Abstract
Background
We examined whether the effects of topiramate and a single nucleotide polymorphism (SNP; rs2832407) in GRIK1, which encodes a kainate receptor subunit, persisted following a 12-week, placebo-controlled trial in 138 heavy drinkers with a treatment goal of reduced drinking. During treatment, topiramate 200 mg/day significantly reduced heavy drinking days and increased the frequency of abstinent days (Kranzler et al. 2014a). In the European-American (EA) subsample (n=122), rs2832407 moderated the treatment effect on heavy drinking.
Methods
Patients were re-interviewed 3 and 6 months after the end of treatment. During treatment, we obtained 92.4% of drinking data, with 89.1% and 85.5% complete data at the 3- and 6-month follow-up visits, respectively. We examined four outcomes over time in the overall sample and the EA subsample: percent heavy drinking days (PHDD), percent days abstinent (PDA), serum γ-glutamyl transpeptidase (GGTP) concentration, and a measure of alcohol-related problems.
Results
In the full sample, the lower PHDD and higher PDA seen with topiramate treatment were no longer significant during follow-up. Nonetheless, the topiramate-treated patients had lower alcohol-related problem scores during treatment and both follow-up periods. Further, in the EA subsample, the greater reduction in PHDD seen during treatment in rs2832407*C-allele homozygotes persisted throughout follow-up, with no significant effects in A-allele carriers. A reduction in GGTP concentration was consistent with the reduction in heavy drinking, but did not reach statistical significance.
Conclusion
There are persistent therapeutic effects of topiramate in heavy drinkers, principally in rs2832407*C-allele homozygotes.
Keywords: Topiramate, Heavy Drinkers, Post-treatment Follow-up, GRIK1, rs2832407
Introduction
The majority of adults in the United States drink regularly (Blackwell et al., 2012). Nearly one-quarter of U.S. adults also report at least occasional heavy drinking (i.e., five or more drinks on an occasion) and alcohol use is one of the country's leading causes of premature death and disability (NIAAA, 2013). Three medications have been approved by the Food and Drug Administration (FDA) to treat alcohol dependence (AD): naltrexone, acamprosate, and disulfiram. Randomized controlled trials (RCTs) of these medications show that they have only a small effect in reducing drinking (Rösner et al., 2010), and comparatively few RCTs included post-treatment follow-up visits. Most of the RCTs with a post-treatment follow-up have involved naltrexone (Anton et al., 2001; Anton et al., 2006; O'Malley et al., 1996), and they consistently show that the differential treatment effects seen during treatment diminish once the medication has been discontinued. In contrast, one study of acamprosate (Sass et al., 1996) showed that the reduced risk of relapse to drinking seen during a 48-week treatment period was sustained for another 48 weeks after the study medication was discontinued.
Topiramate is a promising medication for alcohol treatment that is not approved for that indication. It is a GABA/glutamate modulator that is FDA approved to treat seizure disorder, prevent migraine, and (in combination with phentermine) promote weight loss. Animal (Breslin et al., 2010; Hargreaves & McGregor, 2007) and human studies (Johnson et al., 2003; Johnson et al., 2007; Kranzler et al., 2014a; Miranda et al., 2008) have shown topiramate to be effective in reducing drinking. In RCTs, topiramate has shown a larger effect size than either naltrexone (Baltieri et al., 2008; Florez et al., 2011; Blodgett et al., 2014) or acamprosate (Kenna et al., 2009; Blodgett et al., 2014). The only placebo-controlled study that failed to show an effect of topiramate in the treatment of AD (Likhitsathian et al., 2013) suffered from a high rate of dropout and missing data, limiting its interpretability. However, absent from the growing literature on the use of topiramate to reduce heavy drinking are studies that follow participants to determine whether topiramate's beneficial effects persist after the medication is discontinued.
Recently, we completed a 12-week, parallel-groups, placebo-controlled trial of topiramate in a sample of 138 heavy drinkers whose goal was to reduce their drinking (Kranzler et al., 2014a). Based on a prior study that showed the single nucleotide polymorphism (SNP) rs2832407, a C-to-A non-coding substitution in GRIK1 that encodes the GluK1 kainate receptor subunit, to be associated with alcohol dependence (Kranzler et al. 2009), we examined the moderating effects of the SNP on the response to topiramate treatment. We found that topiramate treatment significantly reduced heavy drinking days and increased abstinent days more than placebo (Kranzler et al., 2014a). Patients receiving topiramate also had lower concentrations of the liver enzyme γ-glutamyl transpeptidase (GGTP) and lower scores on a measure of alcohol-related problems than placebo-treated patients. In a subsample of EA (n=122), patients homozygous for the rs2832407*C allele (n=51, 42%) previously associated with alcohol dependence (Kranzler et al., 2009) showed greater reductions in heavy drinking days when treated with topiramate than placebo. In contrast, rs2832407*A-allele carriers showed no benefit of topiramate treatment compared with placebo. In the present study, we examined the persistence of treatment effects for the six months following the end of the pharmacotherapy trial.
Materials and Methods
Overview
The study was initiated at the University of Connecticut Health Center and completed at the University of Pennsylvania. The institutional review board at each institution approved the study treatment and follow-up procedures. Details of the recruitment process and selection criteria for the treatment trial were reported previously (Kranzler et al., 2014a). Briefly, we randomly assigned 138 treatment-seeking individuals who expressed a desire to reduce their heavy drinking to receive either topiramate (n=67) or placebo (n=71). All patients were medically stable, educated, and with no concomitant serious comorbid psychiatric or substance dependence (except nicotine dependence). Urine toxicological assessment was conducted at baseline. We used an urn randomization (i.e., balancing) procedure to assign patients to treatment groups and double-blind conditions were maintained throughout the study. Patients received treatment with either topiramate, which was initiated at a dosage of 25 mg/d with a gradual increase over six weeks to a maximum of 200 mg/d, or matched placebo. All patients also received medical management, a brief form of counseling that focuses on medication adherence (Pettinati et al., 2004). At baseline, the end of treatment, and each of the two follow-up visits (3 months and 6 months after treatment ended), patients reported on their drinking, drug use, and alcohol-related problems. We measured the concentration of the liver enzyme GGTP five times (at baseline, the midpoint and endpoint of the 12-week treatment trial, and at each of the two follow-up visits).
Post-treatment Assessments
We attempted to contact and evaluate all patients in both medication groups at all of the scheduled time points, regardless of whether they completed treatment. During follow-up visits, patients completed the Timeline Follow-back (TLFB) (Sobell & Sobell, 1992) to estimate the number of abstinent days and heavy drinking days they had during the follow-up periods in a manner similar to that employed at the pretreatment and treatment endpoint visits. Patients for whom more than 90 days had elapsed from their prior visit (including those who missed an assessment) were asked to report on their drinking since the last assessment. Post-treatment assessments also included the recent (i.e., past 3 months) version of the Short Index of Problems (SIP), a 15-item questionnaire that yields alcohol-related problem scores (from 0-45) (Miller & Tonigan, 1995) and a blood sample for measurement of GGTP. Patients were paid $50 to complete the endpoint assessment and each of the two follow-up visits.
Statistical Analysis
We used linear mixed models to examine medication group differences in percent heavy drinking days (PHDD), percent days abstinent (PDA), SIP score, and GGTP concentration across the study periods and whether differences were moderated by rs2832407. Data from the 12-week treatment period and the 3- and 6-month follow-up periods were included in the analysis to determine whether the change in outcomes from treatment to follow-up differed by medication group. Because the length of treatment (12 weeks) and follow-up periods (13 weeks) differed slightly, heavy drinking days and abstinent days were converted to percentages so that the study periods were on a common scale for analysis.
We conducted two sets of analyses. The first analysis examined models that included the effects of study period (treatment and 3-, and 6-month follow-ups), treatment group (topiramate, placebo) and the interaction of these two factors, which tested the change in the difference between groups across study periods. We included data from the treatment period, which were reported previously (Kranzler et al. 2014a), to model changes over time using consistent measures of drinking behavior (i.e., PHDD and PDA). These analyses included all patients who provided data at any time point. Baseline or pretreatment values for each outcome were included as covariates in modeling the respective outcome. GGTP was log transformed prior to analysis because of excessive positive skewness. The second analysis examined models to which rs2832407 genotype (C-allele homozygote vs. A-allele carrier) was added as a factor to test whether it moderated the effects of study period and treatment group on the outcomes.
Results
Descriptive Statistics
The sample was a mean of 51.1 years old (SD=8.3), 62.3% male, and 88.4% EA, and had completed a mean of 15.5 years of education (SD=2.5). During the pretreatment period, patients were abstinent on 12.5% of days (SD=15.4) and they drank heavily (men: >5 drinks in a day; women: >4 drinks in a day) on 66.4% of days (SD=26.7). At baseline, the only significant difference between medication groups was on age; placebo patients were a mean of about 3.5 years older than topiramate patients. Because analysis of the models with and without age as a covariate showed that it was not a significant predictor, the results reported here do not include age as a variable.
Of the 138 patients who started the trial, 117 (84.9%) completed treatment (82.1% topiramate, 87.3% placebo), with 92.4% of TLFB data available. Drinking data were available for 123 (89.1%) individuals at the 3-month post-treatment assessment (89.6% topiramate, 88.7% placebo), and 118 (85.5%) at the 6-month post-treatment assessment (88.1% topiramate, 83.1% placebo). A comparison of patients completing follow-up visits with those not completing the visits on baseline demographic and drinking variables revealed that non-completers were younger (Δ=4.47 years; F=5.07, df=1,137, p=0.026), reported more alcohol-related problems (Δ SIP score=4.65; F=6.26, df=1,137, p=0.014), and reported greater PDA (Δ=9.2%, F=6.42, df=1,137, p=0.012) than patients who completed the follow-up visits. Because some follow-up interviews were conducted by telephone, fewer patients provided a blood sample for GGTP measurement at the 3-month (n=104) and 6-month (n=96) follow-ups than provided self-report information.
For the pharmacogenetic (i.e., moderation) analyses, we limited the sample to EA patients (n=122) because of the large difference in rs2832407 allele frequencies by population. Demographic and pretreatment clinical features of the EA subsample are provided in detail elsewhere (Kranzler et al., 2014a). Briefly, medication groups did not differ on the frequency of the CC genotype (37.5% topiramate, 45.5% placebo, χ2=0.79, p=0.38). Further, comparisons among the four groups resulting from the cross of medication (placebo vs. topiramate) by genotype (CC vs. A-allele carriers) showed no significant differences on any of the demographic or clinical measures.
Within EAs, the completion rate among rs2832407*C homozygotes was 92.3% during treatment and 90.2% at the 3-month follow-up and 90.2% at the 6-month follow-up, with the respective rates among A-allele carriers being 82.7%, 88.7%, and 81.7%. Contingency table analyses revealed no significant difference in completion rates by medication group, study period, genotype, or their interactions.
In the sections that follow, we first present findings for the intent-to-treat sample (n=138) and then present parallel results for the EA subsample (n=122), in which we examined rs2832407 as a moderator variable.
Effects of Topiramate in the Full Sample
Percent Heavy Drinking Days and Percent Days Abstinent
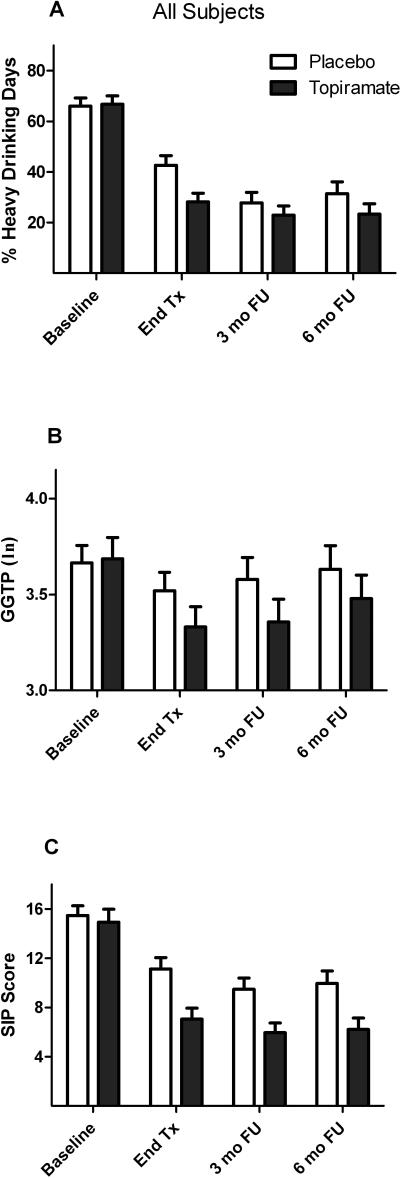
For PHDD, there was a non-significant trend for a main effect of medication group (F=3.21, df=1, 132, p=0.075) and a significant interaction of medication group by study period (F=3.55, df=2, 243, p=0.036). Although topiramate-treated patients had a significantly lower PHDD than placebo patients at the end of treatment (t=2.94, df=220, p=0.004), the medication effect was no longer significant at the 3-month (t=0.55, df=237, p=0.58) or 6-month (t=1.12, df=244, p=0.26) follow-up visits (see Figure 1A). Post hoc analyses revealed that the reduction in PHDD in the topiramate group persisted through the 3- and 6-month follow-up periods. However, following treatment, the placebo group showed a significant decrease in PHDD from treatment to the 3-month (t=4.57, df=244, p<0.001) and 6-month follow-ups (t=3.47, df=245, p=0.001).
Figure 1.
Comparison of topiramate and placebo across study periods in the full sample (n=138). Values are means (SEM).
A: Mean percent heavy drinking days (PHDD). There was a significant interaction of medication group by study period (p=0.03) on PHDD.
B: Mean natural log γ-glutamyltranspeptidase (GGTP) concentration. The interaction of medication group by study period was not significant for GGTP (p=0.18).
C: Mean Short Index of Problems (SIP) score. There was significant main effect of medication group (p=0.001) on SIP score, but no interaction of medication group by study period.
For PDA, there was also no main effect of medication group (F=0.03, df=1, 137, p=0.87) and a significant medication group-by-study period interaction on PDA (F=3.21, df=2,248, p=0.042). Post hoc analyses revealed that, in contrast to what was seen with PHDD, the medication groups were similar on PDA at the end of treatment, but there was a significantly greater increase in PDA from treatment to 3-month follow-up in patients who received placebo (t=2.40, df=250, p=0.017).
γ-Glutamyltranspeptidase Concentration (Figure 1B)
Neither medication group (F=1.42, df=1, 122, p=0.23) nor the interaction of medication group by study period (F=2.09, df=2, 203, p=0.13) showed significant effects on GGTP concentration.
Short Index of Problems Score (Figure 1C)
There was a significant main effect of medication group on SIP score, with topiramate patients reporting lower severity of alcohol-related problems than placebo patients (F=11.77, df=1, 128, p=0.001). The topiramate group had a lower SIP score than the placebo group at the end of treatment (t=3.46, df=240, p=0.001) and at the 3-month (t=2.43, df=256, p=0.016) and 6-month (t=2.48, df=266, p=0.014) follow-ups. The interaction of medication group with study period on SIP score was not significant (F=0.50, df=2, 235, p=0.61), i.e., the medication effect on this outcome was consistent across the study periods.
Moderation of the Effects of Topiramate by rs2832407
Percent Heavy Drinking Days and Percent Days Abstinent
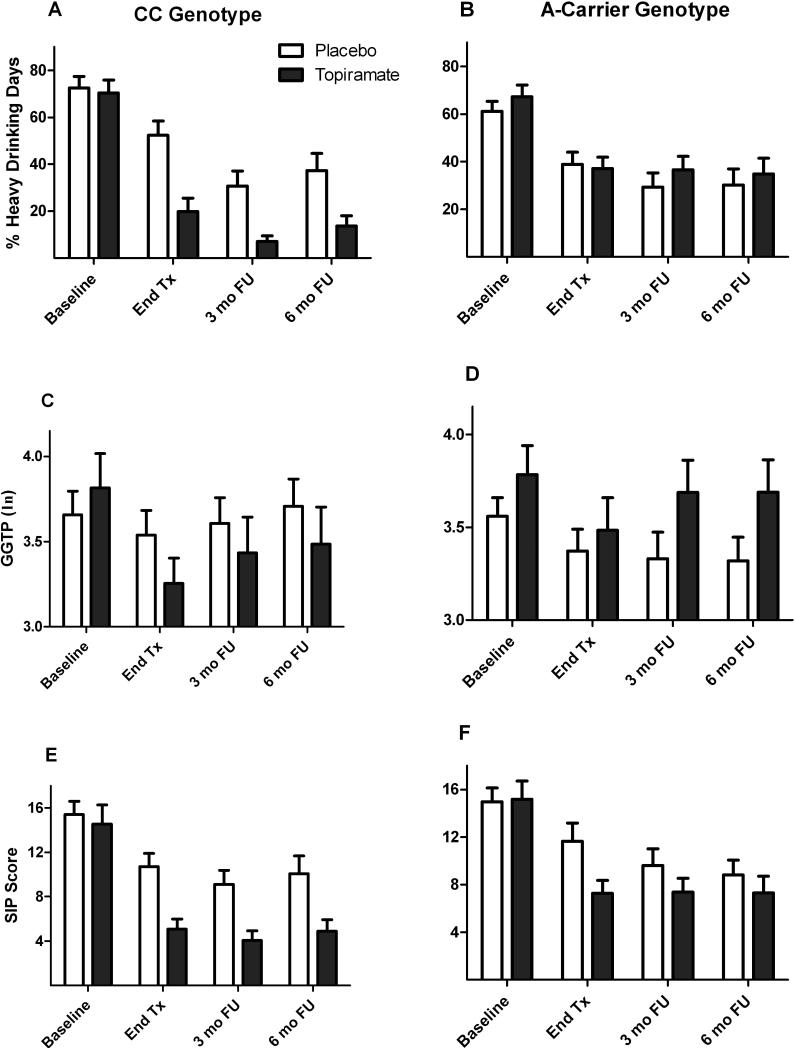
In the EA subsample, the three-way interaction of study period by medication group by genotype group was not significant (F=0.01, df=2, 210, p=0.99). However, there was a significant medication group-by-genotype interaction (F=8.79, df=1, 113, p=0.004) on PHDD. As shown in Figure 2A, topiramate was efficacious in patients with the CC genotype, but not those in the A-allele carrier group. Specifically, there were significant interactions between medication group and genotype group during treatment (t=2.52, df=203, p=0.013) and during the 3-month (t=2.39, df=217, p=0.018) and 6-month (t=2.47, df=221, p=0.014) follow-up periods. Simple effects analysis within rs2832407*C-allele homozygotes showed that the topiramate-treated patients had a lower PHDD than placebo patients during treatment (t=3.80, df=48, p<0.001) and during the 3-month (t=2.97, df=44, p=0.005) and 6-month (t=2.60, df=44, p=0.013) follow-up periods. There were no significant group differences within A-allele carriers (all p-values > 0.30).
Figure 2.
Moderating effects of rs2832407 on the comparison of topiramate and placebo across study periods in the European-American subsample (n=122). Values are means (SEM).
A and B: Mean percent heavy drinking days (PHDD). There were significant interactions between medication group and genotype group during treatment (p=0.013) and during the 3- month (p=0.018) and 6-month (p=0.014) follow-up periods. Simple effects analysis within rs2832407*C-allele homozygotes showed that topiramate-treated patients had a lower PHDD than placebo patients during treatment (p<0.001) and during the 3-month (p=0.005) and 6-month (p=0.013) follow-up periods. There were no significant group differences within A-allele carriers (all p-values > 0.30).
C and D: Mean natural log γ-glutamyltranspeptidase (GGTP) concentration. The interaction of medication group by genotype group across the four time points approached significance for GGTP (p=0.097).
E and F: Mean Short Index of Problems (SIP) score. There was no interaction of medication group by genotype group on SIP score (p=0.30).
The interaction of study period by medication group by genotype group was not significant for PDA (F=0.95, df=2, 215, p=0.39), nor was there an effect of genotype by medication group on PDA (F=0.11, df=1, 117, p=0.74).
γ-Glutamyl Transpeptidase Concentration (Figure 2B)
Although the interaction of medication group by genotype group on GGTP concentration did not vary by study period (F=0.29, df=2, 172, p=0.75), the interaction of medication group by genotype group across the three time points approached significance (F=2.80, df=1, 105, p=0.097). The rs2832407*CC-genotype group treated with topiramate had marginally lower GGTP concentrations than the placebo group.
Short Index of Problems Score (Figure 2C)
There was no evidence that genotype moderate the effect of topiramate on SIP score. Neither the three-way interaction of medication group by genotype group by study period (F=0.28, df=2, 204, p=0.75) nor the interaction of medication group by genotype group (F=1.09, df=1, 111, p=0.30) was significant for SIP score.
Controlling for Potential Confounds
As mentioned above, because the medication groups differed on age, we repeated the analyses with age as a covariate, which did not alter the findings. Further, to address missing data concerns, we conducted multiple imputation using a fully conditional Markov Chain Monte Carlo procedure to generate 10 imputed data sets, and used patient characteristics and drinking behavior to predict missing 3- and 6-month outcomes. In neither case did the results differ from those based on the available data.
Discussion
This is the first study to evaluate the post-treatment effects of topiramate in an alcohol treatment trial. We found that some, but not all, of the benefits produced by topiramate treatment persisted after discontinuation of the medication. Although topiramate treatment significantly reduced heavy drinking days and increased abstinent days more than placebo in the full sample during treatment (Kranzler et al., 2014a), the effect of the medication was no longer significant at the 3- or 6-month follow-up visits. Post hoc analyses revealed that, despite the persistence of topiramate's beneficial effects, the placebo group substantially reduced their PHDD and increased their PDA once the study medication was discontinued. In the full sample, topiramate-treated patients also had lower concentrations of the liver enzyme GGTP at the end of treatment than placebo-treated patients, but the effect was no longer significant at the follow-up visits. In contrast, topiramate's reduction of alcohol-related problems, which was significant during treatment, persisted throughout the 6-month follow-up period.
Interestingly, placebo-treated patients reported a significant reduction in heavy drinking and an increase in abstinent days during the post-treatment follow-up. Despite the convergence in self-reported drinking between the two treatment groups during follow-up, GGTP concentrations in topiramate-treated patients were marginally lower throughout the course of treatment and follow-up. Further, the changes in drinking behavior reported by the placebo-treated patients were not accompanied by similar changes in the severity of alcohol-related problems during follow-up. Some decay in topiramate treatment effects is expected over time and may be reflected in the inconsistent pattern of differences from placebo. It is also likely that non-treatment factors affected the course of drinking and related variables during the follow-up period.
Importantly, however, we found that the robust effect of topiramate during treatment, where it reduced PHDD significantly in rs2832407*C-homozygotes, persisted throughout the 6-month post-treatment period. Specifically, during follow-up, patients with the rs2832407*CC genotype who received topiramate had a significantly lower PHDD than those who received placebo or than A-allele carriers receiving either treatment. This effect is based on self-report, but there is no reason to assume that self-report is less valid in this genotype group than in the A-allele carriers from whom it did not otherwise appear to differ. Further, although the interaction of medication group by genotype on GGTP concentrations did not reach significance, there was a non-significant trend in rs2832407*C-allele homozygotes treated with topiramate to have lower GGTP concentrations than those receiving placebo. There was no such effect in A-allele carriers. Because the number of patients providing a blood sample at the follow-up visits was lower than those providing self-report data, the lack of statistically reliable findings for GGTP concentration may be due, in part, to lower statistical power for that outcome measure than for self-reported drinking.
Overall, these findings provide additional support for the use of topiramate to reduce heavy drinking in EAs with the rs2832407*CC genotype, which is present in approximately 42% of that population group (Kranzler et al., 2009). This effect, however, requires replication, perhaps in the context of a topiramate study for heavy drinking that compares treatment for 12 weeks with that over a longer period of time, and includes a post-treatment follow-up. An adequately powered study using that design could test the moderator effect, define the appropriate duration of treatment, and clarify the impact of treatment duration on the persistence of treatment effects.
The findings reported here are consistent with our examination in this sample of the moderating effect of rs2832407 on topiramate's reduction of positive alcohol expectancies and desire to drink (Kranzler et al., 2014b). In that study, we found that, among rs2832407*C-allele homozygotes, topiramate treatment reduced positive alcohol expectancies and desire to drink significantly more than placebo treatment. Thus, in this heavy drinker sample, the CC genotype was associated with other indicators of response to topiramate treatment.
Pre-clinical research also provides evidence of an interaction of genetic features with the response to topiramate. In a study examining the effects of topiramate (alone or in combination with naltrexone or ondansetron) on alcohol consumption and reinforcement, topiramate reduced alcohol consumption and reinforcement in alcohol preferring (P) rats, but not in Wistar rats (Breslin et al., 2010). Although the mechanisms underlying these effects in different rodent lines are unknown, P rats show differences from non-preferring animals in the cortico-mesolimbic dopamine system, which mediates the reinforcing effects of alcohol (McBride & Li, 1998), and differences in GABAergic and glutamatergic signaling (Kapasova & Szumlinski, 2008; McBride et al., 1990). The use of a rodent line selected for alcohol preference is analogous to the use of a genotype to select patients who are most responsive to topiramate treatment. Thus, research examining the effect of selective antagonism of GluK1-containing kainate receptors in selected rodent lines could provide insights into the mechanism and duration of pharmacological effects on drinking behavior such as those reported with topiramate.
The current findings should be considered in the context of the study's strengths and limitations. This was the first study to examine the persistence of the effects of topiramate in reducing heavy drinking. During treatment, there was a high rate of medication adherence and treatment completion (Kranzler et al., 2014a) and during the post-treatment period there was a high-rate of follow-up, which support the internal validity of the findings. The finding that three months of topiramate treatment in patients selected for a single genotype that is prevalent in EAs can lead to clinically relevant treatment effects six months after treatment is discontinued has important clinical implications. Limitations of the study include a sample size that limited the statistical power to detect some interaction effects reliably. Sample attrition, despite good follow-up rates, also diminished the power available to conduct comparisons during the follow-up periods, particularly for GGTP, which unlike self-reported drinking measures, could not be obtained via telephone. Further, the post-treatment follow-up evaluations did not include a detailed assessment of non-treatment factors that may have diminished the effects of treatment over time. We are currently exploring how topiramate treatment interacts with rs2832407 to modify other aspects of alcohol use and relapse, including a measure of self-efficacy. Future studies with larger sample sizes are needed to confirm our findings and to explore the psychological and biochemical mechanisms of topiramate's effects on heavy drinking and its moderation by rs2832407. Such studies should also examine in detail non-treatment-related factors that could influence post-treatment outcomes.
The current study extends our previous findings of topiramate's effects in heavy drinkers whose goal was to reduce drinking, demonstrating that the beneficial effects of topiramate persist as long as 6 months after the discontinuation of treatment. In the overall sample, the advantage of topiramate over placebo in self-reported PHDD and PDA appeared to diminish over the follow-up period, as placebo-treated patients reported reduced PHDD and increased PDA. However, the finding that heavy drinkers with the rs2832407*CC genotype experienced beneficial effects of topiramate that persist beyond the period of medication treatment underscores the potential for this treatment approach to reduce heavy drinking. Replication and extension of these findings may help to personalize the pharmacological treatment of heavy drinking.
Acknowledgments
Staff members of the Clinical Research and Evaluation Unit of the University of Connecticut Alcohol Research Center and the Center for Studies of Addiction of the University of Pennsylvania Perelman School of Medicine contributed to the conduct of this study.
Supported by NIH grants P60 AA03510 and K24 AA13736
Footnotes
Disclosure
HRK has been a consultant or advisory board member for the following pharmaceutical companies: Alkermes, Lilly, Lundbeck, Otsuka, Pfizer, and Roche. He is a member of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative, which is supported by Lilly, Lundbeck, AbbVie, Pfizer, and Ethypharm. HRK and JC have a U.S. patent pending entitled, “Test for Predicting Response to Topiramate and Use of Topiramate.”
None of the other authors has any disclosure to make.
www.clinicaltrials.gov registration: NCT00626925
References
- Anton RF, Moak DH, Latham PK, Waid LR, Malcolm RJ, Dias JK, Roberts JS. Posttreatment results of combining naltrexone with cognitive-behavior therapy for the treatment of alcoholism. J Clin Psychopharmacol. 2001;21:72–77. doi: 10.1097/00004714-200102000-00013. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, Daro FR, Ribeiro PL, de Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2008;103:2035–2044. doi: 10.1111/j.1360-0443.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- Blackwell D, Lucas J, Clarke T. Summary health statistics for U.S. adults: National health interview survey, 2012. National Center for Health Statistics. Vital Health Stat. 2012;10(260) [PubMed] [Google Scholar]
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate's effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38:1481–1488. doi: 10.1111/acer.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology (Berl) 2010;207:529–534. doi: 10.1007/s00213-009-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez G, Saiz PA, Garcia-Portilla P, Alvarez S, Nogueiras L, Bobes J. Topiramate for the treatment of alcohol dependence: comparison with naltrexone. Eur Addict Res. 2011;17:29–36. doi: 10.1159/000320471. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, McGregor IS. Topiramate moderately reduces the motivation to consume alcohol and has a marked antidepressant effect in rats. Alcohol Clin Exp Res. 2007;31:1900–1907. doi: 10.1111/j.1530-0277.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Harris AH, Kivlahan DR, Bowe T, Humphreys KN. Pharmacotherapy of alcohol use disorders in the Veterans Health Administration. Psychiatr Serv. 2010;61:392–398. doi: 10.1176/ps.2010.61.4.392. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, Swift RM. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Lomastro TL, Schiesl A, Leggio L, Swift RM. Review of topiramate: an antiepileptic for the treatment of alcohol dependence. Curr Drug Abuse Rev. 2009;2:135–142. doi: 10.2174/1874473710902020135. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, Anton RF, Arias AJ, Herman A, Zhao H, Covault J. Association of markers in the 3' region of the GluR5 kainate receptor subunit gene to alcohol dependence. Alcohol Clin Exp Res. 2009;33:925–930. doi: 10.1111/j.1530-0277.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Kampman KM. Topiramate treatment for heavy drinkers: Moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014a;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Feinn R, Tennen H, Gelernter J, Covault J. GRIK1 genotype moderates topiramate's effects on daily drinking level, expectations of alcohol's positive effects and desire to drink. Int J Neuropsychopharmacol. 2014b;30:1–8. doi: 10.1017/S1461145714000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhitsathian S, Uttawichai K, Booncharoen H, Wittayanookulluk A, Angkurawaranon C, Srisurapanont M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: a 12-week, randomized, placebo-controlled trial. Drug Alcohol Depend. 2013;133:440–446. doi: 10.1016/j.drugalcdep.2013.06.032. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Serotonin, dopamine and GABA involvement in alcohol drinking of selectively bred rats. Alcohol. 1990;7:199–205. doi: 10.1016/0741-8329(90)90005-w. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS. The Drinker Inventory of Consequences (DrinC) NIAAA Project MATCH Monograph. 1995:1995. Series Vol. 4 NIH Publ. No. 95-3911. [Google Scholar]
- Miranda R, Jr., MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism [April 29, 2013];NIAAA recognizes alcohol awareness month. 2013 April 1, 2013, from http://www.niaaa.nih.gov/news-events/alcohol-awareness-month-2013.
- O'Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, Rounsaville B. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–224. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Weiss RD, Miller WR, Donovan DM, Ernst DB, BJ R. COMBINE Monograph Series, vol 2: Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD.: 2004. (Publication number, NIH 04-5289) [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010:CD004332. doi: 10.1002/14651858.CD004332.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–680. doi: 10.1001/archpsyc.1996.01830080023006. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ.: 1992. Timeline Follow-back: A technique for assessing self-reported ethanol consumption; pp. 41–72. [Google Scholar]