Abstract
Objectives
The optimal management of locally advanced and metastatic pulmonary carcinoid tumors remains to be determined.
Materials and methods
A retrospective review was conducted on patients with typical and atypical pulmonary carcinoid tumors treated at our institutions between 1990 and 2012.
Results
300 patients were identified with pulmonary carcinoid, (80 patients with atypical carcinoid), of whom 29 presented with metastatic disease (16 atypical). Of evaluable patients, 26 (41%) with stages I–III atypical carcinoid tumors recurred at a median time of 3.7 years (range, 0.4–32), compared to 3 (1%) patients with typical carcinoid (range, 8–12.3). 39 patients were treated with chemotherapy, including 30 patients with metastatic disease (27 atypical), and 7 patients were treated with adjuvant platinum–etoposide chemoradiation (6 atypical, 1 typical, 6 stage IIIA, 1 stage IIB). At a median follow-up of 2 years there were 2 recurrences in the 7 patients receiving adjuvant treatment. Median survival after diagnosis of metastatic disease for patients with atypical pulmonary carcinoid was 3.3 years with a 5-year survival of 24%. Treatment regimens showing efficacy in pulmonary carcinoid include 15 patients treated with octreotide-based therapies (10% response rate (RR), 70% disease control rate (DCR), 15 month median progression-free survival (PFS)), 13 patients treated with etoposide + platinum (23% RR, 69% DCR, 7 month median PFS), and 14 patients treated with temozolomide-based therapies (14% RR, 57% DCR, 10 month median PFS). 8 of 10 patients with octreotide-avid disease treated with an octreotide-based regimen experienced disease control (1 partial response, 7 stable disease) for a median of 18 months (range 6–72 months).
Conclusions
These results support our previous finding that a subset of pulmonary carcinoid tumors are responsive to chemotherapy.
1. Introduction
Patients with pulmonary carcinoid tumors comprise approximately 2% of all primary lung cancers [1], and the relative rarity of these tumors complicates efforts to effectively plan the therapy of patients with locally advanced or metastatic cancer. Pulmonary carcinoid tumors are defined by their typically bland cytomorphology and neuroendocrine features on histologic examination, and immunohistochemical staining for chromogranin, neural cell adhesion molecule (CD56), or synaptophysin [2]. A spectrum of pulmonary neuroendocrine tumors is thought to exist, with carcinoid tumorlets and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) representing less aggressive forms of the neuroendocrine spectrum, and small cell lung cancer the most malignant [2]. Typical carcinoid tumors behave in a more indolent fashion compared to atypical carcinoid tumors, which have a greater propensity for dissemination at the time of presentation and the subsequent development of metastasis. The pathologic criteria that distinguishes typical from atypical carcinoid tumors were proposed by Travis et al., in 1998, and has been widely adopted [3]. Typical carcinoids are distinguished from carcinoid tumorlets by a size larger than 0.5 cm, and have a mitotic rate of <2 mitoses/10 high power field (HPF) with no necrosis [2]. Atypical carcinoids, in contrast, have a mitotic rate of 2–10 mitoses/10 HPF or the presence of necrosis.
Patients with typical carcinoids have an excellent prognosis, with 5- and 10-year survival rates of 85% reported [4]. Atypical carcinoids have a more aggressive course, with 5- and 10-year survival rates in the range of 35–44%, due to a greater frequency of patients presenting with advanced stage and more frequent recurrence [4]. While metastatic disease is rarely reported for typical carcinoid tumors, atypical carcinoid tumors do metastasize in approximately 30–40% of reported cases, with the liver, bone, and brain being the most common sites [4]. Treatment of patients with typical carcinoids has centered on resection, and the vast majority of patients in case-series remain recurrence free for years, and even decades, with little information on systemic treatment [5], [6] and [7].
The more aggressive nature of atypical carcinoid tumors compared to typical carcinoid tumors prompted investigators to administer adjuvant chemotherapy for high-risk disease (i.e. stage IIB/IIIA) based on data showing a response rate of 20% to any chemotherapy [8], although this approach has been questioned by others who feel the role of systemic treatment is limited [9]. Regimens showing antitumor activity against pulmonary carcinoid tumors include octreotide [10], doxorubicin/capecitabine [11], everolimus + cisplatin [12], everolimus + octreotide [13], and etoposide + cisplatin [8]. The efficacy of adjuvant treatment for stages II and III resected typical and atypical carcinoid tumors is extrapolated from these response rates in metastatic disease, and the efficacy of adjuvant chemotherapy in resected stage II and III non-small cell lung cancer trials [14], [15] and [16]. While patients with pulmonary carcinoid tumors have been included in large-scale studies of neuroendocrine tumors [17], very little data exist on the role of chemotherapy in locally advanced typical or atypical metastatic pulmonary carcinoid tumors.
To further define the role of chemotherapy and adjuvant treatment of pulmonary carcinoid tumors, this retrospective study of patients with atypical and typical carcinoid tumors treated at our institutions between 1990 and 2012 was undertaken. This study updates our 2004 effort which demonstrated that pulmonary carcinoid tumors can respond to chemotherapy [8].
2. Materials and methods
This study was approved by the Dana-Farber/Harvard Cancer Center institutional review board. Patients with pulmonary carcinoid tumors were identified in the Brigham and Women's Hospital pathology database, and the Tumor Registry and radiation oncology databases of the Dana-Farber Cancer Institute using the search terms: pulmonary carcinoid, bronchial carcinoid, and lung carcinoid. Approximately 800 records from 1990 to 2012 were reviewed, and those that included (1) a diagnosis of typical or atypical pulmonary carcinoid, and (2) treatment with chemotherapy or surgery, are presented. Records were crosslinked to patients reported in our previous study [8], under which tumor samples that predated the 1999 update in the WHO criteria were re-examined. Patients with pathology reports that failed to mention the number of mitoses and/or the presence/absence of necrosis underwent further pathologic review by a pulmonary pathologist (LS) to classify the tumor as typical or atypical carcinoid. Using these criteria, 300 patients are included in our study.
Data from the medical records was reviewed and entered into a computer database by a single investigator (CRC), including gender, age, smoking status, presenting symptoms, date of diagnosis, stage, site of presentation, date of documented metastatic disease, treatment, response to treatment, time to progression, last time of follow-up, and date and cause of death. The duration of survival for early stage disease was determined from time of first diagnosis to the date of last follow-up or death as ascertained from the medical record or the Social Security Death Index. The duration of survival for patients with metastatic disease was determined from date of documentation of metastatic disease to date of last follow-up or death as ascertained from the medical record or the Social Security Death Index. The results of imaging studies (i.e. PET CT and/or octreotide scans) and specialized lab studies (i.e. serum chromogranin and urinary 5-HIAA) were included if studied and reported in the medical record. Disease control is defined as patients who achieve a complete/partial response or stable disease (minimum of 2 months) with treatment. The imaging studies were reviewed by a board-certified thoracic radiologist (MN) to determine response by Response Evaluation Criteria in Solid Tumors (RECIST) criteria if pretreatment and post-treatment scans were available. Progression for patients in whom pre/post-treatment scans were unavailable was determined based on notes in the medical record. Sites of metastatic disease were noted on scans after disease progression as documented in radiology reports.
3. Results
3.1. Patient characteristics
The characteristics of patients represented in this study are presented in Table 1. Patients with pulmonary carcinoid presented at a median age of ~60 years and were predominantly women. Of patients with atypical or typical carcinoid, 48% and 41% had a smoking history, respectively. The most common reason for diagnosis was an unusual finding on chest radiograph or CT performed for other reasons in a patient who did not have any pulmonary symptoms directly related to the tumor. The next most common symptomatic presentation for both typical and atypical carcinoids were cough and recurrent pneumonia. Many of the presenting symptoms were non-specific, and included chest/back/shoulder pain or dyspnea. Symptoms of carcinoid syndrome, which are commonly seen with GI carcinoids, such as flushing were observed in 4 patients with atypical pulmonary carcinoid (5%) and in no patients with typical pulmonary carcinoid. Pulmonary carcinoid has been associated with Cushing syndrome and multiple endocrine neoplasia [18] and [19]. Typical carcinoid was diagnosed during workup of these diseases in 7 patients.
Table 1.
Patient characteristics.
| Atypical (n = 80) | Typical (n = 220) | |
|---|---|---|
| Median age at diagnosis (range) | 61 years (21–82 years) | 60 years (16–85 years) |
| Male | 31 (39%) | 64 (29%) |
| Stage at diagnosis | ||
| IA | 29 (36%) | 148 (67%) |
| IB | 5 (6%) | 19 (9%) |
| IIA | 12 (15%) | 14 (6%) |
| IIB | 4 (5%) | 10 (5%) |
| IIIA | 14 (18%) | 13 (6%) |
| IIIB | 0 | 3 (1%) |
| IV | 16 (20%) | 13 (6%) |
| Lymph node involvement | ||
| N1 | 11 (14%) | 11 (5%) |
| N2 | 8 (10%) | 14 (6%) |
| Location of tumora | ||
| Left lower lobe | 21 (24%) | 55 (25%) |
| Left upper lobe | 11 (13%) | 40 (18%) |
| Right lower lobe | 19 (22%) | 51 (23%) |
| Right middle lobe | 24 (28%) | 51 (23%) |
| Right upper lobe | 9 (10%) | 43 (20%) |
| History of smoking | 41 (48%) | 91 (41%) |
| Reason for presentation (may overlap) | ||
| Asymptomatic – detected on imaging | 24 (28%) | 96 (44%) |
| Chest pain | 7 (8%) | 10 (5%) |
| Cough | 12 (14%) | 23 (10%) |
| Dyspnea | 8 (10%) | 8 (4%) |
| Flushing | 4 (5%) | 0 |
| Hemoptysis | 5 (6%) | 12 (5%) |
| Musculoskeletal pain (i.e. shoulder or back) | 6 (7%) | 5 (2%) |
| Recurrent pneumonia | 12 (14%) | 26 (12%) |
| Symptoms of metastatic disease (i.e. pathologic fracture, abdominal pain) | 5 (6%) | 0 |
| Upper respiratory tract infection | 0 | 10 (5%) |
| Wheezing/asthma symptoms | 0 | 7 (3%) |
Some patients had tumors in multiple lobes; percentages reported for evaluable patients.
3.2. Diagnostic evaluation
Carcinoid tumors can produce neuroendocrine secretory proteins such as chromogranin and metabolites such as 5-hydroxyindoleacetic acid (5-HIAA) that can enter the circulation and produce symptoms such as flushing, palpitations, and diarrhea. When tested, the chromogranin level was elevated in 20 of 33 patients (61%) with atypical carcinoid, compared to 11 of 32 patients (34%) with typical carcinoid. The urinary 5-HIAA level was elevated above the limit of normal (>8 mg/24 h) in 15 of 33 patients with atypical carcinoid (46%), and in none of the patients with typical carcinoid. Of patients with metastatic atypical carcinoid, the chromogranin level was elevated in 14 of 19 (74%) and the urinary 5-HIAA level was elevated in 12 of 19 (63%). Significantly, of the 4 patients with atypical carcinoid who presented with flushing, 3 had urinary 5-HIAA tested, and the value was elevated in all 3 patients (9, 9.5, 12 mg/24 h). Of the patients who presented with flushing, 3 had metastatic atypical carcinoid, and 2 died of this disease.
Of patients with atypical carcinoid tumors, 15 had the proliferation index measured by Ki67 or MIB-1 staining. The median proliferative index was 10% (range 3–35%). There were insufficient data to correlate the proliferative index with the presence/absence of necrosis, the mitoses per HPF, or disease course.
Considerable efforts are devoted to the imaging of pulmonary carcinoid tumors using PET-CT and labeling with a variety of ligands [20] and [21]. To further define the role of PET-CT and octreotide scintigraphy in the evaluation of pulmonary carcinoid tumors the imaging findings were reviewed when available for our patient cohorts. PET positive disease was defined as radiotracer uptake greater than background values, and this was seen in 28 of 30 patients with atypical carcinoid (93%) and in 40 of 58 (69%) of patients with typical carcinoid. All 17 patients with metastatic atypical carcinoid who had an FDG-avid primary lung lesion had FDG-avid metastatic lesions. Octreotide scans were positive in 9 of 13 patients with atypical carcinoid (69%) and in 13 of 15 patients with typical carcinoid (87%). Of the three patients with atypical pulmonary carcinoid who had both a PET-CT and an octreotide scan, tumor was FDG and octreotide avid in two patients.
3.3. Disease course
Most patients with pulmonary carcinoid were diagnosed at an early stage of disease (IA or IB), while a metastatic presentation was over 3-fold more common for atypical disease (20% vs. 6%) than typical carcinoid. The anatomic distribution of primary tumors was similar between atypical and typical carcinoids. As previously demonstrated in multiple case series, the risk of recurrence was higher for resected atypical compared to typical carcinoids, with 26 of 64 (41%) of resected atypical patients experiencing disease recurrence at a median time from surgery of 3.7 years (Fig. 1A, Table 2A). This was in contrast to 3 patients (1%) with resected typical carcinoid who experienced a recurrence at 8, 9, and 12 years. 5-year survival for atypical carcinoid from time of diagnosis was 95% for patients with stage I disease, 80% for patients with stage II disease, and 76% for patients with stage III disease (Fig. 1B).
Fig. 1.

(A) Time to recurrence for patients with atypical carcinoid tumors. Comparison of time to recurrence between atypical carcinoid patients (by stage) and typical carcinoid patients. p-Value for comparison of recurrence between atypical carcinoid (any stage) and typical carcinoid (any stage) = <0.001 (Log-Rank). (B) Percentage alive of patients with atypical tumors by stage at initial presentation as measured from date of initial diagnosis.
Table 2.
| A. Disease course in patients undergoing resection. | ||
|---|---|---|
| Atypical | Typical | |
| Recurrent disease | 26 (41%) | 3 (1%)a |
| Risk of recurrence by initial stage, median years to recurrence (range) | ||
| IA | 7 of 29 (24%), 3.7 (0.9–32) | 0 |
| IB | 4 of 5 (80%), 4.8 (2.9–5) | 0 |
| IIA | 6 of 12 (50%), 3.5 (1–6.4) | 0 |
| IIB | 1 of 4 (25%), 0.4 | 0 |
| IIIA | 8 of 14 (57%), 3.1 (1.2–11.6) | 1 (33%, 8) |
| IIIB | N/A | 0 |
| B. Sites of metastasis noted on imaging for patients either at presentation or recurrence. | ||
|---|---|---|
| Atypical (n = 34) |
Typical (n = 14) |
|
| Adrenal | 2 (6%) | – |
| Bone | 18 (53%) | 2 (14%) |
| Brain | 6 (18%) | – |
| Liver | 27 (79%) | 1 (7%) |
| Lung, contralateral | 1 (3%) | 11 (79%)a |
| Pancreas | 2 (6%) | – |
| Soft tissue | 3 (9%) | – |
| Spleen | 2 (6%) | – |
Initial stage at diagnosis unavailable for 2 patients.
Cannot exclude synchronous or metachronous tumors.
Metastases were observed in both typical and atypical carcinoid tumors. In cases where typical carcinoid tumors were observed in both lungs, it is unclear whether this represents true metastasis, enlarging tumorlets, or a tendency to form multiple primary pulmonary carcinoid tumors. Sites of involvement for patients with typical carcinoid tumors included the liver (pathologically confirmed in 1 patient) and lytic bone lesions (2 patients). No patient with metastatic typical carcinoid died during the duration of their observation. In contrast, 34 patients with atypical carcinoid experienced metastasis most commonly to the liver in 27 patients (79%) followed by the bone in 18 patients (53%), brain in 6 patients (18%), soft tissue in 3 patients (9%), and adrenal/spleen in 2 patients each (6%) (Table 2B). Once metastatic disease developed in patients with atypical carcinoid, a median survival of 3.3 years was observed (Fig. 2B).
Fig. 2.
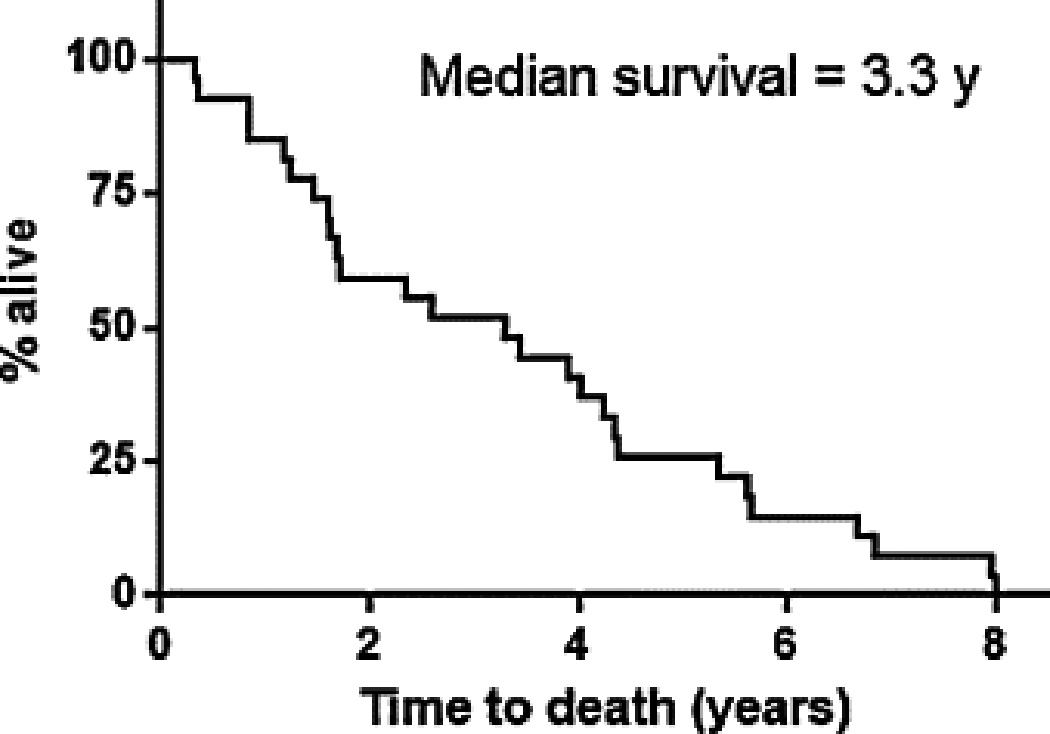
Percentage survival for atypical carcinoid after development of metastatic disease. Ten of 27 patients presented with metastatic disease.
3.4. Response to treatment
Thirty-nine patients were treated with 76 regimens, 35 of whom had atypical carcinoid (Table 3, Supplementary Table 1). Treatment regimens were grouped into 5 categories: etoposide-based (13 patients treated), temozolomide-based (14 patients), other cytotoxic treatments (8 patients), octreotide-based (15 patients), and clinical trials (7 patients). Treatment of atypical carcinoid with any cytotoxic regimen yielded a response in 7/37 regimens (19%, 2 partial responses in 4 neoadjuvant cytotoxic regimens, and 1 complete response and 4 partial responses in 33 regimens used in metastatic disease), a median progression free survival of 7 months, and disease control with 24/37 regimens (65%). Etoposide-platinum regimens are used given the efficacy of this therapy in small cell lung cancer, and previous data showing some efficacy in pulmonary carcinoid tumors [8]. Three of 13 patients (23%) responded to an etoposide-platinum regimen, with a median PFS of 7 months, and 9 of 13 (69%) patients achieved disease control. One patient with metastatic atypical carcinoid experienced a complete response to carboplatin + etoposide. Temozolomide-based treatments resulted in a response in 2 of 14 patients (14%), with a 10 month median PFS, and disease control in 8 of 14 patients (57%). Octreotide-based therapy resulted in a response in 2 of 15 patients treated with 20 regimens (10%), a median PFS of 15 months, and disease control in 14 patients (70%). Octreotide-based scans were performed in 10 of the 15 patients treated with octreotide-based regimens, and in all cases the scan showed octreotide-avid disease. Of patients with octreotide-avid disease, 7 showed either a partial response (1 patient) or stable disease (6 patients) to an octreotide-based regimen, while 2 patients had progressive disease, and one patient who had stable disease with octreotide developed progressive disease by RECIST criteria on pasireotide.
Table 3.
Response to treatment for patients with pulmonary carcinoid.
| Regimen | Response rate |
Disease control rate |
Median PFS (months, range) |
|---|---|---|---|
| Etoposide + platinum (n = 13) | 23% | 69% | 7 (3–13) |
| Temozolomide-based (n = 14) | 14% | 57% | 10 (6–29) |
| Other cytotoxic chemotherapy (n = 11)a | 20% | 70% | 4 (3–18) |
| Any cytotoxic chemotherapy (n = 38) | 18% | 63% | 7 (3–29) |
| First-line cytotoxic chemotherapy (n = 20)b | 20% | 70% | 8 (3–18) |
| Second-line cytotoxic chemotherapy (n = 10) | 20% | 70% | 5 (3–10) |
| Third-line cytotoxic chemotherapy (n = 6) | 16% | 50% | 5 (4–12) |
| Octreotide-based (n = 20)c | 10% | 70% | 15 (6–72) |
11 regimens, 8 patients, including 1 patient treated with neoadjuvant cytotoxic chemotherapy.
Includes 4 patients treated with neoadjuvant cytotoxic chemotherapy.
20 regimens, 15 patients.
Adjuvant treatment was pursued in 7 patients, 6 of whom had atypical carcinoid (Supplementary Table 2). At present, two patients developed metastatic recurrence at 2.1 and 4.6 years, while the other 5 are free of recurrent carcinoid at a median follow-up of 14 months (3–23 months). Neoadjuvant treatment was pursued in 4 patients with stage IIIA atypical carcinoid; all of these patients experienced recurrence and died of metastatic disease.
4. Discussion
This retrospective review was undertaken to further define the role of chemotherapy in the management of locally advanced or metastatic pulmonary carcinoid. Treatment with cytotoxic chemotherapy was associated with an 18% response rate in patients with atypical carcinoid, which is consistent with our previous finding in 2004 of a 22% response rate, also in patients with atypical carcinoid [8]. Only one patient with typical carcinoid was treated with any cytotoxic regimen (temozolomide) and achieved stable disease for 4 months. Three patients with typical carcinoid were treated with octreotide-based regimens and achieved stable disease for 11, 20, and 72 months.
Etoposide plus a platinum agent showed a response in 3 of 13 patients with atypical carcinoid (23%), with a complete response in one patient with metastatic disease. Given the activity of etoposide + platinum based regimens in the metastatic setting, and the high rate of recurrence for stage II and III disease (44% and 57%, respectively), this regimen has been used at times in combination with chest radiotherapy at our institution as an adjuvant treatment for patients with stage III disease. Two of seven patients treated with adjuvant chemo-radiotherapy developed metastatic disease, three patients with atypical disease remain recurrence-free after 17, 18, and 23 months of observation, and one developed an ALK mutant Ewing sarcoma at the completion of adjuvant treatment.
Temozolomide and octreotide-based regimens also showed antitumor activity. A response rate to temozolomide is noted in 2 patients (14%), a disease control rate in 8 patients (57%), and a median PFS of 10 months. This is similar to a recent retrospective report of 31 patients, 14 of whom had typical pulmonary carcinoid, which noted a response rate in 3 of 22 patients evaluable with RECIST (14%), stable disease in 11 patients (52%), a median PFS of 5.3 months, and a median overall survival of 23.2 months from the start of temozolomide treatment [22].
We report two patients treated with everolimus and either pasireotide or sorafenib; both patients achieved stable disease for 24 and 12 months, respectively. The RADIANT-2 trial studied the use of everolimus + octreotide vs. octreotide alone in patients with unresectable or metastatic low to intermediate grade neuroendocrine tumors and a history of carcinoid symptoms [17]. A subset analysis of the 44 patients with pulmonary carcinoid (75% typical) showed treatment with everolimus + octreotide resulted in a trend toward prolonged progression-free survival (13.6 vs. 5.6 months, p = 0.23, HR = 0.72, 95% CI = 0.31–1.68) compared to octreotide alone [13]. Given the indolent nature of typical carcinoid and the fact that only 2–5% of patients with pulmonary carcinoid have carcinoid-type symptoms, it is unclear how these results apply to the patients reported in our study.
Octreotide has been used to treat metastatic atypical carcinoid tumors, and we observed a 10% response rate, a 70% disease control rate, and a median PFS of 15 months in the 12 patients treated. Of patients with resected pulmonary atypical carcinoid presenting with carcinoid syndrome due to recurrence in the liver, octreotide treatment resulted in resolution of carcinoid symptoms and normalization of urinary 5-HIAA levels in 5 of 5 patients [23]. Octreotide treatment was associated with a partial response in 2 of 5 patients, and a complete response in 1 of 5 patients [23]. Treatment with octreotide resulted in control of carcinoid symptoms and a partial tumor response, both of which were maintained for 9 years in a case report of a patient with extensive (>70%) hepatic involvement by a recurrent “classic” type pulmonary carcinoid 12 years after resection [24]. There is a case report of a woman with a resected atypical pulmonary carcinoid treated with 6 cycles of adjuvant carboplatin, etoposide, and epirubicin followed by 10 years of adjuvant octreotide with no evidence of recurrent disease [10].
Other regimens with reported efficacy in pulmonary carcinoid include liposomal doxorubicin + capecitabine (case report of a single patient with typical carcinoid and a partial response) [11], everolimus + cisplatin (of 3 patients with metastatic pulmonary carcinoid, partial response was seen in 2 patients with atypical disease and 1 patient had stable disease) [12], and navitoclax [25].
This study confirms the widely noted observation that typical carcinoids behave in a much more indolent fashion than atypical carcinoid tumors. Metastatic disease for atypical carcinoid tumors was noted primarily in the bone [26], brain [27], and liver [23], which is consistent with previous reports. We note that for typical carcinoid the most common site of metastasis, when metastasis occurs at all, was the liver and bone, also consistent with previous case reports [26], [28] and [29].
Tumors in the contralateral lung were observed in 11 of our patients with typical carcinoid. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia has been noted in both lungs [30], and the bilateral typical pulmonary carcinoids we observe may represent progression of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia or carcinoid tumorlets. Patients with multiple typical bilateral pulmonary carcinoid tumors have been reported in the literature [31], [32], [33] and [34], have an excellent prognosis, and are managed with observation and resection of lesions that cause symptoms such as airway obstruction or hemoptysis.
There are several limitations to our study, such as the number of patients included, the retrospective nature, and lack of long-term follow-up for all patients. The heterogeneity in patients and crossover to clinical trials makes it difficult to directly compare treatment regimens. We were also unable to evaluate the response by RECIST in 25 of 65 courses of treatment (39%). There was also no prospective approach to the use of diagnostic tests (5-HIAA, chromogranin, PET-CT, and octreoscan) at our institution. Future studies may compare the sensitivity/specificity of the octreotide scan vs. PET-CT in evaluation of pulmonary carcinoid tumors, and whether pre-operative chromogranin or 5-HIAA levels may be prognostic in patients with resectable stage I-IIIA disease. The small number of patients receiving adjuvant treatment and the long duration of follow-up needed in this disease makes it difficult to draw conclusions on the impact this approach has in the survival of patients with resected disease.
In summary, these results support our previous finding that a subset of pulmonary carcinoid tumors are responsive to chemotherapy, with platinum + etoposide or temozolomide being the most frequently used effective regimens. The role of adjuvant systemic treatment for resected disease is an area of ongoing study. Larger studies will be necessary to compare the efficacy of treatment regimens to determine the optimal therapy for patients with metastatic disease.
Supplementary Material
Acknowledgements
This work was funded by a Conquer Cancer Foundation of ASCO Young Investigator Award, a postdoctoral fellowship, PF-14-020-01-CDD from the American Cancer Society, and a grant from Uniting Against Lung Cancer to C.R.C. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or the Conquer Cancer Foundation. M.N. was supported by 1K23CA157631 (National Cancer Institute). The authors express appreciation for a gift supporting the study to the Dana-Farber Cancer Institute from the Gallup Foundation.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest related to the work presented in this manuscript.
- Genzyme (post-market royalties for EGFR mutation testing).
- Consulting for Genentech, Pfizer, Chugai, Acceleron, Astra Zeneca, Millenium, Kew, Transgenomic, Veridex, and Teva.
References
- 1.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628–1638. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010;21(Suppl. 7):vii65–vii71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Yuste M, Matilla JM, Cueto A, Paniagua JM, Ramos G, Canizares MA, et al. Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur J Cardiothorac Surg. 2007;31:192–197. doi: 10.1016/j.ejcts.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Cao C, Yan TD, Kennedy C, Hendel N, Bannon PG, McCaughan BC. Bronchopulmonary carcinoid tumors: long-term outcomes after resection. Ann Thorac Surg. 2011;91:339–343. doi: 10.1016/j.athoracsur.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Sato T, Fujinaga T, Sakai H, Miyahara R, Bando T, et al. Surgical management of bronchopulmonary typical carcinoid tumors: an institutional experience. Interact Cardiovasc Thorac Surg. 2010;11:737–739. doi: 10.1510/icvts.2010.247361. [DOI] [PubMed] [Google Scholar]
- 7.Machuca TN, Cardoso PF, Camargo SM, Signori L, Andrade CF, Moreira AL, et al. Surgical treatment of bronchial carcinoid tumors: a single-center experience. Lung Cancer. 2010;70:158–162. doi: 10.1016/j.lungcan.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Wirth LJ, Carter MR, Janne PA, Johnson BE. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung Cancer. 2004;44:213–220. doi: 10.1016/j.lungcan.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Jett JR, Carr LL. Systemic treatment of advanced lung carcinoid tumors: show me the data! Chest. 2013;143:884–886. doi: 10.1378/chest.12-2455. [DOI] [PubMed] [Google Scholar]
- 10.Buonerba C, Gallo C, Di Lorenzo G, Romeo V, Marinelli A. Ten-year adjuvant treatment with somatostatin analogs in a patient with atypical carcinoid of the lung. Anticancer Drugs. 2010;21:465–468. doi: 10.1097/CAD.0b013e32833688a2. [DOI] [PubMed] [Google Scholar]
- 11.Masi G, Fornaro L, Cupini S, Loupakis F, Vasile E, Baldi GG, et al. Refractory neuroendocrine tumor-response to liposomal doxorubicin and capecitabine. Nat Rev Clin Oncol. 2009;6:670–674. doi: 10.1038/nrclinonc.2009.148. [DOI] [PubMed] [Google Scholar]
- 12.Fury MG, Sherman E, Haque S, Korte S, Lisa D, Shen R, et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;69:591–598. doi: 10.1007/s00280-011-1734-5. [DOI] [PubMed] [Google Scholar]
- 13.Fazio N, Granberg D, Grossman A, Saletan S, Klimovsky J, Panneerselvam A, et al. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest. 2013;143:955–962. doi: 10.1378/chest.12-1108. [DOI] [PubMed] [Google Scholar]
- 14.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 15.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 16.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association[ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 17.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Horsch D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 18.Florez JC, Shepard JA, Kradin RL. Case records of the Massachusetts General Hospital. Case 17-2013. A 56-year-old woman with poorly controlled diabetes mellitus and fatigue. N Engl J Med. 2013;368:2126–2136. doi: 10.1056/NEJMcpc1215971. [DOI] [PubMed] [Google Scholar]
- 19.Sachithanandan N, Harle RA, Burgess JR. Bronchopulmonary carcinoid in multiple endocrine neoplasia type 1. Cancer. 2005;103:509–515. doi: 10.1002/cncr.20825. [DOI] [PubMed] [Google Scholar]
- 20.Kruger S, Buck AK, Blumstein NM, Pauls S, Schelzig H, Kropf C, et al. Use of integrated FDG PET/CT imaging in pulmonary carcinoid tumours. J Intern Med. 2006;260:545–550. doi: 10.1111/j.1365-2796.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuyumcu S, Adalet I, Sanli Y, Turkmen C, Ozkan ZG, Yilmazbayhan D. Somatostatin receptor scintigraphy with 111In-octreotide in pulmonary carcinoid tumours correlated with pathological and 18FDG PET/CT findings. Ann Nucl Med. 2012;26:689–697. doi: 10.1007/s12149-012-0628-x. [DOI] [PubMed] [Google Scholar]
- 22.Crona J, Fanola I, Lindholm DP, Antonodimitrakis P, Oberg K, Eriksson B, et al. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology. 2013;98:151–155. doi: 10.1159/000354760. [DOI] [PubMed] [Google Scholar]
- 23.Filosso PL, Ruffini E, Oliaro A, Papalia E, Donati G, Rena O. Long-term survival of atypical bronchial carcinoids with liver metastases, treated with octreotide. Eur J Cardiothorac Surg. 2002;21:913–917. doi: 10.1016/s1010-7940(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 24.Corleto VD, Angeletti S, Schillaci O, Marignani M, Caratozzolo M, Panzuto F, et al. Long-term octreotide treatment of metastatic carcinoid tumor. Ann Oncol. 2000;11:491–493. doi: 10.1023/a:1008398431246. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merle-Vincent F, Chevrel G, Lombard-Bohas C, Felmann C, Meunier PJ. Bone metastases from bronchial carcinoid tumors. Two case-reports. Rev Rhum Engl Ed. 1999;66:46–48. [PubMed] [Google Scholar]
- 27.Kon T, Hara N, Su M, Takahashi H. Multiple brain metastasis of bronchial atypical carcinoid: unusual MR imaging, case report. No Shinkei Geka. 1997;25:815–818. [PubMed] [Google Scholar]
- 28.Canizares MA, Garcia-Fontan EM, Rivo JE, Gonzalez-Pineiro A. Local recurrence and metastatic disease in a typical N1 carcinoid bronchial tumour. Clin Transl Oncol. 2005;7:216–218. doi: 10.1007/BF02712820. [DOI] [PubMed] [Google Scholar]
- 29.Mokuno Y, Katoh T, Yoshida K, Kamiya S, Chigira H, Maeda M. Liver metastasis nineteen years after surgery for typical bronchial carcinoid. Hepatogastroenterology. 1999;46:2961–2964. [PubMed] [Google Scholar]
- 30.Nassar AA, Jaroszewski DE, Helmers RA, Colby TV, Patel BM, Mookadam F. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a systematic overview. Am J Respir Crit Care Med. 2011;184:8–16. doi: 10.1164/rccm.201010-1685PP. [DOI] [PubMed] [Google Scholar]
- 31.Akashiba T, Matsumoto K, Kosaka N, Saito O, Horie T, Nemoto N. Multifocal peripheral bronchial carcinoid tumour. Respirology. 1999;4:199–201. doi: 10.1046/j.1440-1843.1999.00175.x. [DOI] [PubMed] [Google Scholar]
- 32.Aubry MC, Thomas CF, Jr, Jett JR, Swensen SJ, Myers JL. Significance of multiple carcinoid tumors and tumorlets in surgical lung specimens: analysis of 28 patients. Chest. 2007;131:1635–1643. doi: 10.1378/chest.06-2788. [DOI] [PubMed] [Google Scholar]
- 33.Beshay M, Roth T, Stein R, Schmid RA. Synchronous bilateral typical pulmonary carcinoid tumors. Eur J Cardiothorac Surg. 2003;23:251–253. doi: 10.1016/s1010-7940(02)00747-9. [DOI] [PubMed] [Google Scholar]
- 34.Yazici U, Gulhan E, Agackiran Y, Tastepe I, Yaran P. Synchronous bilateral multiple typical pulmonary carcinoid tumors. Ann Thorac Surg. 2010;89:1278–1280. doi: 10.1016/j.athoracsur.2009.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


