Abstract
Objectives
To examine the association of individual income and end of life (EOL) care in older cancer decedents in Taiwan.
Design
Retrospective cohort study.
Setting
National Health Insurance Research Database (NHIRD) in Taiwan.
Participants
28,978 decedents >65 years were diagnosed with cancer and died during 2009-2011 in Taiwan. Of these decedents, 10941, 16535, and 1502 were categorized by individual income as having low, moderate, and high SES, respectively.
Main outcome measures
Indicators of aggressiveness of EOL care: chemotherapy use before EOL, more than one emergency department (ER) visit, more than one hospital admission, hospital length of stay >14 days, intensive care unit (ICU) admission, and dying in a hospital.
Results
Low individual income was associated with more aggressive EOL treatment (estimate -0.30 for moderate income, -0.27 for high income, both p<0.01). The major source of aggressiveness was the tendency for older decedents with low income to die in the acute care hospital. The indicators had an increasing trend from 2009 to 2011, except for hospital stay >14 days.
Conclusions
Low individual income is associated with more aggressive EOL treatment in older cancer decedents. Public health providers should make available appropriate education and hospice resources to these decedents and their families, to reduce the amount of aggressive terminal care such decedents receive.
INTRODUCTION
Cancer has been the leading cause of death in Taiwan for decades [1]. Decedents older than 65 years account for 47.1% of new cancer cases, and 59.8% of cancer deaths [2]. Globally, an estimated 12.7 million new cancer cases and 7.6 million cancer deaths occurred in 2008 [3]. End-of-life (EOL) care is an issue in terminal decedents with cancer, with more aggressive care requiring greater healthcare spending in Taiwan over the last decade [4, 5]. In the United States, treatment for decedents in their last year of life accounted for more than one-quarter of Medicare spending [6]. In Canada, decedents in the final six months of life comprised 1.1% of the population but consumed 21.3% of health care [7]. Thus, evaluating the aggressiveness of EOL care in terminal cancer decedents and defining the determinants of such overuse of care are important for older Taiwanese with cancer, both medically and financially.
Data do not agree about the impact of socioeconomic status (SES) on aggressiveness of EOL care. Some studies show weak negative trends between EOL spending and area level income [8, 9]. Others show a positive association of higher SES with EOL spending [10–12].
Earle et al. has developed a set of indicators to evaluate aggressiveness of EOL care using administrative data [13]. Using the National Health Insurance Research Database (NHIRD), this study explored the association of indictors for aggressive EOL care with SES for older cancer decedents in Taiwan.
METHODS
Study Design and Sample
Database. The data for this study were collected from the Taiwan NHIRD for the years 2009 to 2011. This dataset is organized and managed by the Taiwan National Health Research Institutes but collected by the Taiwan National Health Insurance Program, in place since 1995. Taiwan’s NHI has the unique characteristics of universal insurance coverage, comprehensive services provided, and a single-payer system with the government as sole insurer. Patients have free access to any healthcare facilities they choose. Healthcare systems are reimbursed from Taiwan’s National Health Insurance Administration Ministry of Health and Welfare for services they provided. The program covers approximately 99% of the residents in Taiwan and has contracts with 97% of medical providers nationally. To verify the accuracy of diagnosis, the Taiwan Bureau of National Health Insurance randomly reviews the charts of one per 100 ambulatory and one per 20 inpatient claims[14]. All patient data were reviewed retrospectively.
Our study cohort consisted of older adult decedents (age > 65 years) with cancer as identified by the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]. Diagnosis was verified by using the catastrophic illness dataset. Decedents also had a record of death during the study period (2009–2011).
Measurement
Aggressiveness of EOL care. This study measured the aggressiveness of EOL care as a dependent variable using the following six quality indicators in the last month of life suggested by Earle et al. [13]: chemotherapy use before EOL, more than one emergency department (ER) visit, more than one hospital admission, hospital length of stay >14 days, intensive care unit (ICU) admission, and dying in a hospital. These data were collected from the NHIRD dataset within one month of death. Aggressiveness of EOL care was evaluated by assigning each patient a composite score which was the summation of all indicators. This composite score ranged from 0 to 6, with higher scores indicating more aggressive EOL care [15].
Individual SES. The four-factor Hollingshead scale uses marital status, gender, education and occupation [16]. Because other factors, such as marital status, and education level can’t be extracted from the NHIRD, this study used income-related insurance payment amount as a proxy measure of individual SES, which is an important prognostic factor for cancer [17, 18]. This method had been validated in several studies [19, 20]. The older decedents with cancer diagnosis were classified into three groups: (1) low SES, lower than US $528 per month (New Taiwan Dollars (NT) $1 to $15,840), (2) moderate SES, between US$528 to $833 per month (NT $15,841 to $25,000), and (3) high SES, US$833 per month (NT $25,001) or more [17]. We selected NT$15,840 as the low income level cutoff point because this was the government stipulated minimum wage for full-time employees in Taiwan in 2006.
Patient characteristics. Patient characteristics were recorded, including age, gender, urbanization level, geographic region, disease severity, post-diagnosis survival duration, cancer diagnosis, and primary physician’s specialty. Disease severity was estimated by using the Deyo adaptation of the Charlson Comorbidities Index Score (CCIS), which was derived from inpatient diagnoses in the last six months of life [21, 22]. Diagnosis and metastatic status were combined to identify seven subgroups (I-VII) of cancers that were homogeneous in terms of survival and disease course [15]. Metastatic status was identified by using ICD-9 codes 196.xx to 199.xx. Subgroups included four cancer types: germ cell tumors and prostate cancer; lung, liver, and pancreatic cancer; hematologic malignancies; and all other cancers. Survival time was calculated as the interval (in months) between the date of diagnosis and death, then categorized into 1–2, 3–6, 7–12, 13–24, and 25 or more months. The primary physician’s specialty was retrieved from the code in National Health Insurance claims and was divided into oncologist and other. Hospital characteristics such as accreditation level, case load, urbanization level, and geographic region were recorded.
Statistical analysis
All data were analyzed using SPSS (version 15, SPSS Inc., Chicago, IL). Pearson’s chi-square test was used for categorical variables such as gender, level of urbanization, geographic region of residence, CCIS category, cancer group, and hospital characteristics (teaching level, geographic region, and caseload). Continuous variables were analyzed using one-way ANOVA.
The impact of each explanatory variable on the aggressiveness of EOL care was examined by hierarchical linear regression using a random-intercept model. A multilevel logistic regression model was constructed to explore the association of SES category with each indicator of aggressive EOL care after adjusting for patient characteristics (age, gender, cancer type, post-diagnosis survival, CCIS score, urbanization and geographic area, primary physician specialty, and hospital characteristics. A p-value of P<0.05 was used to indicate statistical significance.
Ethics statement
The Institutional Review Board of Dalin Tzu Chi Hospital, Taiwan approved this study. Review board requirements for written informed consent were waived because all personal identifying information was removed from the NHIRD database prior to data analysis.
RESULTS
A total of 28,978 terminal cancer decedents from 2009 to 2011 were identified. Of these, 10941, 16535, and 1502 were categorized as having low, moderate, and high income, respectively. Their basic characteristics are described in Table 1.
Table 1. Baseline characteristics of older patients (age >65 years) in Taiwan with terminal cancer by years (2009–2011) and total.
| Socioeconomic status | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Total | Low | Moderate | High | p value | ||||
| No. | % | No. | % | No. | % | No. | % | ||
| Total | 28978 | 100 | 10941 | 37.8 | 16535 | 57.1 | 1502 | 5.2 | |
| Gender | <0.001 | ||||||||
| Female | 8770 | 30.3 | 2269 | 20.7 | 6194 | 37.5 | 307 | 20.4 | |
| Male | 20208 | 69.7 | 8672 | 79.3 | 10341 | 62.5 | 1195 | 79.6 | |
| Mean age, years (±SD) | 77.6±7.1 | 79.0±7.1 | 77.2±6.9 | 71.9±6.2 | <0.001 | ||||
| Age group | <0.001 | ||||||||
| 65–74 | 10994 | 37.9 | 3245 | 29.7 | 6639 | 40.1 | 1110 | 73.9 | |
| 74–84 | 13439 | 46.4 | 5514 | 50.4 | 7603 | 46.0 | 322 | 21.4 | |
| 85+ | 4545 | 15.7 | 2182 | 19.9 | 2293 | 13.9 | 70 | 4.7 | |
| CCIS | <0.001 | ||||||||
| 0 or 1 | 12792 | 44.1 | 5079 | 46.4 | 7056 | 42.7 | 657 | 43.7 | |
| 2 | 3813 | 13.2 | 1484 | 13.6 | 2152 | 13.0 | 177 | 11.8 | |
| 3 | 2736 | 9.4 | 990 | 9.0 | 1637 | 9.9 | 109 | 7.3 | |
| 4 | 9637 | 33.3 | 3388 | 31.0 | 5690 | 34.4 | 559 | 37.2 | |
| Cancer group | <0.001 | ||||||||
| I | 464 | 1.6 | 239 | 2.2 | 210 | 1.3 | 15 | 1.0 | |
| II | 811 | 2.8 | 364 | 3.3 | 418 | 2.5 | 29 | 1.9 | |
| III | 5169 | 17.8 | 1778 | 16.3 | 3125 | 18.9 | 266 | 17.7 | |
| IV | 8192 | 28.3 | 2966 | 27.1 | 4736 | 28.6 | 490 | 32.6 | |
| V | 5341 | 18.4 | 2121 | 19.4 | 3001 | 18.1 | 219 | 14.6 | |
| VI | 7997 | 27.6 | 3084 | 28.2 | 4483 | 27.1 | 430 | 28.6 | |
| VII | 1004 | 3.5 | 389 | 3.6 | 562 | 3.4 | 53 | 3.5 | |
| Post-diagnosis survival, months | 0.073 | ||||||||
| ≤6 | 14699 | 50.7 | 5617 | 51.3 | 8370 | 50.6 | 712 | 47.4 | |
| 6.01–12 | 6206 | 21.4 | 2292 | 20.9 | 3567 | 21.6 | 347 | 23.1 | |
| 12.01–24 | 5591 | 19.3 | 2113 | 19.4 | 3159 | 19.1 | 319 | 21.2 | |
| >24.01 | 2482 | 8.6 | 919 | 8.4 | 1439 | 8.7 | 124 | 8.3 | |
| Primary physician’s specialty | 0.001 | ||||||||
| Oncologist | 3798 | 13.1 | 1329 | 12.1 | 2259 | 13.7 | 210 | 14.0 | |
| Other | 25180 | 86.9 | 9612 | 87.9 | 14276 | 86.3 | 1292 | 86.0 | |
| Hospital characteristics | <0.001 | ||||||||
| Medical center | 15387 | 53.1 | 6277 | 57.4 | 8175 | 49.4 | 935 | 62.3 | |
| Regional | 11646 | 40.2 | 3970 | 36.3 | 7147 | 43.2 | 529 | 35.2 | |
| District | 1945 | 6.7 | 694 | 6.3 | 1213 | 7.3 | 38 | 2.5 | |
| Caseload group | <0.001 | ||||||||
| High | 11077 | 38.2 | 4042 | 36.9 | 6586 | 39.8 | 449 | 29.9 | |
| Medium | 9303 | 32.1 | 3133 | 28.6 | 5648 | 34.2 | 522 | 34.8 | |
| Low | 8598 | 29.7 | 3766 | 34.5 | 4301 | 26.0 | 531 | 35.4 | |
| Urbanization | <0.001 | ||||||||
| Urban | 4817 | 16.6 | 3291 | 30.1 | 975 | 5.9 | 551 | 36.7 | |
| Suburban | 10437 | 36.0 | 5443 | 49.7 | 4288 | 25.9 | 706 | 47.0 | |
| Rural | 13724 | 47.4 | 2207 | 20.2 | 11272 | 68.2 | 245 | 16.3 | |
| Geographic Region | <0.001 | ||||||||
| Northern | 12366 | 42.7 | 7017 | 64.1 | 4493 | 27.2 | 856 | 57.0 | |
| Central | 4731 | 16.3 | 1352 | 12.4 | 3159 | 19.1 | 220 | 14.6 | |
| Southern | 10601 | 36.6 | 2103 | 19.2 | 8113 | 49.0 | 385 | 25.6 | |
| Eastern | 1278 | 4.4 | 468 | 4.3 | 769 | 4.7 | 41 | 2.8 | |
Cancer group I: nonmetastatic germ-cell tumors and prostate cancer; II: metastatic germ-cell tumors and prostate cancer; III: nonmetastatic lung, liver, and pancreatic cancer; IV: metastatic lung, liver, and pancreatic cancer; V: all other nonmetastatic cancers; VI: all other metastatic cancers; and VII: hematologic malignancies.
SD, standard deviation.
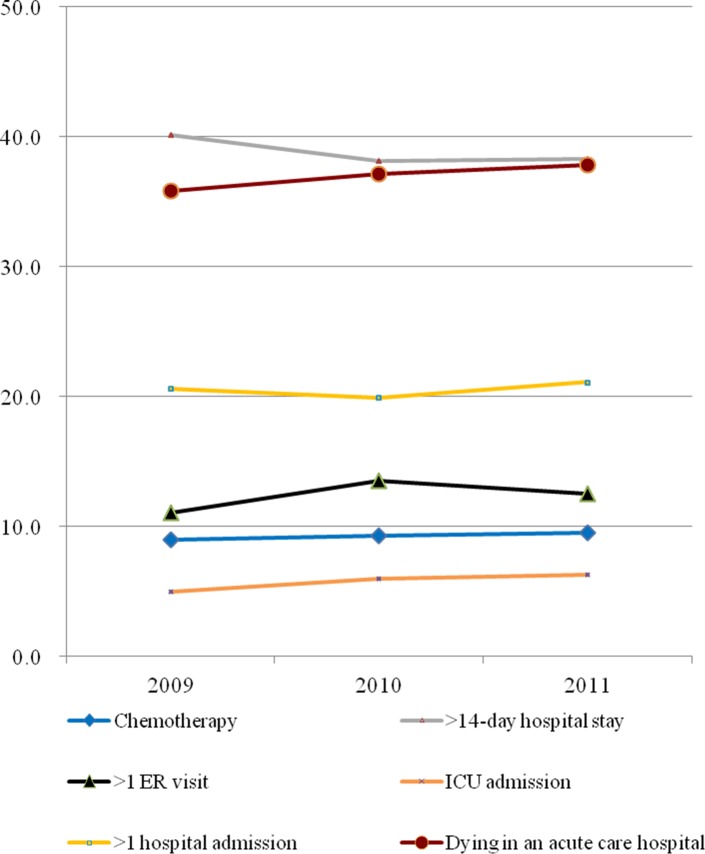
The distribution of indicators for aggressive EOL care is provided in Fig. 1. The indicators had an increasing trend from 2009 to 2011, except for hospital stay >14 days. The number of indicators of aggressive EOL care averaged 1.26±1.16 for all study subjects.
Figure 1. Trends for the six indicators of aggressive end-of-life care for Taiwanese cancer patients age 65 years and above for the period 2009 to 2011.
ER, emergency room; ICU, intensive care unit.
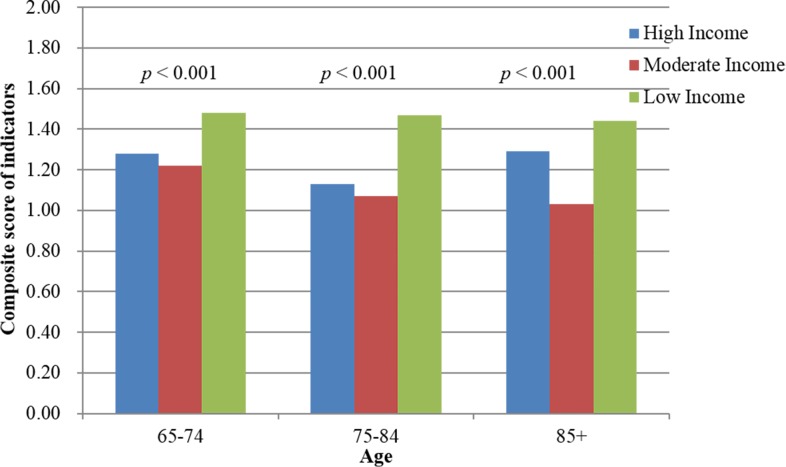
Fig. 2 depicts the association of SES (individual income) and EOL care. Cancer decedents with low income were associated with having more aggressive EOL care. The aggressiveness of EOL care also declined with age. Hierarchical linear modeling using a random-intercept model revealed that, compared with decedents with low income, those with moderate (estimate-0.30, P<0.001) and high (estimate-0.27, P<0.001) income received less aggressive EOL care (Table 2). Male gender, being 65–75 years old, high CCIS, and post-diagnosis survival <6 months were associated with more aggressive EOL care. Furthermore, the aggressiveness of EOL treatment overall increased each year.
Figure 2. The impact of socioeconomic status (SES) on aggressiveness of end-of-life treatment by age.
Table 2. Determinants of aggressive end-of-life care for Taiwanese cancer patients age 65 years and older, 2009–2011 by multivariate analysis using a random-intercept model (average indicator scores = 1.26±1.16).
| Parameter | Estimate | 95%CI | p value |
|---|---|---|---|
| Intercept | 0.71 | (0.51,0.91) | <0.001 |
| SES | |||
| Low | Reference | ||
| Moderate | -0.30 | (-0.33, -0.27) | <0.001 |
| High | -0.27 | (-0.33, -0.20) | <0.001 |
| Gender | |||
| Female | Reference | ||
| Male | 0.10 | (0.07,0.13) | <0.001 |
| Age group | |||
| 65–74 | Reference | ||
| 75–84 | -0.09 | (-0.11, -0.06) | <0.001 |
| 85+ | -0.10 | (-0.14, -0.06) | <0.001 |
| Charlson Comorbidity Index Score | |||
| 0 or 1 | Reference | ||
| 2 | 0.21 | (0.17,0.25) | <0.001 |
| 3 | 0.21 | (0.17,0.26) | <0.001 |
| ≧4 | 0.26 | (0.23,0.29) | <0.001 |
| Cancer group | |||
| I | Reference | ||
| II | 0.41 | (0.28,0.54) | <0.001 |
| III | 0.37 | (0.26,0.48) | <0.001 |
| IV | 0.60 | (0.49,0.70) | <0.001 |
| V | 0.41 | (0.30,0.52) | <0.001 |
| VI | 0.72 | (0.61,0.83) | <0.001 |
| VII | 0.35 | (0.22,0.48) | <0.001 |
| Post-diagnosis survival, months | |||
| ≤6 | Reference | ||
| 6.01–12 | -0.07 | (-0.10, -0.03) | <0.001 |
| 12.01–24 | -0.11 | (-0.15, -0.08) | <0.001 |
| >24 | -0.09 | (-0.14, -0.08) | 0.001 |
| Primary physician’s specialty | |||
| Other | Reference | ||
| Oncologist | 0.004 | (-0.04,0.05) | 0.841 |
| Hospital characteristics | |||
| District | Reference | ||
| Medical center | 0.02 | (-0.10,0.14) | 0.751 |
| Regional | 0.05 | (-0.02,0.13) | 0.180 |
| Caseload group | |||
| High | Reference | ||
| Moderate | 0.06 | (-0.07,0.20) | 0.327 |
| Low | 0.03 | (-0.12,0.17) | 0.672 |
| Urbanization | |||
| Urban | Reference | ||
| Suburban | -0.02 | (-0.06,0.02) | 0.372 |
| Rural | -0.02 | (-0.07,0.03) | 0.422 |
| Geographic Region | |||
| Northern | Reference | ||
| Central | -0.03 | (-0.08,0.03) | 0.371 |
| Southern | 0.02 | (-0.03,0.06) | 0.459 |
| Eastern | 0.11 | (0.02,0.20) | 0.021 |
| Year | |||
| 2009 | Reference | ||
| 2010 | 0.06 | (0.03,0.10) | <0.001 |
| 2011 | 0.08 | (0.05,0.11) | <0.001 |
Cancer group I: nonmetastatic germ-cell tumors and prostate cancer; II: metastatic germ-cell tumors and prostate cancer; III: nonmetastatic lung, liver, and pancreatic cancer; IV: metastatic lung, liver, and pancreatic cancer; V: all other nonmetastatic cancers; VI: all other metastatic cancers; and VII: hematologic malignancies.
SES, socioeconomic status; EC, enrollee category. SD, standard deviation.
Compared to nonmetastatic germ-cell tumors and prostate cancer, decedents with cancer of poor prognosis (such as pancreatic, lung, and liver cancer) received more aggressive EOL care (Table 2). Decedents with distant metastasis cancer received more aggressive EOL care than those without metastasis.
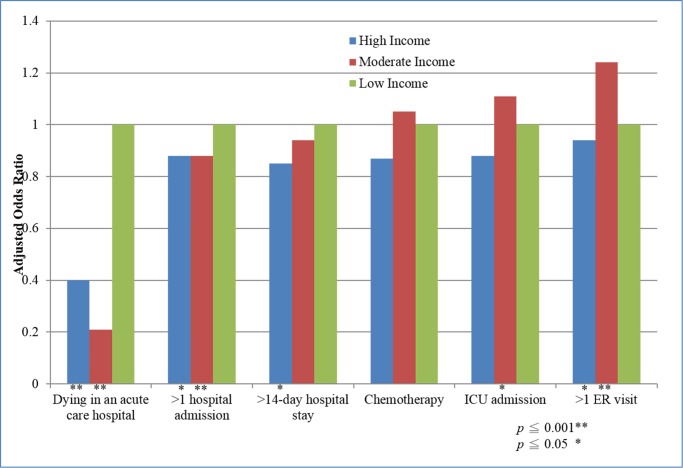
We further examined the association of each type of aggressive EOL care and income. Multilevel logistic regression analysis revealed that older cancer decedents with low income were more likely to stay in the hospital >14 days and to die in an acute hospital (Table 3 and Fig. 3). By contrast, older cancer decedents with moderate or high income visited the ER more than once and were admitted to the ICU more frequently than those with low income.
Table 3. Effects on SES categories on aggressive indicators of EOL care by multilevel logistic regression in older patients with cancer.
| Adjusted* | |||
|---|---|---|---|
| Variable | OR | (95% CI) | P value |
| Dying in an acute care hospital | |||
| SES Low | |||
| SES Moderate | 0.26 | 0.24–0.27 | <0.001 |
| SES High | 0.50 | 0.49–0.52 | <0.001 |
| >1 hospital admission | |||
| SES Low | 1 | ||
| SES Moderate | 0.89 | 0.86–0.92 | <.0001 |
| SES High | 0.93 | 0.90–0.96 | 0.04 |
| >14-day hospital stay | |||
| SES Low | 1 | ||
| SES Moderate | 0.94 | 0.92–0.97 | 0.03 |
| SES High | 0.96 | 0.94–1.00 | 0.20 |
| Chemotherapy | |||
| SES Low | 1 | ||
| SES Moderate | 1.06 | 1.00–1.11 | 0.15 |
| SES High | 1.00 | 0.95–1.05 | 0.90 |
| ICU admission | |||
| SES Low | 1 | ||
| SES Moderate | 1.17 | 1.11–1.25 | 0.005 |
| SES High | 1.06 | 1.00–1.13 | 0.31 |
| >1 ER visit | |||
| SES Low | 1 | ||
| SES Moderate | 1.23 | 1.18–1.28 | <.0001 |
| SES High | 1.10 | 1.05–1.14 | 0.01 |
* Adjusted for patient age, gender, hospital spending index, Charlson Comorbidity Index Score, cancer group, primary physician’s specialty, post-diagnosis survival, hospital characteristics, hospital caseload, urbanization and geographic region.
SES, socioeconomic status; EOL, end-of-life; OR, odds ratio; CI, confidence interval; ER, emergency department; ICU, Intensive care unit;
Figure 3. The differential effects of socioeconomic status (SES) on indicators of aggressive end-of-life care.
DISCUSSION
This study found that low individual income was associated with more aggressive EOL care in older cancer decedents in Taiwan. There was the greater tendency of older decedents with low income to die in the acute care hospital compared to more affluent decedents. Income was found to have differential effects on different indicators of aggressive EOL care. This difference by type of treatment may explain the disparities in results between studies. These results have implications for public health providers, who should offer hospice care to older cancer decedents with low income, to reduce the aggressiveness of the EOL care they receive and lessen the financial and emotional burden generated by such futile treatment.
The strength of our study is that it is a population-based observation study with abundant patient numbers to mitigate the effect of minor confounding factors. The Taiwan Health Insurance Program has covered approximately 99% of island residents for decades, and the validity of the dataset has been confirmed. We observed an influence of individual income on aggressiveness of EOL care in older cancer decedents, and further determined the effect of specific treatments on aggressive EOL care. To our knowledge, no previous studies have done this.
Determinants of place of death for terminal cancer decedents are complex. Factors such as age, gender, ethnicity, functional status, family support, personal and family preferences, hospice home visits, and details of the health care system all affect the choices of these decedents [23–27]. Taylor et al. revealed that decedents who die in an aged/residential care facility are more likely to be poorer than those who die elsewhere [28]. Cohen et al. found that education beyond high school was associated with greater likelihood of dying at home for cancer decedents living in Belgium, Italy, and Norway [27]. Motiwala et al. also showed that higher SES was associated with a slightly greater probability of dying at home [29]. Our study found that cancer decedents with low individual income were more likely than wealthier decedents to die in an acute care hospital, itself a major source of aggressive EOL treatment.
Our study revealed that male gender, high CCSI score, post-diagnosis survival <6 months, living in an urban area, and living in the northern region of Taiwan are associated with more aggressive EOL care. Other studies have already shown a relationship of male gender and post-diagnosis survival <6 months with more aggressive EOL treatment [15, 30, 31]. However, our findings differ from other studies in some respects. Thi et al. found that living in a rural area was associated with more aggressive EOL treatment in Canada [22]. But Lin et al. demonstrated increased hospice care in rural decedents over urban decedents in Taiwan [32]. This differential distribution of hospice care may explain why urban decedents received more aggressive EOL care in this study, since hospice care may reduce the incidence of aggressive EOL care [33].
In Sweden, Randén et al. found that having a high level of education was associated with more chemotherapy use [34]. Among older melanoma decedents, those residing in poorer SES areas were less likely to receive chemotherapy [35]. In decedents with non-small cell lung cancer, Saito et al. found no additional survival benefit from continuing chemotherapy within 14 days of death. In addition, continuing chemotherapy has been associated with a decreased likelihood of receiving hospice care [36]. In our study, SES had little differential effect on whether older decedents diagnosed with cancer continued chemotherapy.
In asthma decedents, lower SES was associated with higher odds of asthma-related ER/urgent care visits [37]. Hu et al. showed that geographical region of residence had a strong association with multiple ER visits in decedents with colorectal cancer in Alberta, Canada [38]. Our study showed that older cancer decedents with low SES have slightly lower likelihood of visiting the ER more than once, compared to more affluent decedents. The co-pay charge for an ER visit ($150) may deter low SES decedents from utilizing such care.
Previous studies have found that both low patient SES and low hospital area socioeconomic profile are associated with longer length of stay [39]. Hollowell et al. found that socioeconomically deprived decedents are more likely to remain in the hospital without morbidity following total knee replacement [40]. In contrast to these findings, this study found no significant difference in length of stay >14 days by SES in older decedents with cancer. It may be that decedents with terminal conditions view hospitalization differently or that their doctors recommend hospital stays differently than is the case with other types of conditions.
One limitation of the present study is that the cancer diagnosis and comorbidities were collected from the National Health Insurance claims using ICD-9 codes. While no administrative dataset is perfect, the National Health Insurance Bureau in Taiwan does randomly review charts and interview decedents to spot-verify the accuracy of diagnosis. Furthermore, some diseases have been validated in the NHIRD [41]. The second limitation is that we gave the same weight to each indicator of aggressive EOL care. Decedents from different cultures and societies may not consider such factors as of equal weight in making decisions about care. Given the robustness of the evidence and the statistical analysis in this study, these limitations are unlikely to compromise the validity of our results.
This study showed that older cancer decedents with low individual income were more likely to receive aggressive EOL care than those with high or medium income. Dying in an acute care hospital was the main factor related to this difference. We also found that the aggressiveness of EOL care for older decedents with cancer increased slowly over the past few years. Public health providers should be encouraged to educate their older cancer decedents on their disease prognosis and the benefits of hospice care, particularly when treating decedents with low income. Such strategies may reduce the rate of aggressive, but futile, EOL care. This reduction may in turn reduce the demand on staff, the emotional toll on decedents and their families, and the financial burden on the healthcare system.
Funding Statement
The authors have no support or funding to report.
REFERENCES
- 1. Ministry of Health and Welfare (2013) 2013 Statistics of causes of death. Available: http://www.mohw.gov.tw/EN/Ministry/DM2.aspx?f_list_no=474&fod_list_no=3443. Accessed 2014 Sep 10.
- 2. Tang ST, Liu T-W, Shyu Y-IL, Huang E-W, Koong SL, et al. (2012) Impact of age on end-of-life care for adult Taiwanese cancer decedents, 2001–2006. Palliat Med 26: 80–88. 10.1177/0269216311406989 [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 4. Tang ST, Wu S-C, Hung Y-N, Huang E-W, Chen J-S, et al. (2009) Trends in quality of end-of-life care for Taiwanese cancer patients who died in 2000–2006. Ann Oncol 20: 343–348. 10.1093/annonc/mdn602 [DOI] [PubMed] [Google Scholar]
- 5. Jen-Chieh Huang W-CT (2009) Factors Affecting the Health Care Expenditure of Cancer Patients in their Last One Year of Life in Taiwan. National Digital Library of Theses and Dissertations in Taiwan.
- 6. Riley GF, Lubitz JD (2010) Long-Term Trends in Medicare Payments in the Last Year of Life. Health Serv Res 45: 565–576. 10.1111/j.1475-6773.2010.01082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fassbender K, Fainsinger RL, Carson M, Finegan BA (2009) Cost Trajectories at the End of Life: The Canadian Experience. J Pain Symptom Manage 38: 75–80. 10.1016/j.jpainsymman.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 8. Hogan C, Lunney J, Gabel J, Lynn J (2001) Medicare Beneficiaries’ Costs Of Care In The Last Year Of Life. Health Aff (Millwood) 20: 188–195. 10.1377/hlthaff.20.4.188 [DOI] [PubMed] [Google Scholar]
- 9. Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E (2009) Racial and ethnic differences in end-of-life costs: Why do minorities cost more than whites? Arch Intern Med 169: 493–501. 10.1001/archinternmed.2008.616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanratty B, Burström B, Walander A, Whitehead M (2007) Inequality in the face of death? Public expenditure on health care for different socioeconomic groups in the last year of life. J Health Serv Res Policy 12: 90–94. 10.1258/135581907780279585 [DOI] [PubMed] [Google Scholar]
- 11. Felder S, Meier M, Schmitt H (2000) Health care expenditure in the last months of life. J Health Econ 19: 679–695. 10.1016/S0167-6296(00)00039-4 [DOI] [PubMed] [Google Scholar]
- 12. Chawla N, Butler EN, Lund J, Warren JL, Harlan LC, et al. (2013) Patterns of Colorectal Cancer Care in Europe, Australia, and New Zealand. JNCI Monographs 2013: 36–61. 10.1093/jncimonographs/lgt009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, et al. (2003) Identifying Potential Indicators of the Quality of End-of-Life Cancer Care From Administrative Data. J Clin Oncol 21: 1133–1138. 10.1200/JCO.2003.03.059 [DOI] [PubMed] [Google Scholar]
- 14. Tseng C-H (2004) Mortality and Causes of Death in a National Sample of Diabetic Patients in Taiwan. Diabetes Care 27: 1605–1609. 10.2337/diacare.27.7.1605 [DOI] [PubMed] [Google Scholar]
- 15. Tang ST, Wu S-C, Hung Y-N, Chen J-S, Huang E-W, et al. (2009) Determinants of Aggressive End-of-Life Care for Taiwanese Cancer Decedents, 2001 to 2006. J Clin Oncol 27: 4613–4618. 10.1200/JCO.2008.20.5096 [DOI] [PubMed] [Google Scholar]
- 16. A H (1975) Four Factor Index of Social Status. Yale University Department of Psychology, New Haven. [Google Scholar]
- 17. Lin H-C, Chao P-Z, Lee H-C (2008) Sudden Sensorineural Hearing Loss Increases the Risk of Stroke: A 5-Year Follow-Up Study. Stroke 39: 2744–2748. 10.1161/STROKEAHA.108.519090 [DOI] [PubMed] [Google Scholar]
- 18. Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, et al. (2010) The impact of health insurance status on the survival of patients with head and neck cancer. Cancer 116: 476–485. 10.1002/cncr.24774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J-Y, Wang C-Y, Juang S-Y, Huang K-Y, Chou P, et al. Low socioeconomic status increases short-term mortality of acute myocardial infarction despite universal health coverage. Int J Cardiol 172: 82–87. 10.1016/j.ijcard.2013.12.082 [DOI] [PubMed] [Google Scholar]
- 20. Chang C-M, Su Y-C, Lai N-S, Huang K-Y, Chien S-H, et al. (2012) The Combined Effect of Individual and Neighborhood Socioeconomic Status on Cancer Survival Rates. PLoS One 7: e44325 10.1371/journal.pone.0044325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 22. Ho TH, Barbera L, Saskin R, Lu H, Neville BA, et al. (2011) Trends in the Aggressiveness of End-of-Life Cancer Care in the Universal Health Care System of Ontario, Canada. J Clin Oncol 29: 1587–1591. 10.1200/JCO.2010.31.9897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang ST (2003) When Death Is Imminent: Where Terminally Ill Patients With Cancer Prefer to Die and Why. Cancer Nurs 26: 245–251. 10.1097/00002820-200306000-00012 [DOI] [PubMed] [Google Scholar]
- 24. Johnson KS, Kuchibhatala M, Sloane RJ, Tanis D, Galanos AN, et al. (2005) Ethnic Differences in the Place of Death of Elderly Hospice Enrollees. J Am Geriatr Soc 53: 2209–2215. 10.1111/j.1532-5415.2005.00502.x [DOI] [PubMed] [Google Scholar]
- 25. Tang ST (2002) Influencing Factors of Place of Death Among Home Care Patients With Cancer in Taiwan. Cancer Nurs 25: 158–166. 10.1097/00002820-200204000-00013 [DOI] [PubMed] [Google Scholar]
- 26. Nakamura S, Kuzuya M, Funaki Y, Matsui W, Ishiguro N (2010) Factors influencing death at home in terminally ill cancer patients. Geriatrics & Gerontology International 10: 154–160. [DOI] [PubMed] [Google Scholar]
- 27. Cohen J, Houttekier D, Onwuteaka-Philipsen B, Miccinesi G, Addington-Hall J, et al. (2010) Which Patients With Cancer Die at Home? A Study of Six European Countries Using Death Certificate Data. J Clin Oncol 28: 2267–2273. 10.1200/JCO.2009.23.2850 [DOI] [PubMed] [Google Scholar]
- 28. Taylor EJ, Ensor B, Stanley J (2012) Place of death related to demographic factors for hospice patients in Wellington, Aotearoa New Zealand. Palliat Med 26: 342–349. 10.1177/0269216311412229 [DOI] [PubMed] [Google Scholar]
- 29. Motiwala SS CR, Guerriere DN, Coyte PC (2006) Predictors of place of death for seniors in Ontario: a population-based cohort analysis. CAN J AGING 25: 363–371. 10.1353/cja.2007.0019 [DOI] [PubMed] [Google Scholar]
- 30. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, et al. (2004) Trends in the Aggressiveness of Cancer Care Near the End of Life. J Clin Oncol 22: 315–321. 10.1200/JCO.2004.08.136 [DOI] [PubMed] [Google Scholar]
- 31. Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, et al. (2008) Aggressiveness of Cancer Care Near the End of Life: Is It a Quality-of-Care Issue? J Clin Oncol 26: 3860–3866. 10.1200/JCO.2007.15.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin Y-H, Chen Y-C, Tseng Y-H, Lin M-H, Hwang S-J, et al. (2013) Trend of Urban-Rural Disparities in Hospice Utilization in Taiwan. PLoS One 8: e62492 10.1371/journal.pone.0062492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang H-M, Koong SL, Hsiao SC, Chen J-S, Liu T-W, et al. (2011) Impact of Availability of an Inpatient Hospice Unit on the Parent Hospital’s Quality of Palliative Care for Taiwanese Cancer Decedents, 2001–2006. J Pain Symptom Manage 42: 400–409. 10.1016/j.jpainsymman.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 34.(2013) Treatment decisions and discontinuation of palliative chemotherapy near the end-of-life, in relation to socioeconomic variables. Acta Oncol 52: 1062–1066. 10.3109/0284186X.2012.758872 [DOI] [PubMed] [Google Scholar]
- 35. Reyes-Ortiz CA, Goodwin JS, Zhang DD, Freeman JL (2011) Socioeconomic Status and Chemotherapy Use for Melanoma in Older People. Canadian Journal on Aging/La Revue canadienne du vieillissement 30: 143–153. 10.1017/S0714980810000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saito A, Landrum M, Neville B, Ayanian J, Earle C (2011) The effect on survival of continuing chemotherapy to near death. BMC Palliat Care 10: 14 10.1186/1472-684X-10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Law HZ OE, Mannino DM (2011) The role of income in reducing racial and ethnic disparities in emergency room and urgent care center visits for asthma-United States, 2001–2009. J Asthma 48: 405–413. 10.3109/02770903.2011.565849 [DOI] [PubMed] [Google Scholar]
- 38. Hu W, Yasui Y, White J, Winget M (2013) Aggressiveness of End-of-Life Care for Patients With Colorectal Cancer in Alberta, Canada: 2006–2009. J Pain Symptom Manage. [DOI] [PubMed] [Google Scholar]
- 39. Perelman J, Closon M-C (2011) Impact of socioeconomic factors on in-patient length of stay and their consequences in per case hospital payment systems. J Health Serv Res Policy 16: 197–202. 10.1258/jhsrp.2011.010047 [DOI] [PubMed] [Google Scholar]
- 40. Hollowell J, Grocott MPW, Hardy R, Haddad FS, Mythen MG, et al. (2010) Major elective joint replacement surgery: socioeconomic variations in surgical risk, postoperative morbidity and length of stay. J Eval Clin Pract 16: 529–538. [DOI] [PubMed] [Google Scholar]
- 41. Cheng C-L, Kao Y-HY, Lin S-J, Lee C-H, Lai ML (2011) Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 20: 236–242. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]