deficits in ventral striatum function in alcoholism may involve post- as well as pre-synaptic effects, since D2/3 receptor densities are decreased in alcohol dependent subjects (Volkow et al. 1996).
The BOLD response from the VS is related to DA function (Knutson and Gibbs 2007; Schott et al. 2008). Our findings are therefore consistent with, and represent a translation of, prior animal data on dependence induced deficits in VS responses to alcohol. We extend those data by demonstrating that in patients, the deficit persists longer than what was observed in the animal studies, presumably due to a longer history of dependence. In attempting to integrates the human and animal studies, a possible interpretation is that a persistent tolerance to positively reinforcing effects of alcohol develops in alcohol dependence, and sets up a powerful incentive for relapse. Animal findings indicate that resumption of alcohol self-administration can restore dependence-induced deficits in accumbal DA levels (Weiss et al. 1996). This sequence of events is consistent with an allostatic process as an important factor for maintaining drug use (Koob and Le Moal 2005).
The interpretation that our present finding represents lasting tolerance to alcohol reinforcement may be complicated by the fact that VS activation in response to alcohol is not unequivocally associated with reinforcing alcohol effects. Prior work from our group found an association between VS responses to alcohol in social drinkers and their subjective reports of “feeling intoxicated”, but not of “liking” or “wanting more” (Gilman et al. 2008). It is therefore possible that the alcohol induced VS activation observed in social drinkers reflects effects of alcohol on processes other than reinforcement, such as e.g. incentive salience. This interpretation is, however, more difficult to reconcile with the apparent tolerance to alcohol induced VS activation we have observed in treatment seeking alcohol dependent patients.
Because brain microdialysis experiments in rats have shown that NTX blocks the DA response to alcohol in the NcAcc (Gonzales and Weiss 1998), we hypothesized that NTX pretreatment would prevent alcohol-induced VS activation as measured by BOLD fMRI. Since alcohol did not activate the VS in our study population, a blockade of alcohol-induced VS activation by NTX could not be evaluated. Interestingly, however, we found that NTX itself increased the activity of the VS, irrespective of alcohol and saline condition. Activity of the NcAcc is under the control of opposing stimulatory µ-opioid and inhibitory κ-opioid tone ( Spanagel et al. 1992). Alcohol reinforcement is primarily mediated by µ-opioid activity (Roberts et al. 2000; Hall et al. 2001). With prolonged brain alcohol exposure, expression of the κ-opioid ligand dynorphin is up-regulated, and with it presumably the strength of inhibitory tone onto the NcAcc (Lindholm et al. 2000). Although therapeutic effects of NTX and its active metabolite 6-β-naltrexol are thought to be predominantly mediated by µ-opioid receptor blockade, NTX is a competitive antagonist both at µ- and κ-opioid receptors (Ray et al. 2010). In a chronic dependent state, in which inhibitory κ-opioid tone is up-regulated, NTX would therefore be expected to activate the VS by removing κ-opioid inhibition.
It is less clear why subjects receiving NTX reported higher craving following exposure to alcohol cues. In alcohol dependent rats, NTX has been reported to produce a robust and selective blockade of cue-induced alcohol seeking (Liu and Weiss 2002), but effects of NTX on cue-reactivity in humans have been variable. In fact, while several studies have shown an effect of NTX on alcohol craving (Rosner et al., 2010), other studies have generated different results (Kranzler et al., 2000; Krystal et al., 2001). Using the cue-reactivity procedure employed in our study, no medication effect on the intensity of alcohol craving was previously found in treatment seeking alcoholics (Monti et al. 1999). In a subsequent study, NTX left alcohol cravings unaffected in patients homozygous for the major OPRM1 118A allele, while increasing them in 118G carriers (McGeary et al. 2006). In contrast, an fMRI study in non-treatment seeking alcoholics found that NTX and ondansetron given together reduced subjective cravings induced by alcohol cues presented during the scan, although NTX alone was insufficient in doing so (Myrick et al. 2008). Finally, in patients with co-morbid alcohol and cocaine dependence, NTX did not affect craving in response to alcohol cues (Modesto-Lowe et al. 1997). Genetic moderators may have contributed to the variability in NTX effects on cue-induced craving in these studies. In our study, however, only a small number of subjects were 118G carriers, and the higher cue-induced craving in NTX treated subjects was present even when OPRM1 A118G variation was controlled for.
While other studies suggest that lowering craving is a biobehavioral mechanism through which NTX reduces alcohol drinking, our present findings suggest the possibility of another mechanisms of NTX action in treatment-seeking alcoholic individuals. Because NTX increased subjective reports of intoxication and high, it is possible that these effects of NTX may amplify the subjective experiences of alcohol consumption, thus reducing individuals’ motivation to drink additional alcohol. This is also consistent with the known ability of NTX to reduce heavy drinking, while its effects are weaker in promoting total alcohol abstinence. The large multi-site COMBINE study (Anton et al., 2006), which was conducted with treatment-seeking alcohol-dependent patients, provided evidence that naltrexone is significantly effective in reducing heavy drinking, but not in promoting total alcohol abstinence. Of special note considering the present findings, a secondary analysis of the COMBINE study indicated that, after excluding all abstainers (only 22% of the patients in the COMBINE trial), individuals who drank more regularly during the trial seemed to benefit most from naltrexone, thus suggesting that naltrexone's beneficial effects among non-abstainers may depend on frequency of drinking (Ray et al., 2010). NTX might be beneficial in alcohol dependent patients in part because its ability to increase VS activity is able to reverse a “reward deficit syndrome” present in this condition ({Koob, 2005 #1}), potentially related to removal of a κ-opioid supporession of Nc. Accumbens function as discussed above.
Finally, we found that BOLD response in amygdala was unaffected by emotional condition (fearful vs. neutral faces), both during alcohol and saline infusion. This hypoactivity of the amygdala in response to fearful stimuli in alcoholic patients fits well with the notion that alcoholics fail to effectively avoid cues that signal danger (Gilman et al., 2008).
In summary, we report that treatment seeking alcoholics have an impaired VS response to moderate alcohol intoxication, corresponding to consumption of 3 – 4 drinks. Our study has some important limitations. Because of ethical considerations, we were unable to explicitly address what level of intoxication, if any, would be sufficient to activate the VS in our population of alcoholic patients. Second, we cannot determine with certainty whether our subjects exhibited an impaired VS response to alcohol as a result of acquired tolerance, pre-existing insensitivity, or both. Moreover, all the subjects enrolled in the present study reported a positive family history of alcoholism, which has been identified as a factor that may affect the response to NTX treatment (Monterosso et al. 2001; Rubio et al. 2005), therefore future larger studies are needed to compare positive vs. negative family history individuals. Finally, since baseline measures of mood or alcohol craving were not collected before the IV session, we could not determine whether these measures were related to neural activity and subjective response to alcohol.
Nevertheless, our finding suggests that escalation into progressively heavier alcohol use in alcohol dependent patients may in part be driven by a desire to regain reward circuitry activation expected to result from alcohol consumption. Furthermore, because the cascade on which NTX is thought to act for its therapeutic effects is not engaged at moderate levels of intoxication in treatment seeking alcoholics, this observation may also help explain why NTX is efficacious to reduce heavy drinking, but not to achieve abstinence.
Acknowledgments
Funding and Disclosure
Supported by the Division of Intramural Clinical and Biological Research, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. None of the authors has any conflict to disclose.
Footnotes
Clinical trials registration
References
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 3. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Chamorro AJ, Marcos M, Miron-Canelo JA, Pastor I, Gonzalez-Sarmiento R, Laso FJ. Association of mu-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addiction biology. 2012;17:505–512. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin Exp Res. 2005;29:1965–1975. doi: 10.1097/01.alc.0000187599.17786.4a. [DOI] [PubMed] [Google Scholar]
- de Wit H, McCracken SG. Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res. 1990;14:63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–1295. [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni S, Bosio A, Di Monda E, Fazzari G, Spano PF, Trabucchi M. Immunoreactive met-enkephalin plasma concentrations in chronic alcoholics and in children born from alcoholic mothers. Life Sci. 1983;33:1581–1586. doi: 10.1016/0024-3205(83)90699-9. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, Dohmen BM, Braus DF, Schumann G, Machulla HJ, Bares R, Mann K. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psychiatry. 2005;62:57–64. doi: 10.1001/archpsyc.62.1.57. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25:198–205. [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O'Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side' of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol. 2007;97:1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR. Pharmacotherapy of alcoholism: gaps in knowledge and opportunities for research. Alcohol Alcohol. 2000;35(6):537–47. doi: 10.1093/alcalc/35.6.537. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Human Interaction Laboratory. San Francisco: University of California; 1988. Japanese and Caucasian facial expressions of emotion and neutral faces (JACFEE and JACNeuF) p. 401. [Google Scholar]
- McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R., Jr Genetic moderators of naltrexone's effects on alcohol cue reactivity. Alcohol Clin Exp Res. 2006;30:1288–1296. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O'Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3002902. 116ra116. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Burleson JA, Hersh D, Bauer LO, Kranzler HR. Effects of naltrexone on cue-elicited craving for alcohol and cocaine. Drug Alcohol Depend. 1997;49:9–16. doi: 10.1016/s0376-8716(97)00134-8. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: the influence of craving and family history. American Journal of Addictions. 2001;10:258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB. Alcohol cue reactivity: effects of detoxification and extended exposure. J Stud Alcohol. 1993;54:235–245. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra V, Kang F, Kim C, Nova AS, Bajaj A, Hall FS, Uhl GR, Gonzales RA. The mu opioid receptor is not involved in ethanol-stimulated dopamine release in the ventral striatum of C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:929–938. doi: 10.1111/j.1530-0277.2010.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Associations among GABRG1, level of response to alcohol, and drinking behaviors. Alcohol Clin Exp Res. 2009;33:1382–1390. doi: 10.1111/j.1530-0277.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS & neurological disorders drug targets. 2010;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane database of systematic reviews. 2010 doi: 10.1002/14651858.CD001867.pub3. CD001867. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Rodriguez-Jiménez R, Jiménez-Arriero MA, Hoenicka J, Palomo T. Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol Alcohol. 2005;40(3):227–33. doi: 10.1093/alcalc/agh151. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiewicz PR, Gulliver SB, Bradizza CM, Rohsenow DJ, Torrisi R, Monti PM. Exposure to negative emotional cues and alcohol cue reactivity with alcoholics: a preliminary investigation. Behav Res Ther. 1997;35:1143–1149. [PubMed] [Google Scholar]
- Tanda G, Di Chiara G. A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, Coiro V, Volpi R, Giannini A, Passeri M. Plasma beta-endorphin, but not met-enkephalin levels are abnormal in chronic alcoholics. Alcohol Alcohol. 1992;27:471–475. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]