Abstract
Background
Despite evidence that prenatal alcohol exposure (PAE) can lead to a wide range of impairments in cognitive, social and emotional behaviors, drinking during pregnancy remains common. Although there is a general understanding that high levels of drinking during pregnancy are unsafe, conflicting evidence regarding the impact of low intake may account for the persistence of this behavior.
Methods
In order to investigate the effects of PAE on learning and executive control we utilized a voluntary paradigm where pregnant mice had access to a saccharin sweetened 10% alcohol solution for 4 hours, during the dark cycle, throughout gestation. Male and female offspring were tested as adults on a touch-screen discrimination and reversal task mediated by corticostriatal circuits.
Results
Consistent with previous findings, PAE did not lead to gross morphological, motor or sensory alterations in offspring. Both PAE and saccharin control female mice were slower to acquire the discrimination than males, but PAE did not impair associative learning in either sex. During reversal, PAE led to a specific and significant impairment in the early phase, where cortical control is most required to flexibly alter choice behavior. PAE mice showed a significant increase in maladaptive perseverative responses but showed intact learning of the new association during late reversal.
Conclusions
Previously, data from clinical studies have suggested that executive control deficits may underlie cognitive, as well as social, problems seen in adolescents with documented PAE. These data demonstrate that even more moderate alcohol exposure during development can lead to impaired cognitive functioning well into adulthood.
Keywords: Executive Function, FASD, Touch-Screen, Mouse
Introduction
It has been over 30 years since the Surgeon General of the United States advised that women who were, or were considering becoming, pregnant abstain from alcohol. Despite a wealth of evidence that consumption of alcohol during pregnancy can have profound and wide-ranging consequences on offspring, rates of drinking during pregnancy remain high. Recent reports suggest that as many as one third of all women drink at some time during pregnancy and between 5–10% report binge drinking behavior (Ethen et al., 2009). This disconnect may be partially explained by conflicting reports regarding the safety of consuming alcohol at low levels, both from the research literature and primary care providers.
There is strong evidence that high levels of prenatal alcohol exposure (PAE) during pregnancy have negative consequences on the physical and cognitive development of offspring (Streissguth et al., 1991). Studies in clinical populations have found that high dose PAE is associated with a wide-range of symptoms that include impaired growth, deficits in cognitive function and executive control (Mattson et al., 1999, Day et al., 2002, Olesen et al., 2004, Willford et al., 2006, Green et al., 2009) and increased behavioral and emotional problems (Richardson et al., 2002). Close comparison of end-points between human patients and rodent ethanol exposure models suggest a congruent effect of blood alcohol content (BAC) on behavioral outcomes across species (Driscoll et al., 1990). Controlled-dose studies using rodent models have underlined the detrimental effects of PAE, demonstrating that high levels of exposure (BAC 300–400mg/dl) can alter motor behavior, impair learning, and decrease cognitive flexibility (Riley et al., 1979, Thomas et al., 2004a, Thomas et al., 2004b, Morton et al., 2014).
While these and other studies have helped to establish a link between high dose exposure and later cognitive and behavioral deficits, the effects of more moderate dose PAE remain controversial (Henderson et al., 2007, Todorow et al., 2010). Clinical studies have shown that lower dose PAE can lead to increased risk of behavioral issues in adolescence including aggression and emotional problems (Sood et al., 2001, Sayal et al., 2007, O’Leary et al., 2010). More moderate PAE has also been associated with cognitive deficits in both children and adolescents (Coles et al., 1991, Burden et al., 2005a, Burden et al., 2005b). In particular, adolescents with fetal alcohol spectrum disorders (FASD) who do not show the hallmark morphological abnormalities associated with Fetal Alcohol Syndrome exhibit impairments in executive control, including, behavioral inflexibility, which can interfere with everyday classroom functioning (Green, 2007). However, other studies failed to find an association between more moderate PAE and impairment in either behavioral or emotional development (Kelly et al., 2009, Bay and Kesmodel, 2011, Kelly et al., 2012). Several preclinical studies have shown that PAE can lead to alterations in brain function and behavior. PAE resulting from maternal daily ethanol consumption resulting in a BAC of 80–90mg/dl, has been shown to impair hippocampal mediated working memory and N-methyl-D-aspartate receptor- (NMDAR) mediated synaptic plasticity (Brady et al., 2012, Brady et al., 2013). Additionally, PAE has been shown to alter cerebellar-motor coordination, cortical organization and social behavior (Valenzuela et al., 2012). However, others have failed to find any impact of moderate PAE on cognition (O’Leary-Moore et al., 2006). Although measures of cortically-mediated cognition have been shown to be sensitive to high dose ethanol (EtOH) exposure during development in rodents (Riley et al., 1979, Thomas et al., 2004a, Thomas et al., 2004b) studies using spatial tasks to measure flexible behavior have had mixed results (Riley et al., 1979, O’Leary-Moore et al., 2006).
Here we show that clinically-relevant levels of PAE can impair cortically mediated behavioral flexibility using a hallmark task of executive control across species: reversal learning. Mice tested on a touch-screen based paradigm during adulthood showed a significant and specific increase in maladaptive perseverative responding on the task. To our knowledge, these are the first data to demonstrate that even moderate PAE can have long-lasting negative impact on executive control.
Materials and Methods
Prenatal Alcohol Exposure Model
Male and female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were used in a limited access PAE paradigm as previously described (Brady et al., 2012, Brady et al., 2013). Briefly, two hours into the dark cycle, female mice were given access to either 0.066% (w/v) saccharin or an ethanol solution (5% w/v for four days, then 10% w/v) sweetened with 0.066% (w/v) saccharin for four hours (from 1000 to 1400 hr). After one week of drinking 10% ethanol or the saccharin control solution, individual females were placed into the cage of a singly-housed male for two hours immediately following the drinking period. Females continued to consume ethanol and saccharin solutions throughout the five-day mating period. Pregnancy was positively determined by monitoring weight gain every 3–4 days. Access to alcohol was withdrawn beginning on post-natal day 0 using a step-down procedure over a 6-day period and offspring were weaned at approximately 23 days of age. We have shown this protocol reliably produces blood ethanol concentrations of 80–90 mg/dL at the end of the 4-hour drinking period (Brady et al., 2012, Brady et al., 2013). Offspring from 20 dams in successive breeding rounds were housed in groupings of 1–2 per cage in a temperature- and humidity- controlled vivarium under a reverse 12 h light/dark cycle (lights off 0800 h) and tested during the dark phase. All behavior was conducted on adult male and female offspring (1.7±.59 mice per litter, n=7–9 per sex/treatment; ~9 weeks at onset of testing, Fig. 1). All experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee.
Figure 1. Experimental timeline of training and behavioral testing.

After limited access voluntary PAE or SAC treatment mice were weaned and allowed to age to 6 weeks. The functional observational battery (FOB) was conducted immediately prior to food restriction and the three stage pre-training paradigm that acclimated mice to initiate and respond to visual stimuli. Next, discrimination learning was tested followed by reversal on the session immediately following criterion performance.
Functional observation battery
PAE and saccharin control mice were assessed for physical, neurological or gross behavioral abnormalities as previously described (Brigman et al., 2010b). Briefly, mice were individually placed in a bare empty home cage and observed over 60 sec for the presence of freezing, trembling, wild running, grooming, sniffing, licking, rearing, jumping, spontaneous seizure, defecation, urination, head bobbing, circling, abnormal gait, and retropulsion. Basic physical health was evaluated by examining for missing whiskers, bald patches, exophthalmus, straub tail, kinked tail, kyphosis, lordosis, body weight, and core body temperature. Simple sensory reflexes were measured via orienting responses to an approaching probe and to physical touch, and via palpebral closure on touch of the eye, twitch of the pinna on touch and an orienting response to tail pinch. Basic motor and neurological functions were assessed by observing the instance of splayed limbs, forepaw clutch and hind limb clutch when mice were tail suspended. Grip strength was measured by placing the mouse on a grid surface made of 2-mm diameter metal rods running lengthwise at 10-mm intervals. This was then slowly rotated and the latency of the mouse to fall was manually recorded (60-sec maximum) (Boyce-Rustay and Holmes, 2006). Observers were blinded to treatment conditions throughout the assessment.
Operant apparatus
Touch-screen discrimination and reversal learning were assessed as previously described (Brigman et al., 2010a). Briefly, operant behavior was conducted in a chamber measuring 21.6 × 17.8 × 12.7 cm (model # ENV-307W, Med Associates, St. Albans, VT) housed within a sound- and light-attenuating box (Med Associates, St. Albans, VT). The standard grid floor of the chamber was covered with a solid acrylic plate to facilitate ambulation. A pellet dispenser delivering 14 mg dustless pellets (#F05684, BioServ, Frenchtown, NJ) into a magazine, a house-light, tone generator and an ultra-sensitive lever was located at one end of the chamber. At the opposite end of the chamber there was a touch-sensitive screen (Conclusive Solutions, Sawbridgeworth, U.K.) covered by a black acrylic aperture plate allowing two 7.5 × 7.5 cm touch areas separated by 1 cm and located at a height of 0.8 cm from the floor of the chamber. Stimulus presentation in the response windows and touches were controlled and recorded by the KLimbic Software Package v1.20.2 (Conclusive Solutions, Sawbridgeworth, U.K.).
Pretraining
Mice were first slowly reduced and then maintained at 85% free-feeding body weight. Prior to testing, mice were acclimated to the 14 mg pellet food reward by provision of ~10 pellets/mouse in the home cage for 3–5 days. Mice were then habituated to the operant chamber and to eating out of the pellet magazine by being placed in the chamber for 30 min with pellets available in the magazine. Mice retrieving 10 pellets within 30 min were moved to a three-stage pre-training regimen. First, mice were able to obtain reward by pressing a lever within the chamber. Mice pressing and collecting 30 rewards in under 30 minutes were moved to touch training. Here, a lever press led to the presentation of a white (variously-shaped) stimulus in 1 of the 2 response windows (spatially pseudorandomized). The stimulus remained on the screen until a response was made. Touches in the blank response window had no response. Mice initiating, touching and retrieving 30 pellets within 30 min were moved to the final stage of pre-training. This stage was identical to touch-training except that responses at a blank window during stimulus presentation now produced a 15 sec timeout (signaled by illumination of the house light) to discourage indiscriminate screen responding. Errors on this stage were followed by correction trials in which the same stimulus and left/right position was presented until a correct response was made. Mice making ≥75% (excluding correction trials) of their responses at a stimulus-containing window over a 30-trial session were moved onto discrimination.
Discrimination and Reversal Learning
Following pre-training all mice were tested on a pairwise discrimination-reversal paradigm during daily sessions of a maximum of 60 minutes. For discrimination learning, 2 novel approximately equiluminescent stimuli were presented in a spatially pseudorandomized manner over 30-trial sessions (5 sec inter-trial interval). Responses at 1 stimulus resulted in reward; responses at the other stimulus resulted in a 15 sec timeout (signaled by illumination of the house light) and were followed by a correction trial. Designation of initially rewarded stimulus was randomized across treatment. Stimuli remained on screen until a response was made. As during pre-training, errors on first presentation trials were followed by correction trials which continued until a correct response was made or the session ended. Mice were trained to a criterion of ≥85% correct responding (excluding correction trials) over 2 consecutive sessions. Reversal training began on the session after discrimination criterion was attained. Here, the designation of stimuli as correct versus incorrect was reversed for each mouse. Mice were trained on 30-trial daily sessions (as for discrimination) to a criterion of ≥85% correct responding (excluding correction trials) over 2 consecutive sessions.
Statistical Analysis
The following dependent measures were taken during discrimination and reversal: total sessions, correct responses made, errors (=incorrect responses made), correction errors (=correction trials made) which are a putative measure of perseveration during reversal (Brigman et al., 2013), stimulus response (=time from trial initiation to touchscreen response) and reward response (=time from touchscreen response to reward retrieval). As correct and incorrect response measures were consistent on all analysis, incorrect responses are shown throughout. Discrimination performance was analyzed across all sessions required to reach criterion (Fig. 2A). In order to examine distinct phases of reversal (early perseverative and late learning) mediated by cortical and striatal subregions respectively, we separately analyzed errors and correction errors for sessions where performance was below 50% and performance from 50% to criterion, as previously described (Brigman et al., 2010a, Brigman et al., 2013) (Fig 3A). Main effects of sex, treatment (PAE vs. SAC) and interaction were compared for all measures using analysis of variance (ANOVA).
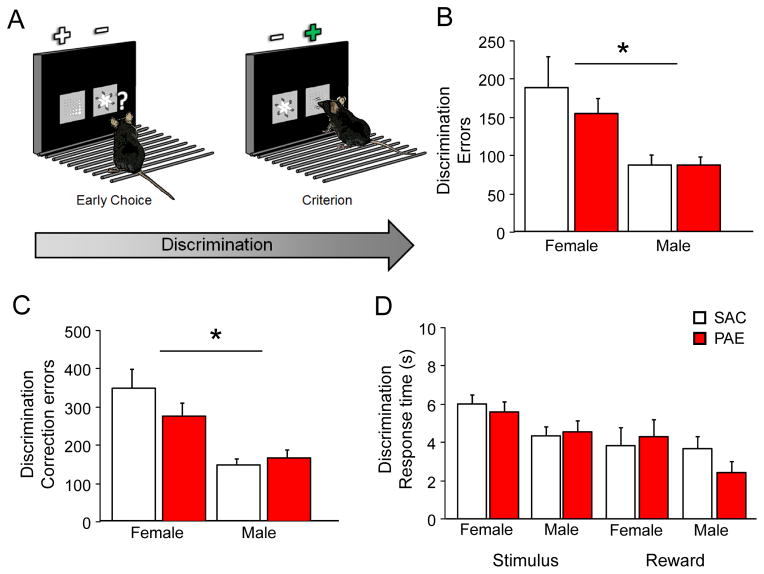
Figure 2. PAE does not alter associative learning, motor or motivation behaviors.
(A) After initially responding at approximately chance, mice learn to respond to a rewarded stimuli (+) and ignore the unrewarded (−) to high level of criterion (85% over 2 consecutive sessions). Female mice required significantly more errors (B) and correction errors (C) than males to reach discrimination criterion but did not significantly differ by treatment. (D) There were no significant differences between sex or treatment on stimulus response or reward response after correct choice across the problem. *=P<.01 main effect of sex.
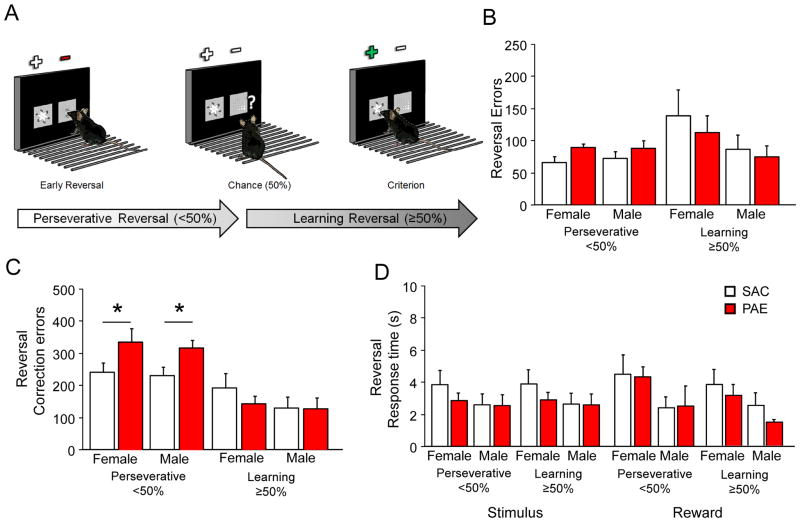
Figure 3. PAE specifically and significantly increases maladaptive perseveration during reversal learning.
(A) Mice show high levels of perseverative responding to the previously rewarded and now unrewarded stimulus (−) before re-attaining chance over several sessions (perseverative phase= sessions <50% correct). Over several sessions mice then learn to respond to the newly rewarded (+) stimuli to high level of criterion (learning phase= sessions ≥50% correct). (B) Female and male PAE and SAC controls did not make significantly different rates of errors during early perseverative or late learning phases of reversal. (C) Both female and male PAE mice made significantly more correction errors during early perseverative sessions, but not late learning reversal performance. (D) There were no significant differences between sex or treatment on stimulus or reward response times after correct choice during either phase of the reversal problem. *=P<.01 main effect of treatment.
Results
We found that the limited access paradigm yielded ethanol consumption levels in dams similar to those producing BACs of approximately 80–90 mg/dL (Brady et al., 2012, Brady et al., 2013). Offspring tested were taken from litters born to dams with an average consumption of 6.31±0.34 g of EtOH/kg of body weight/d. Our analysis of the functional observation battery revealed no obvious physical, neurological or gross behavioral abnormalities in PAE as compared to SAC control animals (Table 1). In correspondence with previously reported findings PAE mice also showed no significant differences in ad lib weight at time of testing versus saccharin control mice (Male SAC=24.2±1.0 Female SAC=21.6±0.8; Male PAE=25.1±1.2 Female PAE=22.2 ±0.7).
Table 1.
PAE mice were normal on measures of physical health, sensory reflexes, neurological functions, and empty cage behaviors compared to SAC controls. Data denote the percentage of animals showing a response unless specified otherwise in parenthesis.
| SAC | PAE | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Physical health | ||||
| Bald patches | 0 | 0 | 0 | 0 |
| Body weight (g) | 24.2 ± 1.0 | 21.6 ± 0.8 | 25.1 ± 1.2 | 22.2± 0.7 |
| Exophthalmus | 0 | 0 | 0 | 0 |
| Kinked tail | 0 | 0 | 0 | 0 |
| Kyphosis | 0 | 0 | 0 | 0 |
| Lordosis | 0 | 0 | 0 | 0 |
| Missing whiskers | 0 | 0 | 0 | 0 |
| Straub tail | 0 | 0 | 0 | 0 |
| Empty cage behaviors | ||||
| Abnormal gait | 0 | 0 | 0 | 0 |
| Circling | 0 | 0 | 0 | 0 |
| Defecation | 38 | 44 | 40 | 40 |
| Freezing | 0 | 0 | 0 | 0 |
| Head bobbing | 0 | 0 | 0 | 0 |
| Jumping | 0 | 0 | 0 | 0 |
| Licking | 0 | 0 | 0 | 0 |
| Rearing | 100 | 100 | 100 | 100 |
| Seizure | 0 | 0 | 0 | 0 |
| Sniffing | 100 | 100 | 100 | 100 |
| Trembling | 0 | 0 | 0 | 0 |
| Retropulsion | 0 | 0 | 0 | 0 |
| Urination | 0 | 0 | 0 | 10 |
| Wild running | 0 | 0 | 0 | 0 |
| Sensory reflexes | ||||
| Approach responses | 100 | 100 | 100 | 100 |
| Touch responses | 100 | 100 | 100 | 100 |
| Palpebral responses | 100 | 100 | 100 | 100 |
| Pinna reflex | 100 | 100 | 100 | 100 |
| Tail Pinch Reflex | 60 | 55 | 65 | 60 |
| Motor, neurological | ||||
| Forepaw clutch | 50 | 50 | 50 | 45 |
| Hanging Wire (sec) | 60.0 ± 0.0 | 54.2 ± 6.0 | 58.6 ± 2.0 | 50.8 ± 8.2 |
| Hindpaw clutch | 0 | 0 | 0 | 0 |
| Splayed limbs | 0 | 0 | 0 | 0 |
All mice were able to successfully complete the three-stage pre-training and no significant differences were seen by sex or treatment (Table 2). We next examined PAE and SAC performance on the pairwise visual discrimination task (Fig. 2A). There was a main effect of sex (F1,25=16.12, P<.001) but no main effect of treatment and no interaction (ANOVA: ns) on number of sessions to achieve discrimination criterion with females (20.2±2.5) requiring significantly more sessions than males (10.3±0.9) to learn the problem. Similarly, there was a significant main effect of sex on errors (F1,25=16.79, P<.001; Fig. 2B) and correction errors (F1,25=25.14, P<.001; Fig. 2C) to discrimination criterion with females making significantly more of both error types. No main effect of treatment and no significant interaction was seen on either errors or correction errors to attain criterion performance on the discrimination problem (ANOVA: ns). No main effects of sex, treatment or interactions were seen on motivation to respond to visual stimuli or work for food reward as measured by stimulus or reward response time respectively (Fig. 2D).
Table 2.
PAE male and female mice demonstrated normal motivation to retrieve food reward and performance across all three pre-training stages compared to SAC control mice (data= sessions to criterion ± SEM).
| SAC | PAE | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Stage 1 (Bar Press) | 7.62 ± 1.2 | 8.00 ± 0.6 | 8.90 ± 1.2 | 7.00 ± 1.5 |
| Stage 2 (Touch) | 3.75 ± 0.4 | 5.00 ± 1.5 | 4.43 ± 0.6 | 5.44 ± 0.7 |
| Stage 3 (Punish) | 1.12 ± 0.1 | 1.25 ± 0.3 | 1.42 ± 0.2 | 3.11 ± 0.9 |
Analysis of performance across both stages of reversal (early perseverative and later learning phases; Fig. 3A) revealed no significant main effect of sex or treatment on sessions, total errors, correction errors or response times (ANOVA: ns). However, analysis of reversal performance divided by phase revealed a profound perseverative impairment in PAE mice. Although PAE did not perform more sessions (PAE=5.8±0.5; SAC=5.05±1.3) during the early perseverative phase (sessions <50% correct), they made significantly more correction errors, or repeated incorrect responses after an initial error (F1,27=8.40, P<.01; Fig. 2C), but not initial errors (ANOVA: ns; Fig. 3B) during this stage. During the choice re-learning phase (sessions ≥50% correct) PAE performance was intact with no significant difference in sessions (PAE=11.4±1.9; SAC=12.6±2.0), total errors or correction errors between treatments (ANOVA: ns). Importantly, the significant increase in correction errors, a measure of maladaptive perseveration, during early reversal was not due to motivation to respond or retrieve reward as measured by stimulus and reward response times on either phase of the reversal stages (ANOVA: ns; Fig. 2D).
Discussion
Although there is increasing awareness of the detrimental effects of heavy alcohol intake during pregnancy, the effects of more moderate drinking are still controversial. The current results demonstrate that lower, but still clinically relevant, doses can lead to impairments in executive control that persist into adulthood. These higher-order mental processes, which include attention, working memory, future planning and behavioral flexibility are essential to succeed in a complex, constantly changing environment (Mattson et al., 1999, Green et al., 2009). Not surprisingly, impairments in executive control are associated with reduced quality of life due to negative impact on employment, managing finances and personal relationships (Royall et al., 2002). The specific impairment seen in the current study is particularly intriguing given evidence from the clinical literature that executive control is impaired in individuals with Fetal Alcohol Syndrome (McGee et al., 2008). Further, executive functioning has been shown to predict level of social skill, suggesting that alterations in these domains may underlie a wide range of deficits after PAE (Schonfeld et al., 2006).
Using a previously established PAE model, we found that dams drank to levels previously shown to induce BACs that correspond to a physiological relevant moderate dose (Driscoll et al., 1990, Brady et al., 2012). In agreement with previously published results in this model, PAE did not lead to gross morphological, motor or sensory alterations in offspring. Importantly, this moderate exposure paradigm has previously been shown not to alter dam-pup interactions, as measured by time-on-nest and pup retrieval time by dams (Brady et al., 2012). Additionally, adult PAE mice did not show alterations in ad lib weight or feeding behavior prior to, or after the cessation of behavioral testing that might significantly alter performance in an appetitive operant paradigm. This is consistent with previous findings that our PAE model does not significantly alter growth in the offspring as measured by pup weights at birth, PD 7, 14 and 23 (Brady et al., 2012). Our analysis of pairwise visual discrimination learning showed that female mice required more errors and correction errors to learn the problem than males, although all animals of both sexes were able to learn the problem to criterion at levels comparable to non-treated control strain performance (Izquierdo et al., 2006). The effect did not persist into either phase of the reversal, suggesting that sex differences in weight prior to training may have initially driven lower motivation to perform for food reward that increased as food reduction was adjusted for normal growth through the problem sequence. Despite the lack of interaction with PAE treatment, this sex difference is potentially important given the common practice of using combined groups of male and female mice in behavioral studies of cognition.
Previous studies using the mouse PAE model have demonstrated robust impairments in hippocampal plasticity and hippocampal dependent spatial learning behaviors (Brady et al., 2012, Brady et al., 2013). Here we found that PAE did not impact visual associative learning, either during discrimination or during the learning phase of reversal. While the hippocampus likely plays a role in the working memory necessary for visual discrimination performance, associative learning has been shown to be mediated by the dorsal striatum both during initial learning and later re-learning of the new association in late reversal (Featherstone and McDonald, 2004, Yin et al., 2004, Brigman et al., 2013). Although high-dose exposures in adult mice have been shown to prime associative processes, our PAE model did not alter discrimination learning, suggesting non-spatial tasks may be uniquely suited for examining flexible behavior after intact learning in these models (Depoy et al., 2013). In contrast, early perseverative reversal learning in rodents is mediated by cortical subregions, particularly the lateral orbitofrontal cortex (lOFC) (Schoenbaum et al., 2002, Chudasama and Robbins, 2003, Izquierdo et al., 2013). The lOFC has been hypothesized to be critically important in monitoring expected reward value and signaling when expectations are altered or violated (Rudebeck et al., 2013). We have recently shown that optimal early-reversal in the touch-screen paradigm specifically recruits lOFC in the mouse and that this region is functionally necessary for optimal behavioral flexibility on the task (Graybeal et al., 2011, Brigman et al., 2013). Together, the profile of intact discrimination learning and increased maladaptive perseveration shown here, suggest that PAE may be primarily altering cortical development, leading to a loss of top-down control of striatal-subregions. This hypofrontality in turn leads to continued responding to a previously learned cue even when it ceases to be beneficial, due to a failure to monitor expected outcomes and update reward contingencies as needed. This is consistent with previous findings in PAE models which have been shown to alter neocortical development, alter immediate early gene expression and decrease social behavior in the rat (Cuzon et al., 2008, Hamilton et al., 2010a, Hamilton et al., 2010b). Recent evidence in both rat and mouse PAE showing both alterations in neurochemistry and spatial flexibility further support the current findings and suggest that even moderate exposure can alter cortical function long-term (Allan et al., 2014, Hamilton et al., 2014).
In conclusion, our study provides the first evidence using a highly-translatable touchscreen learning paradigm that prenatal alcohol exposure can impair behavioral flexibility, a common measure cortically-mediated executive control. These data provide strong support for both the voluntary prenatal exposure model and operant behavioral measures for investigating cortical alterations after developmental insult. More importantly, given reports of executive control impairments in adolescents with FASD, the present data provide strong evidence that even a low amount of alcohol consumption during pregnancy may have detrimental effects on cognition that last well into adulthood.
Acknowledgments
We are very grateful to C. Fernando Valenzuela for comments on this manuscript. Supported by National Institutes of Health grants 1K22AA020303-01, P20-AA17068 and P50-AA022534-01.
References
- Allan AM, Goggin SL, Caldwell KK. Prenatal alcohol exposure modifies glucocorticoid receptor subcellular distribution in the medial prefrontal cortex and impairs frontal cortex-dependent learning. PLoS One. 2014;9:e96200. doi: 10.1371/journal.pone.0096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay B, Kesmodel US. Prenatal alcohol exposure - a systematic review of the effects on child motor function. Acta Obstet Gynecol Scand. 2011;90:210–226. doi: 10.1111/j.1600-0412.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res. 2012;36:457–466. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK. Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. J Neurosci. 2013;33:1062–1067. doi: 10.1523/JNEUROSCI.1217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nature Neuroscience. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or Genetic Inactivation of the Serotonin Transporter Improves Reversal Learning in Mice. Cereb Cortex. 2010a doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010b;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005a;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005b;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol. 1991;13:357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28:1854–1864. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26:1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- Depoy L, Daut R, Brigman JL, Macpherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a stimulus-response-based instrumental discrimination task, while sparing conditioned place preference learning. Neuroscience. 2004;124:23–31. doi: 10.1016/j.neuroscience.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nature neuroscience. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, Reynolds JN. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB) J Child Psychol Psychiatry. 2009;50:688–697. doi: 10.1111/j.1469-7610.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Green JH. Fetal Alcohol Spectrum Disorders: understanding the effects of prenatal alcohol exposure and supporting students. The Journal of school health. 2007;77:103–108. doi: 10.1111/j.1746-1561.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010a;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S, Savage DD. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res. 2014;269C:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav Brain Res. 2010b;214:66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. Bjog. 2007;114:243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Darling C, Manos N, Pozos H, Kim C, Ostrander S, Cazares V, Stepp H, Rudebeck PH. Basolateral amygdala lesions facilitate reward choices after negative feedback in rats. J Neurosci. 2013;33:4105–4109. doi: 10.1523/JNEUROSCI.4942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy, a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38:129–140. doi: 10.1093/ije/dyn230. [DOI] [PubMed] [Google Scholar]
- Kelly YJ, Sacker A, Gray R, Kelly J, Wolke D, Head J, Quigley MA. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66:41–48. doi: 10.1136/jech.2009.103002. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- McGee CL, Schonfeld AM, Roebuck-Spencer TM, Riley EP, Mattson SN. Children with heavy prenatal alcohol exposure demonstrate deficits on multiple measures of concept formation. Alcohol Clin Exp Res. 2008;32:1388–1397. doi: 10.1111/j.1530-0277.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RA, Diaz MR, Topper LA, Valenzuela CF. Construction of Vapor Chambers Used to Expose Mice to Alcohol During the Equivalent of all Three Trimesters of Human Development. J Vis Exp. 2014 doi: 10.3791/51839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, Hannigan JH. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38:99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- O’Leary CM, Bower C, Zubrick SR, Geelhoed E, Kurinczuk JJ, Nassar N. A new method of prenatal alcohol classification accounting for dose, pattern and timing of exposure: improving our ability to examine fetal effects from low to moderate alcohol. J Epidemiol Community Health. 2010;64:956–962. doi: 10.1136/jech.2009.091785. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR, Baldwin J. Response perseveration in rats exposed to alcohol prenatally. Pharmacol Biochem Behav. 1979;10:255–259. doi: 10.1016/0091-3057(79)90097-2. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, LaFrance WC, Jr, Coffey CE. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature neuroscience. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayal K, Heron J, Golding J, Emond A. Prenatal alcohol exposure and gender differences in childhood mental health problems: a longitudinal population-based study. Pediatrics. 2007;119:e426–434. doi: 10.1542/peds.2006-1840. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychol. 2006;12:439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, Janisse J, Martier S, Sokol RJ. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose-response effect. Pediatrics. 2001;108:E34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. Jama. 1991;265:1961–1967. [PubMed] [Google Scholar]
- Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004a;175:189–195. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004b;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Todorow M, Moore TE, Koren G. Investigating the effects of low to moderate levels of prenatal alcohol exposure on child behaviour: a critical review. J Popul Ther Clin Pharmacol. 2010;17:e323–330. [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends in neurosciences. 2012;35:284–292. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30:1051–1059. doi: 10.1111/j.1530-0277.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]