Abstract
Concurrent use of mephedrone (4-methylmethcathinone) (MEPH) and established drugs of abuse is now commonplace, but knowledge about interactions between these drugs is sparse. The present study was designed to test the hypothesis that prior MEPH exposure enhances the locomotor-stimulant effects of cocaine and methamphetamine (METH). For cocaine experiments, rats pretreated with saline, cocaine (15 mg/kg), or MEPH (15 mg/kg) for 5 days were injected with cocaine after 10 days of drug absence. For METH experiments, rats pretreated with saline, METH (2 mg/kg), or MEPH (15 mg/kg) were injected with METH after 10 days of drug absence. Cocaine challenge produced greater locomotor activity following pretreatment with cocaine or MEPH than following pretreatment with saline. METH challenge produced greater locomotor activity following METH pretreatment than following saline pretreatment; however, locomotor activity in rats pretreated with MEPH or saline and then challenged with METH was not significantly different. The locomotor response to MEPH (15 mg/kg) was not significantly affected by pretreatment with cocaine (15 mg/kg) or METH (0.5, 2 mg/kg). The present demonstration that cocaine-induced locomotor activation is enhanced by prior MEPH exposure suggests that MEPH cross-sensitizes to cocaine and increases cocaine efficacy. Interestingly, MEPH cross-sensitization was not bi-directional and did not extend to METH, suggesting the phenomenon is sensitive to specific psychostimulants.
Keywords: mephedrone, cathinone, bath salt, cocaine, methamphetamine, sensitization, rat
Introduction
Mephedrone (MEPH) shares structural and pharmacological features with abused psychostimulants and is highly popular among recreational drug users, especially in the UK, where it was recently identified as the sixth most frequently used drug of abuse (Winstock et al., 2011). MEPH produces locomotor activation in rats but possesses a weaker locomotor stimulus than methamphetamine (METH) (Baumann et al., 2013; Huang et al., 2012). MEPH increases extracellular dopamine and serotonin (5-HT) in the rat nucleus accumbens, with greater augmentation of 5-HT (Baumann et al., 2012, 2013; Kehr et al., 2011). Consistent with its reported psychoactive properties, MEPH produces conditioned place preference (CPP) (Lisek et al., 2012), is self-administered (Hadlock et al., 2011; Aarde et al., 2013), and displays weak locomotor-sensitizing properties (Gregg et al., 2013) in laboratory animals. Polydrug abuse is commonly practiced among those taking MEPH with greater than 80% of MEPH users reporting the use of cocaine, amphetamine derivatives, alcohol, tobacco, and cannabis (Prosser and Nelson, 2012). The present study was designed to test the hypothesis that prior MEPH exposure enhances the locomotor-stimulant effects of cocaine and METH.
Methods
Subject
Male Sprague-Dawley rats (260–290 g) (Harlan Laboratories, Indianapolis, IN) were housed 2 per cage and maintained on a 12-hour light-dark cycle. Food and water were freely available. Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and Institutional Guidelines for the Care of Animals.
Procedure
Doses, dosing schedules, and experimental paradigms were based on our previous work (Gregg et al., 2013; Kovalevich et al., 2012). To investigate effects of prior MEPH exposure on cocaine efficacy, rats were injected for 5 days with saline, cocaine (15 mg/kg), or MEPH (15 mg/kg) and challenged with cocaine (15 mg/kg) 10 days later. To examine effects of prior cocaine exposure on MEPH efficacy, rats were injected for 5 days with cocaine (15 mg/kg) or saline were challenged with MEPH (15 mg/kg) 10 days later. MEPH/METH interactions were investigated in separate experiments. To investigate effects of prior MEPH exposure on METH efficacy, rats were injected with saline, METH (2 mg/kg), or MEPH (15 mg/kg) and challenged with METH (2 mg/kg) 10 days later. Effects of METH exposure on MEPH efficacy were investigated by injecting rats with saline or METH (0.5, 2 mg/kg) for 5 days and then challenging them with MEPH (15 mg/kg) 10 days later.
Locomotor activity was measured following psychostimulant challenge on the last day of injections. Rats were placed individually into activity chambers and allowed to acclimate for 60 min. Basal activity was then recorded for 30 min, followed by drug injection and recording of activity for at least 60 min. The Digiscan DMicro system measured ambulatory activity as consecutive beam breaks resulting from horizontal movement and non-ambulatory activity as repetitive-beam breaks (Lisek et al., 2012). Eight rats per group were used.
Drugs
Racemic MEPH was synthesized by Dr. Allen Reitz. Cocaine hydrochloride and METH were provided by NIDA. Drugs were dissolved in saline and injected intraperitoneally (ip).
Statistical analysis
Temporal data were analyzed with a two-way ANOVA (treatment, time) or Student’s t-test. Cumulative data were analyzed by a one-way ANOVA or Student’s t-test. Group differences were identified with a Bonferroni or Dunnett’s test. p < 0.05 was considered statistically significant.
Results
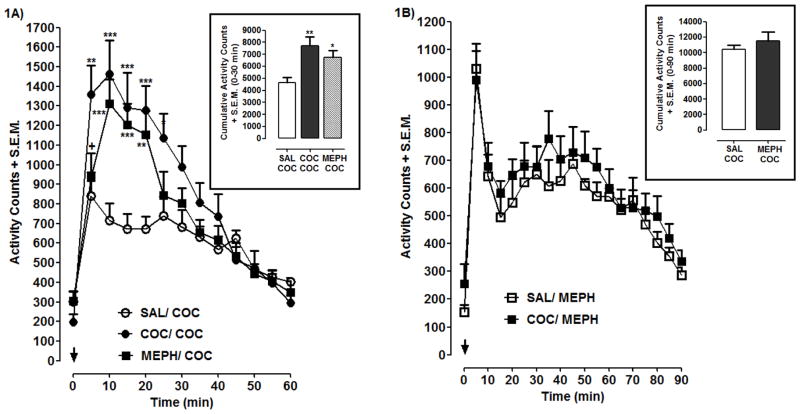
Effects of MEPH or cocaine exposure on cocaine-induced locomotor activity are shown in Fig. 1A. Significant main effects of treatment [F(2, 21) = 23.52, p < 0.001] and time [F(12, 273) = 32.67, p < 0.001] effects, and a significant interaction [F(24, 273) = 3.39, p < 0.001] were identified for temporal data. A significant main effect [F(2, 21) = 6.65, p < 0.01] was identified for cumulative data (30 min post-injection) (Fig. 1A, box). Post-hoc analysis of cumulative data indicated that cocaine produced greater locomotor activity in MEPH- (p < 0.05) or cocaine- (p < 0.01) exposed rats compared to previously drug-naïve rats; further, no difference in cocaine-induced locomotor activity was detected between rats previously treated with cocaine or MEPH (p > 0.05). Similar effects were observed for time-course data, in that MEPH- or cocaine-exposed rats displayed enhanced locomotor activation following cocaine challenge compared to saline-pretreated controls. Results of the converse situation (prior cocaine exposure on MEPH efficacy) are presented in Fig 1B. Aanalysis of temporal and cumulative data revealed that locomotor activity induced by MEPH challenge was not different in rats pretreated with saline or cocaine (p > 0.05).
Fig. 1. Effects of MEPH and cocaine exposure on locomotor activity.
1A) Rats pretreated with saline (SAL), cocaine (COC) (15 mg/kg) or MEPH (15 mg/kg) were challenged with COC (15 mg/kg). 1B) Rats pretreated with saline or COC (15 mg/kg) were challenged with MEPH (15 mg/kg). Temporal data are expressed as activity counts + S.E.M. following COC (1A) or MEPH (1B) injection (arrow). Cumulative data (box) are expressed as total activity counts following COC (0–30 min) or MEPH (0–90) injection. N=8 rats/group. ***p < 0.001, **p < 0.01 or *p < 0.05 compared to SAL/COC; +p < 0.05 compared to COC/COC.
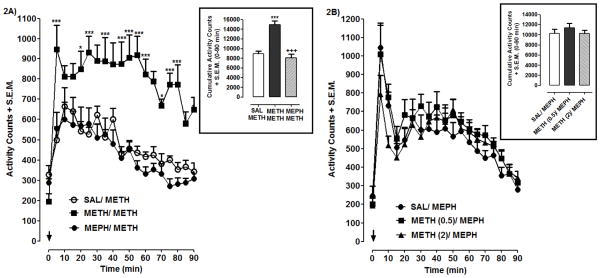
Effects of METH/MEPH interactions are shown in Fig. 2. For METH-induced hyperlocomotion (Fig. 2A), significant main effects of prior MEPH or METH treatment [F(2, 21) = 177.9, p < 0.001] and time [F(18, 399) = 9.85, p < 0.001] effects, and a significant interaction [F(36, 399) = 1.80, p < 0.01], were identified. For cumulative data (60 min post-injection) a significant main effect [F(2, 21) = 27.52, p < 0.001] was identified (Fig. 2A, box). Post-hoc analysis indicated that METH produced greater locomotor activity in previously METH-treated rats than in rats previously treated with MEPH or saline (p < 0.001). Locomotor activity induced by METH challenge was not different following pretreatment with MEPH or saline (p < 0.05). For the converse situation (prior METH exposure on MEPH efficacy) (Fig 2B), analysis of temporal and cumulative data indicated that locomotor activity induced by MEPH challenge (15 mg/kg) was not different following pretreatment with saline or METH (0.5, 2 mg/kg) (p > 0.05).
Fig. 2. Effects of MEPH and METH exposure on locomotor activity.
2A) Rats pretreated with saline (SAL), METH (2 mg/kg) or MEPH (15 mg/kg) were challenged with METH (2 mg/kg). 2B) Rats pretreated with saline or METH (0.5, 2 mg/kg) were challenged with MEPH (15 mg/kg). Temporal data are expressed as activity counts + S.E.M. following METH (2A) or MEPH (2B) injection (arrow). Cumulative data (box) are expressed as total activity counts following METH (0–90 min) or MEPH (0–90) injection. N=8 rats/group. ***p < 0.001, **p < 0.01 or *p < 0.05 compared to SAL/METH; +++p < 0.001 compared to METH/METH.
Discussion
Our study revealed that prior MEPH exposure enhances the locomotor-stimulant properties of cocaine and MEPH cross-sensitizes to cocaine. Behavioral cross-sensitization has been established between several psychostimulants, including cocaine and amphetamine (Brandon et al., 2001), methylphenidate and amphetamine (Itzhak et al., 2003), and methylphenidate and cocaine (Achat-Mendes et al., 2003). The mechanism underlying MEPH cross-sensitization with cocaine is unclear. A general explanation for two drugs that cross-sensitize is that they act through overlapping mechanisms, but the phenomenon, even for established psychostimulants, is poorly understood. Cocaine itself displays strong behavioral sensitizing properties (Pierce et al., 1996; Steketee and Kalivas, 2011) whereas MEPH possesses weaker motor-sensitizing properties, with preferential effects on stereotypical activity, that are nonetheless consistent across multiple doses, dosing schedules and contexts (Gregg et al., 2013). MEPH and cocaine enhance brain stimulation reward to similar extents (Robinson et al., 2012). In drug discrimination studies, MEPH fully substitutes for the discriminative stimulus effects of cocaine (Gatch et al., 2013), and cocaine partially substitutes for the discriminative stimulus effects of MEPH (Varner et al., 2013). One explanation for the locomotor cross-sensitization observed here is that cocaine and MEPH produce overlapping increases in extracellular dopamine but through separable mechanisms (Kehr et al., 2011); MEPH is a substrate of plasma membrane monoamine transporters and produces depolarizing currents at the dopamine transporter similar to dopamine-releasing agents (e.g. METH) whereas cocaine is a dopamine uptake blocker (Cameron et al., 2012). Additive, or synergistic, drug-drug interactions are often most pronounced in cases in which individual drugs administered in combination produce qualitatively similar effects but through distinguishable pharmacological mechanisms (Tallarida, 2012). Thus, in the present case, a synergistic increase in dopamine transmission following combined administration of a dopamine-releaser (MEPH) and dopamine-uptake blocker (cocaine) may have facilitated MEPH cross-sensitization with cocaine.
Counter to our hypothesis, MEPH and METH did not display cross sensitization. The reason for the lack of locomotor interaction is unknown, but it should be noted that the neuropharmacological profile of MEPH is often compared to that of MDMA (ecstasy), and that crossover effects of MDMA with cocaine are more consistent than with amphetamine derivatives (Modi et al., 2006). For example, MDMA pretreatment enhances behavioral responses to cocaine (Kalivas et al., 1998; Itzhak et al., 2003; Achat-Mendes et al., 2003; Fletcher et al., 2001; Morgan et al., 1997), while crossover between MDMA and amphetamines is more variable, with no effect (Modi et al., 2006; Cornish et al., 2003; Cole et al., 2003) and positive (Callaway and Geyer, 1992) effects reported. The lack of interaction, as discussed above, may be related to the fact that MEPH and METH are both dopamine transporter substrates, as opposed to the combination of MEPH and cocaine that consists of dopamine releaser and uptake blocker. Finally, the most parsimonious explanation may be that cross-sensitization studies are highly dependent on dose, dosing schedule, experimental paradigm (e.g. repeated exposure interval, withdrawal period, administration context, etc.), and species/strain. The identification of behavioral crossover, such as that observed between cocaine and MEPH, can indeed provide a foundation for studying underlying mechanism. Conversely, despite the use of relatively equi-effective doses of MEPH and METH, and testing of low and high doses of METH, the possibility that different doses or paradigms might have yielded a different outcome cannot be excluded.
In summary, our behavioral data indicate that MEPH cross-sensitizes to cocaine and that prior exposure to MEPH increases the locomotor effects of cocaine. Although these results suggest that MEPH interacts additively or synergistically with cocaine in vivo, more rigorous dose-combination studies directed toward specific endpoints (e.g. addiction, neurotoxicity, cardiotoxicity) are now required to better quantify the MEPH-cocaine interaction and determine if the interaction poses enhanced health risks to polydrug abusers that take cocaine and MEPH. For example, assays such as self-administration and conditioned place preference could be used to determine if the positive reinforcing efficacy of a combination of MEPH and cocaine is greater than that of either cocaine or MEPH by itself. Finally, because drug abusers often consume a cocktail of designer cathinones, experimental studies testing the impacts of combinations of synthetic cathinones, such as MEPH + methylone or MEPH + methylenedioxypyrovalerone (MDPV), are needed to better understand the clinical hazards of bath salts.
Acknowledgments
Funding Sources: National Institute on Drug Abuse grants DA025314, DA01342, and DA032718
Footnotes
Conflicts of Interest: None declared
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013 doi: 10.1111/adb.12038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Anderson KL, Itzhak Y. Methylphenidate and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropharmacology. 2003;45:106–115. doi: 10.1016/s0028-3908(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol. 2013;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine1B agonist. J Pharmacol Exp Ther. 1992;263:318–326. [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of ‘bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013 doi: 10.1007/s00213-013-2967-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR, O’Shea E, Marsden CA. Effects of MDMA exposure on the conditioned place preference produced by other drugs of abuse. Psychopharmacology (Berl) 2003;166:383–390. doi: 10.1007/s00213-002-1374-x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, McGregor IS. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol. 2003;482:339–341. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Robinson SR, Slippoy DL. Pre-exposure to (+/−)3,4-methylenedioxy-methamphetamine (MDMA) facilitates acquisition of intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2001;25:195–203. doi: 10.1016/S0893-133X(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013 doi: 10.1097/FBP.0b013e328364166d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R, Tallarida CS, Reitz A, McCurdy C, Rawls SM. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug and Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.06.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF, Achat CN, Anderson KL. Relevance of MDMA (“ecstasy”)-induced neurotoxicity to long-lasting psychomotor stimulation in mice. Psychopharmacology (Berl) 2003;166:241–248. doi: 10.1007/s00213-002-1320-y. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology. 1998;18:469–479. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Corley G, Yen W, Rawls SM, Langford D. Cocaine-induced loss of white matter proteins in the adult mouse nucleus accumbens is attenuated by administration of a β-lactam antibiotic during cocaine withdrawal. Am J Pathol. 2012;181:1921–1927. doi: 10.1016/j.ajpath.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi GM, Yang PB, Swann AC, Dafny N. Chronic exposure to MDMA (Ecstasy) elicits behavioral sensitization in rats but fails to induce cross-sensitization to other psychostimulants. Behav Brain Funct. 2006;2:1. doi: 10.1186/1744-9081-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AE, Horan B, Dewey SL, Ashby CR. Repeated administration of 3,4-methylenedioxymethamphetamine augments cocaine’s action on dopamine in the nucleus accumbens: a microdialysis study. Eur J Pharmacol. 1997;331:R1–3. doi: 10.1016/s0014-2999(97)01035-2. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res. 2012;234:76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berl) 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]