Abstract
Background
Prenatal exposures to alcohol, cigarettes, and other drugs of abuse are associated with numerous adverse consequences for affected offspring, including increased risk for substance use and abuse. However, maternal substance use during pregnancy appears to occur more often in those with a family history of alcohol dependence. Utilizing a sample that is enriched for familial alcohol dependence and includes controls selected for virtual absence of familial alcohol dependence could provide important information on the relative contribution of familial risk and prenatal exposures to offspring substance use.
Methods
A sample of multigenerational families specifically ascertained to be at either high or low risk for developing alcohol dependence (AD) provided biological offspring for a longitudinal prospective study. High-Risk families were selected based on the presence of two alcohol dependent sisters. Low-Risk families were selected on the basis of minimal first and second degree relatives with AD. High-Risk (HR=99) and Low-Risk offspring (LR=110) were assessed annually during childhood and biennially in young-adulthood regarding their alcohol, drug, and cigarette use. At the first childhood visit mothers were interviewed concerning their prenatal use of substances.
Results
High-Risk mothers were more likely to use alcohol, cigarettes, and other drugs during pregnancy than Low-Risk control mothers, and to consume these substances in greater quantities. Across the sample, prenatal exposure to alcohol was associated with increased risk for both offspring cigarette use and substance use disorders (SUD), and prenatal cigarette exposure was associated with increased risk for offspring cigarette use. Controlling for risk status by examining patterns within the HR sample, prenatal cigarette exposure remained a specific predictor of offspring cigarette use, and prenatal alcohol exposure was specifically associated with increased risk for offspring SUD.
Conclusions
Women with a family history of SUD are at increased risk for substance use during pregnancy. Both familial loading for alcohol dependence and prenatal exposure to alcohol or cigarettes are important risk factors in the development of offspring substance use. An inadequate assessment of family history may obscure important interactions between familial risk and prenatal exposures on offspring outcomes.
Keywords: Family history of alcoholism, prenatal alcohol exposure, prenatal cigarette exposure, offspring substance use
Introduction
Prenatal exposures to alcohol, cigarettes, and other drugs of abuse are associated with numerous adverse consequences for affected offspring, including increased risk for substance use and abuse (Baer et al., 2003, Ernst et al., 2001, Glantz and Chambers, 2006, Streissguth et al., 2004). Animal studies have demonstrated physiological effects of prenatal exposures that could plausibly contribute to offspring substance use disorders (SUD), including abnormalities of neural reward systems (Malanga and Kosofsky, 2003). However, the causal effect of prenatal exposures on human offspring is less clear as maternal substance use during pregnancy frequently co-occurs with other environmental and genetic risk factors (Wendell, 2013). Epidemiological studies indicate that prenatal drug and alcohol exposure are modest direct contributors to increased substance abuse vulnerability (Glantz and Chambers, 2006), though some studies suggest that these associations are accounted for by correlated risks such as socioeconomic status or familial history of substance use disorders (D’Onofrio et al., 2012, Ellingson et al., 2012).
Research using animal models has documented plausible neural pathways through which prenatal exposure to cigarettes, alcohol, and other drugs could cause offspring substance use and abuse. Animals prenatally exposed to alcohol show greater preference for alcohol and consume significantly more alcohol than unexposed controls (Spear and Molina, 2005), and animals prenatally exposed to nicotine show long-term alterations in the density of nicotinic acetylcholine receptors and demonstrate increased nicotine self-administration (Slotkin, 2008). These prenatal exposures are also associated with morphological and neurochemical alterations of midbrain areas mediating reward, indicating that the reward system and reward-driven behaviors may be altered by intrauterine exposure in ways that predispose animals to increased self-administration of drugs (Lidow, 2003, Malanga and Kosofsky, 2003). These studies provide evidence that prenatal drug exposure, independent of familial behavioral and environmental factors, can result in increased behavior related to drug reinforcement.
Human studies on the effect of prenatal exposures on offspring substance use may be confounded by co-occurring environmental and genetic risk factors for offspring substance use and problems. Mothers who continue to smoke, drink, and use illicit substances during pregnancy have lower levels of educational attainment, less annual income, higher rates of antisocial behavior, and more substance use problems (Gilman et al., 2008, Smith et al., 1987, Wendell, 2013). Given that offspring of substance-abusing parents are at increased risk for SUD (Dawson and Grant, 1998, Grant, 1998, Hill et al., 2008, Hill et al., 2011), a family history of SUD may be a particularly important factor to consider in the relation between prenatal exposures and offspring substance use problems.
Both a family history of alcoholism and prenatal exposure to alcohol, cigarettes, and other drugs of abuse are associated with an increased risk of drug and alcohol problems in adolescence and adulthood (Baer et al., 1998, Baer et al., 2003, Hill et al., 2008, Hill et al., 2011). However, disentangling the impact of these two factors on the etiology of substance use disorders has proved challenging because (1) maternal substance use during pregnancy frequently co-occurs with disadvantaged prenatal care, lower socioeconomic status and familial risk for alcohol dependence or substance use disorders (Wendell, 2013), and (2) mothers with substance use disorders may be more likely to use substances while pregnant (Smith et al., 1987, Hill et al., 2000).
Previous studies examining the relation between prenatal exposures and offspring substance use disorders have used differing methodologies and have yielded contrasting results. Some studies reporting an association between offspring substance use and very heavy levels of prenatal alcohol and/or drug exposure have not been able to assess a family history of alcohol dependence because the severity of the mothers’ substance abuse has led to their offspring being adopted away or reared by others. Studies that have assessed family history of SUD typically involve a more restricted range of drinking behavior with no more than moderate levels of prenatal exposure. Nevertheless, they indicate a relationship between prenatal alcohol exposure and offspring alcohol-use problems in adolescence and young adulthood (Baer et al., 1998, Baer et al., 2003, Yates et al., 1998), and prenatal cigarette exposure with offspring substance use disorders (Brennan et al., 2002) that remain significant after accounting for parental substance use disorders. Reviews of such studies have interpreted the relationship between prenatal exposures and offspring outcomes as causal because these associations are robust to the use of statistical controls, including a family history of substance use problems (Ernst et al., 2001, Glantz and Chambers, 2006).
Family-based quasi experimental designs are well-suited to testing gene by environmental exposures of the type seen where offspring outcome may be influenced by both genetic factors and environmental prenatal exposure (D’Onofrio et al., 2013). Comparisons of siblings with varying levels of prenatal cigarette exposure have indicated that the relationship between prenatal cigarette exposure and offspring substance use outcomes are not significantly linked, suggesting that the association between prenatal exposure to cigarettes and offspring substance use problems is more likely due to familial background factors rather than a direct causal influence of prenatal exposure (D’Onofrio et al., 2012, Ellingson et al., 2012). However, this approach has not been utilized to study the effects of prenatal alcohol exposure.
Another method for disentangling the relation between the direct, causal effects of prenatal exposures and indirect, genetic effects on offspring outcomes is to use samples enriched for a high familial density of alcohol dependence in which offspring carry a much higher genetic risk for substance use problems. This design can provide information on the link between prenatal exposures and offspring outcomes among individuals with high genetic loading for SUD. Accordingly, the current study sought to examine the relative contribution of having a multiplex, familial history of alcohol dependence and prenatal exposure to alcohol and/or cigarettes on offspring smoking and drinking behavior. The goals of the study were: (1) to compare rates of maternal substance use in women varying in family density of alcohol dependence; (2) to determine if prenatal exposure to alcohol and cigarettes would increase offspring use of these substances; and (3) to determine if exposure to these substances would increase the rate of offspring substance use controlling for familial risk. The latter goal was carried out by contrasting exposed and non-exposed offspring within the High-Risk sample. It was hypothesized that mothers from a High-Risk background would be more likely to consume alcohol and cigarettes during pregnancy, and among mothers who continued to drink and/or smoke during pregnancy, women from High-Risk backgrounds would consume greater quantities of these substances. Also, consistent with previous studies, it was expected that prenatal exposures would be associated with increased risk of offspring cigarette use and substance use problems. Finally, it was hypothesized that High-Risk offspring prenatally exposed to cigarettes and/or alcohol would have higher rates of both substance use disorders and childhood smoking than non-exposed High-Risk youth.
Materials and Methods
Participants
The present report is based on an analysis of data for 209 third generation offspring who are part of an ongoing family study that selected families through their parents’ generation. The offspring were evaluated during childhood and adolescence at approximately annual intervals and biennially during young adulthood. A total of 99 High-Risk offspring from maternal multiplex families were studied (HR; 55 females and 44 males) along with 110 Low-Risk offspring (LR; 50 females and 60 males). The study has ongoing approval from the University of Pittsburgh Institutional Review Board. All participants provided consent at each visit. Children provided assent with parental consent.
Risk Group Status
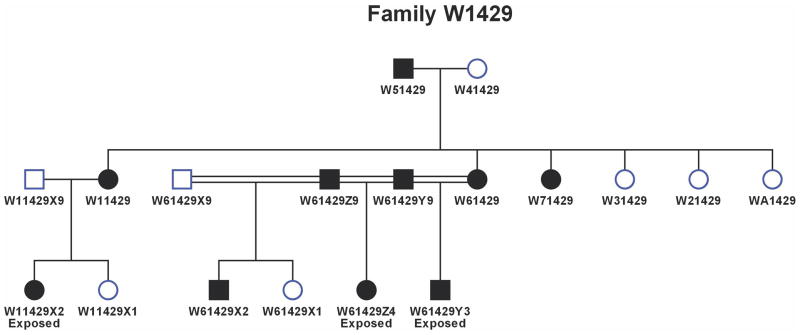
The high-risk families were identified through a proband pair of alcohol dependent sisters. One member of the proband pair was in a substance abuse treatment facility in the Pittsburgh area at the time of recruitment. Both members of the proband pair were screened using an in-person structured interview (Diagnostic Interview Schedule) (DIS; Robins et al., 1981) to determine the presence of alcohol dependence and other Axis I psychopathology. This ascertainment strategy results in a high density of alcohol dependence (Figure 1). The DIS was also administered to all available first-degree relatives. Unavailable or deceased relatives were diagnosed by family history report. Families were excluded if the proband or her first-degree relatives met criteria for primary recurrent major depression, bipolar disorder, primary drug dependence (i.e. drug dependence preceded alcohol dependence by 1 or more years) or schizophrenia. Available spouses were diagnosed using the same methods as members of the target families.
Figure 1.
This pedigree illustrates the high density of alcohol dependence seen in the high-risk families in this study. Families were selected through women in the second generation depicted here. Solid circles (females) or squares (males) indicated affected (alcohol dependence) status by the time of the last follow up. In this pedigree, ‘exposed’ refers to prenatal alcohol exposure. Third generation offspring from this pedigree were first seen at an average age of 10.2 ± 2.2 years and were 31.3 ± 2.9 years of age at last follow up.
Selection of control families was based on availability of families with children between the ages of 8–18. Each control family was selected on basis of residence within a census track from which a high risk family had been selected with an attempt made to yoke each control family to a high risk family in the study. Random phone calls were made to homes within the census track to determine if the family had available children and were interested in participating in the study. Those expressing an interest were sent a letter of introduction. Parents were screened for absence of alcohol and drug dependence using the DIS, though other psychopathology was free to vary. Diagnostic data were also collected for all first degree relatives using either an in-person DIS interview or by obtaining multiple family history reports to ensure an absence of a family history of alcohol dependence. All control families had a low density of familial alcohol dependence. Most low-risk control families had an absence of alcohol or drug dependence among all first-degree relatives. However, three low-risk mothers were identified as having past alcohol abuse or dependence in late adolescence or early adulthood but had been free of any substance use disorder for numerous years (i.e. ≥ 6) before study entry. In addition, one father who married into the target pedigree family had drug dependence.
Evaluation of Prenatal Exposures
Each mother was administered a structured interview (Drinking and Drug Use During Pregnancy) at the time her child was entered into the follow up study. The interview covered her alcohol, cigarette, and other drug use during each of her pregnancies so that the quantity and frequency of these substances could be determined. The interview format was developed in our laboratory and was designed to measure typical and maximal daily use by obtaining information for each of several substances, noting the quantity per occasion and the frequency of use. Daily use was multiplied by the number of days in each trimester and accumulated for all three trimesters, allowing for the total amount used throughout pregnancy to be calculated. Because drug use involved varying quantities taken by various routes (smoking, intravenous, and inhalation), no attempt was made to analyze these data using quantity estimates. Rather, the number of days any drug was used was calculated and used in the analyses. If the mother had multiple children, she was queried concerning each child separately.
Clinical Assessment of Children/Adolescents
Each child/adolescent and his/her parent were separately administered the Schedule for Affective Disorders and Schizophrenia (K-SADS) (Chambers et al., 1985) by trained, Masters’ level clinical interviewers and an advanced resident in child psychiatry at each annual evaluation. Using DSM-III criteria that have been used throughout the follow-up, K-SADS interviewers and the resident independently provided diagnostic data. A best-estimate diagnosis based on these four blinded interviews was completed in the presence of a third clinician who facilitated discussion to resolve diagnostic disagreements if needed. Offspring cigarette/tobacco use was also assessed with the K-SADS at each annual evaluation. Young adult assessments were conducted biennially using the Composite International Diagnostic Interview (CIDI) (Janca et al., 1992) and CIDI-Substance Abuse Module (CIDI-SAM) (Cottler et al., 1989). Substance use disorders included alcohol abuse, alcohol dependence, drug abuse, and drug dependence as assessed by DSM-III (childhood) or DSM-IV (young adult) criteria.
Socioeconomic Status
Socioeconomic status (SES) was determined at each annual evaluation with the Hollingshead Four-Factor Index of Socioeconomic Status (Hollingshead, 1975). An average of SES between the ages of 8 and 18 was used in the current analyses.
Results
Offspring Demographics
Demographic data were analyzed with Pearson chi-square (categorical variables) or linear mixed models (continuous variables) (Table 1). The HR and LR offspring did not significantly differ in gender composition or length of follow-up. HR offspring had a significantly earlier age of study entry (F(1,207)=12.95, p<.001), though age at last follow-up was not significantly different. The HR group had statistically significantly lower SES (F(1,207)=22.08, p<.001) than LR controls, though this may not reflect meaningful differences because both groups fall within the same Hollingshead SES social strata (medium size business, minor professional, technical). The success of the selection strategy used to define the high and low risk groups can be seen in the differing number of first and second degree relatives with AD. HR offspring had an average of 4.61 first- and second-degree relatives with alcohol dependence, compared to an average of 0.64 affected relatives in LR controls.
Table 1.
Offspring Demographic Data
| Low Risk (n=110) | High Risk (n=99) | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Males | 60 | 54.5 | 44 | 44.4 | n.s. |
| M | SD | M | SD | ||
| Age at Study Entry | 13.25 | 5.0 | 11.18 | 3.0 | <0.001 |
| Age at Last Follow-up | 23.05 | 5.6 | 22.1 | 6.5 | n.s. |
| Length of Follow-up | 9.80 | 5.1 | 10.9 | 6.2 | n.s. |
| SESa | 49.13 | 11.7 | 41.2 | 12.0 | <0.001 |
| 1st and 2nd degree relatives with ADb | 0.64 | 1.10 | 4.61 | 2.30 | <0.001 |
Socioeconomic Status (SES) was determined with the Hollingshead Four-Factor Index of Socioeconomic Status and averaged over childhood visits. The Hollingshead Index categorizes SES into five social strata with strata I (unskilled laborers, menial service workers) being the lowest and strata V (major business and professional) the highest. Scores between 40 and 54 are considered to be within strata IV (medium business, minor professional, and technical).
Alcohol Dependence
Maternal Substance Use During Pregnancy
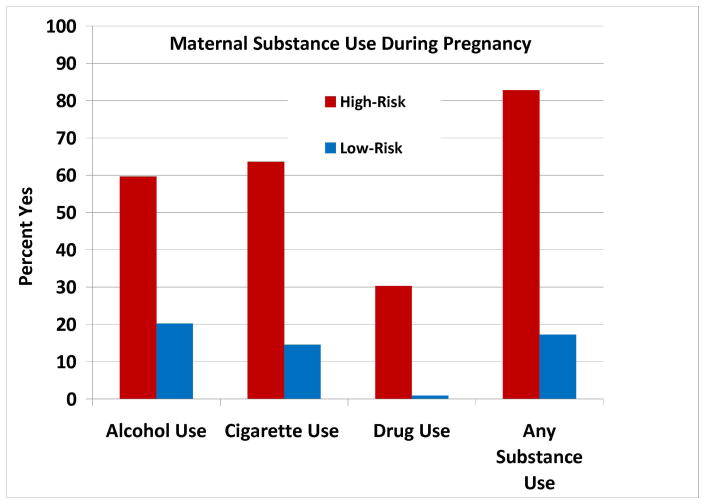
Maternal substance use during pregnancy data were analyzed with Pearson chi-square (categorical variables) or linear mixed models (continuous variables) (Table 2). Maternal report occurred at an average of 12.27 years postpartum. Mothers with a family history of SUD were significantly more likely to use any substance (alcohol, cigarettes, and/or drugs) during pregnancy than LR control mothers (χ2=59.89, p<.001). The HR mothers consumed more alcohol (χ2=33.90, p<.001), cigarettes (χ2=53.41, p<.001), and other drugs (χ2=35.64, p<.001) than mothers from LR families (Figure 2). Among women who used substances during pregnancy, HR mothers reported consuming greater amounts of alcohol (F(1,79)=6.28, p=.014) and cigarettes (F(1,77)=4.54, p=.036) over the course of pregnancy than LR mothers. High-risk mothers who drank during pregnancy consumed an average of 6.03 drinks per week across pregnancy, compared to an average of 0.56 drinks per week among low-risk mothers. High-risk mothers who smoked cigarettes during pregnancy consumed an average of 4.35 packs per week across pregnancy, compared to an average of 2.34 packs per week among low-risk mothers. Because only one LR mother used other drugs during pregnancy, we were unable to compare the number of days of drug use between risk groups.
Table 2.
Maternal Substance Use during Pregnancy
| Low-Risk (n=110) | High-Risk (n=99) | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Any Substance Use | 32 | 29.4 | 82 | 82.8 | <0.001 |
| Alcohol Use | 22 | 20.2 | 59 | 59.6 | <0.001 |
| Cigarette Use | 16 | 14.5 | 63 | 63.6 | <0.001 |
| Drug Use | 1 | 0.9 | 30 | 30.3 | <0.001 |
| Polysubstance Use | 6 | 18.8 | 48 | 58.5 | <0.001 |
| M | SD | M | SD | ||
| Number of Alcoholic Drinksb | 21.14 | 17.70 | 229.22 | 387.91 | 0.014 |
| Number of Cigarettesb | 1779.00 | 1539.77 | 3306.19 | 2752.10 | 0.036 |
| Number of Days of Drug Useb | ----a | ---- | 87.80 | 105.30 | ---- |
Drug use during pregnancy was only reported by one Low-Risk mother
Means reflect total consumption across the duration of pregnancy
Figure 2.
Mothers of High-Risk offspring (n=99) were significantly more likely than Low-Risk mothers to use substances during pregnancy, including alcohol, cigarettes, and other abusable drugs.
Maternal substance use during pregnancy data were also analyzed by trimester. High-risk mothers were more likely to use alcohol and cigarettes and to consume these substances in greater quantities during each trimester (all p<.01). High-risk mothers who drank during pregnancy consumed an average of 12.71 drinks per week in the first trimester (n=57), 5.81 per week in the second trimester (n=31), and 5.09 per week in the third trimester (n=32). Low-risk mothers who drank during pregnancy consumed an average of 1.60 drinks per week in the first trimester (n=19), 0.44 per week in the second trimester (n=7), and 0.41 per week in the third trimester (n=8). High-risk mothers who smoked cigarettes during pregnancy consumed an average of 5.24 packs per week in the first trimester (n=60), 4.78 packs per week in the second trimester (n=54), and 4.71 packs per week in the third trimester (n=53). Low-risk mothers who smoked cigarettes during pregnancy consumed an average of 3.46 packs per week in the first trimester (n=15), 3.91 packs per week in the second trimester (n=9), and 3.51 packs per week in the third trimester (n=8).
HR mothers were also more likely to use more than one substance than LR control mothers (χ2=14.62, p<.001). Among HR mothers who used at least one substance during pregnancy, 26.8% (n=22) used alcohol, cigarettes, and other drugs, while 24.4% (n=20) used both alcohol and cigarettes, 3.7% (n=3) used both alcohol and other drugs, and 3.7% (n=3) used both cigarettes and other drugs. Some mothers reported using only one substance, with 17.1% (n=14) using only alcohol, 22% (n=18) using cigarettes only, and 2% (n=2) using a single other drug during pregnancy. Among LR mothers who used at least one substance during pregnancy, 3% (n=1) used alcohol, cigarettes, and other drugs, 15.6% (n=5) used both alcohol and cigarettes, 50% (n=16) only drank alcohol, and 31.3% (n=10) only smoked cigarettes.
For High-Risk mothers who used drugs during pregnancy, the majority reported use of marijuana (19.1%) or cocaine (13.1%), with a small number using stimulants (3.0%), prescription opioids (3.0%), or prescription sedatives (1.0%). Eight of these mothers reported using more than one drug. In addition, one Low-Risk mother reported cocaine use during pregnancy. Because of the heterogeneity of maternal drug use during pregnancy, it was not possible to assess the main effect of these exposures. Prenatal drug exposure was used as a covariate in subsequent analyses.
Prenatal Exposures and Offspring Outcomes
Separate Cox Regression survival analyses were conducted to assess the effects of prenatal exposure to alcohol or cigarettes on offspring SUD or smoking outcome using relevant covariates (SES, gender, and other prenatal exposures). In order to determine the relative contribution of risk and prenatal exposures to offspring substance use outcomes, survival analyses were conducted within the HR group in which offspring share a similar genetic loading for SUD but varying prenatal exposures. Given the collinearity between risk group status and substance use during pregnancy (i.e. lower rates and amounts of prenatal exposures among low-risk offspring), a statistical interactive design between risk and prenatal exposure could not be performed. Because offspring from multiplex, alcohol dependent families are at increased risk for both alcohol and drug use disorders (Hill et al., 2011), initial analyses assessed the broader category of offspring SUD. Significant relationships between prenatal exposures and SUD were subsequently assessed separately by alcohol and drug use disorders.
For each substance used during pregnancy, a median for the entire group of mothers reporting any use was calculated. The median split was then used to categorize each mother’s use of that substance during pregnancy. A third group was formed from those without any use. Across the entire sample, for mothers who drank during pregnancy, the median number of drinks consumed was 48. Using the median split, thirty-nine HR mothers (66%) were above the median compared to only 1 (4.5%) of the LR mothers. The median number of cigarettes used by HR and LR was 2700, with 41% of HR mothers reporting smoking in the range above the median, compared to 31% of the LR mothers.
Prenatal Alcohol Exposure
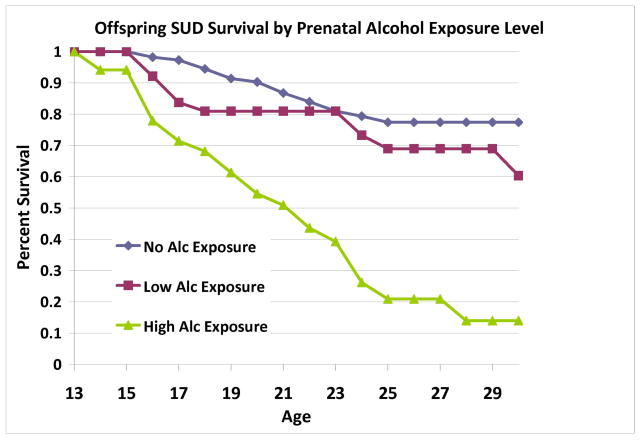
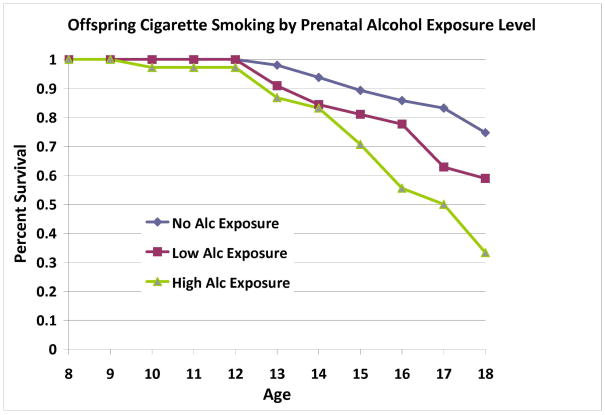
Survival analysis revealed that offspring substance use disorder outcome was influenced by prenatal alcohol exposure after controlling for SES, gender, prenatal cigarette exposure, and prenatal exposure to other drugs (Wald=10.12, p=0.001) (Table 3a). Prenatal alcohol exposure influenced both alcohol use disorders (Wald=14.64, p<.001) and drug use disorders (Wald=8.24, p=.004). Offspring cigarette use by young adulthood was also influenced by prenatal exposure to alcohol after controlling for SES, gender, prenatal cigarette exposure and other drugs (Wald=4.74, p=0.029) (Table 4a). Alcohol-exposed offspring had higher rates and earlier ages of cigarette use and SUD (Figures 3–4). No covariates were significantly associated with offspring substance use outcomes. To determine the appropriateness of including offspring of mothers who had previously met criteria for SUD, analyses were repeated excluding the offspring of three low-risk mothers. This analysis showed that inclusion or exclusion of these three mothers did not alter the results obtained for offspring outcomes.
Table 3a.
Substance Use Disorder Outcome as a Function of Prenatal Alcohol Exposure in Offspring with a Family History of Alcohol Dependence versus Low-Risk Controls
| Offspring with SUD [n (%)] | |||
|---|---|---|---|
| Prenatal Alcohol Exposurea | Low-Risk (n=109) | High-Risk (n=99) | Overall (n=208) |
| None (n=127) | 10 (11.5) | 9 (22.5) | 19 (15.0) |
| Low (n=41) | 5 (23.8) | 6 (30.0) | 11 (26.8) |
| High (n=40) | ---b | 23 (59.0) | 23 (57.5) |
| Overall (n=208) | 15 (13.7) | 38 (38.4) | 53 (25.4) |
Exposure level was determined by conducting a median split of total number of alcoholic drinks consumed during pregnancy (median = 48 alcoholic drinks across pregnancy).
Only one Low-Risk mother was above the median for number of drinks consumed during pregnancy.
Table 4a.
Cigarette Use as a Function of Prenatal Alcohol Exposurein Offspring with a Family History of Alcohol Dependence versus Low-Risk Controls
| Offspring Using Cigarettes [n (%)] | |||
|---|---|---|---|
| Prenatal Alcohol Exposurea | Low-Risk (n=92) | High-Risk (n=98) | Overall (n=190) |
| None (n=116) | 10 (13.2) | 10 (25.0) | 20 (17.2) |
| Low (n=35) | 3 (20.0) | 9 (45.0) | 12 (34.3) |
| High (n=39) | ---b | 14 (36.8) | 14 (35.9) |
| Overall (n=190) | 13 (14.1) | 33 (33.7) | 46 (24.6) |
Exposure level was determined by conducting a median split of total number of alcoholic drinks consumed during pregnancy (median = 48 alcoholic drinks across pregnancy).
Only one Low-Risk mother was above the median for number of drinks consumed during pregnancy
Figure 3.
Across the sample, prenatal alcohol exposure was significantly associated with offspring substance use disorders. Exposure level was determined by conducting a median split of total number of alcoholic drinks consumed during pregnancy (median = 48 alcoholic drinks across pregnancy).
Figure 4.
Across the sample, prenatal alcohol exposure was significantly associated with offspring cigarette use in childhood and adolescence. Exposure level was determined by conducting a median split of total number of alcoholic drinks consumed during pregnancy (median = 48 alcoholic drinks across pregnancy).
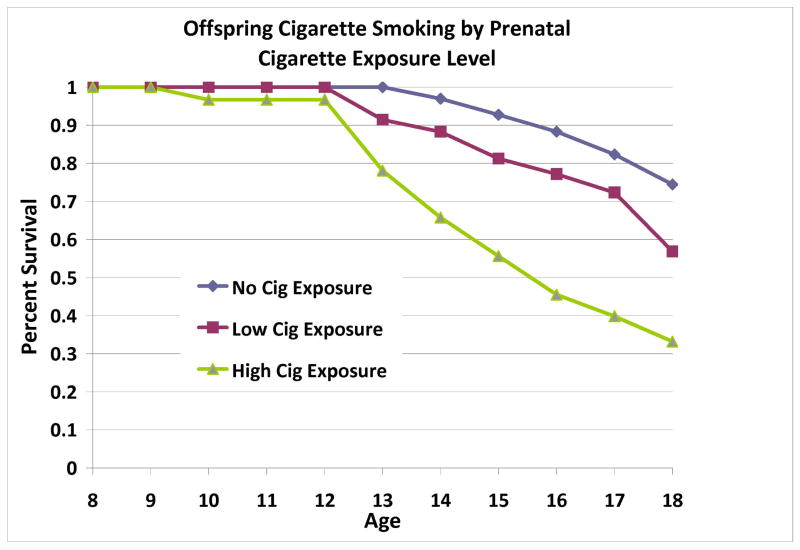
Prenatal Cigarette Exposure
Offspring cigarette use, but not substance use disorder, was influenced by prenatal use of cigarettes after controlling for SES, gender, prenatal alcohol or other drug exposure (Wald=8.04, p=.005) (Tables 3b & 4b). Cigarette-exposed offspring had higher rates and earlier ages of cigarette use (Figure 5). Prenatal alcohol exposure was the only significant covariate (Wald=4.74, p=0.029). Analyses were repeated excluding the offspring of three low-risk mothers who had previously met criteria for SUD. The significance of results remained consistent.
Table 3b.
Substance Use Disorder Outcome as a Function of Prenatal Cigarette Exposure in Offspring with a Family History of Alcohol Dependence versus Low-Risk Controls
| Offspring with SUD [n (%)] | |||
|---|---|---|---|
| Prenatal Cigarette Exposurea | Low-Risk (n=110) | High-Risk (n=99) | Overall (n=209) |
| None (n=130) | 10 (10.6) | 11 (30.6) | 21 (16.2) |
| Low (n=48) | 3 (27.3) | 12 (32.4) | 15 (31.3) |
| High (n=31) | 2 (40.0) | 15 (57.7) | 17 (54.8) |
| Overall (n=209) | 15 (13.6) | 38 (38.4) | 53 (25.4) |
Exposure level was determined by conducting a median split of total number of cigarettes consumed during pregnancy (median = 2700 cigarettes across pregnancy).
Table 4b.
Cigarette Use as a Function of Prenatal Cigarette Exposure in Offspring with a Family History of Alcohol Dependence versus Low-Risk Controls
| Offspring Using Cigarettes [n (%)] | |||
|---|---|---|---|
| Prenatal Cigarette Exposurea | Low-Risk (n=93) | High-Risk (n=98) | Overall (n=191) |
| None (n=117) | 12 (14.8) | 9 (25.0) | 21 (17.9) |
| Low (n=44) | 1 (12.5) | 10 (27.8) | 11 (25.0) |
| High (n=30) | 1 (25.0) | 14 (53.8) | 15 (50.0) |
| Overall (n=191) | 14 (15.1) | 33 (33.7) | 47 (24.6) |
Exposure level was determined by conducting a median split of total number of cigarettes consumed during pregnancy (median = 2700 cigarettes across pregnancy).
Figure 5.
Across the sample, prenatal cigarette exposure was significantly associated with offspring cigarette use in childhood and adolescence. Exposure level was determined by conducting a median split of total number of cigarettes consumed during pregnancy (median = 2700 cigarettes across pregnancy).
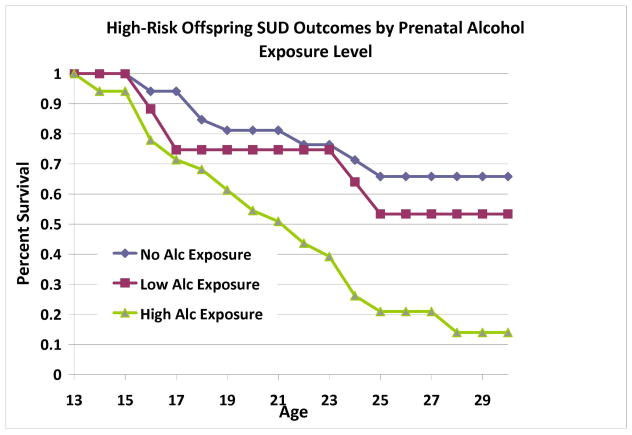
Analyses within the High-Risk Group: Prenatal Alcohol Exposure
Among HR offspring, level of prenatal alcohol exposure was significantly associated with offspring SUD outcomes after controlling for SES, gender, prenatal cigarette exposure, and prenatal exposure to other drugs (Wald=5.54, p=.019) (Figure 6). Prenatal alcohol exposure influenced both alcohol use disorders (Wald=8.61, p=.003) and drug use disorders (Wald=4.52, p=.03). However, prenatal alcohol exposure was not associated with offspring cigarette use, and no covariates were significantly associated with offspring substance use outcomes.
Figure 6.
Among offspring from multiplex, alcohol dependent families, prenatal alcohol exposure was significantly associated with offspring substance use disorders. Exposure level was determined by conducting a median split of total number of alcoholic drinks consumed during pregnancy (median = 48 alcoholic drinks across pregnancy).
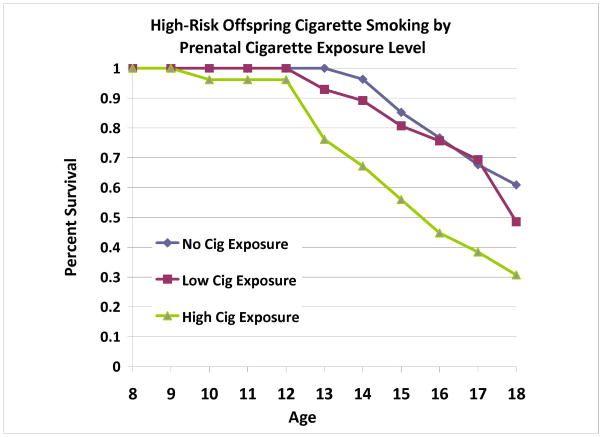
Analyses within the High-Risk Group: Prenatal Cigarette Exposure
Among HR offspring, level of prenatal cigarette exposure was associated with increased risk for cigarette use after controlling for SES, gender, prenatal alcohol exposure, and prenatal exposure to other drugs (Wald=4.27, p=.039) (Figure 7). Prenatal cigarette exposure was not associated with offspring SUD, and no covariates were significantly associated with offspring substance use outcomes.
Figure 7.
Among offspring from multiplex, alcohol dependent families, prenatal cigarette exposure were significantly associated with offspring cigarette use during childhood and adolescence. Exposure level was determined by conducting a median split of total number of cigarettes consumed during pregnancy (median = 2700 cigarettes across pregnancy).
Discussion
The current study sought to characterize maternal substance use during pregnancy in a sample of women at high familial risk for substance use disorders in comparison to women with low familial risk and to examine the relative contribution of prenatal exposure to alcohol or cigarettes and a family history of alcohol dependence on offspring substance use outcomes. Both high- and low-risk women decreased their substance use across trimesters, a pattern previously reported in other studies (Fried et al 1985; Robles and Day, 1990; Hill et al., 2000). Women with a family history of SUD are significantly more likely to use alcohol, cigarettes, and other drugs of abuse during pregnancy, and consume greater quantities of these substances than women without a family history. This finding has important implications for public health and highlights the importance of obtaining information on family history of SUD, as well as the mother’s pattern of substance use prior to pregnancy recognition, to determine the relative risk of maternal substance use during pregnancy.
Across High and Low-Risk participants, prenatal exposure to either alcohol or cigarettes increased the risk of offspring cigarette smoking, whereas prenatal alcohol exposure increased the risk of SUD. Within the High-Risk sample, specificity of type of prenatal exposures and offspring outcomes was seen. Prenatal exposure to alcohol was associated with increased rates of substance use disorders including alcohol use disorders, but not cigarette smoking. Similarly, prenatal exposure to cigarettes was associated with increased rates of cigarette smoking, but not substance use disorders. Thus, prenatal exposures increased the risk for offspring substance use and abuse above and beyond the effect of familial risk for SUD.
These findings are an important contribution to the extant literature on the etiology of substance use disorders by attempting to disentangle the direct, teratogenic effects of prenatal alcohol, cigarette, and drug exposures from the indirect effects of associated familial background factors on offspring substance use outcomes. Although several studies examining the relationship between offspring substance use and prenatal exposure to alcohol and cigarettes have controlled for a family history of substance use, the drastically different patterns of substance use during pregnancy shown in our High-Risk sample indicate that these statistical procedures may not be sufficient for separating direct, prenatal effects from inherited familial factors that are associated with maternal substance use during pregnancy. Furthermore, studies examining the association between prenatal cigarette exposure and offspring substance use have yielded contrasting results depending on their methodology. Those studies controlling for a family history of SUD support a causal influence of prenatal exposure on offspring substance use (Ernst et al., 2001, Glantz and Chambers, 2006), whereas family-based, quasi-experimental designs indicate that familial background factors explain this association (D’Onofrio et al., 2012, Ellingson et al., 2012). Studies controlling for a family history of SUD implicate a causal influence of prenatal alcohol exposure on offspring substance use problems (Baer et al., 1998, Baer et al., 2003), but this association has not been examined within a family-based research design. To our knowledge, neither the effect of prenatal alcohol nor cigarette exposure in the context of ultra-high familial risk has been examined. The assessment of offspring outcomes in individuals with similarly high genetic loading for SUD but varying prenatal exposures provides a unique opportunity to disentangle the impact of these factors on offspring outcomes. Previous studies utilizing offspring at ultra-high familial risk for alcohol dependence have yielded important findings on the association between prenatal exposures and adolescent psychiatric disorders (Hill et al., 2000) and body mass index (BMI) (Hill et al., 2005). The present results extend the previous observations by relating prenatal exposure and familial risk to offspring outcome now that the sample has now reach young adulthood.
The current study has demonstrated more specific effects of prenatal exposures within the High-Risk offspring than across the general sample, such that prenatal alcohol exposure was associated with offspring alcohol problems, and prenatal cigarette exposure was associated with offspring cigarette use. The specificity of prenatal exposure type and offspring alcohol or cigarette use is a novel finding. Previous studies examining the effect of prenatal alcohol exposure on offspring substance use outcomes find support for increases in alcohol, nicotine, and other drug use (Pfinder et al., 2014, Yates et al., 1998). Similarly, studies examining maternal smoking during pregnancy also demonstrate increases in both alcohol and tobacco use by affected offspring (Ernst et al., 2001). However, evidence from animal research points to plausible mechanisms by which prenatal exposures may increase the risk that offspring use or abuse the substance of exposure. Prenatal exposure to nicotine is associated with increased numbers of nicotinic binding sites in the brain both pre- and post-natally(Hellstrom-Lindahl and Nordberg, 2002), which may predispose the brain to the subsequent addictive influence of nicotine consumed later in life. Similarly, prenatal exposure to alcohol in rodents has been found to increase offspring preference for alcohol in both infancy and adulthood, though the specific mechanism accounting for this relationship remains unclear (Abel et al., 1981, Bond and Di Giusto, 1976, Chotro and Molina, 1990).
In addition to disentangling the relative contributions of direct and indirect effects of prenatal exposures on offspring outcomes, this study also has potential implications for identifying individuals who are at the highest risk for substance use and abuse. Though offspring of alcoholics are at increased risk for early onset of drinking and development of SUD, not all HR adolescents go on to develop drug or alcohol problems. The current findings indicate that individuals from a high-risk background who are also prenatally exposed to alcohol and/or cigarettes during pregnancy are especially likely to use and abuse substances, and may have the greatest need for identification and intervention.
Study Limitations
Retrospective collection of data on maternal substance use during pregnancy has been considered less accurate or precise than concurrent reports by some investigators. However, comparison of prospective and retrospective data for drinking during pregnancy has shown retrospective data to be valid (Griesler and Kandel, 1998). Furthermore, some evidence suggests that retrospective report may actually be more valid among women reporting heavy alcohol use (Czarnecki et al., 1990). Women tend to report higher levels of alcohol use during pregnancy retrospectively than concurrently (Ernhart et al., 1988, Jacobson et al., 1991), indicating that concurrently reported alcohol consumption during pregnancy is probably under-reported (Alvik et al., 2006). Furthermore, retrospective reports acquired fourteen years postpartum have been shown to relate to significantly more birth and teen outcomes than antenatal report (Hannigan et al., 2010).
A limitation of this study is that we were unable to assess the direct effect of prenatal exposure to illicit drugs. Like alcohol and cigarettes, prenatal exposure to marijuana or cocaine has been associated with increased rates of offspring use (Minnes et al., 2014, Porath and Fried, 2005, Richardson et al., 2013). In the current study, patterns of maternal drug use during pregnancy were heterogeneous with regard to the substance or substances consumed, the route of administration, and the quantity and frequency of use. Therefore, it was not possible to detect the singular effects of any particular drug.
Women who use substances during pregnancy may be more likely to continue to drink, smoke, or use illicit drugs after the birth of her child. Previous research informed by social learning theory indicates that living with a parent with SUD predicts offspring substance use, with one recent study demonstrating that post-natal exposure to parental SUD increases offspring substance use after controlling for a family history of SUD (Yule et al., 2013). Furthermore, some evidence suggests that current substance use may influence maternal report of prenatal use (Ernhart, 1988). Although parental updates of psychiatric diagnoses including substance use disorder was completed at each yearly offspring follow-up, quantity and frequency of substance use by the parent was not assessed, limiting the available information on parental substance use during offspring childhood and adolescence.
Use of ultra-high-risk AD families can be viewed as either a strength or weakness of our findings. On the positive side, use of these families to assess the impact of prenatal substance use provides a unique opportunity to increase the familial/genetic susceptibility for substance use disorders while examining the specific drug use behaviors of women during their pregnancies. However, it must be acknowledged that these families are not representative of AD families in the general population. Follow-up of offspring from these multiplex families indicates an exceptionally high rate of AD and substance use by young adulthood (Hill et al., 2008, Hill et al., 2011). Although these families may not be representative of AD families in the general population, the study of multiplex families provides an efficient means for identifying risk factors that can then be taken to population samples for replication.
This study compared maternal substance use during pregnancy between women at ultra-high familial risk for substance use disorders and women selected for an absence of familial risk for alcohol dependence and other substance use disorders. In addition, this study design allowed us to uniquely assess the relative contributions of prenatal exposures to drugs of abuse and a family history of alcoholism on offspring substance use outcomes. Our results indicate that women from families with a high prevalence of substance use disorders are more likely to continue to use and abuse substances during pregnancy. Furthermore, High-Risk offspring who are exposed to alcohol and cigarettes in utero are at especially high risk for early-onset cigarette smoking and substance use disorders. From a public health perspective, it is essential that pregnant women be advised to abstain from both alcohol and cigarettes during pregnancy.
Acknowledgments
Funding for this study was provided by NIAAA Grants AA018289, AA05909, AA08082, and AA015168 to SYH
References
- Abel EL, Bush R, Dintcheff BA. Exposure of rats to alcohol in utero alters drug sensitivity in adulthood. Science. 1981;212:1531–1533. doi: 10.1126/science.7233243. [DOI] [PubMed] [Google Scholar]
- Alvik A, Haldorsen T, Groholt B, Lindemann R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Bond NW, Di Giusto EL. Effects of prenatal alcohol consumption on open-field behaviour and alcohol preference in rats. Psychopharmacologia. 1976;46:163–165. doi: 10.1007/BF00421386. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Grekin ER, Mortensen EL, Mednick SA. Relationship of maternal smoking during pregnancy with criminal arrest and hospitalization for substance abuse in male and female adult offspring. Am J Psychiatry. 2002;159:48–54. doi: 10.1176/appi.ajp.159.1.48. [DOI] [PubMed] [Google Scholar]
- Chambers W, Puig-Antich J, Hirsch M, Paez P, Ambrosini P, Tabrizi M, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview: test–retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Acute ethanol contamination of the amniotic fluid during gestational day 21: postnatal changes in alcohol responsiveness in rats. Dev Psychobiol. 1990;23:535–547. doi: 10.1002/dev.420230608. [DOI] [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D. Five-year reliability of self-reported alcohol consumption. J Stud Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- Cottler L, Robins L, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Rickert ME, Langstrom N, Donahue KL, Coyne CA, Larsson H, Ellingson JM, Van Hulle CA, Iliadou AN, Rathouz PJ, Lahey BB, Lichtenstein P. Familial confounding of the association between maternal smoking during pregnancy and offspring substance use and problems. Arch Gen Psychiatry. 2012;69:1140–1150. doi: 10.1001/archgenpsychiatry.2011.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103(Suppl 1):S46–55. doi: 10.2105/AJPH.2013.301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF. Family history of alcoholism and gender: Their combined effects on DSM-IV alcohol dependence and major depression. J Stud Alcohol. 1998;59:97–106. doi: 10.15288/jsa.1998.59.97. [DOI] [PubMed] [Google Scholar]
- Ellingson JM, Rickert ME, Lichtenstein P, Langstrom N, D’Onofrio BM. Disentangling the relationships between maternal smoking during pregnancy and co-occurring risk factors. Psychol Med. 2012;42:1547–1557. doi: 10.1017/S0033291711002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res. 1988;12:506–511. doi: 10.1111/j.1530-0277.1988.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Fried PA, Barnes MV, Drake ER. Soft drug use after pregnancy compared to use before and during pregnancy. AMer J Obstet Gynecol. 1985;151:787–792. doi: 10.1016/0002-9378(85)90520-4. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. Social factors, psychopathology, and maternal smoking during pregnancy. Am J Public Health. 2008;98:448–453. doi: 10.2105/AJPH.2006.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz MD, Chambers JC. Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Dev Psychopathol. 2006;18:893–922. doi: 10.1017/s0954579406060445. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence - Results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Griesler PC, Kandel DB. The impact of maternal drinking during and after pregnancy on the drinking of adolescent offspring. J Stud Alcohol. 1998;59:292–304. doi: 10.15288/jsa.1998.59.292. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, Delaney-Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44:583–594. doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation? Respiration. 2002;69:289–293. doi: 10.1159/000063261. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lowers L, Locke-Wellman J, Shen SA. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J Stud Alcohol. 2000;61:661–668. doi: 10.15288/jsa.2000.61.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke Wellman J, Rickin E, Lowers L. Offspring from families at high risk for alcohol dependence: increased body mass index in association with prenatal exposure to cigarettes but not alcohol. Psychiatry Res. 2005;135:203–216. doi: 10.1016/j.psychres.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry Res. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Tessner KD, McDermott MD. Psychopathology in offspring from families of alcohol dependent female probands: a prospective study. J Psychiatr Res. 2011;45:285–294. doi: 10.1016/j.jpsychires.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four-factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW, Kaplan MG. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol Teratol. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Janca A, Robins L, Cottler L, Early T. Clinical observation of assessment using the Composite International Diagnostic Interview (CIDI). An analysis of the CIDI field trials B wave II at the St Louis site. Br J Psychiatry. 1992;160:815–818. doi: 10.1192/bjp.160.6.815. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Brain Res Dev Brain Res. 2003;147:23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Dev Brain Res. 2003;147:47–57. doi: 10.1016/j.devbrainres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Minnes S, Singer L, Min MO, Wu M, Lang A, Yoon S. Effects of prenatal cocaine/polydrug exposure on substance use by age 15. Drug Alcohol Depend. 2014;134:201–210. doi: 10.1016/j.drugalcdep.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfinder M, Liebig S, Feldmann R. Adolescents’ use of alcohol, tobacco and illicit drugs in relation to prenatal alcohol exposure: modifications by gender and ethnicity. Alcohol Alcohol. 2014;49:143–153. doi: 10.1093/alcalc/agt166. [DOI] [PubMed] [Google Scholar]
- Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol. 2005;27:267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL. Adolescent initiation of drug use: effects of prenatal cocaine exposure. J Am Acad Child Adolesc Psychiatry. 2013;52:37–46. doi: 10.1016/j.jaac.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Robles N, Day NL. Recall of alcohol consumption during pregnancy. J Stud Alcohol. 1990;51:403–407. doi: 10.15288/jsa.1990.51.403. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Smith IE, Lancaster JS, Moss-Wells S, Coles CD, Falek A. Identifying high-risk pregnant drinkers: biological and behavioral correlates of continuous heavy drinking during pregnancy. J Stud Alcohol. 1987;48:304–309. doi: 10.15288/jsa.1987.48.304. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Wendell AD. Overview and Epidemiology of Substance Abuse in Pregnancy. Clin Obstet Gynecol. 2013;56:91–96. doi: 10.1097/GRF.0b013e31827feeb9. [DOI] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]
- Yule AM, Wilens TE, Martelon MK, Simon A, Biederman J. Does exposure to parental substance use disorders increase substance use disorder risk in offspring? a 5-year follow-up study. Am J Addict. 2013;22:460–465. doi: 10.1111/j.1521-0391.2013.12048.x. [DOI] [PubMed] [Google Scholar]