Abstract
Oxygen tension is a known regulator of mesenchymal stem cell (MSC) plasticity, differentiation, proliferation, and recruitment to sites of injury. Materials capable of affecting the MSC oxygen-sensing pathway, independently of the environmental oxygen pressure, are therefore of immense interest to the tissue engineering (TE) and regenerative medicine community. In this study, we describe the evaluation of the effect of hypoxia inducible factor (HIF)-stabilizing bioactive glasses (BGs) on human MSCs. The dissolution products from these hypoxia-mimicking BGs stabilized HIF-1α in a concentration-dependent manner, altered cell proliferation and metabolism, and upregulated a number of genes involved in the hypoxic response (HIF1A, HIF2A, and VHL), MSC survival (SAG and BCL2), extracellular matrix remodeling (MMP1), and angiogenesis (VEGF and PDGF). These HIF-stabilizing materials can therefore be used to improve MSC survival and enhance regeneration in a number of TE strategies.
Introduction
Mesenchymal stem cells (MSCs) have an important role in tissue regeneration in vivo1,2 and have been used extensively in a number of cell therapy and tissue engineering (TE) strategies.3,4 Their differentiation capability, immunomodulatory activity, and ability to be recruited to sites of injury are the main characteristics that together suggest these cells can act as a “natural in vivo system for tissue repair.”4
Oxygen pressure (pO2) is a known regulator of the behavior of several stem/progenitor cells, including MSCs.5,6 The physiological pO2 value in bone marrow has been estimated to vary from 1% to 7%, with well-oxygenated marrow sinuses and more hypoxic endosteal regions.7 At the commonly used in vitro pO2 of 21%, MSCs have diminished growth potential and typically become senescent after a few passages.8 For this reason, the effect of pO2 on ex vivo expansion of MSCs has been extensively studied. When MSCs are expanded under hypoxia (1–3% pO2), their lifespan was extended and senescence was avoided.8,9 In addition, a low pO2 value has been shown to maintain MSCs in an undifferentiated state9–11 as well as to increase the population homogeneity.12 Interestingly, hypoxia also stimulates the angiogenic properties of MSCs. Migration of these multipotent cells in vitro as well as tropism to sites of injury in vivo have been described.13–15 The matrix metalloproteinase (MMP) activity and endothelial cell tubule formation ability were enhanced as MSCs were cultured in vitro at pO2 values of lower than 3%.16,17 Mechanistically, hypoxia is known to enhance the stabilization and activity of the hypoxia-sensing transcription factor, hypoxia inducible factor 1 alpha (HIF-1α).18 In MSCs, the HIF-1α activity induces expression of a plethora of proangiogenic factors such as VEGF, HGF, and FGF2.9,13,16,19–21 Hypoxia induces these processes through autocrine and paracrine effects following protein secretion. In most cases, HIF-1α stabilization has been shown to be an essential factor and a sequential contributor, making HIF-1α a target for regulation by materials for regenerative therapies.
An expanding palette of small molecules and ions is, currently, widely used for the activation of cellular responses.22 The most well-described example in the field of TE is through the use of bioactive glass (BG). The release of both calcium and soluble sílica from the glass has been shown to facilitate bone regeneration, by upregulating expression of genes involved in pathways essential for osteoblast differentiation.23 More recently, we have shown that strontium can positively impact bone repair, promoting osteoblast activity and reducing osteoclast survival.24 Compared to growth factor release, the main advantages of using ions for TE purposes lie not only on the cost but, more importantly, also on the fact that their release can be easily controlled and a single ion, contrarily to single growth factors, can activate specific cellular responses that would require a plethora of growth factors to be delivered in a very precise manner.25 Moreover, the activity of growth factors is limited by their half-life within the body as well as the narrow concentration range within which the response they activate is not aberrant.
We have previously described the development of a series of hypoxia-mimicking BGs.26 These glasses enable the controlled release of cobalt ions (Co2+) (Fig. 1), a cation known to activate the hypoxia pathway through inhibition of HIF-1α degradation.18,27 Making use of this strategy, we intend to stabilize the hypoxia-sensing transcription factor, which will subsequently activate a variety of genes crucial for regeneration, regardless of the local environmental oxygen pressure. In this study, the effect of hypoxia-mimicking BGs on the biology and behavior of human MSCs (hMSCs) was assessed. hMSCs were treated with hypoxia-mimicking BG-conditioned media, after which the activity of the hypoxia pathway, cell viability, and proliferation were evaluated. To better understand the biological effect of the BG, an array of known hypoxia-regulated genes was assessed.
FIG. 1.
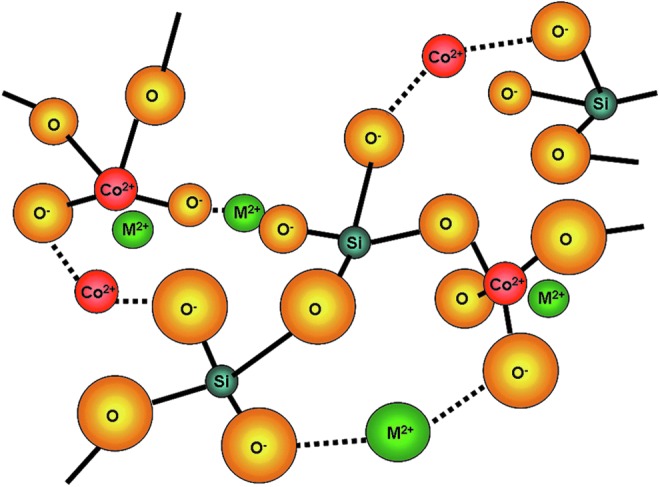
The structure of bioactive glasses (BGs) is based on SiO4 tetrahedral network (as described previously26), in which the 3D continuous network is formed by SiO4 tetrahedral connected to each other by oxygen atoms in the corners. The addition of alkali and alkali-earth oxides (e.g., CaO, Na2O, and CoO) reduces the degree of connectivity of the network, which facilitates the degradation of the glass structure when immersed in aqueous solutions or in body fluids. Varying the type and concentration of alkali and alkali-earth oxides added to the glass structure enables the controlled release of cations of interest. The lines represent network forming-like bonding and broken lines represent network modifying bonding. Color images available online at www.liebertpub.com/tea
Materials and Methods
Cell culture
hMSCs were purchased from Cambrex. They were expanded in low glucose Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% (v/v) fetal bovine serum (Gibco), and 50 U/mL penicillin, and 50 μg/mL streptomycin (Gibco). Initial culture was performed in 75-cm2 flasks at 37°C in a humidified atmosphere with 5% CO2, and the medium was changed every 2–3 days. Experiments were performed using cells at passage 5–8. hMSCs were treated for different lengths of time with BG-conditioned media. The medium containing CoCl2 was used as a positive control for cobalt ions.
Preparation of BG-conditioned media
BGs were manufactured, as previously described,26 with compositions, as listed in Table 1. The glass frit was ground and sieved to less than 38 μm. BGs were named BG0Co, BG0.5Co, BG1Co, BG2Co, and BG4Co, as they contained, respectively, 0, 0.5, 1, 2, and 4 mol% of Co2+ in their composition. BG-conditioned media were prepared by incubating BG particles (<38 μm) in DMEM at a concentration of 1.5 mg/mL, with continuous rolling, for 4 h at 37°C, followed by filtration (0.2-μm-pore filters). To stabilize pH, conditioned media were incubated (37°C, 5% CO2) in a vented flask for 2 h before use. The concentration of the ions released from the BG to the conditioned media was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) (Table 2). A control CoCl2-containing medium was prepared with a final Co2+ concentration of 100 μM. The Co2+ concentration was confirmed by ICP-OES (Table 2).
Table 1.
Composition of the Bioactive Glass Used in This Study
| BGs | SiO2 (mol%) | P2O5 (mol%) | CaO (mol%) | Na2O (mol%) | CoO (mol%) |
|---|---|---|---|---|---|
| BG0Co | 49.46 | 1.07 | 23.08 | 26.38 | |
| BG0.5Co | 49.46 | 1.07 | 22.58 | 26.38 | 0.50 |
| BG1Co | 49.46 | 1.07 | 22.08 | 26.38 | 1.00 |
| BG2Co | 49.46 | 1.07 | 21.08 | 26.38 | 2.00 |
| BG4Co | 49.46 | 1.07 | 19.08 | 26.38 | 4.00 |
BG, bioactive glass.
Table 2.
Elemental Concentrations of the Ionic Dissolution Products of the BG (Si, Ca, Na, P, Co) in the BG-Conditioned DMEM (ppm) Determined by ICP-OES
| Si (g·L−1) | Ca (g·L−1) | Na (g·L−1) | P (g·L−1) | Co (g·L−1) | Co (μM) | |
|---|---|---|---|---|---|---|
| DMEM | 0.0±0.2 | 81.3±6.6 | 2920±420 | 39.1±3.0 | 0.03±0.05 | 0.51±0.85 |
| BG0Co | 73.7±13.0 | 95.7±27.2 | 3047±508 | 15.1±7.0 | 0.02±0.03 | 0.33±0.51 |
| BG0.5Co | 69.3±6.6 | 103.5±14.7 | 2916±413 | 19.2±7.6 | 2.18±0.40 | 36.97±6.78 |
| BG1Co | 76.8±12.9 | 106.1±17.1 | 2996±487 | 16.0±7.6 | 5.35±0.89 | 90.74±15.09 |
| BG2Co | 76.9±18.1 | 110.3±18.3 | 2993±489 | 16.8±9.7 | 10.55±2.06 | 178.93±34.94 |
| BG4Co | 80.6±17.6 | 109.8±17.2 | 2995±523 | 16.5±7.4 | 22.97±2.06 | 389.57±34.94 |
| CoCl2 [100 μM Co] | 0.0±0.9 | 81.5±8.3 | 2928±475 | 38.7±4.0 | 6.61±0.62 | 112.11±10.52 |
The Co concentrations presented in the shadowed column were converted to μM. The values presented here are the mean±SD of all batches used for the experiments.
DMEM, Dulbecco's modified Eagle's medium; ICP-OES, inductively coupled plasma–optical emission spectrometry.
Evaluation of ion release from BGs
The prepared BG samples (<38 μm particles) were incubated in DMEM (45 mg of glass per 50 cm3 DMEM) at 37°C, in an orbital shaker at 120 rpm. After 4 h, 5 cm3 samples were collected, centrifuged, and filtered. ICP-OES was performed on the resulting solutions, and the concentrations of silicon, calcium, sodium, phosphorus, and cobalt were calculated, based on standard solutions. ICP was performed using a Thermo Scientific iCAP 6000 Series ICP Spectrometer and each sample read in triplicate.
AlamarBlue assay
To determine cell viability, hMSCs were seeded in 96-well plates (50,000 cells/cm2) in BG-conditioned media. After 2, 4, or 7 days, the cell culture medium was replaced with 150 μL of 10% (v/v) alamarBlue in DMEM with no phenol red (Gibco). After a 2-h incubation at 37°C, 100 μL of the reaction product was transferred to a black 96-well plate to measure fluorescence at an excitation wavelength of 540 nm and an emission wavelength of 585 nm in a microplate reader (SpectraMax M5; Molecular Devices).
Lactate dehydrogenase assay
The colorimetric lactate dehydrogenase (LDH) assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay; Promega) was performed to assess the effect of BG-conditioned medium on hMSC metabolism. hMSCs were seeded in 96-well plates (50,000 cells/cm2) in BG-conditioned media. After 2, 4, or 7 days, adherent cells were lysed. Fifty microliters of reconstituted substrate mix was added to 50 μL of the cell lysate and incubated for 30 min. The reaction was stopped with 50 μL of stop solution, and absorbance was read at 490 nm in a microplate reader (SpectraMax M5; Molecular Devices). The LDH activity was normalized to total DNA content that was determined using the Hoechst staining method.
HIF-1α activity assay
The TransAM HIF-1 assay kit (Active Motif) was used to assess the HIF-1α activity. hMSCs were seeded (50,000 cells/cm2) in 25-cm2 flasks and cultured until they were ∼90% confluent. Cells were then incubated for 8 h in BG-conditioned media and the assay was performed according to the manufacturer's instructions. Briefly, nuclear extracts were prepared and 8 μg was added to a 96-well plate precoated with hypoxia-responsive element-specific oligonucleotides. A primary antibody specific for the bound and active form of the transcription factor was then added, followed by incubation with a secondary antibody conjugated to horseradish peroxidase and a substrate solution. Absorbance was measured in a microplate reader (SpectraMax M5; Molecular Devices) at a wavelength of 450 nm and a reference wavelength of 655 nm.
Human HIF-regulated cDNA profiling
The effect on human HIF-regulated cDNA was performed using a human HIF-regulated cDNA profiling kit from Signosis. hMSCs were seeded (50,000 cells/cm2) in 25-cm2 flasks and cultured until they were ∼90% confluent. Cells were then incubated for 24 h in BG-conditioned media and the assay was performed according to the manufacturer's instructions. Briefly, cellular lysates were prepared using the lysis buffer provided, and cDNA synthesis was performed. The plate hybridization was performed using the cDNA prepared in combination with the provided buffer. After overnight incubation at 45°C, the plate was washed and a streptavidin-HRP conjugate was added. The detection was performed after addition of the substrate solution using a luminescence microplate reader (SpectraMax M5; Molecular Devices).
Analysis of VEGF expression
A VEGF-A165 ELISA kit (Quantikine Human VEGF immunoassay; R&D Systems) was performed to assess VEGF-A165 secretion. hMSCs were seeded in 24-well plates (50,000 cells/cm2) in BG-conditioned media. After 2, 4, or 7 days, the cell culture supernatant was collected and the VEGF concentration determined. Briefly, cell culture supernatant samples or standards together with assay diluent were added to the provided 96-well plate (precoated with a monoclonal antibody specific for VEGF-A165). An enzyme-linked polyclonal antibody specific for VEGF was then added to the wells, followed by a substrate solution. Absorbance was measured in a microplate reader (SpectraMax M5; Molecular Devices) at 450 nm with a reference wavelength of 540 nm.
Statistical analysis
All experimental data shown are expressed as mean±SD and were obtained from experiments performed at least twice in quadruplicates. All data analysis was performed in Excel. Statistical analysis was by one-way analysis of variance using multiple comparisons (Bonferroni test) using SPSS, with p-values <0.05 considered significant.
Results
Low pO2 or hypoxia affects MSC metabolism, proliferation, differentiation, and survival.28 Although Co2+ has been shown to activate the hypoxia pathway,29,30 its effect on the biology of hMSCs is incompletely characterized. In this study, hypoxia-mimicking BGs that release Co2+ are investigated for their effect on hMSC proliferation, metabolism, and activation of the hypoxia pathway.
Effect of the hypoxia-mimicking BGs on hMSC viability
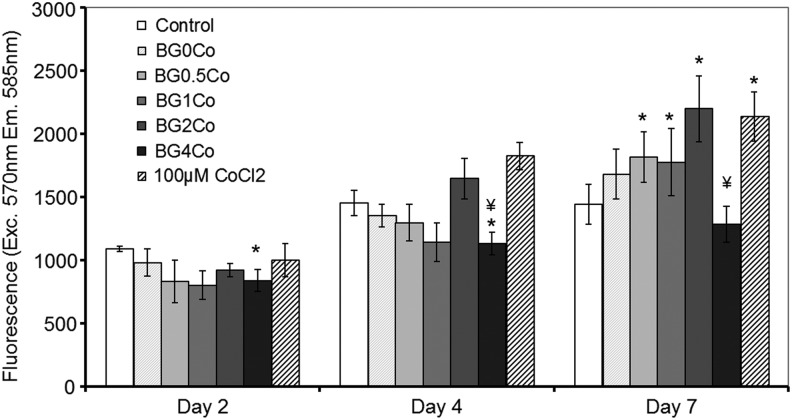
An alamarBlue assay was used to assess the viability and metabolic activity of hMSCs after exposure to the hypoxia-mimicking BGs (Table 1) after 2, 4, and 7 days (Fig. 2). Up to 4 days, there was no significant difference in the viability/metabolic activity of hMSCs treated with either BG-conditioned or control media. After 7 days, the cell number increased in correlation with the concentration of Co2+ ions present. However, the conditioned medium with the highest Co2+ concentration (BG4Co) had no positive effect and the cell number was statistically similar to cells cultured in the control medium. A medium supplemented with CoCl2 showed a similar effect to the BG1Co and BG2Co BGs, which could be explained by the similar Co2+ concentrations in these three conditions.
FIG. 2.
Effect of hypoxia-mimicking BGs on hMSC viability. An alamarBlue assay was performed after hMSCs were treated with BG-conditioned media (1.5 mg/mL) for 1, 2, 4, and 7 days. (*) Indicates that the difference between the marked bar and the control (cells cultured with standard cell culture media, with no BG) for the same time period is statistically significant. (¥) Indicates that the difference between the marked bar and the sample treated with the dissolution products of BG0Co for the same time period is statistically significant (p<0.05). hMSC, human mesenchymal stem cell.
Effect of the hypoxia-mimicking BG on hMSC metabolism
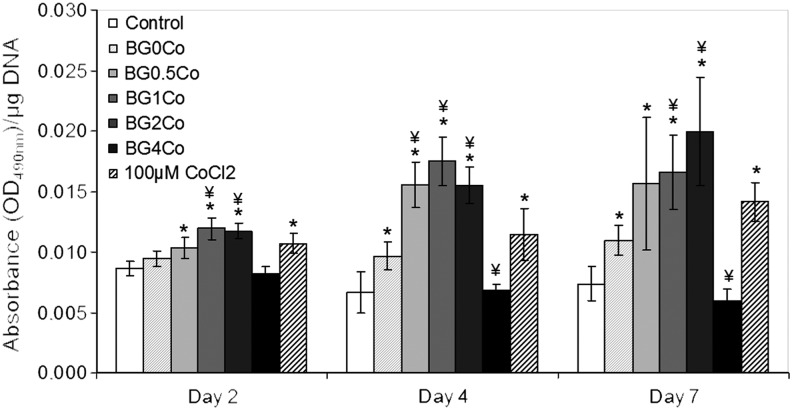
When cells experience hypoxia, they alter their metabolism through the activation of the hypoxia pathway and the reduction of pyruvate to lactate by LDH becomes their main energy source.31 To assess the effect of the hypoxia-mimicking BGs on hMSC metabolism, the LDH activity was measured after 2, 4, and 7 days (Fig. 3). At every time point evaluated, the LDH activity (normalized to total DNA) was higher in the presence of the BG-conditioned medium in a concentration-dependent manner. Over time, the difference between cells exposed to the BG-conditioned medium and control medium increased even further, revealing a metabolic switch that occurs when hMSCs are exposed to the hypoxia-mimicking BG-conditioned media. Only cells exposed to the BG4Co-conditioned medium did not follow this trend, with the LDH activity significantly decreased as hMSCs were treated with the dissolution products of the BG with the highest Co2+ concentration, suggesting toxicity at high concentrations. The LDH activity of the cells treated with the CoCl2-supplemented media was similar to that of cells treated with the BG1Co- and BG2Co-conditioned media, which can be explained by similar Co2+ concentrations (Table 2). At days 4 and 7, BG with no Co2+ was also able to enhance the LDH activity, although to a lower degree, suggesting a small effect due to the release of other BG ions (e.g., Ca2+).
FIG. 3.
Effect of hypoxia-mimicking BGs on hMSC metabolism. An LDH assay was performed after hMSCs were treated with BG-conditioned media (1.5 mg/mL) for 2, 4, and 7 days. (*) Indicates that the difference between the marked bar and the control (cells cultured with standard cell culture media, with no BG) for the same time period is statistically significant. (¥) Indicates that the difference between the marked bar and the sample treated with the dissolution products of BG0Co for the same time period is statistically significant (p<0.05). LDH, lactate dehydrogenase.
Effect of hypoxia-mimicking BG on the activation of the hypoxia pathway
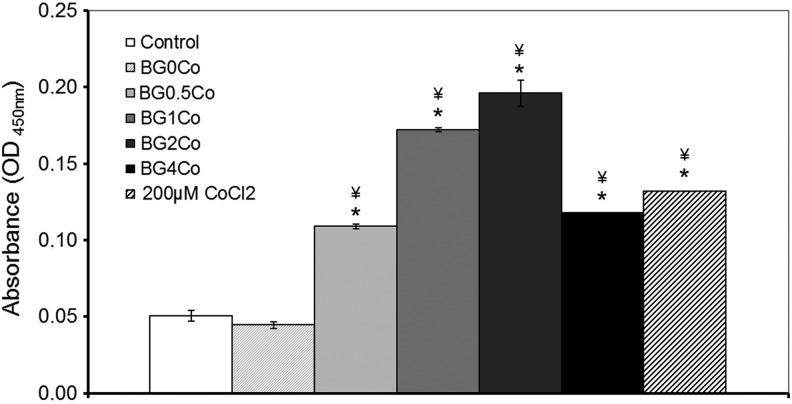
The HIF-1 pathway is the main regulator of the cellular response to hypoxia. In particular, HIF-1α is activated by translocation to the nucleus in response to low pO2, after which it initiates the subsequent cellular responses. To determine whether the hypoxia-mimicking BGs were activating, the hypoxia pathway nuclear HIF-1α was quantified by ELISA. The HIF-1α activity was significantly enhanced in a concentration-dependent manner when hMSCs were incubated with the hypoxia-mimicking BG-conditioned media (Fig. 4). BG0.5Co-conditioned media doubled the HIF-1α activity compared to the control, while BG2Co-conditioned media quadrupled the HIF-1α activity. The enhancement of HIF-1α activity after exposure of hMSCs to BG4Co-conditioned media was lower than when the same cells were treated with BG1Co- and BG2Co-conditioned media, suggesting toxicity due to high Co2+ concentrations.
FIG. 4.
Effect of hypoxia-mimicking BGs on the hypoxia pathway: HIF-1α activity. The HIF-1α activity was determined using an immunoassay after hMSCs were treated with BG-conditioned media (1.5 mg/mL) for 8 h. (*) Indicates that the difference between the marked bar and the control (cells cultured with standard cell culture media, with no BG) for the same time period is statistically significant. (¥) Indicates that the difference between the marked bar and the sample treated with the dissolution products of BG0Co for the same time period is statistically significant (p<0.05). HIF-1α, hypoxia inducible factor 1 alpha.
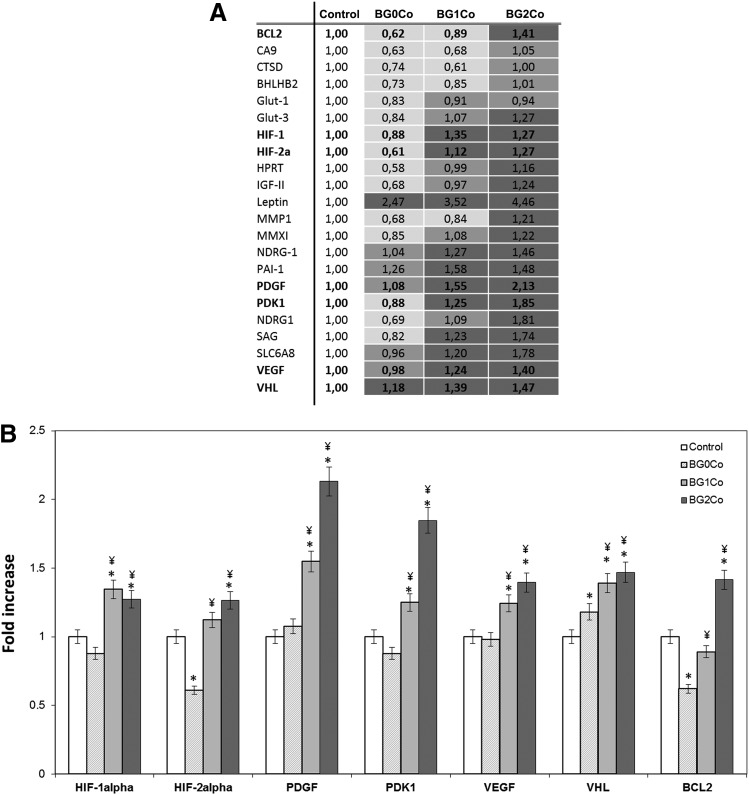
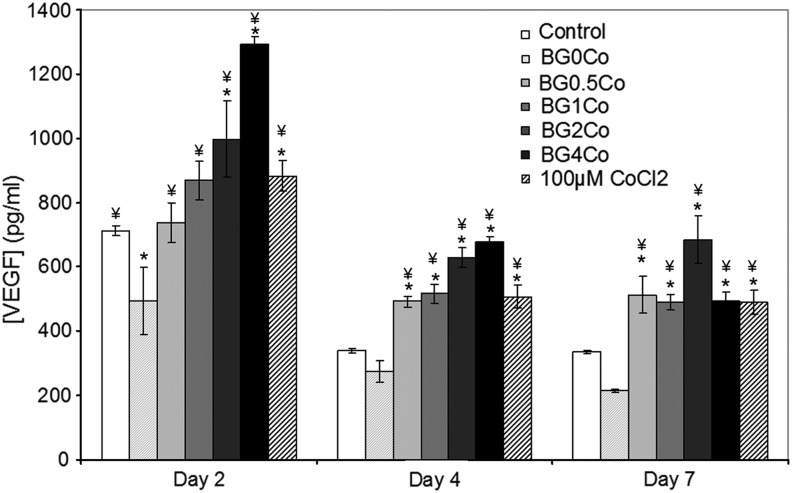
To establish the effect of the hypoxia-mimicking BGs on the expression of HIF-regulated genes, a gene array was performed (Fig. 5). Several hypoxia-responsive genes, including PDGF, PDK1, VEGF, VHL, and BCL2, were upregulated when hMSCs were treated with the Co2+-containing BGs (Fig. 5A), suggesting a global activation of the hypoxia pathway. The upregulation of HIF1A and HIF2A, genes directly involved in the HIF pathway, confirmed its activation. The upregulation of the von Hippel–Lindau tumor suppressor factor (VHL), a protein responsible for the degradation of HIF-1α, suggests a cellular response to regulate the HIF pathway, although the HIF-1α activity remained high (Fig. 4). Genes involved in cellular proliferation and death (BCL2, IGF2, NDRG1, and PDGF), as well as metabolism (SLC2A3, LEP, and PDK1) and differentiation (BHLHB2 and NDRG1), were also upregulated, as were several genes essential for angiogenesis, including MMP1, MMX-1, PDGF, and VEGF. To validate the results of the gene array at the protein level, VEGF expression was evaluated after hMSCs were treated with the BG-conditioned media for 2, 4, and 7 days (Fig. 6). At every time point assessed, VEGF expression was significantly enhanced when cells were incubated with the hypoxia-mimicking BG-conditioned media.
FIG. 5.
Effect of hypoxia-mimicking BGs on several hypoxia-regulated genes. The expression of several hypoxia-regulated genes was determined using a human HIF-regulated cDNA profiling kit (Signosis) after hMSCs were treated with BG-conditioned media (1.5 mg/mL) for 24 h. (A) Table indicating the fold increase in expression of the analyzed genes in comparison to control (no BG). (B) Graphical representation of the fold increase in expression of selected genes in comparison to control (no BG). (*) Indicates that the difference between the marked bar and the control (cells cultured with standard cell culture media, with no BG) for the same time period is statistically significant. (¥) Indicates that the difference between the marked bar and the sample treated with the dissolution products of BG0Co for the same time period is statistically significant (p<0.05).
FIG. 6.
Effect of hypoxia-mimicking BGs on VEGF expression. The VEGF concentration was determined using a VEGF immunoassay after hMSCs were treated with BG-conditioned media (1.5 mg/mL) for 2, 4, and 7 days. (*) Indicates that the difference between the marked bar and the control (cells cultured with standard cell culture media, with no BG) for the same time period is statistically significant. (¥) Indicates that the difference between the marked bar and the sample treated with the dissolution products of BG0Co for the same time period is statistically significant (p<0.05).
Discussion
Regulation of the hypoxia pathway is of great interest to direct hMSC behavior to promote tissue regeneration. We synthesized a series of BGs that could mimic the effects of low oxygen by the controlled release of cobalt ions, which are known to activate the hypoxia pathway through the inhibition of HIF-1α degradation, independently of the environmental oxygen pressure. Using this strategy, we intend to regulate/activate cellular responses crucial for tissue regeneration, including cell survival, migration, and angiogenesis, in normoxic conditions. In this study, we describe the effect of these materials on the biological activity of hMSCs.
The literature presents a somewhat contradictory description of how hypoxia affects hMSC viability and proliferation. Some authors report no significant effect of hypoxia on hMSC survival,21 some show growth arrest after relatively short time periods,10 while others show increased proliferation.30 Recent articles tend to agree that hypoxia increases the lifespan of MSCs ex vivo.8,9,11 In addition, hypoxia has been shown to upregulate genes involved in proliferation and cell survival,17 decrease cell death, increase colony forming units,9 increase culture homogeneity,12 and increase the number of population doublings.8,9,11 It is thought that culturing MSCs in hypoxia better mimics their in vivo environment in bone marrow, where the pO2 is known to vary from 1% to 7%, which is significantly lower than the 21% commonly used in cell culture.6 Indeed, high pO2 increases reactive oxygen species (ROS) production, which causes DNA damage and subsequent cell death.32 These positive effects of hypoxia are also apparent in vivo, where MSCs expanded under hypoxic conditions in vitro have enhanced tissue regeneration capabilities.33 For use in TE or regenerative therapies, HIF stabilization strategies could help overcome the limitations associated with the low numbers of MSCs present in the adult body by increasing their expansion and lifespan ex vivo.
In contrast, there are only a few studies on the effect of the hypoxia-mimicking ion, Co2+ on MSC viability and proliferation.29,30 In those studies, proliferation was shown to decrease with exposure to the CoCl2 supplemented medium. Pacary et al. suggested this result was not due to toxicity but simply to growth arrest as expression of p21 and PC3 mRNA (antiproliferative proteins) is enhanced and cyclin D1 is reduced.29 The effect is probably dependent on hypoxia levels since MSCs at very low pO2 levels (1%) have been shown to accumulate in the G1 phase.10 Conversely, when these cells are incubated at higher pO2 levels (8%), proliferation is enhanced.10 Our results corroborate previous findings on the effect of Co2+ on MSC viability and proliferation. hMSC viability was unaffected by the hypoxia-mimicking BG except when the cells were treated with the BG containing the highest Co2+ concentration (Fig. 2). Co2+-induced toxicity has been observed with high Co2+ concentrations and is likely due to the formation of ROS.32 To maintain MSC viability under exposure to the hypoxia-mimicking BGs, Co2+ levels should therefore be accurately controlled both in terms of concentration and duration of exposure. On the other hand, the BG0Co was never shown to decrease cell viability, indicating that BG with no Co2+ does not diminish the growth of hMSCs.
In normoxia, HIF-1α is degraded in the cytoplasm and is not typically found in the nucleus. In hypoxia, however, HIF-1α is accumulated in the cytoplasm and has an overall increased expression.10,15 A similar effect has previously been shown when hMSCs are exposed to Co2+, a hypoxia-mimicking ion.29,30,36 The hypoxia-mimicking BGs in this study were able to reproduce these findings (Fig. 4).
We were interested in the effect of the BGs on VEGF, since it is a HIF-1α-regulated gene and indicates the activation of the hypoxia response.34 The upregulation of this growth factor has been shown at both mRNA and protein levels at various time periods, under a series of different pO2 values, and in various species.9,16,17,19,27,35 VEGF expression has been shown to be upregulated after exposure to Co2+.29,30 Our data are in agreement with these findings, with the Co2+-containing BGs enhancing VEGF expression by hMSCs in a concentration-dependent manner (Fig. 6).
Beyond indicating the activation of the hypoxia pathway, VEGF is also important in a number of processes essential for tissue regeneration, for example, the formation of new blood vessels (vasculogenesis and angiogenesis),37 and has been used in a number of TE constructs. Biodegradable poly(dl-lactic acid) scaffolds with encapsulated VEGF and MSCs were implanted in mouse femur defects for 4 weeks, after which enhanced bone regeneration, including the synthesis of new bone matrix was observed.38 A separate study using VEGF-releasing scaffolds coated with BG showed significant enhancement of blood vessel density as early as 2 weeks.39 After 12 weeks, the density of mineral bone was also significantly enhanced in the VEGF-releasing scaffolds.
In addition to VEGF, the upregulation of other hypoxia-regulated growth factors critical for angiogenesis (PDGF, MMP-1, and MMX-1) was detected (Fig. 5). The expression of many other growth factors known to be enhanced by HIF-1α under hypoxia (bFGF, HGF, TNF-α, IL-1, and IL-69,17,28) might also be upregulated by the Co2+-containing BGs. However, it is not only the secretion of soluble growth factors that is increased at low pO2 values but also the increased expression of several membrane receptors involved in MSC migration and homing to damaged sites, including CXCR4 and CXCR7, which has been reported in hypoxic conditions or when cells were exposed to CoCl2.15 Several mechanisms critical for tissue regeneration, including angiogenesis, migration, and immunomodulatory activity, could then be enhanced under hypoxia through the trophic or paracrine activity of MSCs.
Hypoxia has been shown to control and direct progenitor cell stemness and differentiation through regulation of the expression of key factors such as Oct4 by HIF-1α.40 This is also the case for MSCs, where the plasticity and differentiation potential of these progenitor cells have been shown to be reduced at low pO2 values in most cases.9,10,11,14,21 Only the chondrogenic differentiation potential was enhanced with hypoxia.28 The hypoxia-mimicking BGs could potentially help maintain MSCs in an undifferentiated state in normoxic conditions through the activation of HIF-1α, which is essential for ex vivo expansion before implantation for regenerative therapies, or for promoting differentiation into the chondrocytic lineage. The effect of BGs on stem cell (including MSCs) differentiation is still controversial.41 Although several authors have reported BGs to promote differentiation of MSCs toward an osteogenic lineage,42 others were simply able to show enhanced osteoblastic activity.43 Others, however, saw no effect at all of BGs on the differentiation of MSCs.44 Therefore, it would be interesting to study the combinatorial effect of the hypoxia-mimicking BGs on the differentiation potential of hMSCs. Finally, preconditioning of hMSCs under hypoxia has been shown to enhance their survival in hypoxic environments, such as after implantation in cardiac tissue after myocardial infarction.33,45 The hypoxia-mimicking BGs may then activate several cellular processes in the hMSCs that are critical to enhance cell survival and tissue regeneration.
Conclusion
This study shows the activation of the hypoxia pathway in hMSCs by BGs releasing hypoxia-mimicking Co2+ ions. Both the expression and activity of HIF-1α were significantly enhanced in hMSCs exposed to the hypoxia-mimicking BG-conditioned media in a concentration-dependent manner, independently of the local oxygen pressure. Several HIF-regulated genes involved in cellular proliferation, death, metabolism, and differentiation were upregulated in the same conditions. These results are enticing since various cellular processes, essential for physiological tissue repair and regeneration, are activated by the hypoxia pathway. In particular, our results demonstrate that several genes crucial for angiogenesis were overexpressed, including VEGF at both the transcript and protein levels, in a concentration-dependent manner. This simple strategy to stabilize the hypoxia-sensing transcription factor (HIF-1α) through the controlled release of ions from BGs is able to induce the activation of a variety of genes crucial for tissue regeneration and could be of great potential for use in a variety of tissue regenerative therapies.
Acknowledgments
This work was supported by EPSRC and Fundação para a Ciência e Tecnologia, Portugal (SFRH/BD/36864/2007) for MMA. MMS thanks ERC Individual Investigator grant “Naturale.”
Disclosure Statement
GJ, MMS, and MA are listed as among the inventors on International Patent Application No. PCT/GB2009/001323 titled “Hypoxia Inducing Factor (HIF) stabilising glasses.” No other competing financial interests exist.
References
- 1.Nombela-Arrieta C., Ritz J., and Silberstein L.E.The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 12,126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohishi M., and Schipani E.Bone marrow mesenchymal stem cells. J Cell Biochem 109,277, 2010 [DOI] [PubMed] [Google Scholar]
- 3.DiMarino A., Caplan A., and Bonfield T.Mesenchymal stem cells in tissue repair. Front Immunol 4,201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan A.I.Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213,341, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Simon M.C., and Keith B.The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9,285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forristal C.E., Wright K.L., Hanley N.A., Oreffo R.O.C., and Houghton F.D.Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 139,85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow D.C., Wenning L.A., Miller W.M., and Papoutsakis E.T.Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J 81,685, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y., et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun 391,1471, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Tamama K., Kawasaki H., Kerpedjieva S.S., Guan J., Ganju R.K., and Sen C.K.Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem 112,804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzwarth C., Vaegler M., Gieseke F., Pfister S.M., Handgretinger R., Kerst G., and Müller I.Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol 11,11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehrer C., Brunauer R., Laschober G., Unterluggauer H., Reitinger S., Kloss F., Gülly C., Gassner R., and Lepperdinger G.Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 6,745, 2007 [DOI] [PubMed] [Google Scholar]
- 12.D'Ippolito G., Diabira S., Howard G., Menei P., Roos B., and Schiller P.Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 117,2971, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Annabi B., Lee Y.-T., Turcotte S., Naud E., Desrosiers R., Champagne R.R., Eliopoulos N., Galipeau J., and Béliveaua R.Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells 21,337, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Rochefort G.Y., Delorme B., Lopez A., Hérault O., Bonnet P., Charbord P., Eder V., and Domenech J.Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 24,2202, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Liu H., Xue W., Ge G., Luo X., Li Y., Xiang H., Ding X., Tian P., and Tian P.Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Commun 401,509, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Kinnaird T., Stabile E., Burnett M.S., Lee C.W., Barr S., Fuchs S., and Epstein S.E.Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 94,678, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Yasuda S.O.T., Kitamura S., and Nagaya N.Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells 25,1166, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Semenza G.Life with oxygen. Science 318,62, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann J., Glassford A.J., Doyle T.C., Robbins R.C., Schrepfer S., and Pelletier M.P.Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia. Thorac Cardiovasc Surg 58,136, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Okuyama H., Krishnamachary B., Zhou Y.F., Nagasawa H., Bosch-Marce M., and Semenza G.L.Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem 281,15554, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Potier E., Ferreira E., Andriamanalijaona R., Pujol J.P., Oudina K., Logeart-Avramoglou D., and Petite H.Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 40,1078, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Place E., Evans N., and Stevens M.Complexity of biomaterials for tissue engineering. Nat Mater 8,457, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Xynos I.D., Edgar A.J., Buttery L.D.K., Hench L.L., and Polak J.M.Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res 55,151, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Gentleman E., Fredholm Y.C., Lotfibakhshaiesh N., O'Donnell M.D., Hill R.G., and Stevens M.M.The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 31,3949, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Hench L.L., and Polak J.M.Third-generation biomedical materials. Science 295,1014, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Azevedo M.M., Jell G., O'Donnell M.D., Law R.V., Hill R.G., and Stevens M.M.Synthesis and characterization of hypoxia-mimicking bioactive glasses for skeletal regeneration. J Mater Chem 20,8854, 2010 [Google Scholar]
- 27.Yuan Y., Hilliard G., Ferguson T., and Millhorn D.E.Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau Protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem 278,15911, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Das R., Jahr H., van Osch G., and Farrell E.The role of hypoxia in bone marrow–derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B 16,159, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Pacary E., Legros H., Valable S., Duchatelle P., Lecocq M., Petit E., Nicole O., and Bernaudin M.Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci 119,2667, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ren H., Cao Y., Zhao Q., Li J., Zhou C., Liao L., Jia M., Zhao Q., Cai H., Han Z.C., Yang R., Chen G., and Zhao R.C.Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun 347,12, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nelson D.L., and Cox M.M.Lehninger Principles of Biochemistry, Third edition. W.H. Freeman, Chapter 15, p. 542, 2000, New York [Google Scholar]
- 32.Catelas I., Petit A., Zukor D.J., and Huk O.L.Cytotoxic and apoptotic effects of cobalt and chromium ions on J774 macrophages—implications of caspase-3 in the apoptotic pathway. J Mater Sci Mater Med 12,949, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Hu X., Yu S.P., Fraser J.L., Lu Z., Ogle M.E., Wang J.A., and Wei L.Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135,799, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Forsythe J.A., et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16,4604, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y., Xu M., Wang Y., Pasha Z., Li T., and Ashraf M.HIF-1α induced-VEGF over-expression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol 42,1036, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters K., Unger R.E., Barth S., Gerdes T., and Kirkpatrick J.Induction of apoptosis in human microvascular endothelial cells by divalent cobalt ions. Evidence for integrin-mediated signalig via the cytoskeleton. J Mater Sci Mater Med 12,955, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Risau W.Mechanisms of angiogenesis. Nature 386,672, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Kanczler J.M., et al. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials 29,1892, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Leach J.K., Kaigler D., Wang Z., Krebsbach P.H., and Mooney D.J.Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 27,3249, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ji L., Liu Y.X., Yang C., Yue W., Shi S.S., Bai C.X., Xi J.F., Nan X., and Pai X.T.Self-renewal and pluripotency is maintained in human embryonic stem cells by co-culture with fetal liver stromal cells expressing hypoxia inducible factor 1 alpha. J Cell Physiol 221,54, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Jones J.R.Review of bioactive glass: from Hench to hybrids. Acta Biomater 9,4457, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Bosetti M.C.The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials 26,3873, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Gough J.E., Jones J.R., and Hench L.L.Nodule formation and mineralisation of human primary osteoblasts cultured on a porous bioactive glass scaffold. Biomaterials 25,2039, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Tsigkou O., Labbaf S., Stevens M.M., Porter A.E., and Jones J.R.Monodispersed bioactive glass submicron particles and their effect on bone marrow and adipose tissue-derived stem cells. Adv Healthc Mater 3,115, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Mao X., Zeng Q., Wang X., Cao L., and Bai Z.Angiogenic potency of bone marrow stromal cells improved by ex vivo hypoxia prestimulation. J Huazhong Univ Sci Technolog Med Sci 24,566, 2004 [DOI] [PubMed] [Google Scholar]