Abstract
Local delivery of lipid mediators has become a promising new approach for therapeutic angiogenesis and regenerative medicine. In this study, we investigated how gradient stimulation (either abluminal/distal or luminal/proximal) of engineered microvessels with sphingosine 1-phosphate (S1P) receptor-subtype-targeted molecules affects endothelial sprout growth using a microfluidic device. Our studies show that distal stimulation of microvessels with FTY720, an S1P1/3 selective agonist, promotes both arterial and venular sprout growth, whereas proximal stimulation does not. Using novel pharmacological antagonists of S1P receptor subtypes, we further show that S1P3 functionality is necessary for VEGF-induced sprouting, and confirmed these findings ex vivo using a murine aortic ring assay from S1P3-deficient mice. S1P3 agonist stimulation enhanced vascular stability in both cell types via upregulation of the interendothelial junction protein VE-cadherin. Lastly, S1P3 activation under flow promoted endothelial sprouting and branching while decreasing migratory cell fate in the microfluidic device. We used an in vivo murine dorsal skinfold window chamber model to confirm S1P3's role in neovascular branching. Together, these data suggest that a distal transendothelial gradient of S1P1/3-targeted drugs is an effective technique for both enhancing and stabilizing capillary morphogenesis in angiogenic applications.
Introduction
Angiogenesis, the formation of new blood vessels from existing ones, is central to many different diseases, disorders, and pathologies including cancer, peripheral arterial disease, and ischemic stroke. It is influenced by a variety of soluble biomolecules, including growth factors,1–4 matrix metalloproteinases,5,6 chemokines,7,8 and lipid mediators.9–11 As small molecules, lipid mediators, such as sphingosine 1-phosphate (S1P), are receiving increasing interest in recent years as tools for developing pro-angiogenic and immunomodulatory therapies in regenerative medicine, due to their relative stability and ease of use with regard to synthesis and delivery.12–16 S1P signals through five G protein-coupled receptors designated S1P1–5, which vary in their downstream signaling effects, including proliferation, migration, and differentiation.15,17 Thus, the ability to determine and target specific receptors responsible for angiogenic responses is critical for therapeutic applications. S1P1 and S1P3 are most heavily expressed in endothelial cells, while smooth muscle cells primarily express S1P3.11 S1P is critical in the regulation of sprout formation, stabilization, and vessel permeability,18–20 and numerous studies have shown that S1P works cooperatively with vascular endothelial growth factor (VEGF) to regulate endothelial sprout formation and stabilization, via VE-cadherin.18,21,22
Erythrocytes maintain a high S1P concentration in the bloodstream (up to 1 μM), and, thus, there is a sharp concentration gradient between the blood and the surrounding tissue.15,23 The interaction between S1P and known angiogenic growth factors has only recently become appreciated. When endothelial cells are stimulated with VEGF, VE-cadherin becomes phosphorylated and internalized via clatherin-coated pits, thereby increasing the permeability of the endothelial barrier.21,24 In contrast, S1P stimulation inhibits the VEGF-induced signaling and stabilizes VE-cadherin localization at interendothelial junctions.25 S1P1 and S1P3 stimulation independently promote VE-cadherin trafficking and adherens junction assembly via the non-Gi-dependent activation of the small GTPases Rac (through S1P1) and Rho (through S1P3).25 Although many have proposed a role for S1P in secondary or paracrine signaling between endothelial and mural cells,26,27 more recent studies suggest that its primary effects in the regulation of microvascular growth and remodeling are on endothelial cells themselves.25,28
In this work, we sought to study how gradient presentation of S1P receptor agonists affects endothelial cell morphogenesis. Using a microfluidic device, we sought to investigate how the directionality of S1PR agonist gradients and receptor subtype activation affect arterial and venular endothelial sprouting in a controlled microenvironment. Together, our results implicate that, independent of endothelial interaction with other blood or stromal cell types, the delivery method of S1P1/3 receptor-targeted drugs may be critical for improving angiogenesis both alone and in the presence of growth factors such as VEGF.
Materials and Methods
Microfluidic device fabrication and gel filling
A two-channel microfluidic device (Fig. 1A) cast into PDMS (Dow Corning, Washington, D.C.) on silicon wafers was used for all experiments. Device fabrication, surface modification, and measurements of gel regions are explained elsewhere.29–32 For gel filling, 2.5 mg/mL collagen I (pH 7.4) was prepared as described by Das et al.29 Twenty-four hours after gel filling, green fluorescent protein (GFP)-expressing human umbilical vein or aortic artery endothelial cells (HUVECs or HAAECs) (Angio-Proteomie, Boston, MA) were seeded in a single channel, hereafter referred to as the “cell channel.” Cells were allowed to form a monolayer on all sides of the cell channel, three of whose boundaries were the walls of the device and the fourth was the collagen I gel. Angiogenic behavior at the cell–gel interface is analogous to the blood vessel wall–tissue interface, and was, thus, the focus of our microfluidic studies.
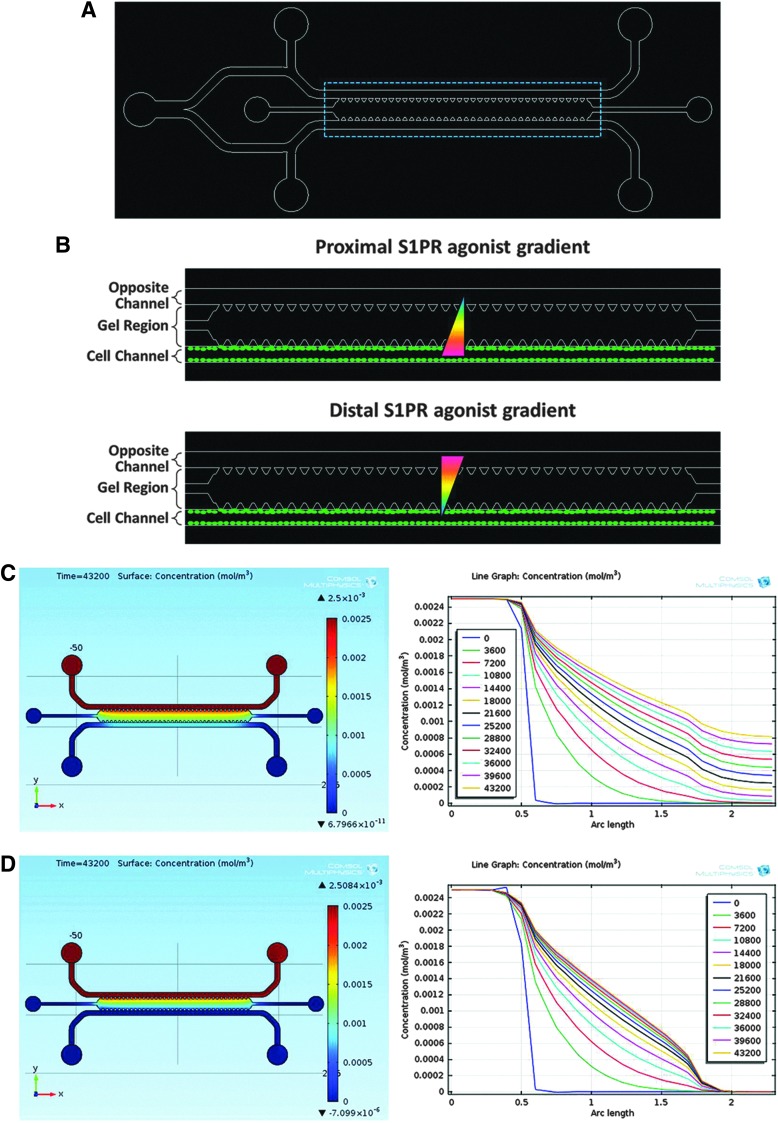
FIG. 1.
Experimental setup and S1PR agonist diffusion model. (A) Microfluidic device. (B) Schematic of outlined area in (A), showing proximal (top image) and distal (bottom image) sphingosine 1-phosphate (S1P) receptor agonist gradients (red, high concentration; blue, low concentration; green ovals, cells). (C) Computational model of static S1PR agonist diffusion profile at 12 h (left image) and concentration versus distance plot (right image) for t=0–12 h. (D) Diffusion profile of S1PR agonist at 12 h (left image) and concentration versus distance plot (right image) for t=0–12 h under 1 μL/min flow.
Computational modeling
The COMSOL Microfluidics Module (COMSOL Group, Stockholm, Sweden) was used to simulate molecular diffusion within the microfluidic device in order to analyze how often drug solutions needed to be replenished for maintenance of concentration gradients. Parameters of the microfluidic device, including geometry and mechanical properties of the collagen gel, were taken from Farahat et al.,30 where appropriate, and S1P parameters (e.g., molecular weight=379.47 g/mol) were used for sphingolipid-specific simulations. For static simulations, one channel was filled with 250 nM S1P solution and diffusion was allowed to take place across the collagen gel toward the other channel. For flow analysis, 1 μL/min unidirectional fluid flow was simulated in both channels, with one channel replenished with 250 nM solution and the other channel replenished with fluid only.
Capillary formation assay
All cell cultures were maintained as described elsewhere.29 Once confluent in 250 mL flasks, cells were seeded at a density of 2×106 per mL into devices; 1 h after seeding the cells in basal media (Angio-proteomie), the media was replenished to ensure that all unattached cells were washed away. This was recorded as time point 0. At 24 h, the growth media was replaced by conditioned media (40 ng/mL VEGF [Life Technologies, Carlsbad, CA] or 250 nM S1PR agonist or the combination of VEGF and S1PR agonist). Forty nanogram per milliliter VEGF was chosen based on studies by Das et al.29 and Farahat et al.30; 250 nM S1PR agonists (i.e., FTY720 or VPC01091) were chosen based on the concentration of S1P in studies by Farahat et al., which used this device to quantify overall cytosolic signal in the gel region.30 FTY720 was purchased from Cayman Chemical (Ann Arbor, MI). VPC01091 was generously provided by Dr. Kevin Lynch and Dr. Timothy Macdonald at the University of Virginia.
For static studies, media was changed every 12 h. For flow studies, a syringe pump (World Precision Instruments, Sarasota, FL) was used to push media at a constant flow rate of 1 μL/min through polyurethane tubing into the devices. For all such studies, flow in each channel had the same directionality.
Immunofluorescent staining
After 6 days in vitro, cells were fixed using 4% PFA for 30 min and then stained for extracellular VE-cadherin expression. Devices were rinsed thrice with wash buffer (5% bovine serum albumen [BSA] in 1× phosphate-buffered saline [PBS]) before incubating with block buffer (5% BSA+10% goat serum in 1× PBS) for 2 h at room temperature. Human VE-cadherin primary antibody (MAB9381; R&D Systems, Minneapolis, MN) was added at a concentration of 25 μg/mL in dilution buffer (1% BSA, 1% goat serum in 1×PBS), and devices were kept at 4°C overnight. After washing thrice (20 min each) with wash buffer, cells were incubated with the fluorescent-conjugated secondary antibody (NL008; R&D Systems) at a concentration of 1:100 in dilution buffer for 2 h at room temperature. Devices were rinsed thrice with 1×PBS and stored in the dark at 4°C until they were imaged.
Imaging and analysis
Cells were imaged at 2, 4, and 6 days after seeding using confocal microscopy (Zeiss, Oberkochen, Germany; Nikon EZ-C1 software, Melville, NY). All images shown are at 6 days postseeding, as determined to be an optimum time point to enable maximum sprout formation without the regression observed at later time points (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). This regression is due to the isotropic stiffness of the gel itself, and, thus, all groups show a noticeably similar decrease in sprout density after day 6. Longer time points may be achievable with solid posts or beads dispersed throughout the gel region, and will, therefore, be the focus of future studies. For experiments shown here, three devices were used for each condition. Six gel regions (each defined as the region between two posts) from a device were randomly chosen for each of the conditions. At each gel region, 40 z-stack images were taken at 20× magnification (each 5 μm apart) to observe sprouting in a 3D volume. Sprout density (i.e., number of sprouts per gel region) and number of individual cells migrating into the gel were calculated manually using ImageJ software package (National Institute of Health website, http://rsb.info.nih.gov/ij/). VE-cadherin expression was also quantified for cell monolayer and sprouts using ImageJ.
Mice
Animal experiments were performed using sterile techniques in accordance with an approved protocol from the University of Virginia Animal Care and Use Committee. All mice used were male and between 8 and 12 weeks and weighing between 18 and 25 g. Control mice were C57BL/6 (Harlan, Indianapolis, IN), hereafter referred to as “wild-type,” and S1P3−/− mice (a kind gift of Dr. Richard Proia [NIH]) were also on a C57BL/6 background.
Aortic ring assay
In a supine position, the thoracic cavity was opened with scissors, and the aorta was carefully lifted and excised from euthanized age-matched wild-type or S1P3−/− mice. The excised aorta was placed in cold sterile PBS. The periaortic fibroadipose tissue was carefully removed. With a sharp scalpel, the aorta was cut into rings (length ∼1 mm) and extensively rinsed in consecutive cold sterile PBS washes. The rings were individually embedded in wells of a 24-well plate containing a 300 μL synthetic basement membrane (Matrigel; BD Biosciences, San Jose, CA). The rings were incubated for 30 min at 37°C. Ten micromolar VEGF was applied to the wells, and the rings were incubated in 37°C incubators for 7 days. Imaging of the rings was taken on a daily basis using a Zeiss inverted microscope in order to observe growth. Vessel sprouting was traced using the paintbrush tool in Photoshop, and traced images were converted to 8-bit black and white images in ImageJ. Traced images were thresholded in ImageJ to reveal the sprouting vessels and to visualize the aortic rings more clearly.
Fabrication of drug-loaded PLAGA thin films
Poly-lactic-co-glycolic acid (PLAGA) thin films were fabricated using a solvent-casting technique and loaded with drug as described elsewhere.33 Briefly, FTY720 and VPC01091 were chosen as S1P receptor-targeted drugs. Films were loaded with a loading ratio of 1:200 (wt./wt., drug:PLAGA). For implantation in vivo, films with a diameter of 1 mm were extracted using a 1 mm biopsy punch (Acuderm, Inc., Ft. Lauderdale, FL), rinsed in 70% ethanol for ∼30 s, and then washed in sterile Ringer's solution for an additional 30 s (137.9 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.9 mM CaCl2, and 23 mM NaHCO3). Films had an average thickness of 517±41 μm, as measured with calipers (L.S. Starrett Co., Athol, MA).
Dorsal skinfold window chamber surgical procedure and intravital image acquisition
Mice were implanted with dorsal skinfold window chambers (APJ Trading Company, Inc., Ventura, CA) according to Sefcik et al.34 PLAGA thin films were implanted into the window chamber at 7 days postsurgical implantation, hereafter referred to as day 0. On day 0 and 3, the mouse was mounted to a microscope stage and imaged noninvasively using a 4× objective on an Axioskop 40 microscope (Zeiss). Images were captured using an Olympus MicroFire color digital camera and PictureFrame image acquisition software (Optronics, Goleta, CA).
Quantitative microvascular metrics
Intravital microscopy montages of entire vascular windows at day 0 and 3 were analyzed using a combination of Adobe Photoshop CS and ImageJ software packages for different treatment conditions. Circles with a diameter of 5 mm (or 2 mm concentric radius from outer edge of one film) were cropped around each film, with no overlap of the two circles. The number of venular branch points was quantified by marking a point of bifurcation on a blood vessel at days 0 and 3. This vessel branch was then followed, and bifurcations were marked. These new branches were also tracked and marked at points of bifurcations; thus, branch points three degrees of freedom away from the parent vessel were quantified. The number of branch points at day 3 was normalized to the number present at day 0.
Statistical analysis
Where appropriate, Student's t-test or a one-way ANOVA with Tukey's post hoc analysis was used to calculate the statistical significance of the different conditions on sprout metrics. Significance was asserted at α=0.05.
Results
Device characterization and diffusion of S1P receptor-targeted drugs
In order to study the effects of gradient directionality on endothelial cell fate in a microfluidic device (Fig. 1A), S1PR agonists were replenished either in the cell channel (i.e., proximally, Fig. 1B [top image]) or in the opposite channel (i.e., distally, Fig. 1B [bottom image]). When included in a study, VEGF was always replenished in the opposite channel. In order to evaluate the diffusion profile of S1PR agonists across the gel region, COMSOL computational software was used (Fig. 1C, D). Under static conditions, the concentration gradient at the cell barrier is maintained at 0.93±0.05 mol/m4 (mean±SEM) for the first 12 h after changing media, and by hour 12 has dropped to only 7.0% from the mean (Fig. 1C). Therefore, to maintain concentration gradients under static conditions, media was changed every 12 h. As expected under flow, the concentration gradient reaches and maintains a nearly linear diffusive profile (R2 ∼ 0.95) within 12 h of initiating flow (Fig. 1D). Note that VEGF diffusion in this microfluidic device was previously modeled by Farahat et al.30 Together, this model shows that concentration gradients can be established and maintained in the microfluidic device.
Proximal S1P3 antagonism inhibits VEGF-induced sprouting
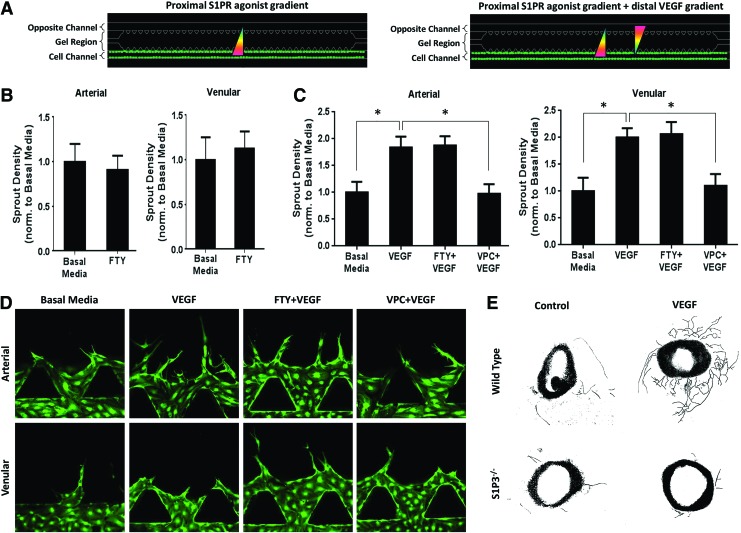
The gradient of S1P between the blood and tissues is important in maintaining the balance of cells between circulation and tissues.16 We wished to investigate how proximal S1P1/3 stimulation via FTY720 on arterial (HAAEC) and venular (HUVEC) endothelial cells affects the number of sprouts formed (Fig. 2A [left], 2B). We observed no significant difference in the number of arterial or venular sprouts under FTY720 stimulation compared with basal media (containing 5 ng/mL VEGF but no gradient of VEGF). We then looked at the effect of proximal S1PR agonist stimulation in the presence of VEGF (Fig. 2A [right], C, D). As expected, VEGF induced a significantly greater sprout density in both arterial (1.84±0.20-fold change) and venular (2.0±0.17-fold change) cells compared with basal media, and was not significantly different from the number of spouts formed in the FTY720+VEGF group. Notably, for both HAAECs and HUVECs, proximal S1P3 antagonism via VPC01091 significantly reduced VEGF-induced sprouting by 46.91% and 45.31%, respectively (Fig. 2C). The finding that S1P3 antagonism reduced endothelial sprouting even in the presence of VEGF was interesting and suggested that S1P3 activation is required for VEGF-induced sprouting. We recently showed that mice deficient of S1P3 in their tissues had impaired FTY720-induced vascular remodeling, in the presence of VEGF.16 In order to firmly establish a role for S1P3 in VEGF-induced sprouting, we performed an aortic ring assay. Briefly, mouse aortas were harvested and sectioned into 1 mm pieces, embedded in Matrigel, and treated with VEGF. After 7 days ex vivo, we observed that VEGF stimulation promoted greater sprout formation from aortic rings in wild-type mice than in those without VEGF (Fig. 2E). This sprouting was noticeably reduced in S1P3−/− mice. Together, these results demonstrate that S1P3 activity on endothelial cells is required for sprout formation in the presence of VEGF.
FIG. 2.
Proximal stimulation with S1P3 inhibitors decreases vascular endothelial growth factor (VEGF)-induced endothelial sprouting. (A) Schematic of proximal S1PR agonist stimulation without (left image) and with (right image) VEGF. (B) Sprout density, normalized to basal media control, for human aortic artery endothelial cells (HAAECs) and human umbilical vein endothelial cells (HUVECs) in the absence of VEGF. (C) Sprout density for HAAECs and HUVECs in the presence of VEGF, normalized to basal media. (D) Representative images of sprout density shown in (C). Magnification=20×. Green color indicates GFP. (E) Representative images of ex vivo aortic ring assay with vessel tracing. Aorta diameter ∼1 mm. Error bars represent standard error. *p<0.05. Color images available online at www.liebertpub.com/tea
Distal S1P1/3 stimulation promotes arterial and venular sprout formation
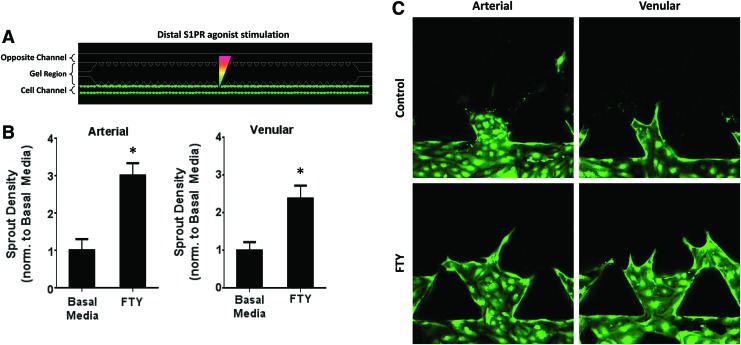
Previous work has shown that S1P1 and S1P3 activation enhances microvascular growth and remodeling by the delivery of FTY720 from polymeric thin films.16,33 We sought to examine whether distal S1PR agonist stimulation, representative of a spatial gradient of S1P, would have an effect on endothelial sprouting in the microfluidic device (Fig. 3A). In contrast to proximal S1PR stimulation, both HAAECs and HUVECs under distal S1P1/3 activation with FTY720 displayed a significantly greater number of sprouts (3.00±0.33- and 2.39±0.33-fold change, respectively) than those in basal media (Fig. 3B, C). Note that this increase in sprout density was observed even in the absence of VEGF, suggesting that FTY720 alone has the potential for therapeutic angiogenesis.
FIG. 3.
Distal S1P1/3 stimulation promotes sprout formation in arterial and venular endothelial cells. (A) Schematic of distal S1PR agonist stimulation in the absence of VEGF. (B) Sprout density of HAAECs and HUVECs under distal agonist stimulation, normalized to basal media. (C) Representative images of (B). Magnification=20×. Green color indicates green fluorescent protein (GFP). Error bars represent standard error. *p<0.05. Color images available online at www.liebertpub.com/tea
Distal S1P3 activation is required to increase sprout stability
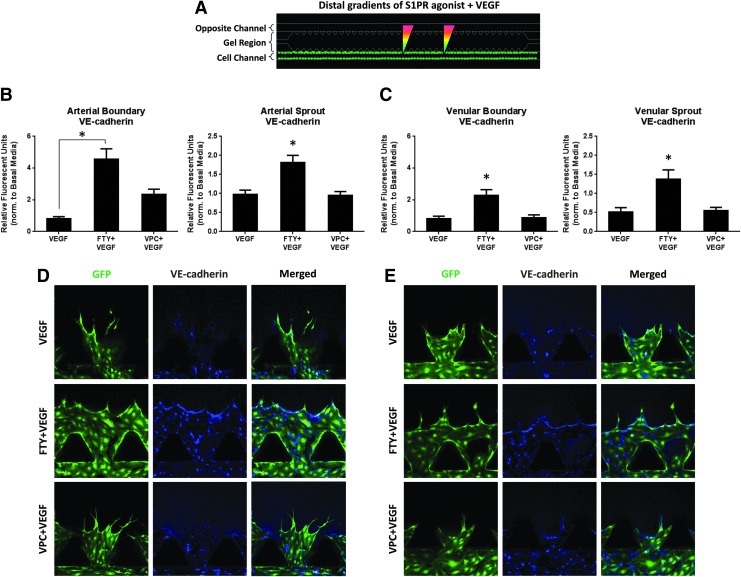
In order for endothelial cells to sprout into new vessels, cell-to-cell contacts need to be loosened.21,35 Given that distal S1P1/3 activation promotes sprout formation, we wished to investigate how S1PR-targeted drugs impact barrier stability between endothelial cells via VE-cadherin expression. Our findings agree with literature in that VE-cadherin expression is consistently reduced (17.83%±11.92% and −19.31%±16.22%) under VEGF stimulation compared with basal media within arterial and venular cell boundaries, respectively (Fig. 4B, C. Basal media has value of unity, bar not shown). Notably, for both HAAECs and HUVECs, S1P1/3 stimulation in the presence of VEGF significantly increased the expression of VE-cadherin in both the cell boundary (4.57±0.64 and 2.29±0.35 A.U., respectively) and the sprouts (1.82±0.18 and 1.45±0.24 A.U., respectively) compared with VEGF alone (0.82±0.12 and 0.81±0.16 for boundaries, 0.97±0.12 and 0.51±0.12 for sprouts, A.U.). For both arterial and venular sprouts, as well as for the venular boundary, FTY720 stimulation significantly increased VE-cadherin expression compared with VPC01091 stimulation (1.82±0.18 vs. 0.95±0.10, 1.45±0.24 vs. 0.55±0.08, 2.29±0.35 vs. 0.87±0.18 A.U., respectively). Although not significant, FTY720 promoted a 95.99% increase in VE-cadherin expression compared with VPC01091 in the cell boundary for arterial cells. Together, these results demonstrate that VEGF reduces VE-cadherin expression, whereas distal S1P1/3 stimulation significantly increases VE-cadherin expression throughout the endothelial barrier and sprouts for both arterial and venular cells, even in the presence of VEGF.
FIG. 4.
Distal S1P1/3 stimulation stabilizes vasculature via VE-cadherin. (A) Schematic of distal S1PR agonist stimulation in the presence of VEGF. (B) VE-cadherin expression (relative fluorescent units [RFU]) in the arterial cell boundary (left) and sprouts (right) per region, normalized to basal media (value of unity, bar not shown). (C) VE-cadherin expression (RFU) in the venular cell boundary (left) and sprouts (right) per region, normalized to basal media (value of unity, bar not shown). (D, E) Representative images showing GFP and VE-cadherin channels, as well as merged images for HAAECs (D) and HUVECs (E). Magnification=20×. Green color indicates GFP and blue color indicates VE-cadherin. Error bars represent standard error. *p<0.05. Color images available online at www.liebertpub.com/tea
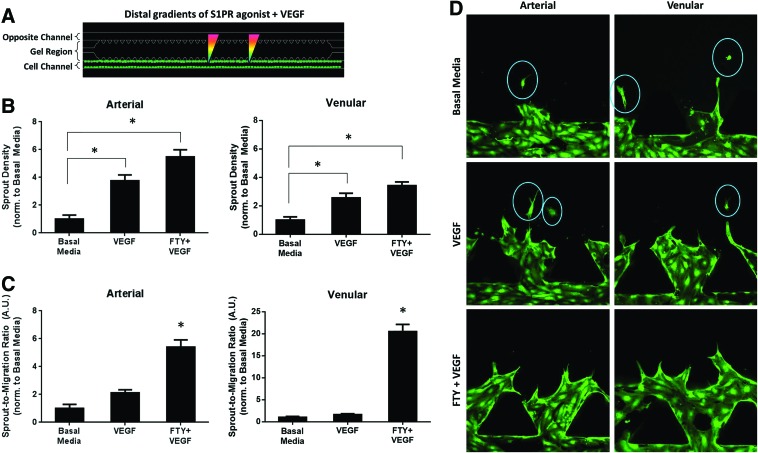
Distal S1P1/3 stimulation directs endothelial cell fate toward sprout formation, not migration
The ability to develop endothelial sprouts that maintain their multi-cell structure without disassembling into single cells is critical for functional microvascular remodeling. To that end, we sought to characterize arterial and venular development via sprouting and migration analysis (distal stimulation schematic shown in Fig. 5A). A migrating cell was defined as an individual cell that has moved into the gel region and is not attached to the cell boundary or existing sprouts (e.g., migrating cells circled in representative images, Fig. 5D). Since we have shown that S1P3 receptor subtype activation (i) is required for VEGF-induced sprouting and (ii) upregulates VE-cadherin junctions to a greater degree than VEGF downregulates them, we hypothesized that the combination of FTY720 and VEGF would promote the greatest sprouting compared with basal media or VEGF alone, and, thus, may have the greatest potential. As expected, both VEGF and FTY720+VEGF stimulation promoted a significantly greater number of sprouts (3.75±0.43- and 5.48±0.52-fold change, respectively) in arterial cells than in basal media (Fig. 5B). Notably, FTY720+VEGF promoted a 46.01% increase in arterial sprout density compared with VEGF. Similarly for venular cells, VEGF and FTY720+VEGF treatment induced significantly greater sprout density (2.56±0.33- and 3.42±0.27-fold change, respectively) compared with basal media (Fig. 5B). Importantly, FTY720+VEGF promoted a 25.19% increase in sprouting compared with VEGF alone.
FIG. 5.
Distal S1P1/3 stimulation promotes and stabilizes sprouting in endothelial cells. (A) Schematic of static distal S1PR agonist stimulation in the presence of VEGF. (B) Sprout density normalized to basal media under static distal agonist stimulation for arterial (left) and venular (right) endothelial cells. (C) Sprout-to-migration ratio (A.U.) for HAAECs (left) and HUVECs (right), normalized to basal media. (D) Representative images of sprouting and migrating cell data shown in (B, C), with migrating cells circled. Magnification=20×. Green color indicates GFP. Error bars represent standard error. *p<0.05. Color images available online at www.liebertpub.com/tea
However, high sprout count alone does not necessarily translate to stable angiogenesis. Thus, we normalized the sprout density data to the number of migrating cells per group. In agreement with our hypothesis, S1P1/3 stimulation in the presence of VEGF promoted the greatest sprout-to-migration ratio (5.41±0.51 arterial, 20.5±1.60 venular) compared with VEGF alone (2.10±0.24 arterial, 1.64±0.21 venular) and basal media (value of unity) (Fig. 5C). Together, these data suggest that distal S1P1/3 stimulation promotes and stabilizes VEGF-induced sprout formation in both arterial and venular endothelial cells.
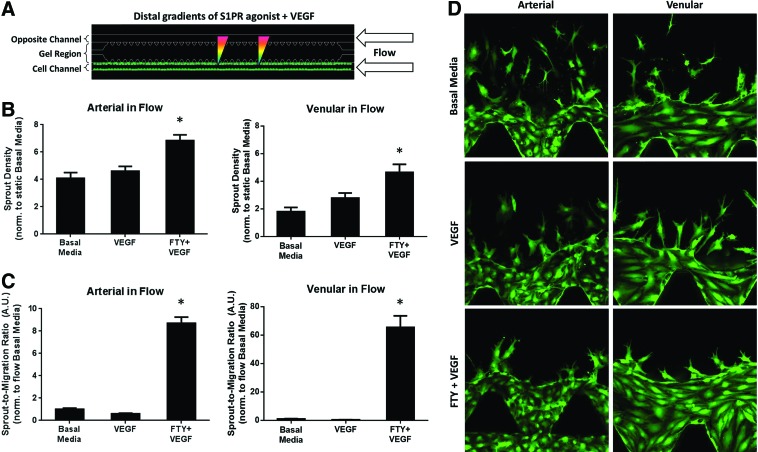
Distal S1P1/3 activation under flow promotes developed vasculature
Flow has been shown to induce vascular sprouting and remodeling.36,37 Under 1 μL/min flow (Fig. 6A), distal VEGF+FTY720 stimulation significantly enhanced sprout formation (6.83±0.43 arterial, 4.64±0.58 venular) compared with VEGF alone (4.61±0.35 arterial, 2.79±0.37 venular) and basal media (4.09±0.41 arterial, 1.79±0.31 venular) (Fig. 6B, values normalized to static basal media, bar not shown). Note that flow induced higher sprout formation in all groups than in the static basal media group, which agrees with findings in literature.38 A distal gradient of FTY720 was chosen here based on static studies described earlier, in which no increase in sprout density was observed under a proximal FTY720 gradient (Fig. 2). We also show that S1P1/3 stimulation, even in the presence of VEGF, significantly increases the ratio of sprouts-to-migrating cells (8.70±0.55 arterial, 65.38±8.17 venular) compared with VEGF alone (0.61±0.05 arterial, 0.44±0.06 venular) and basal media (value of unity) (Fig. 6C).
FIG. 6.
Distal S1P1/3 stimulation under flow promotes stable endothelial sprout formation. (A) Schematic of distal S1PR agonist stimulation in the presence of VEGF and 1 μL/min flow. (B) Sprout density for arterial (left) and venular (right) cells under flow, normalized to static basal media (value of unity, bar not shown). (C) Sprout-to-migration ratio (A.U.) for HAAECs (left) and HUVECs (right) under flow normalized to flow basal media. (D) Representative images of sprouting and migration data shown in (B, C). Magnification=20×. Green color indicates GFP. Error bars represent standard error. *p<0.05. Color images available online at www.liebertpub.com/tea
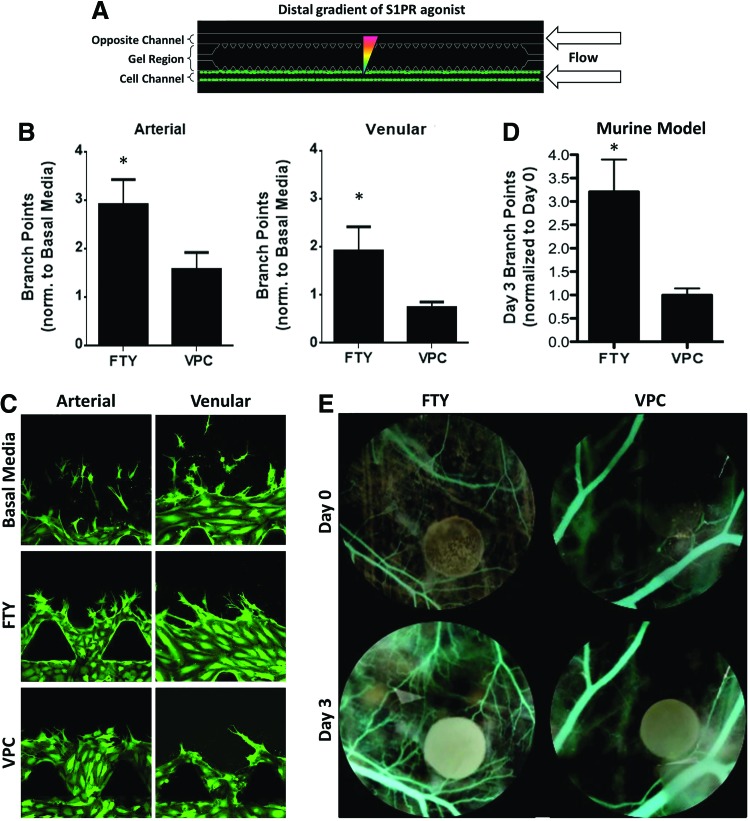
As a final metric, we quantified the number of branch points between distal FTY720 and VPC01091 treatments under flow (Fig. 7A). FTY720 and VPC01091 were chosen in order to compare microfluidic branching to that observed using an in vivo animal model; that is, since VEGF is a naturally occurring, endogenously expressed protein, we sought to observe vascular responses to the exogenous sphingolipids FTY720 and VPC01091. In the microfluidic device, distal S1P1/3 stimulation was found to promote a significantly greater degree of branching in arterial cells than S1P1 agonism/S1P3 antagonism (2.92±0.52 vs. 1.57±0.35 branches, normalized to basal media, value of unity) (Fig. 7B). Similarly, venular cells exhibited significantly higher branch points under FTY720 stimulation (1.92±0.51) than cells under VPC01091 stimulation (0.737±0.12). To compare these microfluidic findings under flow to a physiological environment, we used a murine dorsal skinfold window chamber model. We have previously shown that S1P receptor compounds can be delivered to microvascular networks in vivo, at concentrations that promote endothelial cell proliferation and migration, in a sustained fashion for as long as 7 days with PLAGA thin film encapsulation.34 Our in vivo data demonstrate that FTY720 stimulation produced a significantly greater number of sprout branches (3.21±0.69) than did VPC01091 (1.00±0.14) (Fig. 7D), in agreement with our microfluidic observations. Together, these results support our hypothesis that S1P3 stimulation is required to promote the most developed vasculature both in a microfluidic device under flow and within an in vivo mouse model.
FIG. 7.
Distal S1P1/3 agonism under flow promotes endothelial branching. (A) Schematic of distal S1PR agonist stimulation in the presence of 1 μL/min flow. (B) Number of branch points of HAAECs (left) and HUVECs (right) under flow in the microfluidic device, normalized to basal media (value of unity, bar not shown). (C) Representative images of branching data shown in (B). (D) Change in number of branch points within a dorsal skinfold window chamber model between 0 and 3 days post-implantation. (E) Representative pseudocolored images of (D) under FTY720 or VPC01091 stimulation. Circular film diameter=1 mm. Magnification=20×. Green color indicates GFP. Error bars represent standard error. *p<0.05. Color images available online at www.liebertpub.com/tea
Discussion
Creating and maintaining mature vascular networks is a critical need in many pathological contexts.39–41,42–46 The ability to establish and manipulate lipid gradients poses a promising new approach in therapeutic angiogenesis and regenerative medicine. The purpose of this study was to evaluate how the direction of S1P-targeted drug gradients alters endothelial sprout morphology using a microfluidic device, consisting of two parallel channels surrounding a central type-I collagen gel region, which enables control over gradient directionality between the channels as well as unidirectional fluid flow through the channels.29,30 The design also allows control of temporal, mechanical, and chemical cues within physiologically relevant length scales, and it establishes stable concentration gradients in a 3D context.
In the regulation of angiogenesis, not only is the absolute concentration of a signaling biomolecule important, but also the direction of its gradient with regard to blood vessels and the surrounding tissue.47–49 To study the effect of sphingolipid gradient on endothelial sprouting, we used a parallel-channel microfluidic device in which the channels are analogous to pre-existing blood vessels and the central collagen gel represents tissue. Although proximal S1P1/3 stimulation caused no increase in sprout density, distal S1P1/3 stimulation in all studies promoted sprout formation, even to a greater degree than VEGF. The effectiveness of a distal S1P gradient to induce sprouting has also been shown by Farahat et al., who used a microfluidic device seeded with human microvascular endothelial cells to demonstrate that S1P amplifies VEGF-induced sprouting.30 Clinically, a distal gradient of angiogenic therapeutics is more physiologically relevant in procedures such as biomaterial implants that require blood vessel ingrowth for functional vascular integration. Our data suggest that the high concentration of S1P found in the bloodstream under normal physiological conditions may serve to prevent unnecessary sprouting. Indeed, if proximal S1P receptor activation induced angiogenesis, one would expect hypersprouting to occur throughout the body, significantly altering cardiovascular stability and perfusion. It is also important to note that distal stimulation with FTY720 significantly enhanced sprout growth in the absence of VEGF, which suggests that S1P1/3-targeted drug therapies may induce angiogenesis while avoiding possible side effects of VEGF administration.50
For applications that require VEGF, our data suggest that specific sphingolipid receptors should also be functional. We first showed that S1P3 antagonism inhibits VEGF-induced sprouting in a microfluidic device (Fig. 2). The proximal gradient of S1P receptor-targeted drug (i.e., high concentration of VPC01091 in the cell channel) was chosen in order to maximally block S1P3, without the decrease in concentration that the cells would have experienced had a distal gradient been used. Indeed, the dependence of VEGF-induced sprouting on S1P3 was confirmed ex vivo using an aortic ring assay in which S1P3−/− aortic cross-sections showed noticeably less sprouting under VEGF stimulation than wild-type mice. Others have studied the possibility of cross-talk between VEGF and S1P or S1PR-targeted drugs.18,51,52 For example, Lee et al. were able to show not only that S1P administration enhanced VEGF-induced sprouting but also that antisense phosphorothioate oligonucleotide of S1P1 and S1P3 suppressed mature sprout formation in vivo.18 Although our data agree with findings in literature, further studies need to be done to elucidate the exact mechanisms of cross-talk between VEGF and S1P signaling with regard to angiogenesis.
It is understood that VEGF induces sprouting from pre-existing vasculature53; however, the stability of those newly formed vessels may be just as important as sprout growth for regenerative medicine applications. Here, we have shown that VE-cadherin expression in both arterial and venular cells is S1PR specific. VE-cadherin analysis was done on groups where VEGF was present, because VEGF has been shown to increase barrier permeability by destabilizing VE-cadherin junctions.21 For both the cell boundary and the sprouts, S1P1 agonism/S1P3 antagonism did not increase VE-cadherin expression for either cell type, suggesting that S1P3 activation is more important to VE-cadherin upregulation than S1P1 stimulation. This difference in downstream protein expression is plausible, because S1P1 and S1P3 have independent signaling cascades that lead to VE-cadherin regulation.26 It is important to note that S1P3 stimulation did not merely negate the VEGF-induced decrease in VE-cadherin expression, but significantly upregulated interendothelial junctions in both the cell boundary and the sprouts. Interestingly, S1P3-induced VE-cadherin expression in the cell boundary would be expected to decrease barrier permeability (thereby decreasing sprout formation), but the data suggest that S1P3 stimulation, instead, reduces the tendency of cells to migrate away from the cell boundary, even under a VEGF gradient. In addition, VE-cadherin junctions were upregulated in the sprouts, suggesting that S1P3 activation in the presence of VEGF creates more stable sprouts than VEGF alone. It is important to note that attenuated endothelial migration did not suppress capillary morphogenesis, as distal S1P1/3 stimulation in the presence of VEGF was still found to increase sprout formation. Thus, distal stimulation of S1P3-targeted drugs and VEGF may provide an ideal balance for increased sprout formation, enhanced barrier stability, and inhibition of migratory cell fate.
One limitation of microfluidic devices is that, although they provide a high level of spatiotemporal control over the microenvironment, the results of these systems may not translate well to tissue responses in vivo. To address this concern, we introduced 1 μL/min flow into the microfluidic device to simulate physiological blood flow and compared our findings with in vivo dorsal skinfold window chamber analysis. Our in vivo results confirm that S1P3 functionality is important for achieving maximal sprout branching. Sefcik et al. were also able to demonstrate that S1P3 stimulation is critical for increasing vascular branching in the dorsal skinfold window chamber model.33 In addition, the microfluidic data under flow reinforce our hypothesis that S1P1/3 stimulation promotes and stabilizes sprout formation. In fact, under flow, distal FTY720+VEGF treatment induces significantly more sprouts than VEGF alone, even to a greater extent than under static conditions. In addition, S1P1/3 stimulation under flow produced the greatest sprout-to-migration ratio in the presence of VEGF compared with VEGF alone, suggesting that even under flow the sprouts formed in response to a distal FTY720+VEGF gradient remain more stable than those under only a VEGF gradient. Based on these findings, we expect a drug delivery system that promotes a high degree of sprout formation while inhibiting individual cell migration to most effectively deliver oxygen and nutrients to surrounding tissue, and, thus, be a promising strategy in therapeutic angiogenesis.
Conclusion
We have shown that the gradient direction of S1PR-targeted drugs is critical for eliciting an angiogenic response from endothelial cells. Distal S1P3 activation was shown both in vitro and in vivo to be necessary for improved sprout formation and stabilization even in the absence of endocrine or mural cues. Together, these data suggest that S1P gradient and receptor subtype activation are important criteria to be considered when developing novel angiogenic therapies.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 DE019935 and NIH R01 AR056445. Master silicon wafer fabrication was done at the Stanford Microfluidics Foundry (Stanford University, 450 Serra Mall, Stanford, CA 94305). Special thanks are due to Carol Bampoe, BS, and Lauren Sefcik, PhD, for their assistance with the aortic ring assay and dorsal skinfold window chamber surgeries, respectively.
Disclosure Statement
No competing financial interests exist.
References
- 1.Roberts A.B., Sporn M.B., Assoian R.K., Smith J.M., Roche N.S., Wakefield L.M., Heine U.I., Liotta L.A., Falanga V., and Kehri J.H.Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A 83,4167, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J., and Klagsbrun M.Angiogenic factors. Science 235,442, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N., and Alitalo K.Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 5,1359, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., and Holash J.Vascular-specific growth factors and blood vessel formation. Nature 407,242, 2000 [DOI] [PubMed] [Google Scholar]
- 5.John A., and Tuszynski G.The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res 7,14, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Rundhaug J.E.Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9,267, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belperio J.A., Keane M.P., Arenberg D.A., Addison C.L., Ehlert J.E., Burdick M.D., and Strieter R.M.CXC chemokines in angiogenesis. J Leukoc Biol 68,1, 2000 [PubMed] [Google Scholar]
- 8.Salcedo R., and Oppenheim J.J.Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 10,359, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Fierro I.M., Kutok J.L., and Serham C.N.Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A4 and Lipoxin A4. J Parmacol Exp Ther 300,385, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Ogretmen B., and Hannun Y.A.Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4,604, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Takuwa Y., Du W., Qi X., Okamoto Y., Takuwa N., and Yoshioka K.Roles of sphingosine-1-phosphate signaling in angiogenesis. World J Biol Chem 1,298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takabe K., Paugh S.W., Milstien S., and Spiegel S.“Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60,181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen H., Gonzalez-Cabrera P., Marsolais D., Cahalan S., Don A.S., and Sanna M.G.Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol Rev 223,221, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Marsolais D., and Rosen H.Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeautic molecules. Nat Rev Drug Discov 8,297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiegel S., and Milstien S.The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 11,403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awojoodu A.O., Ogle M.E., Sefcik L.S., Bowers D.T., Martin K., Brayman K.L., Lynch K.R., Peirce-Cottler S.M., and Botchwey E.Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A 110,13785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kono M., Mi Y., Liu Y., Sasaki T., Allende M.L., Wu Y., Yamashita T., and Proia R.L.The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem 279,29367, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lee M.J., Thangada S., Claffey K.P., Ancellin N., Liu C.H., Kluk M., Sha'afi R.I., and Hla T.Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99,301, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Lee O.H., Kim Y.M., Lee Y.M., Moon E.J., Lee D.J., Kim J.H., Kim K.W., and Kwon Y.G.Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun 264,743, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Wada R., Yamashita T., Mi Y., Deng C.X., Hobson J.P., Rosenfeldt H.M., Nava V.E., Chae S.S., Lee M.J., Liu C.H., Hla T., Spiegel S., and Proia R.L.Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106,951, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esser S., Lampugnani M.G., Corada M., Dejana E., and Risau W.Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111,1853, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Fleming W.H.Endothelial cell-specific markers: going…going… gone. Blood 106,769, 2005 [Google Scholar]
- 23.Hänel P., Andréani P., and Gräler M.H.Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J 21,1202, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ngai C.Y., and Yao X.Vascular responses to shear stress: the involvement of mechanosensors in endothelial cells. Open Circ Vasc J 3,85, 2010 [Google Scholar]
- 25.Gaengel K., Niaudet C., Hagikura K., Laviña B., Muhl L., Hofmann J.J., Ebarasi L., Nyström S., Rymo S., Chen L.L., Pang M.F., Jin Y., Raschperger E., Roswall P., Schulte D., Benedito R., Larsson J., Hellström M., Fuxe J., Uhlén P., Adams R., Jakobsson L., Majumdar A., Vestweber D., Uv A., and Betsholtz C.The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 23,587, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Gaengel K., Genové G., Armulik A., and Betsholtz C.Endothelial-mural cell signaling in vascular development and angiogenesis. Aterioscler Thromb Vasc Biol 29,630, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Anelli V., Gault C.R., Snider A.J., and Obeid L.M.Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J 24,2727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkerson B.A., Grass G.D., Wing S.B., Argraves W.S., and Argraves K.M.S1P carrier-dependent regulation of endothelial barrier: HDL-S1P prolongs endothelial barrier enhancement as compared to albumin-S1P via effects on levels, trafficking and signaling of S1P1. J Biol Chem 287,44645, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das A.Lauffenburger, D., Asada, H., and Kamm, R. Determining cell fate transition probabilities to VEGF/Ang 1 levels: relating computational modeling to microfluidic angiogenesis studies. Cell Mol Bioeng 3,345, 2010 [Google Scholar]
- 30.Farahat W.A., Wood L.B., Zervantonakis I.K., Schor A., Ong S., Neal D., Kamm R.D., and Asada H.H.Ensemble analysis of angiogenic growth in three-dimensional microfluidic cell cultures. PLoS One 7,e37333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vickerman V., Blundo J., Chung S., and Kamm R.Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8,1468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das A., Lauffenburger D., Asada H., and Kamm R.D.A hybrid continuum-discrete modelling approach to predict and control angiogenesis: analysis of combinatorial growth factor and matrix effects on vessel-sprouting morphology. Philos Trans A Math Phys Eng Sci 368,2937, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Sefcik L.S., Aronin C.E., Awojoodu A.O., Shin S.J., Mac Gabhann F., MacDonald T.L., Wamhoff B.R., Lynch K.R., Peirce S.M., and Botchwey E.A.Selective activation of sphingosine 1-phosphate receptors 1 and 3 promotes local microvascular network growth. Tissue Eng Part A 17,617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sefcik L.S., Petrie Aronin C.E., Wieghaus K.A., and Botchwey E.A.Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials 29,2869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vestweber D.VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol 28,223, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Rissanen T.T., Korpisalo P., Markkanen J.E., Liimatainen T., Ordén M.R., Kholová I., de Goede A., Heikura T., Gröhn O.H., and Ylä-Herttuala S.Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation 112,3937, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Song J.W., and Munn L.L.Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A 108,15342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung B., Obinata H., Galvani S., Mendelson K., Ding B.S., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., and Hla T.Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell 23,600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krupinski J., Kaluza J., Kumar P., Kumar S., and Wang J.M.Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25,1794, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Wei L., Keogh C.L., Whitaker V.R., Theus M.H., and Yu S.P.Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology 12,47, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Arenillas J.F., Sobrino T., Castillo J., and Dávalos A.The role of angiogenesis in damage and recovery from ischemic stroke. Curr Treat Options Cardivasc Med 9,205, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Isner J.M., Walsh K., Symes J., Pieczek A., Takeshita S., Lowry J., Rossow S., Rosenfield K., Weir L., Brogi E., and Schainfeld R.Arterial gene therapy for therapeutic angiogenesis in patients with peripheral artery disease. Circulation 91,2687, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Collinson D.J., and Donnelly R.Therapeutic angiogenesis in peripheral artery disease: can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg 28,9, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Troidl K., and Schaper W.Arteriogenesis versus angiogenesis in peripheral artery disease. Diabetes Metab Res Rev 28,27, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Grochot-Przeczek A., Dulak J., and Jozkowicz A.Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene 525,222, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Frangogiannis N.G.Stromal cell-derived factor-1-mediated angiogenesis for peripheral arterial disease. Circulation 123,1267, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Knighton D.R., Silver I.A., and Hunt T.K.Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery 90,262, 1981 [PubMed] [Google Scholar]
- 48.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., and Betsholtz C.VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161,1163, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schipani E., Wu C., Rankin E.B., and Giaccia A.J.Regulation of bone marrow angiogenesis by osteoblasts during bone development and homeostasis. Front Endocrinol (Lausanne) 4,85, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eskens F.A., and Verweij J.The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGR) targeting angiogenesis inhibitors; a review. Eur J Cancer 42,3127, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Licht T., Tsirulnikov L., Reuveni H., Yarnitzky T., and Ben-Sasson S.A.Induction of pro-angiogenic signaling by a synthetic peptide derived from the second intracellular loop of S1P3 (EDG3). Blood 102,2099, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Carmeliet P., and Jain R.K.Molecular mechanisms and clinical applications of angiogenesis. Nature 473,298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrne A.M., Bouchier-Hayes D.J., and Harmey J.H.Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Med 9,777, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.