Abstract
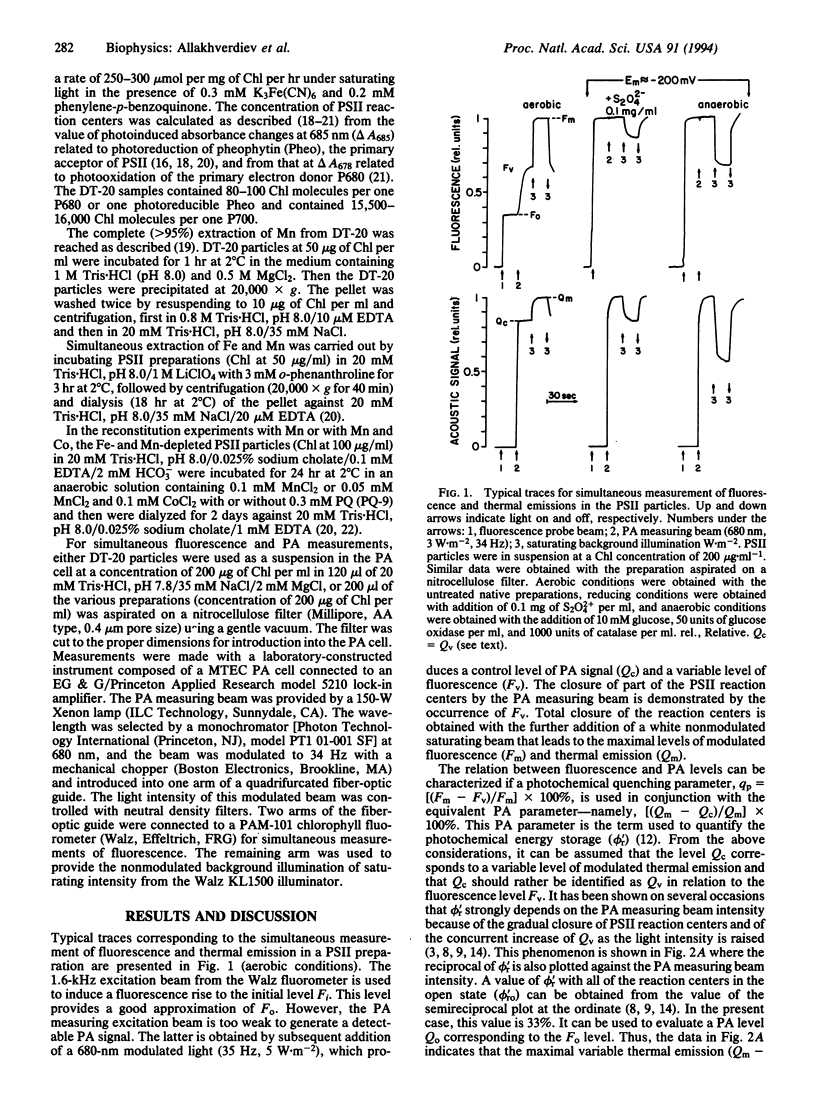
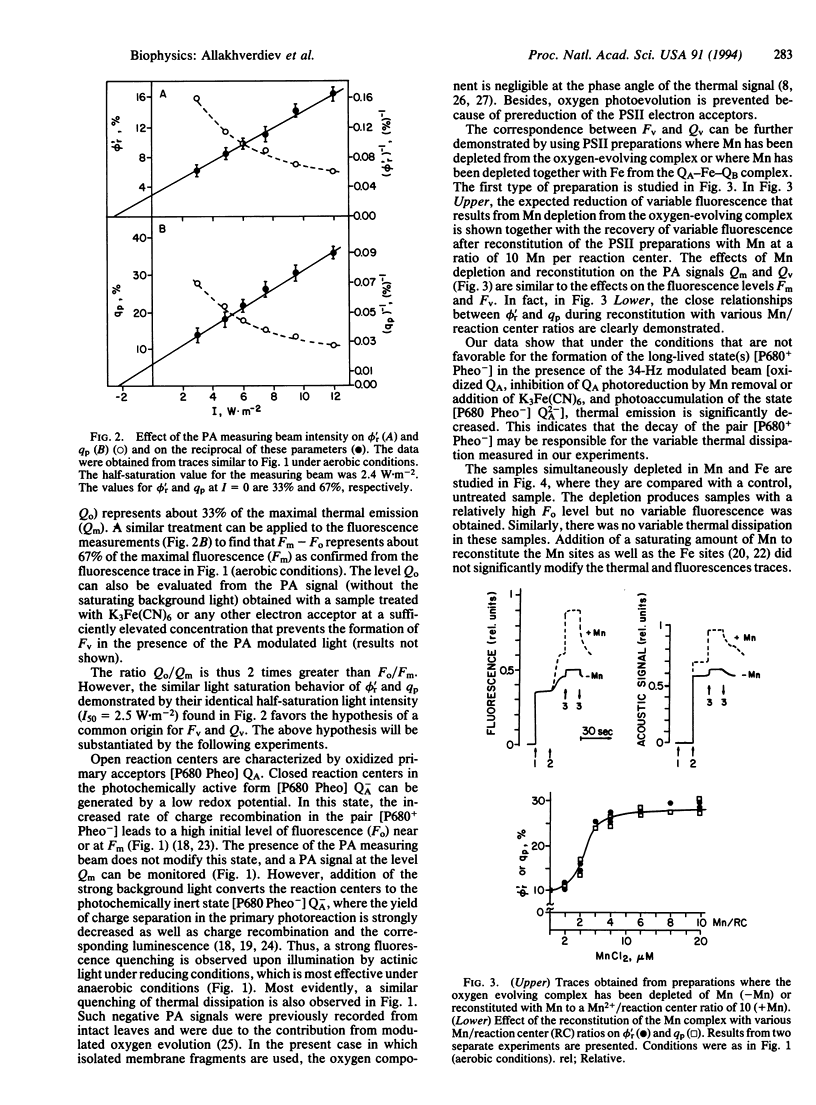
In photosynthetic systems, the absorbed light energy is used to generate electron transport or it is lost in the form of fluorescence and thermal emission. While fluorescence can be readily measured, the detection of thermal deactivation processes can be achieved by the photoacoustic technique. In that case, the pressure wave generated by the thermal deactivations in a sample irradiated with modulated light is detected by a sensitive microphone. The relationships between the yield of fluorescence and thermal emissions measured simultaneously were analyzed by using a spinach photosystem II (PSII)-enriched preparation. It is shown that the quenching of fluorescence due to the photochemical activity of the preparations (photochemical quenching) increases in proportion to the fraction of thermal deactivations that is not immediately released as heat but is stored in photochemical intermediates (energy-storage yield) as the intensity of the photoacoustic modulated measuring beam (35 Hz) is decreased. Maximal levels of fluorescence and thermal emissions were both decreased in similar proportions upon photoreduction of pheophytin (Pheo), the primary acceptor of PSII. The variable components of fluorescence and thermal emissions were strongly decreased upon depletion of Mn from the Mn complex that catalyzes water oxidation and were recovered proportionally during reconstitution with Mn2+ at various Mn2+/reaction center ratios. Finally, depletion of Mn from the Mn complex together with the Fe of the QA-Fe-QB complex that is composed of the secondary quinone acceptors of PSII resulted in an increased initial level of fluorescence Fo and in the loss of the variable components of fluorescence and thermal emissions. The initial Fo and the variable components could be partially recovered by reconstitution of both donor and acceptor sides with Mn2+, Co2+, HCO3- and plastoquinone. It is concluded that the photochemical fluorescence quenching is correlated with a simultaneous "quenching" of a variable component of thermal emission. It is proposed that the measured component of variable thermal emission is related to the decay of the pair [P680+ Pheo-]. The suggestion is also made that a bicarbonate-induced protonation of reduced QA or QB or conformational change in the PSII complex, or both, adds an additional entropic factor to the variable thermal emission component.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braslavsky S. E. Photoacoustic and photothermal methods applied to the study of radiationless deactivation processes in biological systems and in substances of biological interest. Photochem Photobiol. 1986 Jun;43(6):667–675. doi: 10.1111/j.1751-1097.1986.tb05645.x. [DOI] [PubMed] [Google Scholar]
- Carpentier R., Larue B., Leblanc R. M. Photoacoustic spectroscopy of Anacystis nidulans. III. Detection of photosynthetic activities. Arch Biochem Biophys. 1984 Feb 1;228(2):534–543. doi: 10.1016/0003-9861(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Carpentier Y. A., Richelle M., Rubin M., Rössle C., Dahlan W., Bosson D., Fürst P. D. Stabilisation of plasma substrate concentrations: A model for conducting metabolic studies. Clin Nutr. 1990 Dec;9(6):313–318. doi: 10.1016/0261-5614(90)90003-b. [DOI] [PubMed] [Google Scholar]
- Debus R. J., Feher G., Okamura M. Y. Iron-depleted reaction centers from Rhodopseudomonas sphaeroides R-26.1: characterization and reconstitution with Fe2+, Mn2+, Co2+, Ni2+, Cu2+, and Zn2+. Biochemistry. 1986 Apr 22;25(8):2276–2287. doi: 10.1021/bi00356a064. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Dolan E., Shaw E. R., Ke B. Interaction between the intermediary electron acceptor (pheophytin) and a possible plastoquinone-iron complex in photosystem II reaction centers. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7227–7231. doi: 10.1073/pnas.77.12.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser-Ross N., Malkin S., Cahen D. Photoacoustic detection of photosynthetic activities in isolated broken chloroplasts. Biochim Biophys Acta. 1980 Dec 3;593(2):330–341. doi: 10.1016/0005-2728(80)90070-5. [DOI] [PubMed] [Google Scholar]
- Popovic R., Beauregard M., Leblanc R. M. Study of Energy Storage Processes in Bundle Sheath Cells of Zea mays. Plant Physiol. 1987 Aug;84(4):1437–1441. doi: 10.1104/pp.84.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I., Styring S. Spectroscopic characterization of triplet forming states in photosystem II. Biochemistry. 1992 Jul 7;31(26):5957–5963. doi: 10.1021/bi00141a002. [DOI] [PubMed] [Google Scholar]


