Abstract
The intestinal microbiota is firmly implicated not only in the pathogenesis of inflammatory bowel disease (IBD) but increasingly also in the development of inflammation at extraintestinal tissue sites. Significant clinical, genetic, immunological, and microbio-logical overlap exists between IBD and spondyloarthritis (SpA), which indicates that pathophysiological mechanisms are shared between these diseases and may center on the intestinal micro-biota. Recently, culture-independent techniques have enabled the microbiota in health and disease to be described in increasing detail. Moreover, functional studies have identified myriad host effector and regulatory pathways that shape or are shaped by this microbial community. We consider the complex relationship between SpA pathogenesis and gut microbes, with a discussion of how manipulation of the gut microbiota itself may be a promising future target for SpA therapy.
Keywords: Microbiota, Dysbiosis, Spondyloarthritis, Ankylosing spondylitis, Reactive arthritis, Inflammatory bowel disease
Introduction
The potential of the human microbiota to redefine our understanding of spondyloarthritic diseases or spondyloarthritides (SpAs) is discussed within this review. We begin with an overview of the microbiota, moving to potential relationships between the intestinal microbiota and SpA pathogenesis as this has been a major and ongoing focus of research efforts. This discussion includes consideration of different inflammatory pathways that may intersect with an altered gut microbiota, a phenomenon termed “dysbiosis”. We further consider how extraintestinal translocation of intestinal microbes or microbial products may contribute to SpA-related disease, in addition to microbiota-related immune pathways that may link gut and joint pathology. Finally, we review therapeutic manipulation of the microbiota and future research directions for both clinicians and basic scientists. The authors acknowledge the valuable contributions of many to this field, especially those that could not be cited in this review due to space constraints.
The human microbiome
The past decade has seen the advent of high-throughput sequencing approaches to characterize the human microbiota in increasing detail. The human body provides a plethora of habitats for the colonization of trillions of microbes. This is manifest in the high degree of inter-site variation in the community structure of microbiota. For instance, anaerobic Firmicutes/Bacteroidetes spp. dominate the intestine, whereas Actinobacteria and Proteobacteria spp. are found in high abundance on the skin [1]. Indeed, even individual teeth may have a distinct microbial community structure [2]. It is also evident that there is considerable interindividual variation in the microbiota at least at the species level. However, despite this species diversity, it appears that there is an aggregate selection towards species with similar functional gene profile at specific tissue sites [1].
The microbiota is rapidly acquired post partum and persists in a state of flux for the first few years of life. A myriad of factors influence the early structure of the microbiota including delivery method, parenteral nutrition, early infection, and antibiotic use in infancy. As the first few years of life represent a critical window of microbial exposure and immune education of the host, it is therefore feasible (albeit unproven) that early changes to the microbiota may be relevant to the pathogenesis of SpA-related diseases, despite their adult onset.
By roughly 3 years of age, the microbiota appears to stabilize, more closely resembling that of an adult. Nonetheless, this stability is relative. For instance, consumption of a strongly plant- or animal-based diet by human subjects can lead to changes within the microbiota within 24 h, although reversion to a pre-diet community structure is observed within days of returning to a “normal” dietary intake [3]. Following antibiotic treatment, the microbiota is also rapidly impacted, but returns to a pre-antibiotic state consistent with the idea of ecological memory. The intrinsic stability of the microbiota remains an ongoing area of research, but it is possible that a failure to return to a “normal” gut ecosystem following a disturbance of the microbiota in SpA-susceptible individuals may be functionally relevant to disease pathogenesis.
The definition of what constitutes a “normal” gut microbiome, however, is far from resolved. Recently, many efforts are undertaken to study the composition and function of the human gut microbiome as a new strategy to understand IBD. The European MetaHIT consortium identified three different metagenomes from fecal samples from four European countries [4]. These so-called enter-otypes were not nation or gender specific. Each enterotype had a dominant bacterial genus: enterotype 1 was dominated by the genus Bacteroides, enterotype 2 by Prevotella, and enterotype 3 by Ruminococcus. These enterotypes appeared to be functionally different and showed differences in substrate fermentation [4]. However, this concept has not been confirmed by others, such as the National Institutes of Health Human Microbiome Project.
Links between SpA and bowel disease
SpA refers to a group of clinically and genetically related disorders, whose entities include anky-losing spondylitis (AS), psoriatic arthritis (PsA), juvenile SpA, reactive arthritis (ReA), and inflammatory bowel disease (IBD)-related arthritis. Dependent on the predominant symptoms, SpA can be classified as axial SpA, including both AS and non-radiographical axial SpA (nr-axSpA), or as peripheral SpA. The typical clinical features include inflammatory back pain, sacroiliitis, oligoarticular asymmetric synovitis, enthesitis, and frequent extra-articular symptoms such as anterior uveitis, psoriasis, and gut inflammation.
IBD including ulcerative colitis and Crohn's colitis is arguably the disease category in which dysbiosis of the microbiome is most strongly implicated in disease causation. Notably, there is significant overlap between IBD and SpA.
In the 1980s, Mielants et al. performed colonoscopies in SpA patients without bowel symptoms, and they found that up to half of these patients showed microscopic signs of bowel inflammation. Two types were distinguished based on morphological characteristics: an acute type resembling infectious enterocolitis and chronic inflammation in which normal mucosal architecture is disturbed. This type of inflammation was in fact indistinguishable from early Crohn's disease (CD). These pioneering findings have since then been confirmed by others, and include an association between CD-like inflammation and AS, ReA, or PsA.
A similar presence of microscopic gut inflammation was also seen in the GIANT (Ghent Inflammatory Arthritis and spondylitis) cohort, a prospective follow-up study including newly diagnosed patients fulfilling the Assessment of SpondyloArthritis International Society (ASAS) criteria for axial and/or peripheral SpA [5]. Asymptomatic gut inflammation was present in 46.2% of patients including 16.9% acute inflammation and 29.2% chronic inflammation.
Based on this cohort, a predictive model was developed for axial SpA. This model showed that a younger age, progressive disease, male sex, high disease activity as measured by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and restricted spinal mobility as measured by the Bath Ankylosing Spondylitis Metrology Index (BASMI) were all independently associated with microscopic gut inflammation [5]. The prevalence of gut involvement in the GIANT cohort was higher in axial than in peripheral SpA, but similar in nr-axSpA and AS.
Long-term follow-up showed that 6.5% of SpA patients develop clinically overt IBD in a duration of 5 years. Patients with chronic inflammation at the initial biopsy, who were also human leukocyte antigen (HLA) B27 negative, had the highest risk (up to 20%) of developing IBD. This suggests that this type of inflammation represents a preclinical form of CD.
Conversely, 30% of patients with IBD develop articular symptoms with a pattern similar to those in SpA including oligoarticular peripheral arthritis, or even AS [6]. Up to 9% of IBD patients have been reported to develop uveitis [7]. The follow-up study by Mielants et al. also showed a clear clinical association between gut and joint: Remission of joint inflammation was associated with disappearance of gut inflammation and vice versa [8]. Importantly, initial chronic gut inflammation also implied a higher risk of evolution to AS. Recent results from the GIANT cohort showed that chronic gut inflammation is linked with more extensive bone marrow edema (BME) of the sacroiliac joints on magnetic resonance imaging (MRI) [9]. This underscores the impact on mucosal inflammation on disease extent and prognosis in SpA patients.
Links between bacteria and SpA
Beyond the links between IBD and SpA, multiple sets of observations implicate bacteria as being causally related to SpA pathogenesis.
Bacteria trigger ReA
Perhaps the clearest indication of the relationship between gut and joint in SpA is the triggering of ReA by antecedent gastrointestinal infections that include Salmonella, Shigella, Yersinia, and Campylobacter. Moreover, up to 20% of patients with ReA develop AS within 10e20 years [10]. ReA is a classical member of the SpA family of diseases. It shares with AS an association with HLA B27 and involvement of the spine, peripheral joints, entheses, and the uveal tract. Urethral infections are also a trigger for ReA. Many authorities cite Chlamydia as being arthritogenic, although some studies found that other urethral pathogens were equally likely to trigger ReA [11]. Importantly, bacterial products from the antecedent infection have been recovered from the joints of those who develop ReA [12,13].
HLA B27 itself may cross-react with Gram-negative bacteria
Any theory to explain the pathogenesis of SpA needs to incorporate an explanation as to how HLA B27 contributes to these diseases, as it is such a strong genetic risk factor. Many have called attention to the potential for a direct interaction between HLA B27 and Gram-negative bacteria. For example, more than three decades ago, Geczy and colleagues reported that antibodies directed at some isolates of Klebsiella were cytotoxic for HLA B27+ lymphocytes [14]. Others had reported that bowel colonization with Klebsiella heralded flares of AS [15] or acute anterior uveitis [16]. These data have been controversial and difficult to reproduce. However, monoclonal antibodies to HLA B27 detect cross-reactive antigens in Shigella flexneri, Klebsiella pneumoniae, and Yersinia enterocolitica [17]. Schwimmbeck and Oldstone have reported a six-amino-acid sequence in HLA B27, which is identical to a sequence present in a nitrogenase from Klebsiella [18]. Scofield and colleagues searched databases available in 1993 and concluded that HLA B27 was unique in possessing amino acid sequences that were identical to sequences present in proteins from Gram-negative bacteria [19].
Others have proposed that HLA B27 affects the response to Gram-negative bacteria without invoking mimicry as the mechanism. For example, Granfors and colleagues have studied a monocyte cell line transfected with HLA B27 [20]. The presence of HLA B27 appears to affect the intracellular longevity of Salmonella. This, in turn, may affect genes expressed by the bacteria and cytokines produced by the cell line. An emerging hypothesis is that HLA B27 affects dendritic cell function [21], which in turn, of course, would impact the immune response to bacteria.
Bacteria are convincingly implicated in animal models that replicate aspects of SpA
Taurog has generated several lines of rats that express HLA B27 and human beta-2 microglobulin [22]. Although the phenotype differs somewhat in the alternative derivations, these rodents develop spondylitis, peripheral arthritis that includes dactylitis, colitis, and psoriasiform skin lesions. Raising the rats in a germ-free environment markedly reduces colitis, arthritis, and skin disease. Certain bacterial strains can be fed to the germ-free rats such that remission is maintained, while other bacterial strains cause the diarrheal illness to return [23].
Adjuvant arthritis is induced in susceptible strains of rats by injection of complete Freund's adjuvant consisting of mineral oil and killed mycobacteria. The bacteria are an essential component of the arthritogenicity of the immunization. The disease has multiple similarities to SpA including an asymmetric arthritis characterized by new bone formation, spondylitis, uveitis, and urethritis.
Thus, multiple lines of evidence firmly implicate bacteria as a major contributor to SpA. While it is possible that the pathogenesis of bowel disease is entirely separate from the cause of joint disease, a more parsimonious argument is that the causation overlaps. PsA can also manifest as a form of SpA. In this disease to date, however, the skin microbiome has been more strongly implicated than the gut.
Dysbiosis in IBD and SpA
A convincing body of data ports the concept that the gut microbiota is altered in IBD patients, although the identification of dysbiotic changes in SpA patients remains in its infancy. Nonetheless, given the significant disease overlap between these diseases, it is reasonable to hypothesize that they may share common changes to the intestinal microbiota. The most reproducible findings in CD patients are the outgrowth of enterobacteria and a reduction in anaerobes (reviewed by Ref. [24]). The loss of clostridial species is especially marked, particularly of the clades IV and XIVa during active inflammation. These findings are ported by the reduced abundance of clostridial commensals, Faecalibacterium and Roseburia, in ileal CD patients. Whether these changes are secondary to inflammation remains equivocal, yet a consensus has increasingly emerged that IBD is associated with decreased overall microbial diversity, with a concomitant loss of microbes that may promote gut homeostasis.
In contrast to organisms that elicit a regulatory response, the so-called colitogenic flora capable of inducing inflammation may be expanded. Of note, Prevotellaceae were amongst the expanded Bacteroidetes phyla responsible for increased severity of dextran sodium sulfate (DSS) colitis in NLPR6 inflammasome-deficient mice [25], and were also found to be linked to periodontal disease [26]. Interestingly, Prevotella was recently also linked to disease in new-onset rheumatoid arthritis, which suggests that, even though gut inflammation is not associated with this disease, the intestinal microbiome could play a pivotal role in inflammatory diseases traditionally viewed as strictly auto-immune in nature [27]. Despite the identification of promising candidates such as adherent/invasive Escherichia coli, Helicobacter spp., Bacteroides vulgatus, K. pneumoniae, and Proteus mirabilis, which either consistently expand with colitis or induce disease in rodent models; to date, no single pathogenic organism has been identified as inducing the development of IBD.
One of the earliest attempts to identify dysbiotic changes in SpA patients used the sequencing-independent technique of polymerase chain reaction and denaturing gradient gel electrophoresis (DGGE) to compare the gut microbiota of 15 AS patients and controls [28]. This study did not find consistent differences between groups, with high interindividual variation, but it did find that a significantly higher proportion of AS fecal samples had sulfate-reducing bacteria. A similar approach was used in HLA-B27/β2m rats and healthy controls and found a number of unknown or uncultivable species present in the inflamed colon of HLA-B27+ animals [29]. More recently, we have used high-throughput 16s sequencing techniques and identified the significant expansion of Prevotella spp. and an outgrowth of B. vulgatus in the cecum of HLA-B27+ve rats [30]. This was accompanied by a loss of Rikenellaceae spp. Global dysbiotic changes in this system manifest with a loss of members of the dominant Bacteroidetes phylum, as seen in IBD patients, and expansion of less abundant proteo-bacterial and verrucomicrobial species (Ref. [31] and unpublished observations). Importantly, these changes occurred in the absence of bowel inflammation, which indicates that dysbiosis may also be observed using high-resolution molecular approaches in sufficiently powered studies of SpA patients, irrespective of colitis phenotype.
Pathways of dysbiosis and inflammation
The main hypothesis in CD pathogenesis is an aberrant immune response to intestinal commensal bacteria due to environmental and genetic factors. Very convincing evidence for the importance of microbial–emucosal interactions in IBD was the discovery of nucleotide-binding oligomerization domain-containing protein 2 (NOD2)/caspase-associated recruitment domain 15 (CARD15) polymorphisms that predispose to CD. NOD2 is a pattern recognition receptor (PRR) that recognizes bacterial peptidoglycans and, upon activation, leads to stimulation of the nuclear factor kappa B (NFκB) pathway, often associated with inflammation. The spectrum of genetic polymorphisms has increased continuously, expanding to other pathways such as autophagy and endoplasmic reticulum (ER) stress. These have enforced an increasingly important role of perturbed bacterial handling in IBD pathogenesis. Some of the findings have been extended to SpA. Herein, we discuss pathways that may intersect with dysbiosis and disease development.
Disrupted first defenses
The epithelial monolayer of the intestine is a dynamic frontier between gut-resident microbes and the underlying stromal and immune cells of the intestinal lamina propria. Beyond the physical barrier afforded by the intestinal epithelium, many mediators are secreted into the intestinal lumen including mucus, antimicrobial peptides (AMPs), and secretory immunoglobulin A (IgA). Studies of both SpA and CD intestinal tissue indicate that these first defenses may be disturbed relative to healthy controls.
The mucus layer is a major physical and chemical barrier that excludes luminal microbes from the intestinal epithelium, and dysregulated mucus production has been reported in the intestine of both CD and SpA patients [32]. Mice deficient in mucus production also develop a spontaneous colitis phenotype, and altered mucus synthesis accompanies the phenotype of B27+ transgenic rats with an SpA phenotype [33,34]. Mucus may modulate the host microbiota directly, acting as a nutrient source for some bacteria. Moreover, it has recently been reported that the major mucus protein muc2 is a bioactive mediator and can condition tolerogenic responses in both intestinal epithelial cells (IECs) and dendritic cells [35]. Together, this indicates that disrupted mucus production impacts the microbiota and/or promotes a loss of immune tolerance to gut microbes.
AMPs such as defensins, C-type lectins, and cathelicidins are constitutively expressed in the intestine and act to contain the colonization of commensal microbes. Paneth cells, and to a lesser extent conventional enterocytes, are a significant source of intestinal AMPs whose expression is regulated by the recognition of microbial products by innate immune receptors such as NOD2. Interestingly, while Paneth cell deficiency and NOD2 polymorphisms have been associated with reduced production of defensins and ileal CD, these associations have not been observed in the majority of AS patients [36]. Indeed, ileal biopsies from AS patients exhibit an upregulation of Paneth cell AMP expression, and myeloid cells from SpA-susceptible B27 rats exhibit a transcriptional profile with markedly increased AMP expression [36,37]. Further studies should examine whether elevated AMP expression either leads to the loss of protective commensal species in the gut of SpA-susceptible individuals and/or creates an ecological niche exploited by pathogenic/in-flammatory gut microbes.
Secretory IgA production is another critical barrier defense mechanism. It is apparent that AS (and IBD patients) have increased levels of anti-Klebsiella and anti-enterobacterial secretory IgA (reviewed in Ref. [38]), which as mentioned potentially may cross-react with self-antigens in the joint such as HLA B27. Elevated IgA levels have also been reported in the blood and synovial tissue of AS patients [39,40]. In light of numerous reports of the co-occurrence of AS and selective IgA deficiency (SIgAD), however, it is possible that these antibodies are not an etiopathogenic mechanism at all, but merely indicative of increased bacterial translocation from the gut in SpA patients. Indeed, given the role of sIgA in containing and shaping the composition of the intestinal microbiota, it is tempting to speculate that a defective sIgA response to undefined members of the microbiota could plausibly contribute to AS pathogenesis. Consistent with this hypothesis is the severe AS observed in some SIgAD patients [41].
Microbial sensing and barrier function
Constitutive sensing of local microbial products plays an intrinsic role in epithelial integrity and the maintenance of barrier function. Microbes stimulate signaling pathways such as NFκB, mitogen-activated protein (MAP) kinase, and inflammasome/caspase signaling, which modulate several aspects of IEC biology and the activation of local immune cells. These include epithelial proliferation and differentiation, expression of adhesion molecules/tight junction proteins, and secretion of chemokines and cytokines that are key local signals in the immune milieu of the gut.
These factors are plausibly dysregulated in SpA patients. For instance, the blunted and fused villi of ileal SpA inflammation are indicative of the perturbed proliferative responses of resident IEC [42]. Cadherin/catenin molecules mediate celle–cell adhesion, and increased E-cadherin expression by IECs has been observed in SpA patients with subclinical bowel inflammation [43]. Moreover, colonic T cells from AS patients exhibit enhanced expression of αEb7, an E-cadherin ligand that helps mediate T celleepithelial cell interactions. Conversely, however, the IBD phenotype of mice with disrupted N-cadherin function indicates that a diminished expression of adhesion molecules may be a primary pathogenic event [44], and these changes may be secondary to ongoing intestinal inflammation.
Further port for the hypothesis that the intestinal epithelium is perturbed in SpA patients is the observation that both SpA patients and their first-degree relatives exhibit increased bowel permeability [45]. Increased intestinal permeability is also observed in B27+ transgenic rats, although no association was found between HLA-B27 status and permeability in a human study [46,47]. This long-muted “leaky gut” hypothesis hinges on the idea that an intact epithelium limits the exposure of microbial antigen to mucosal immune cells, which may otherwise break oral tolerance. Furthermore, systemic translocation may also permit the accumulation of microbial antigens or adjuvants to sites of peripheral SpA inflammation (discussed further below). Innate immune cells and IECs recognize invading microorganisms through microbe-associated molecular patterns (MAMPs). These are highly conserved microbial structures that are recognized by PRRs on these cells, such as TLR or receptors of the NOD family. With a few exceptions, mice deficient in PRRs including Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), and C-type lectin receptors (CLRs) and their downstream signaling components do not exhibit spontaneous bowel inflammation, but exhibit impaired epithelial repair responses and bowel inflammation following acute challenge to the intestinal epithelium. The nature of this acute challenge in IBD or AS patients remains to be determined, but is consistent with a multi-hit model of pathogenesis where environment and host genotype intersect to drive disease. The polymorphisms in NOD2 that predispose to CD are primarily in the leucine-rich repeat region of the protein, a region responsible for bacterial sensing. The NOD2 knockout mouse might thus be a model with features that resemble the effect of NOD2 polymorphisms predisposing to CD. Infection of mice lacking flagellin sensor TLR5 with adherent/invasive E. coli, a gut microbe itself associated with CD-like lesions, notably led to sustained inflammation even after clearance of the offending microbe [48]. Importantly, this emphasizes that a failure to observe differences in the microbiota of SpA patients and controls may not preclude a role for gut microbes in SpA pathogenesis.
CARD9 has been identified as an AS and CD susceptibility gene in multiple studies and mediates signals from dectin-1 and dectin-2 receptors, which recognize β-glucan, a component of fungal and bacterial cell walls [49]. CARD9 also augments muramyl-dipeptide activation of NOD2 (CARD15), in addition to signaling by the DNA damage/viral DNA sensor Rad50 [50]. This underscores the potential of diverse prokaryotic, eukaryotic, and viral members of the microbiota to modulate host responses via CARD9. Interestingly, following acute disruption to the intestinal epithelium by chemical agents or intestinal pathogens, CARD9-deficient mice exhibited diminished epithelial restitution and repair and more severe intestinal inflammation [51]. An inflammatory role for CARD9 signaling, however, is implicated by the spondyloarthritic phenotype of SKG mice following intraperitoneal administration of β-glucan [52]. These dual roles of CARD9 signaling are indicative that inflammatory innate signaling pathways in the periphery may have been largely co-opted in the gut towards homeostatic functions to avoid chronic microbially induced inflammation.
Beyond microbial sensing, certain endogenous tissue-derived stress factors bind to the same PRRs. These factors are called damage-associated molecular patterns (DAMPs) and can be considered as “alarmins”, danger signals that indicate cell stress or damage. The fact that PAMPs and DAMPs share the same receptors for innate immune activation may also provide a link between gut (with microbial-derived PAMPs) and joint (with tissue-derived DAMPs) inflammation. An additional trigger for release of DAMPs in the joint or entheses might be mechanical stress [53].
An example of DAMPs are calgranulins, a set of cytosolic proteins from the S100 family that are expressed by phagocytic myeloid cells (granulocytes and monocytes). They consist of S100A8, S100A9, and S100A12. S100A8/9 form a complex that is also called calprotectin. As they represent initial cells undergoing stress, and are involved in the early developing inflammatory reaction, they could be promising markers for monitoring local disease activity [54].
Bacterial handling – autophagy and ER stress
In addition to defects in microbial sensing, diminished bacterial handling by host immune and nonimmune cells may also lead to an altered intestinal microbiota and/or the propagation of inflammatory responses to intestinal microbes.
Autophagy promotes cellular immunity by the degradation of intracellular pathogens and homeostasis by the degradation and recycling of cellular organelles. Autophagy inhibits reactive oxygen species generation, which may directly cause tissue damage itself or trigger inflammatory signaling pathways such as the NLRP3 inflammasome. Autophagy is also known to limit pyroptosis, an in-flammatory form of caspase-1-dependent cell death. In chronically inflamed AS intestinal tissue, increased expression of multiple autophagy proteins has been reported, including LC3II, ATG5, and ATG12 [55]. This may reflect that the handling of intestinal microbes by autophagic pathways is dys-regulated in SpA patients. Epithelial cell autophagy also limits dissemination of invasive bacteria and commensals to extraintestinal sites [56]. Whereas autophagy has been firmly implicated in the pathogenesis of IBD, including primary defects in bacterial handling leading to ileitis, the CD-associated autophagy genes ATG16L and immunity-related guanosine triphosphatase family M protein (IRGM) have not been associated with AS so far.
ER stress responses are another homeostatic pathway that may be disturbed in the inflamed intestine. The findings that polymorphisms in genes associated with or proximal to ER stress pathways are associated with CD and AS, and the increased susceptibility of mice with genetic lesions in the ER stress pathway such as XBP-1 and IRE1 to bowel inflammation indicate that perturbed ER stress responses may also be pertinent to IBD and SpA pathogenesis [57,58]. Interestingly, extensive studies by Colbert and others have identified that HLA B27 is susceptible to protein misfolding and ER accumulation, serving as a potent trigger of the ER stress response (referenced in Ref. [58]). In B27-expressing macrophages, microbial signals such as lipopolysaccharide (LPS) augment this response, and elaborate the T helper 17 (Th17)-associated cytokine, IL-23. Thus, innate sensing of gut bacteria or their products may be particularly primed to intersect with ER-stress pathways promoting inflammation. ER-stress-induced IL-1α production has also been shown to promote osteoclast formation, which may be particularly relevant with respect to the joint [58]. Nonetheless, a limited number of studies have not yet reached consensus as to how the ER stress response may be dysregulated in SpA patient samples.
Chronic immune activation and migration to joint
A dysfunctional interaction between gut bacteria and the mucosal immune system could also play an important role in the initiation and/or perpetuation of SpA. Following this hypothesis, an aberrant reaction to luminal antigens and/or bacteria in the gut leads to mucosal inflammation. This would then ultimately lead to joint disease through a process that is only partially understood. Suggested mechanisms based on histological and immunological studies involve uncontrolled immune activation and/or deficient immunoregulation resulting in chronic inflammation. Immune activation may also lead to aberrant migration of intestinal macrophages and lymphocytes from inflamed gut mucosa to the joint or other SpA-affected tissue. As noted above, bacterial fragments have been discovered many years ago in the synovial tissue and fluid of patients with ReA and other forms of SpA. Furthermore, stromal cells in gut and joints were reported to respond to pathologic levels of tumor necrosis factor (TNF), thereby contributing to combined gut and joint pathologies. Indeed, the enhanced messenger RNA (mRNA) stability of TNF in mice leads to the development of inflammation at both of these sites, further suggesting a common TNF-dependent mechanism in disease causation [59].
The innate immune system seems to play an important role in SpA pathogenesis. A notable finding in this regard, which also highlights the importance of the gute–joint axis, is the fact that macrophages expressing CD163 were increased in the gut of both CD and SpA. The same set of macrophages was selectively increased in the SpA synovium [60]. A number of studies also indicate that synovial T cells may also originate in the gut mucosa. Whereas naïve T cells readily recirculate between different lymphatic organs, immunoblasts express homing receptors that favor their migration to lymphoid tissue where they encountered an antigen. Salmi and colleagues demonstrated that mucosal T cells bound synovial high endothelial venules (HEV), which allow extravasation from blood into joint tissue, with comparable efficiency to mucosal HEV [61]. Lymphocytes isolated from SpA synovial tissue were also found to be enriched for integrin α4β7, a ligand for intestinal addressing MadCAM-1, present on vascular endothelium and integrin αEβ7, which binds to the IEC-expressed E-cadherin (reviewed in Ref. [62]). However, while indicative that these cells may have intestinal origins, upregulation of these integrins may also be a result of T cell activation or transforming growth factor beta (TGFβ) exposure [63]. T cell clones specific for enteric pathogens, Salmonella typhi and Y. enterocolitica, have also been isolated from the synovial tissue of ReA patients, although whether these T cells are primed in the gut or their microbial versus host specificity remains uncertain [64,65].
The IL-23/Th17 axis
IL-23 and Th17 signature cytokines, IL17 and IL-22, also provide another link between mucosal and joint immunity. IL-23 and IL-17 expression has been reported to be upregulated in the gut, peripheral blood, and synovium of SpA patients, although with significant variations that may reflect disease activity or duration and the tissue or cell types examined (reviewed by Ref. [66]). Nonetheless, these cytokines drive a number of processes that may be relevant to pathogenesis in the joint, including leukocyte accumulation and differentiation, osteoblast proliferation, and tissue remodeling. Interestingly, entheseal-resident IL-23R+ve RORgt+ve positive cells have recently been identified in a murine model of AS. These cells may be critical mediators of SpA [67].
The intestine is a key inductive site for Th17 generation. Microbiota-derived signals including adenosine triphosphate (ATP) and MAMPs such as LPS and flagellin induce the release of IL-1β, IL-6, and IL-23 by intestinal mononuclear phagocytes creating a tissue microenvironment favorable to Th17 differentiation. These responses may be particularly potent if associated with infection with intestinal pathogens. For instance, Salmonella enteritidis robustly induces local Th17 responses in animal infection models, which may also manifest in ReA-like disease [68]. Under homeostatic conditions, selected gut microbes such as segmented filamentous bacteria (SFB) may also induce Th17 generation in the intestinal lamina propria, with high specificity for SFB [69]. Indeed, a critical link between this specific microbe and extraintestinal disease was provided by the finding that monoassociation of K/BxN mice with SFB was sufficient to induce arthritogenic T cells in this model [70]. Intriguingly, using a transgenic reporter system, intestinal Th17 cells exhibited high migratory capacity and were evident in the spleen of K/BxN mice coincident with their development of arthritis [71].
In addition to these specific examples from animal models, it is also worth noting that, in inflamed AS gut samples, an expansion of IL-17-producing T cells has not been readily observed in contrast to IBD patients [72]. Moreover, Th1 and Th22 cells were also found at comparable frequency in AS gut tissue and controls, despite their expansion in CD [73]. Thus, T cell-independent sources of IL-17 and IL-22 likely warrant further scrutiny in SpA patients. Indeed, one report indicates that NKp44+ innate lymphoid cells (ILCs) are a dominant source of IL-22 in AS patients, and are significantly expanded relative to levels in CD patients and controls [32]. As NKp44 ligands include microbial products [74], this population is plausibly directly activated by gut-resident bacteria.
Other studies of IL-22-producing ILCs show their profound impact on the hostemicrobiota relationship. IL-22R itself is expressed on stromal cells, and many IL-22-dependent effects on the microbiota may be exerted by IL-22 on the intestinal epithelium. These include the production of AMPs, fucosylation of epithelial cells, and mucus production [75–77]. Deficiency in the ILC/IL-22 axis may allow the outgrowth or extraintestinal dissemination of gut commensals such as Alcali-genes spp. or intestinal pathogens [75,78]. Moreover, ILC may also limit the expansion of commensal reactive Th17 responses and favor the induction of regulatory T cells (Tregs) [79,80]. Thus, the expansion of IL-22+ NKp44 cells in the intestine of SpA patients may reflect an attempt to restore these homeostatic modules and limit intestinal inflammation. Conversely, NKp44 agonism has also been shown to elicit TNF and other inflammatory cytokines [81]. Moreover, ILCs also clearly drive disease in some murine IBD models [82]. Thus, a putative pathogenic role for ILC in SpA remains to be excluded.
Innate-like T cells with regulatory potential have also been invoked in SpA pathogenesis. Invariant natural killer T (iNKT) cells appear to dampen TNF-driven joint and gut inflammation [83]. At least in the intestine, iNKT cell engagement of CD1d on IEC promotes the expression of immune regulatory cytokine IL-10 and immune homeostasis, a negative feedback that may be impaired in IBD patients who exhibit diminished CD1d expression [84]. Curiously, however, the balance between their role as regulators versus effector cells seems to be tightly regulated as iNKT cells are the main effectors of IL-13-dependent oxazolone-induced colitis, a mouse model of ulcerative colitis [85]. Similarly, γδ T cells are nonconventional T cells that, like iNKT cells, respond more rapidly than “traditional” lymphocytes, can recognize microbial antigens, and exhibit divergent roles in the promotion or amelioration of IBD in animal models. With respect to SpA, their potential pathogenic role is highlighted by the expansion of γδ T cells in the peripheral blood of AS patients, many of which express a Th17 signature [86]. Indeed, the same study indicated that gd T cells may be a more potent source of IL-17 than conventional ab T cell receptor-positive (TCR+ve) CD4 T cells. As iNKT, γδ T cells, and ILC populations may sense differences in the gut microbiome, it will be interesting to understand how dysbiotic changes may alter the balance between regulation and effector function. Indeed, their innate-like capacity to rapidly respond may render them exquisitely sensitive to the microbial and cytokine milieu of their host tissue and explain their complex role in SpA and IBD pathogenesis.
Several teams have also explored the role of Forkhead box P3 (FoxP3)-positive regulatory T cells in modifying gut and joint inflammation. These master regulators of tolerance were shown to be dysfunctional in preclinical mouse models of IBD [87], but the role of Tregs in human disease remains unclear. In SpA patients, data are conflicting with some reports describing unaltered or decreased frequencies of Tregs in peripheral blood, but rather increased frequencies in synovial fluid [88], suggesting local accumulation within inflamed joints, which mirrors earlier reports on increased frequencies in terminal ileum. These increases likely reflect regulatory feedback loops induced by chronic inflammation.
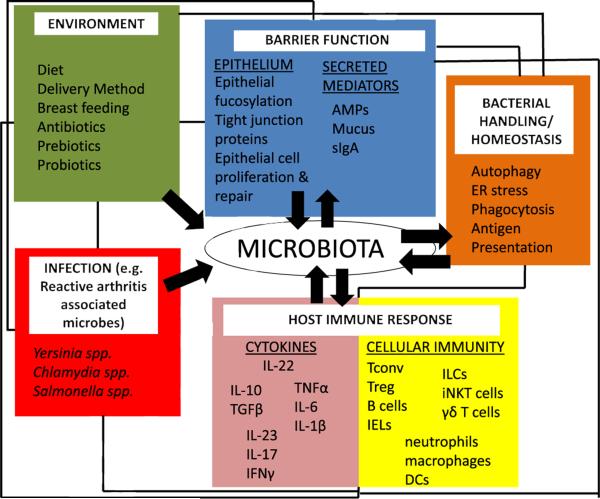
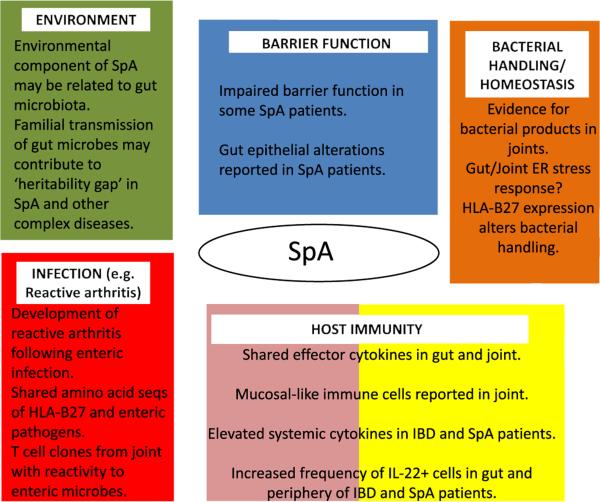
In summary, the intimate relationship between “normal” gut microbes and both adaptive and innate immune cells likely dictates the homeostatic threshold of the gut. This delicate balance enables the gut immune system to remain poised to respond rapidly to intestinal pathogens, under a dominant background of immune regulation. However, dysbiotic changes to the gut, for instance, mediated by putative changes in barrier defense, microbial sensing, or bacterial handling may shift this balance towards inflammatory effector pathways. In turn, this may further amplify dysbiotic changes and disruption of gut homeostasis. Currently, links between gut and joint inflammation remain unclear, but, under this setting, extraintestinal translocation of microbial products, migration of intestinal immune cells, or systemic cytokine release may create a joint microenvironment favorable to SpA pathogenesis (Figs. 1 and 2).
Fig. 1.
Multiple pathways implicated in IBD and SpA pathogenesis impact the hostemicrobiota relationship. The gut microbiota is shaped by both external factors (e.g., environmental factors or infection) and by the host itself. Barrier function, bacterial handling/homeostatic pathways (such as autophagy or ER stress), and host immune responses may contribute to the composition of the microbiota, but are also shaped by the microbiota itself (black arrows). The immune dialog between the host and the microbiota is particularly complex, as effector and regulatory responses must coexist to allow robust immunity to enteric pathogens while avoiding chronic inflammatory responses to commensal microbes (see main text). Black lines denote that these various pathways are interconnected. For instance, manipulation of diet could indirectly modulate the microbiota through direct effects on barrier function or host immunity. AMPs – antimicrobial peptides, Tconv – conventional T cell, Treg – regulatory T cell, IEL – intraepithelial lymphocytes, ILC – innate lymphoid cell, iNKT – invariant natural killer T cell, and DCs – dendritic cells.
Fig. 2.
Evidence that links microbiota-associated pathways with spondyloarthropathy. Mechanisms that link the microbiota with SpA pathogenesis remain to be elucidated. Nonetheless, several lines of evidence implicate pathways that relate to the microbiota (Fig. 1) in SpA pathogenesis.
Therapeutic manipulation of the gut microbiota
Probiotics
While there is a large, mostly unregulated market for probiotics, rigorous scientific study of the putative benefits of these agents and the mechanism of action is sparse. However, studies have shown that even a single bacterial species in the gut can bias the homeostatic balance of the immune system in either direction. Bacteroides fragilis, a common culturable commensal microorganism, ports anti-inflammatory responses by activating IL-10-producing Tregs through its polysaccharide A component, which subsequently dampens Th17 responses [89]. Intestinal clostridia species can induce colonic Treg development and promote resistance to colitis and systemic immunoglobulin E responses in adult mice [90]. Given that decreased proportions of IL-10-producing Tregs are thought to contribute to disease development in HLA-B27 rats [91], treatment with Treg-inducing microorganisms or bacterial components is potentially an innovative modality to treat SpA and related diseases.
Common commercially available probiotics often contain Lactobacillus species and Bifidobacterium. One study showed that ingestion of either heat-killed or live Lactobacillus rhamnosus GG caused amelioration of experimental arthritis induced by adjuvant or tropomyosin in rats [92]. The mechanism of action of L. rhamnosus GG in treating arthritis is thought to be via its inhibition of the p38 MAPK pathway, which results in inhibition of COX2 expression [93]. VSL#3 is a commercially available pro-biotic that contains eight strains of bacteria including several Lactobacillus and Bifidobacterium species. Administration of VSL#3 to IL-10-deficient mice resulted in decreased TNF-α and IFN-α by the gut mucosa, both of which are cytokines important in the pathogenesis of SpA [94]. Despite the preclinical data porting probiotic use in SpA, one randomized controlled trial of a probiotic containing Streptococcus salivarius, Bifidobacterium lactis, and Lactobacillus acidophilus did not demonstrate benefit over placebo in ameliorating symptoms in active SpA patients [95]. Similarly, in an Internet-based randomized controlled trial, a probiotic containing Lactobacillus salivarius, Lactobacillus paracasei, Bifido-bacterium infantis, and Bifidobacterium bifidum did not improve well-being or bowel symptoms over placebo in SpA patients, although this trial was relatively short (12 weeks) [96]. It is important to note, however, that the lack of efficacy in these clinical trials may be due to the wrong probiotics being used, small study size, or a number of study design-related factors.
Prebiotics/diet
The gut microbiota is profoundly affected by diet, which is sometimes termed a prebiotic, whereas synbiotics is the therapeutic combination of diet and probiotics. Several studies show that long-term diet markedly affects the gut microbiota; however, more recently, studies in both animals and humans have shown that shifting diet macronutrients can change the gut microbiota consistently in a rapid fashion [3]. In fact, David et al. demonstrated an association between short-term consumption of dietary fat and outgrowth of microorganisms that can trigger IBD [3]. Despite this information, there is a dearth of data to port specific dietary interventions that alter disease activity in SpA. One study evaluated a prebiotic regimen containing inulin and oligofructose in HLA-B27 transgenic rats and concluded that the treatment was effective in reducing colitis, and it was associated with a change in the gut microbiota and decreased tissue inflammatory cytokines [97]. This study did not examine the specific effect of the prebiotic on arthritis disease activity. Another study showed that HLA-B27 transgenic rats had decreased inflammatory mediators, colitis, and B. fragilis, when fed apples high in polyphenols [98]. There are very few publications reporting diet changes in SpA patients.
Antibiotics and fecal transplant
It is well known that antibiotics can dramatically decrease the biodiversity of the gut after prolonged use. Because of the relationship of SpA to certain Gram-negative bacteria, a number of clinical trials have assessed the utility of antibiotics to treat ReA. In a meta-analysis of these clinical trials excluding unblinded trials, it was unclear whether or not antibiotics were effective for joint disease, although antibiotics were associated with increased gastrointestinal adverse events [99].
Due to the success of fecal transplant in the treatment of Clostridium difficile infection compared to traditional methods with antibiotics [100], it is now known that this treatment can result in reestablishment of gut biodiversity and the healthy state of the gut. It is plausible that fecal transplant could be successful in the treatment of other diseases associated with decreased biodiversity of the gut such as IBD and, potentially, SpA.
Summary and future directions
Our knowledge of the microbiota in SpA pathogenesis will undoubtedly benefit from increased resolution of the intestinal microbiota. Community-wide analysis of the microbiota by 16s sequencing will be facilitated by rapidly increased sequencing speeds, reduced sequencing cost, and ability to multiplex hundreds of samples in the same sequencing run. Whereas 16s sequencing largely provides sequencing data at the genus level, whole bacterial genome sequencing or metagenomics allows resolution of microbes on the species and even strain level. Importantly, this approach allows eukaryotic and viral nucleic acid sequences to be uncovered, gaining insight into novel parasitic, fungal, and viral constituents of the microbiota that may also modulate host immunity [7]. A recent example is provided by the identification of the crAssphage virus, itself a bacteriophage in the intestine predicted to infect members of the Bacteroides genus [101]. Moreover, gene-level resolution provides further targets for transcriptional or metabolomic profiling of the intestinal microbiota – research areas themselves still in their relative infancy.
Functional studies have also begun to facilitate the identification of previously unculturable human fecal microbiota members that transmit immune phenotypes to germ-free rodents [102]. The high translational potential of this approach is striking, and this indicates that animal models will continue to be a mainstay of future microbiota research. Nonetheless, human studies of the microbiota of patients and controls would greatly contribute to our understanding of SpA pathogenesis. Longitudinal studies in SpA patients would also help in this regard with the potential to identify microbiota-related biomarkers of disease progression or treatment response. In short, the world of clinical opportunity created by the microbial world within us is difficult to overstate.
Practice points.
Approximately one-half of SpA patients exhibit signs of microscopic gut inflammation.
Chronic gut inflammation predicts a higher risk of evolving to AS.
Remission of joint disease is associated with reduced bowel inflammation and vice versa. Therapies targeting inflammation at both sites may, therefore, be beneficial in SpA patients with bowel inflammation.
Despite compelling evidence for a major role for the microbiota in SpA and CD pathogenesis, there are currently insufficient data from human studies to make clinical recommendations with respect to therapeutic modulation of the microbiota by diet, probiotics, antibiotics, or other means.
Research agenda.
The use of high-resolution molecular approaches to identify putative dysbiotic changes in the microbiota of SpA patient populations. Preferably, these studies will be longitudinal, allowing the evolution of the microbiota with SpA disease to be established.
Identification of microbes or microbial products that modify SpA-like disease in animal models.
Further identification of host inflammatory or regulatory pathways that are modulated by the microbiota.
Clinical trials for therapeutic manipulation of the microbiota or the administration of micro bial products for the treatment of SpA patients.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- *1.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon-Soro A, Tomas I, Cabrera-Rubio R, et al. Microbial geography of the oral cavity. J Dent Res. 2013;92(7):616–21. doi: 10.1177/0022034513488119. [DOI] [PubMed] [Google Scholar]
- 3.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *5.Van Praet L, Van den Bosch FE, Jacques P, et al. Microscopic gut inflammation in axial spondyloarthritis: a multi-parametric predictive model. Ann Rheum Dis. 2013;72(3):414–7. doi: 10.1136/annrheumdis-2012-202135. [DOI] [PubMed] [Google Scholar]
- 6.Shivashankar R, Loftus EV, Jr, Tremaine WJ, et al. Incidence of spondyloarthropathy in patients with Crohn's disease: a population-based study. J Rheumatol. 2012;39(11):2148–52. doi: 10.3899/jrheum.120321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norman JM, Handley SA, Virgin HW. Kingdom-agnostic metagenomics and the importance of complete characterization of enteric microbial communities. Gastroenterology. 2014;146(6):1459–69. doi: 10.1053/j.gastro.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielants H, Veys EM, Cuvelier C, et al. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J Rheumatol. 1995;22(12):2279–84. [PubMed] [Google Scholar]
- *9.Van Praet L, Jans L, Carron P, et al. Degree of bone marrow oedema in sacroiliac joints of patients with axial spondyloarthritis is linked to gut inflammation and male sex: results from the GIANT cohort. Ann Rheum Dis. 2014;73(6):1186–9. doi: 10.1136/annrheumdis-2013-203854. [DOI] [PubMed] [Google Scholar]
- 10.Leirisalo-Repo M. Prognosis, course of disease, and treatment of the spondyloarthropathies. Rheum Dis Clin North Am. 1998;24(4):737–51. viii. doi: 10.1016/s0889-857x(05)70039-9. [DOI] [PubMed] [Google Scholar]
- 11.Keat AC, Maini RN, Nkwazi GC, et al. Role of Chlamydia trachomatis and HLA-B27 in sexually acquired reactive arthritis. Br Med J. 1978;1(6113):605–7. doi: 10.1136/bmj.1.6113.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Granfors K, Jalkanen S, Lindberg AA, et al. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990;335:685–8. doi: 10.1016/0140-6736(90)90804-e. [DOI] [PubMed] [Google Scholar]
- 13.Merilahti-Palo R, Soderstrom KO, Lahesmaa-Rantala R, et al. Bacterial antigens in synovial biopsy specimens in Yersinia triggered reactive arthritis. Ann Rheum Dis. 1991;50(2):87–90. doi: 10.1136/ard.50.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Geczy AF, Alexander K, Bashir HV, et al. A factor(s) in Klebsiella culture filtrates specifically modifies an HLA-B27 associated cell-surface component. Nature. 1980;283(5749):782–4. doi: 10.1038/283782a0. [DOI] [PubMed] [Google Scholar]
- 15.Rashid T, Ebringer A. Ankylosing spondylitis is linked to Klebsiella – the evidence. Clin Rheumatol. 2007;26(6):858–64. doi: 10.1007/s10067-006-0488-7. [DOI] [PubMed] [Google Scholar]
- 16.Ebringer R, Cawdell D, Ebringer A. Klebsiella pneumoniae and acute anterior uveitis in ankylosing spondylitis. Br Med J. 1979;1(6160):383. doi: 10.1136/bmj.1.6160.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Bohemen CG, Grumet FC, Zanen HC. Identification of HLA-B27M1 and -M2 cross-reactive antigens in Klebsiella, Shigella and Yersinia. Immunology. 1984;52(4):607–10. [PMC free article] [PubMed] [Google Scholar]
- 18.Schwimmbeck PL, Oldstone MB. Molecular mimicry between human leukocyte antigen B27 and Klebsiella. Consequences for spondyloarthropathies. Am J Med. 1988;85(6A):51–3. doi: 10.1016/0002-9343(88)90385-3. [DOI] [PubMed] [Google Scholar]
- 19.Scofield RH, Warren WL, Koelsch G, et al. A hypothesis for the HLA-B27 immune dysregulation in spondyloarthropathy: contributions from enteric organisms, B27 structure, peptides bound by B27, and convergent evolution. Proc Natl Acad Sci U S A. 1993;90(20):9330–4. doi: 10.1073/pnas.90.20.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge S, He Q, Granfors K. HLA-B27 modulates intracellular growth of Salmonella pathogenicity island 2 mutants and production of cytokines in infected monocytic U937 cells. PLoS One. 2012;7(3):e34093. doi: 10.1371/journal.pone.0034093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagg AJ, Breban M, Hammer RE, et al. Defective dendritic cell (DC) function in a HLA-B27 transgenic rat model of spondyloarthropathy (SpA). Adv Exp Med Biol. 1995;378:557–9. doi: 10.1007/978-1-4615-1971-3_125. [DOI] [PubMed] [Google Scholar]
- 22.Taurog JD. Animal models of spondyloarthritis. Adv Exp Med Biol. 2009;649:245–54. doi: 10.1007/978-1-4419-0298-6_18. [DOI] [PubMed] [Google Scholar]
- 23.Dieleman LA, Goerres MS, Arends A, et al. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut. 2003;52(3):370–6. doi: 10.1136/gut.52.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40(6):843–54. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar PS, Griffen AL, Barton JA, et al. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82(5):338–44. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- *27.Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stebbings S, Munro K, Simon MA, et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology (Oxford) 2002;41(12):1395–401. doi: 10.1093/rheumatology/41.12.1395. [DOI] [PubMed] [Google Scholar]
- 29.McBurney W, Mangold M, Munro K, et al. PCR/DGGE and 16S rRNA gene library analysis of the colonic microbiota of HLA-B27/beta2-microglobulin transgenic rats. Lett Appl Microbiol. 2006;42(2):165–71. doi: 10.1111/j.1472-765X.2005.01811.x. [DOI] [PubMed] [Google Scholar]
- *30.Lin P, Bach M, Asquith M, et al. HLA-B27 and human beta2-microglobulin affect the gut microbiota of transgenic rats. PLoS One. 2014;9(8):e105684. doi: 10.1371/journal.pone.0105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciccia F, Accardo-Palumbo A, Alessandro R, et al. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 2012;64(6):1869–78. doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]
- 33.Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–29. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Faure M, Moennoz D, Mettraux C, et al. The chronic colitis developed by HLA-B27 transgenic rats is associated with altered in vivo mucin synthesis. Dig Dis Sci. 2004;49(2):339–46. doi: 10.1023/b:ddas.0000017462.75257.70. [DOI] [PubMed] [Google Scholar]
- 35.Shan M, Gentile M, Yeiser JR, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342(6157):447–53. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laukens D, Peeters H, Marichal D, et al. CARD15 gene polymorphisms in patients with spondyloarthropathies identify a specific phenotype previously related to Crohn's disease. Ann Rheum Dis. 2005;64(6):930–5. doi: 10.1136/ard.2004.028837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fert I, Cagnard N, Glatigny S, et al. Reverse interferon signature is characteristic of antigen-presenting cells in human and rat spondyloarthritis. Arthritis Rheumatol. 2014;66(4):841–51. doi: 10.1002/art.38318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashid T, Wilson C, Ebringer A. The link between ankylosing spondylitis, Crohn's disease, Klebsiella, and starch consumption. Clin Dev Immunol. 2013;2013:872632. doi: 10.1155/2013/872632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veys EM, van Leare M. Serum IgG, IgM, and IgA levels in ankylosing spondylitis. Ann Rheum Dis. 1973;32(6):493–6. doi: 10.1136/ard.32.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Revell PA, Mayston V. Histopathology of the synovial membrane of peripheral joints in ankylosing spondylitis. Ann Rheum Dis. 1982;41(6):579–86. doi: 10.1136/ard.41.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomes MM, Herr AB. IgA and IgA-specific receptors in human disease: structural and functional insights into pathogenesis and therapeutic potential. Springer Semin Immunopathol. 2006;28(4):383–95. doi: 10.1007/s00281-006-0048-x. [DOI] [PubMed] [Google Scholar]
- 42.Cuvelier C, Barbatis C, Mielants H, et al. Histopathology of intestinal inflammation related to reactive arthritis. Gut. 1987;28(4):394–401. doi: 10.1136/gut.28.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demetter P, Baeten D, De Keyser F, et al. Subclinical gut inflammation in spondyloarthropathy patients is associated with upregulation of the E-cadherin/catenin complex. Ann Rheum Dis. 2000;59(3):211–6. doi: 10.1136/ard.59.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270(5239):1203–7. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 45.Mielants H, De Vos M, Goemaere S, et al. Intestinal mucosal permeability in inflammatory rheumatic diseases. II. Role of disease. J Rheumatol. 1991;18(3):394–400. [PubMed] [Google Scholar]
- 46.Kerr SW, Wolyniec WW, Filipovic Z, et al. Repeated measurement of intestinal permeability as an assessment of colitis severity in HLA-B27 transgenic rats. J Pharmacol Exp Ther. 1999;291(2):903–10. [PubMed] [Google Scholar]
- 47.Vaile JH, Meddings JB, Yacyshyn BR, et al. Bowel permeability and CD45RO expression on circulating CD20+ B cells in patients with ankylosing spondylitis and their relatives. J Rheumatol. 1999;26(1):128–35. [PubMed] [Google Scholar]
- 48.Chassaing B, Koren O, Carvalho FA, et al. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2014;63(7):1069–80. doi: 10.1136/gutjnl-2013-304909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pointon JJ, Harvey D, Karaderi T, et al. Elucidating the chromosome 9 association with AS; CARD9 is a candidate gene. Genes Immun. 2010;11(6):490–6. doi: 10.1038/gene.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth S, Rottach A, Lotz-Havla AS, et al. Rad50-CARD9 interactions link cytosolic DNA sensing to IL-1beta production. Nat Immunol. 2014;15(6):538–45. doi: 10.1038/ni.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sokol H, Conway KL, Zhang M, et al. Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice. Gastroenterology. 2013;145(3):591–601. e3. doi: 10.1053/j.gastro.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benham H, Rehaume LM, Hasnain SZ, et al. Interleukin-23 mediates the intestinal response to microbial beta-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol. 2014;66(7):1755–67. doi: 10.1002/art.38638. [DOI] [PubMed] [Google Scholar]
- 53.Jacques P, Lambrecht S, Verheugen E, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014;73(2):437–45. doi: 10.1136/annrheumdis-2013-203643. [DOI] [PubMed] [Google Scholar]
- 54.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58(6):859–68. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- 55.Ciccia F, Accardo-Palumbo A, Rizzo A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benjamin JL, Sumpter R, Jr, Levine B, et al. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe. 2013;13(6):723–34. doi: 10.1016/j.chom.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaser A, Adolph TE, Blumberg RS. The unfolded protein response and gastrointestinal disease. Semin Immunopathol. 2013;35(3):307–19. doi: 10.1007/s00281-013-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57(1):44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 60.Baeten D, Demetter P, Cuvelier CA, et al. Macrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. J Pathol. 2002;196(3):343–50. doi: 10.1002/path.1044. [DOI] [PubMed] [Google Scholar]
- 61.Salmi M, Andrew DP, Butcher EC, et al. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995;181(1):137–49. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacques P, Elewaut D. Joint expedition: linking gut inflammation to arthritis. Mucosal Immunol. 2008;1(5):364–71. doi: 10.1038/mi.2008.24. [DOI] [PubMed] [Google Scholar]
- 63.Austrup F, Rebstock S, Kilshaw PJ, et al. Transforming growth factor-beta 1-induced expression of the mucosa-related integrin alpha E on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol. 1995;25(6):1487–91. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 64.Hermann E, Mayet WJ, Poralla T, et al. Salmonella-reactive synovial fluid T-cell clones in a patient with post-infectious Salmonella arthritis. Scand J Rheumatol. 1990;19(5):350–5. doi: 10.3109/03009749009096790. [DOI] [PubMed] [Google Scholar]
- 65.Probst P, Hermann E, Meyer zum Buschenfelde KH, et al. Multiclonal synovial T cell response to Yersinia enterocolitica in reactive arthritis: the Yersinia 61-kDa heat-shock protein is not the major target antigen. J Infect Dis. 1993;167(2):385–91. doi: 10.1093/infdis/167.2.385. [DOI] [PubMed] [Google Scholar]
- 66.Smith JA, Colbert RA. Review: the interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 2014;66(2):231–41. doi: 10.1002/art.38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18(7):1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 68.Noto Llana M, Sarnacki SH, Aya Castaneda Mdel R, et al. Consumption of Lactobacillus casei fermented milk prevents Salmonella reactive arthritis by modulating IL-23/IL-17 expression. PLoS One. 2013;8(12):e82588. doi: 10.1371/journal.pone.0082588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goto Y, Panea C, Nakato G, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal th17 cell differentiation. Immunity. 2014;40(4):594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *70.Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morton AM, Sefik E, Upadhyay R, et al. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc Natl Acad Sci U S A. 2014;111(18):6696–701. doi: 10.1073/pnas.1405634111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciccia F, Bombardieri M, Principato A, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60(4):955–65. doi: 10.1002/art.24389. [DOI] [PubMed] [Google Scholar]
- 73.Ciccia F, Guggino G, Rizzo A, et al. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren's syndrome. Ann Rheum Dis. 2012;71(2):295–301. doi: 10.1136/ard.2011.154013. [DOI] [PubMed] [Google Scholar]
- 74.Esin S, Batoni G, Counoupas C, et al. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008;76(4):1719–27. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonnenberg GF, Monticelli LA, Alenghat T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–5. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goto Y, Obata T, Kunisawa J, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345(6202):1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner JE, Stockinger B, Helmby H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog. 2013;9(10):e1003698. doi: 10.1371/journal.ppat.1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tumanov AV, Koroleva EP, Guo X, et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 2011;10(1):44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498(7452):113–7. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mortha A, Chudnovskiy A, Hashimoto D, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343(6178):1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glatzer T, Killig M, Meisig J, et al. RORgammat(+) innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity. 2013;38(6):1223–35. doi: 10.1016/j.immuni.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 82.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–5. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacques P, Venken K, Van Beneden K, et al. Invariant natural killer T cells are natural regulators of murine spondylarthritis. Arthritis Rheum. 2010;62(4):988–99. doi: 10.1002/art.27324. [DOI] [PubMed] [Google Scholar]
- 84.Olszak T, Neves JF, Dowds CM, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014;509(7501):497–502. doi: 10.1038/nature13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heller F, Fuss IJ, Nieuwenhuis EE, et al. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17(5):629–38. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 86.Kenna TJ, Davidson SI, Duan R, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive gamma/delta T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012;64(5):1420–9. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 87.Ishikawa D, Okazawa A, Corridoni D, et al. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn's disease. Mucosal Immunol. 2013;6(2):267–75. doi: 10.1038/mi.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao D, van Vollenhoven R, Klareskog L, et al. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6(4):R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *90.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Araujo LM, Fert I, Jouhault Q, et al. Increased production of interleukin-17 over interleukin-10 by treg cells implicates inducible costimulator molecule in experimental spondyloarthritis. Arthritis Rheumatol. 2014;66(9):2412–22. doi: 10.1002/art.38737. [DOI] [PubMed] [Google Scholar]
- 92.Baharav E, Mor F, Halpern M, et al. Lactobacillus GG bacteria ameliorate arthritis in Lewis rats. J Nutr. 2004;134(8):1964–9. doi: 10.1093/jn/134.8.1964. [DOI] [PubMed] [Google Scholar]
- 93.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277(52):50959–65. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121(3):580–91. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 95.Jenks K, Stebbings S, Burton J, et al. Probiotic therapy for the treatment of spondyloarthritis: a randomized controlled trial. J Rheumatol. 2010;37(10):2118–25. doi: 10.3899/jrheum.100193. [DOI] [PubMed] [Google Scholar]
- 96.Brophy S, Burrows CL, Brooks C, et al. Internet-based randomised controlled trials for the evaluation of complementary and alternative medicines: probiotics in spondyloarthropathy. BMC Musculoskelet Disord. 2008;9:4. doi: 10.1186/1471-2474-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoentjen F, Welling GW, Harmsen HJ, et al. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11(11):977–85. doi: 10.1097/01.mib.0000183421.02316.d5. [DOI] [PubMed] [Google Scholar]
- 98.Castagnini C, Luceri C, Toti S, et al. Reduction of colonic inflammation in HLA-B27 transgenic rats by feeding Marie Menard apples, rich in polyphenols. Br J Nutr. 2009;102(11):1620–8. doi: 10.1017/S0007114509990936. [DOI] [PubMed] [Google Scholar]
- 99.Barber CE, Kim J, Inman RD, et al. Antibiotics for treatment of reactive arthritis: a systematic review and metaanalysis. J Rheumatol. 2013;40(6):916–28. doi: 10.3899/jrheum.121192. [DOI] [PubMed] [Google Scholar]
- 100.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 101.Dutilh BE, Cassman N, McNair K, et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat Commun. 2014;5:4498. doi: 10.1038/ncomms5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahern PP, Faith JJ, Gordon JI. Mining the human gut microbiota for effector strains that shape the immune system. Immunity. 2014;40(6):815–23. doi: 10.1016/j.immuni.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]