Abstract
Data are very limited on vitamin D and lung cancer prevention in high-risk populations. The authors investigated whether estimated vitamin D intake was associated with lung cancer risk and whether effect modification by vitamin A existed among current/former heavy smokers and workers with occupational exposure to asbestos. A case-cohort study selected 749 incident lung cancers and 679 non-cases from the Carotene and Retinol Efficacy Trial (CARET), 1988–2005. The active intervention was supplementation of 30 mg β-carotene+25,000 IU retinyl palmitate/day. Baseline total intake including both diet (from food frequency questionnaire) and personal supplements (from brand names linked to the labeled potencies) was assessed. Hazard ratios (HR) were estimated by Cox proportional hazard models. No significant association of total vitamin D intake with lung cancer was observed overall. However, total vitamin D intake ≥600 versus <200 IU/d was associated with a lower risk of non-small cell lung cancer among former smokers (HR=0.36, 95% confidence interval [CI]=0.13–0.96). Total vitamin D intake ≥400 versus <400 IU/d was associated with a lower risk of total lung cancer among participants who received the CARET active intervention (HR=0.56, 95% CI=0.32–0.99) and among those who had total vitamin A intake ≥1,500 μg/d Retinol Activity Equivalent (RAE; HR=0.46, 95% CI=0.23–0.91). The beneficial associations were attenuated among those who did not receive the CARET active intervention or who had total vitamin A intake <1,500 μg/d RAE (P-interaction=0.02 for current smokers). Our observation suggests that vitamin A may assist vitamin D in preventing lung cancer among smokers.
Keywords: Lung cancer, estimated vitamin D intake, vitamin A, smoking, chemoprevention
INTRODUCTION
Lung cancer has been a major disease burden in the United States for six decades. It is estimated that more than 226,000 new cases of lung cancer occurred in 2012.1 Vitamin D has received increasing attention as a potential chemopreventive agent against lung cancer because it enhances innate immunity in the lung and inhibits several signaling pathways of lung carcinogenesis.2-5
Epidemiological evidence has emerged for vitamin D in relation to lung cancer prevention. Studies have linked high serum concentrations of 25-hydroxyvitamin D, the standard biomarker for assessing vitamin D status, to lower lung cancer risk in men and women.6, 7 In addition, analyses suggest that vitamin D is inversely associated with lung cancer risk in nonsmokers.8 However, data are very limited on smokers, a population with high risk for lung cancer, as analyses showed no association8, 9 or associations only in subgroups such as participants whose serum 25-hydroxyvitamin D was tested in winter or who had higher vitamin D intake.10 The subgroup findings are difficult to interpret and subject to false positive bias. It is important to study the vitamin D-lung cancer association in smokers because 90% of lung cancer occurs in this high risk population.11
Vitamin A (retinol) plays a crucial role in lung development and cell differentiation and signaling the vitamin D pathway.12 Vitamin D receptor (VDR) must form a heterodimer complex with retinoid X receptor (RXR) to regulate gene transcription.13, 14 9-cis-retinoic acid, an active vitamin A metabolite and the ligand of RXR, assists VDR signaling and suppresses the degradation of circulating vitamin D.15, 16 The consumption of vitamin A supplements and animal liver that often contains high levels of vitamin A results in elevated concentrations of active vitamin metabolites including 9-cis-retinoic acid in the body.17 However, due to its strong affinity to RXR, excess levels of 9-cis-retinoic acid can form RXR-RXR homodimers and interrupt the dimerization of RXR-VDR and VDR-related transcription.18 It is unknown whether high-dose vitamin A would assist or counteract the association of vitamin D with lung cancer among smokers.
The primary objective of this study was to investigate whether high estimated vitamin D intake from food and dietary supplements was associated with a lower risk of lung cancer among heavy smokers and workers with occupational exposure to asbestos in the Carotene and Retinol Efficacy Trial (CARET), a multicenter chemoprevention trial for lung cancer.19 The secondary objective of the current study was to investigate whether the active intervention and vitamin A intake from diet and supplements modified the association of vitamin D intake with lung cancer. CARET provided a unique opportunity for this investigation because the “supraphysiologic” dose of vitamin A in the active intervention likely led to an increase in circulating and cellular 9-cis-retinoic acid concentrations.17 We therefore hypothesized that an inverse association of vitamin D intake with lung cancer would be stronger among participants who did not receive the CARET active intervention or consumed a lower level of vitamin A from diet and supplements, compared to that among those who received the intervention or consumed a higher level of vitamin A.
MATERIALS AND METHODS
Study population
The detailed methodology of CARET has been described elsewhere.19 Briefly, eligible participants were men and women aged 50-69 years who were current or former (quitting within the previous 6 years) smokers with a history of at least 20 pack-years of cigarette smoking (n=14,254), and men aged 45-69 years who were current or former (quitting up to 15 years) smokers and exposed to asbestos in the workplace beginning at least 15 years prior (n=4,060). The active intervention was 30 mg β-carotene plus 25,000 IU retinyl palmitate supplementation daily or placebo. Recruitment began with a pilot phase during 1985 and the end of 1988 and was expanded to the full-scale efficacy trial in 1989. The capsule consumption rate was 93% through 5 years of the trial period. The efficacy trial was stopped early in 1996 due to an increase in lung cancer risk in the treatment arm. Subsequently, 94% of participants remained in a non-interventional, active follow-up, which discontinued in 2005 when funding for this additional follow-up ended.19 The Institutional Review Board of the Fred Hutchinson Cancer Research Center and each of the five other participating institutions approved all procedures for the study; participants provided written informed consent at recruitment and throughout the trial.
Case and subcohort selection
The current study was a case-cohort design. The cases were CARET participants who developed lung cancer during the efficacy trial and post-intervention follow-up. A “subcohort” serving as the comparison group was a random sample of the same size as the cases from all participants at baseline. Because enhanced dietary and supplemental vitamin data collection questionnaires were added under the efficacy trial protocol, for the present study baseline is defined as the date of transition to the efficacy protocol for those enrolled during the pilot phase. As of September 30, 2005, 1,445 lung cancer cases were ascertained among all CARET participants. After excluding those who did not successfully transition from the pilot to the efficacy protocol (n=39), who never completed a supplemental vitamin inventory (n=7) or who completed an inventory after a lung cancer diagnosis (n=6), and who attended the clinical center in Portland, Oregon (due to no access to their charts, n=377), 1,016 cases were selected. All cases were centrally adjudicated by physicians. From 13,457 participants in the other centers (Seattle, Washington; Irvine, California; New Haven, Connecticut; San Francisco, California; Baltimore, Maryland) with baseline charts available for review, a subcohort of 1,016 participants was randomly selected. Because vitamin D intake was measured as the exposure, and oral vitamin D needs to be absorbed by intestines and converted by enzymes in the liver and kidney into 1,25-dihydroxyvitamin D, the biologically active metabolite,20 we excluded cases and subcohort members who had a history of diseases in intestines, liver, and kidney (n=425); participants with no data on disease history at baseline (n=34) were also excluded. In addition, we excluded those who had no completed food frequency questionnaire (FFQ) through follow-up (n=47), who had an incomplete FFQ (i.e., any full page of the questionnaire was left blank) or implausible estimated energy intake (i.e., <800 or >5,000 kcal for males and <600 or >4,000 kcal for females) (n=50), who completed a FFQ only after lung cancer diagnosis (n=5), who had implausibly low or high body mass index (<15.0 or >50.0 kg/m2) at baseline (n=13), and whose supplemental vitamin use chart was missing (n=1). These exclusions were made regardless the case-subcohort status so that selection bias was unlikely. Consequently, 749 cases (including 44 also selected in the subcohort) and 679 non-cases entered statistical analyses.
Dietary intake assessment
Dietary intake data over the previous year were collected by a self-administered FFQ at baseline and even annual visits. Baseline data were targeted in the current study; the next available FFQ was used when the baseline questionnaire was missing. The questionnaire was designed to be especially sensitive to major sources of fat and carotenoids based on an early version of the National Cancer Institute/Block FFQ.21 CARET FFQ consisted of three sections: (1) 110 food items, with questions on the frequency of use (from “never or less than once per month” to “2+ per day” for foods and “6+ per day” for beverages) and portion size (small, medium, or large, compared to the stated medium portion size); (2) two summary questions on the usual consumption of fruits and vegetables, which were used to reduce the measurement bias toward over-reporting total food consumption resulting from the long list of fruit and vegetables; and (3) seven adjustment questions on types of foods and preparation techniques and one summary question on fat used in cooking. The food items included 7 major vitamin D food sources (tuna, fish other than tuna and shell fish, eggs, animal livers, cottage cheese [regular and low-fat], yogurt, milk [whole fat, 2%, and 1%/skim]), 14 fruit line items, 19 vegetable line items, and 12 line items of mixed foods made with vegetables (e.g., pizza, stew). The nutrient database was derived from the University of Minnesota Nutrition Coordinating Center database (version 4.02, food and nutrient database version 30) and the 1999 U.S. Department of Agriculture– Nutrition Coordinating Center Carotenoid Database for United States foods.22
Personal supplemental vitamin use
Information on the use of personal supplemental vitamins was collected by an inventory method during clinical visits. A personal supplemental vitamin was defined as a dietary supplement used by participants other than the CARET active intervention. Participants were asked to bring their currently used vitamin bottles to clinic centers. First, participants were queried whether they took any supplemental vitamins. For participants who brought bottles, interviewers recorded up to 6 brand names and doses of vitamin A, β-carotene, and vitamin E in international units as specified on the supplement bottles. If participants used more than 6 different supplements, priority of recording was given to vitamin A or β-carotene supplements that participants took regularly followed by those used occasionally. The daily dosage was calculated based on the dose on the label and frequency of use per week. For the supplements that participants did not bring in, the brand names and doses were recorded based on participants’ self-report. At baseline and each intervention phase clinic visit, participants were advised to keep personal supplemental vitamin A intake under 5,500 IU (1,650 μg) per day and to take no β-carotene supplements.
To obtain the doses of vitamin D contained in personal supplements, we retrospectively extracted all the brand names on the baseline charts of the cases and subcohort members who indicated any supplement use (n=813, less one chart that was not available for review). A total of 175 extracted brands were identified as single or multivitamin supplements containing vitamin D. The dosage of vitamin D of each brand was obtained via Physicians’ Desk Reference for Nonprescription Drugs and Dietary Supplements,23 Dietary Supplement Label Database,24 and internet searches. For brands unidentifiable from the above sources (n=96 out of 175 extracted brands, 55%), 400 IU, the most common dosage of vitamin D supplements,23 was assigned. For charts without any information on brand names or vitamin D doses (n=25 out of 812 charts, 3%), 0 IU were assigned. The investigator (TYC) extracting and entering data was blinded to the case-subcohort status. Another investigator (MLN) reviewed a 10% (n=81) random sample of the charts for the quality control; the agreement rate was 98.8%.
We calculated total vitamin D and vitamin A by summing food and personal supplement intake together. Vitamin A intake was expressed as μg Retinol Activity Equivalent (RAE) because it consists of a wide range of compounds including retinol and carotenoids. The calculations of RAE for dietary and supplemental intake were:
Dietary intake = μg retinol + (μg β-carotene equivalent/12), where β-carotene equivalent= μg β-carotene + ½ (μg α-carotene + μg β-cryptoxanthin);
Based on the conversion, the dosage of CARET active intervention was 22,500 μg RAE (7,500 μg of retinol and 15,000 μg RAE of β-carotene)
Covariates
Standardized, self-administered questionnaires were used to collect age, gender, race/ethnicity, education level, smoking habits, number of years in high-risk (asbestos) trade, and medical history at baseline. Participants were queried about whether a doctor had told them they had any medical conditions appearing on a comprehensive list. Conditions related to the absorption and utilization of vitamin D from oral intake included intestinal conditions (colitis and diverticulosis), liver diseases (yellow jaundice, hepatitis, and cirrhosis), and kidney diseases (nephritis, kidney infection, kidney stones, and kidney failure). Current smokers were defined as those who smoked any cigarettes in the past month. Number of cigarettes smoked per day and years as a regular smoker were also queried. Alcohol consumption was assessed by the food frequency questionnaire. Height and weight were measured at baseline clinic visits.
Statistical analysis
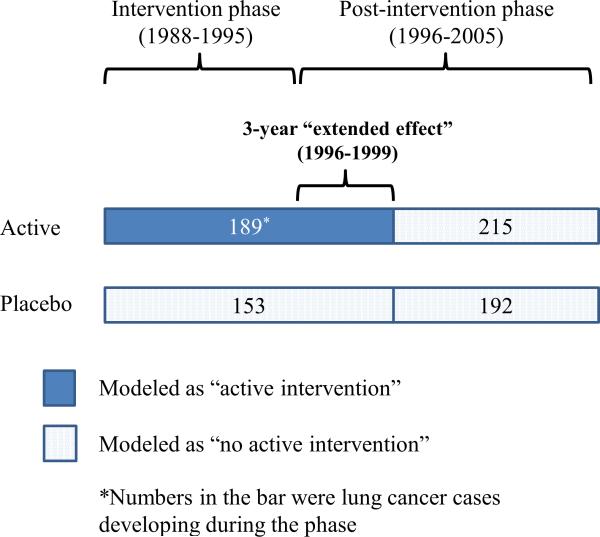
Baseline characteristics between cases and non-cases were examined using t-tests for continuous variables or χ2 tests for categorical variables. Dietary variables were natural-log transformed for t-tests to improve normality. Lung cancer risks were estimated for categorical (<200, 200 – <400, 400 – <600, 600 – <800, and ≥800 IU/d) and linear (per 100 IU/d increment) total vitamin D intake in separate models. We chose these cutoffs because they are reference intakes.26 The categories were re-stratified into fewer categories for analysis by histology and for effect modification analyses to maintain sufficient numbers of lung cancer cases in each stratum. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated by Cox's proportional hazards models with the Self-Prentice method computing robust standard error estimates to account for the case-cohort design.27 Participants contributed follow-up time from the randomization of CARET to the date of lung cancer diagnosis, date of death from causes other than lung cancer, the last documented follow-up contact, or September 30, 2005, whichever came first. The proportionality assumption was examined by testing whether scaled Schoenfeld residuals for total vitamin D intake were associated with survival time;28 the assumption was fulfilled. Multivariate models included baseline covariates that were chosen a priori: age (continuous), study center, race/ethnicity (White, Black, Hispanic/other), education level (no high school diploma, high school, college degree or higher, unknown), enrolled as asbestos exposure worker (yes or no), number of years in high-risk trade (0, 1–20, ≥21), CARET active intervention (yes or no), body mass index (continuous), smoking status (current or former), amount of smoking (<40, 40–59, ≥60 pack-years), total vitamin A intake (<800, 800 – <1,500, 1,500 – <2,500, ≥2,500 μg/d RAE), and energy intake (continuous). The CARET active intervention was modeled as a time-dependent variable (Figure 1), to allow the exposure to vary 3 years (1999 onward) after the trial.28 We classified the first 3 years of post-intervention follow-up as the active intervention because the increased lung cancer risk had remained significant until year 4 post-intervention.29 We also evaluated whether dietary calcium intake and history of asbestosis, chronic bronchitis, and emphysema were confounders.30, 31 They made no changes to risk estimates so were not included in the final model. Linear trends of risk estimates were examined by Wald tests of an ordinal variable of total vitamin D intake categories. Cox models were performed for all participants and by a priori smoking status subgroups (current and former smokers) and for histological subtypes of lung cancer among cases who had complete histological data (n=592, 79% of all cases). We evaluated whether pre-clinical lung cancer affected vitamin D intake by excluding lung cancer cases diagnosed within the first two years of the efficacy trial.
Figure 1.
Illustration of modeling the active intervention (30 mg β-carotene plus 25,000 IU retinyl palmitate daily) and placebo during (1988-1995) and after (1996-2005) the β-Carotene and Retinol Efficacy Trial (CARET). “CARET active intervention” was modeled as time-dependent covariate with a 3-year extended effect in the post-intervention phase.
To evaluate effect modification, we stratified the associations of total vitamin D intake by the CARET active intervention and total vitamin A intake. Two cutoffs–1,000 and 1,500 μg/d RAE based on prior research9 and approximately the 75th quartile of all participants, respectively–were chosen for total vitamin A intake. We reported the result stratified as <1,500 or ≥1,500 μg/d RAE because it showed a more significant effect on modifying the association compared to that using 1,000 μg/d RAE as the cutoff. Statistical evidence of interaction was examined by Wald tests of the cross-product term of total vitamin D intake categories and CARET active interaction or total vitamin A intake categories (all ordinal variables; 1 degree of freedom). Interaction between vitamin D intake and smoking status was also examined by the same approach. All statistical tests were two-sided; statistical significance was defined as P<0.05. Statistical analyses were conducted using STATA (12.0, College Station, Texas).
RESULTS
Lung cancer cases were older, had a lower education attainment, had more current smokers, smoked more pack-years of cigarette, had a higher number of years working in asbestos trade, and had lower body mass index, compared with non-cases (all P<0.05; Table 1). Estimated total vitamin D intake levels were 261 IU/d among cases and 265 IU/d among non-cases. Approximately a quarter of both cases (25.5%) and non-cases (26.1%) used supplemental vitamin D with a median value of 400 IU/d.
Table 1.
Baseline characteristics among the lung cancer cases and non-cases in the Carotene and Retinol Efficacy Trial (CARET)
| Characteristic | Lung cancer casesa | Non-cases | P-valueb | ||
|---|---|---|---|---|---|
| All participants (n, %)c | 749 | (100.0) | 679 | (100.0) | |
| Age, y (mean, SD) | 60.8 | ±5.7 | 57.8 | ±6.1 | <0.001 |
| Female | 206 | (27.5) | 195 | (28.7) | 0.61 |
| Race/ethnicity | |||||
| White | 695 | (92.8) | 621 | (91.5) | 0.26 |
| Black | 33 | (4.4) | 28 | (4.1) | |
| Hispanic/other | 21 | (2.8) | 30 | (4.4) | |
| Education | |||||
| No high school diploma | 114 | (15.2) | 71 | (10.5) | 0.03 |
| High school diploma | 181 | (24.2) | 159 | (23.4) | |
| Some college degree or above | 319 | (42.6) | 326 | (48.0) | |
| Unknown | 135 | (18.0) | 123 | (18.1) | |
| CARET randomization assignment | |||||
| Active | 404 | (53.9) | 349 | (51.4) | 0.34 |
| Placebo | 345 | (46.1) | 330 | (48.6) | |
| Smoking status | |||||
| Current | 527 | (70.4) | 364 | (53.6) | <0.001 |
| Formerd | 222 | (29.6) | 315 | (46.4) | |
| Smoking pack-years | |||||
| <40 | 178 | (23.7) | 276 | (40.7) | <0.001 |
| 40 to 59 | 307 | (41.0) | 246 | (36.2) | |
| ≥60 | 264 | (35.3) | 157 | (23.1) | |
| Enrolled as asbestos exposure worker | 208 | (27.8) | 193 | (28.4) | 0.79 |
| Years in high-risk (asbestos) trade | |||||
| 0 | 577 | (77.0) | 518 | (76.3) | 0.04 |
| 1-20 | 58 | (7.8) | 76 | (11.2) | |
| 21+ | 114 | (15.2) | 85 | (12.5) | |
| Alcohol intake | |||||
| Non-drinkers (intake <0.39 g) | 254 | (33.9) | 219 | (32.3) | 0.30 |
| <10 g/d | 191 | (25.5) | 198 | (29.2) | |
| ≥10 g/d | 304 | (40.6) | 262 | (38.5) | |
| Body mass index, kg/m2 (mean, SD) | 26.9 | ±4.4 | 27.9 | ±4.9 | <0.001 |
| History of asbestosis | 99 | (13.2) | 83 | (12.2) | 0.57 |
| History of asthma | 55 | (7.3) | 44 | (6.5) | 0.52 |
| History of bronchitis or emphysema | 111 | (14.8) | 68 | (10.0) | 0.006 |
| Any supplemental vitamin use | 305 | (40.7) | 292 | (43.0) | 0.38 |
| Total vitamin A intake, μg RAE (median, IQR)e | 835 | (535-1,412) | 892 | (568-1,584) | 0.07 |
| Dietary vitamin A intake, μg RAE (median, IQR) | 724 | (502-1,078) | 731 | (511-1,087) | 0.78 |
| Vitamin A/β-carotene supplement userse | 127 | (17.0) | 126 | (18.6) | 0.43 |
| Supplemental vitamin A intake among the users, μg RAE (median, IQR)e | 3,000 | (1,500-3,000) | 3,000 | (1,500-3,000) | 0.91 |
| Total vitamin D intake, IU/d (median, IQR) | 261 | (160-512) | 265 | (157-519) | 0.57 |
| Dietary vitamin D intake, IU/d (median, IQR) | 201 | (139-295) | 205 | (130-308) | 0.93 |
| Supplemental vitamin D users | 191 | (25.5) | 177 | (26.1) | 0.81 |
| Supplemental vitamin D intake among the users, IU/d intake (median, IQR) | 400 | (400-400) | 400 | (400-400) | 0.12 |
Abbreviations: SD, standard deviation; IQR, interquartile range; RAE, Retinol Activity Equivalent.
Including 44 lung cancer cases arising in the subcohort.
t-tests for continuous variables and χ2 tests for categorical variables. Dietary variables were natural-log transformed for t-tests to improve normality.
Numbers are number of participants and percentages unless otherwise noted.
Including 7 never smokers, representing <1% of all participants. They were recruited in CARET because of their occupational asbestos exposure.
The level did not include the CARET active intervention (30 mg β-carotene plus 25,000 IU retinyl palmitate daily)
There were no significant associations of estimated total vitamin D intake with lung cancer risk among all participants or current smokers in either the linear (for every 100 IU/d increment) or categorical models (Table 2). However, among former smokers, there was an inverse pattern with borderline significance between total vitamin D intake and lung cancer (P-trend=0.06). Former smokers with ≥800 versus <200 IU/d of total vitamin D intake had a 74% lower risk of lung cancer although the risk reduction was not statistically significant (HR=0.26, 95% CI=0.06-1.09). In the analysis of histological subtypes of lung cancer (Table 3), total vitamin D intake ≥600 versus <200 IU/d was significantly associated with a lower risk of non-small cell lung cancer among former smokers (HR=0.36, 95% CI=0.13-0.96). The linear association (HR=0.89, 95% CI=0.78-1.02 per 100 IU) and trend test (P=0.08) across the full range of total vitamin D intake showed only borderline significance. The associations of vitamin D intake with total lung cancer and non-small cell lung cancer significantly differed between current and former smokers (P-interaction=0.002 and 0.003, respectively). The observed associations did not materially change after excluding lung cancer cases diagnosed within the first two years of the efficacy trial (data not shown).
Table 2.
Associations of estimated total vitamin D Intake with lung cancer risk in the CARET, 1988-2005b
| Total Vitamin D Intake (IU/d) |
|||||||
|---|---|---|---|---|---|---|---|
| Per 100 IU | <200 | 200 to <400 | 400 to <600 | 600 to <800 | ≥800 | P-trend | |
| All participants | |||||||
| No. Cases/p-y in subcohort | 749/8,232 | 281/2,897 | 209/2,341 | 145/1,724 | 84/871 | 30/399 | |
| HR (95% CI) | 0.98 (0.92-1.05) | 1.00 (Ref) | 0.76 (0.54–1.07) | 0.74 (0.49-1.13) | 0.83 (0.50-1.40) | 0.67 (0.32-1.39) | 0.26 |
| Current smokers | |||||||
| No. Cases/p-y in subcohort | 527/4,398 | 191/1,666 | 145/1,268 | 106/890 | 59/393 | 26/181 | |
| HR (95% CI) | 1.01 (0.93-1.10) | 1.00 (Ref) | 0.75 (0.48-1.15) | 0.71 (0.41-1.24) | 1.07 (0.55-2.09) | 0.93 (0.37-2.34) | 0.89 |
| Former smokersa | |||||||
| No. Cases/p-y in subcohort | 222/3,834 | 90/1,231 | 64/1,073 | 39/834 | 25/478 | 4/218 | |
| HR (95% CI) | 0.92 (0.81-1.03) | 1.00 (Ref) | 0.87 (0.49-1.54) | 0.67 (0.33-1.36) | 0.51 (0.22-1.21) | 0.26 (0.06-1.09) | 0.06 |
Abbreviations: CI, confidence interval; HR, hazard ratio; p-y, person-years; ref, reference.
P-value for interaction between total vitamin D intake categories and smoking =0.002 (both ordinal variables; Wald test).
Adjusted for age, study center, race/ethnicity, education, enrolled as asbestos exposure worker, number of years in high-risk trade, smoking status (for all participants only), smoking pack-years, body mass index, energy intake, total vitamin A intake, and CARET active intervention (time-dependent covariate with a 3-year extended effect post-intervention).
Table 3.
Associations of estimated total vitamin D intake with lung cancer risk by histological subtype of tumorb
| Total Vitamin D Intake (IU/d) |
||||||
|---|---|---|---|---|---|---|
| Per 100 IU | <200 | 200 to <400 | 400 to <600 | ≥600 | P-trend | |
| Non-small cell lung cancerc | ||||||
| All participants | ||||||
| No. Cases/p-y in subcohort | 476/8,107 | 181/2,871 | 126/2,286 | 97/1,688 | 72/1,262 | |
| HR (95% CI) | 0.95 (0.88-1.03) | 1.00 (Ref) | 0.69 (0.47–1.01) | 0.77 (0.49-1.22) | 0.68 (0.39-1.17) | 0.16 |
| Current smokers | ||||||
| No. Cases/p-y in subcohort | 324/4,298 | 121/1,650 | 79/1,228 | 69/854 | 55/566 | |
| HR (95% CI) | 0.98 (0.89-1.08) | 1.00 (Ref) | 0.62 (0.40-1.01) | 0.73 (0.39-1.35) | 0.92 (0.46-1.87) | 0.60 |
| Former smokersa | ||||||
| No. Cases/p-y in subcohort | 152/3,809 | 60/1,221 | 47/1,058 | 28/834 | 17/696 | |
| HR (95% CI) | 0.89 (0.78-1.02) | 1.00 (Ref) | 1.05 (0.56-1.98) | 0.80 (0.37-1.72) | 0.36 (0.13-0.96) | 0.08 |
| Adenocarcinoma | ||||||
| All participants | ||||||
| No. Cases/p-y in subcohort | 198/7,965 | 81/2,806 | 52/2,254 | 38/1,667 | 27/1,238 | |
| HR (95% CI) | 1.01 (0.91-1.11) | 1.00 (Ref) | 0.80 (0.50–1.29) | 0.96 (0.54-1.70) | 0.97 (0.48-1.95) | 0.88 |
| Current smokers | ||||||
| No. Cases/p-y in subcohort | 132/4,193 | 50/1,615 | 33/1,196 | 26/840 | 23/542 | |
| HR (95% CI) | 1.05 (0.92-1.19) | 1.00 (Ref) | 0.82 (0.44-1.52) | 0.92 (0.43-1.97) | 1.47 (0.61-3.58) | 0.58 |
| Former smokersa | ||||||
| No. Cases/p-y in subcohort | 66/3,772 | 31/1,190 | 19/1,058 | 12/828 | 4/696 | |
| HR (95% CI) | 0.95 (0.78-1.15) | 1.00 (Ref) | 1.12 (0.49-2.59) | 0.94 (0.34-2.59) | 0.42 (0.09-1.87) | 0.45 |
| Squamous cell carcinoma | ||||||
| All participants | ||||||
| No. Cases/p-y in subcohort | 143/7,987 | 49/2,817 | 41/2,257 | 28/1,662 | 25/1,251 | |
| HR (95% CI) | 0.94 (0.84-1.07) | 1.00 (Ref) | 0.68 (0.38–1.21) | 0.70 (0.35-1.43) | 0.65 (0.28-1.51) | 0.30 |
| Current smokers | ||||||
| No. Cases/p-y in subcohort | 104/4,206 | 37/1,611 | 28/1,204 | 20/837 | 19/554 | |
| HR (95% CI) | 0.97 (0.83-1.12) | 1.00 (Ref) | 0.62 (0.30-1.27) | 0.65 (0.26-1.62) | 0.86 (0.31-2.43) | 0.64 |
| Former smokersa | ||||||
| No. Cases/p-y in subcohort | 39/3,781 | 12/1,207 | 13/1,053 | 8/825 | 6/696 | |
| HR (95% CI) | 0.86 (0.68-1.07) | 1.00 (Ref) | 0.97 (0.32-2.88) | 0.74 (0.20-2.81) | 0.33 (0.07-1.62) | 0.18 |
| Small-cell lung cancer | ||||||
| All participants | ||||||
| No. Cases/p-y in subcohort | 116/7,919 | 38/2,779 | 36/2,253 | 23/1,645 | 19/1,242 | |
| HR (95% CI) | 1.08 (0.96-1.22) | 1.00 (Ref) | 0.99 (0.55–1.79) | 1.13 (0.54-2.34) | 1.33 (0.56-3.18) | 0.51 |
| Current smokers | ||||||
| No. Cases/p-y in subcohort | 88/4,155 | 28/1,597 | 30/1,185 | 17/827 | 13/546 | |
| HR (95% CI) | 1.10 (0.95-1.28) | 1.00 (Ref) | 1.16 (0.58-2.33) | 1.22 (0.49-3.04) | 1.53 (0.54-4.38) | 0.45 |
| Former smokersa | ||||||
| No. Cases/p-y in subcohort | 28/3,764 | 10/1,181 | 6/1,068 | 6/818 | 6/697 | |
| HR (95% CI) | 1.07 (0.85-1.34) | 1.00 (Ref) | 0.35 (0.08-1.50) | 0.64 (0.15-2.76) | 0.88 (0.17-4.67) | 0.95 |
Abbreviations: CI, confidence interval; HR, hazard ratio; p-y, person-years; ref, reference.
P-interaction between total vitamin D intake and smoking status: 0.003 for non-small cell lung cancer, 0.001 for adenocarcinoma, 0.21 for squamous cell carcinoma, and 0.45 for small-cell lung cancer. The P-values were obtained by Wald tests of the cross-product term of total vitamin D intake categories and smoking status (both ordinal variables; Wald test).
Adjusted for age, study center, race/ethnicity, education, enrolled as asbestos exposure worker, number of years in high-risk trade, smoking status, smoking pack-years, body mass index, energy intake, total vitamin A intake, and CARET active intervention (time-dependent covariate with a 3-year extended effect post-intervention).
Non-small cell lung cancer included adenocarcinoma, squamous cell carcinoma, and non-small cell lung cancer not otherwise specified or subtypes other than small-cell lung cancer.
In effect modification analyses (Table 4), when stratifying by the CARET active intervention, total vitamin D intake ≥400 versus <400 IU/d was significantly associated with a lower risk of lung cancer among all participants (HR=0.56, 95% CI=0.32-0.99) and among former smokers (HR=0.25, 95% CI=0.08-0.76) who received the CARET active intervention. This inverse association was not observed among those who did not receive the CARET active intervention. When stratifying by estimated total vitamin A intake from diet and personal supplements, total vitamin D intake ≥400 versus <400 IU/d was significantly associated with a lower risk of lung cancer among all participants with total vitamin A intake ≥1,500 μg/d RAE (HR=0.46, 95% CI=0.23-0.91), but not those with total vitamin A intake <1,500 μg/d RAE. The total vitamin D intake-lung cancer association significantly differed by total vitamin A intake among current smokers (P-interaction=0.02).
Table 4.
Associations of estimated total vitamin D intake with lung cancer risk, stratified by receiving the CARET active intervention (30 mg β-carotene plus 25,000 IU retinyl palmitate or 22,500 μg RAE daily) during the trial and total vitamin A intake levels for all Participants, current smokers, and former smokersa
| Total Vitamin D Intake (IU/d) |
|||||
|---|---|---|---|---|---|
| <400 |
≥400 |
||||
| Main effects and stratifications | No. cases/p-y in subcohort | HR (95% CI) | No. cases/p-y in subcohort | HR (95% CI) | P-interactionc |
| All participants, main effect | 490/5,238 | 1.00 (Ref) | 259/2,994 | 0.91 (0.65-1.27) | |
| CARET active intervention b | |||||
| Yes | 129/1,548 | 1.00 (Ref) | 60/950 | 0.56 (0.32-0.99) | 0.26 |
| No | 361/3,690 | 1.00 (Ref) | 199/2,044 | 1.07 (0.75-1.54) | |
| Total vitamin A intake | |||||
| ≥1,500 μg/d RAE | 39/411 | 1.00 (Ref) | 138/1,702 | 0.46 (0.23-0.91) | 0.08 |
| <1,500 μg/d RAE | 451/4,827 | 1.00 (Ref) | 121/1,292 | 1.07 (0.74-1.56) | |
| Current smokers, main effect | 336/2,934 | 1.00 (Ref) | 191/1,463 | 0.97 (0.62-1.53) | |
| CARET active intervention b | |||||
| Yes | 86/865 | 1.00 (Ref) | 45/471 | 0.55 (0.26-1.16) | 0.62 |
| No | 250/2,069 | 1.00 (Ref) | 146/992 | 1.10 (0.68-1.78) | |
| Total vitamin A intake | |||||
| ≥1,500 μg/d RAE | 29/166 | 1.00 (Ref) | 104/839 | 0.38 (0.14-1.07) | 0.02 |
| <1,500 μg/d RAE | 307/2,768 | 1.00 (Ref) | 87/624 | 1.33 (0.82-2.15) | |
| Former smokers, main effect | 154/2,304 | 1.00 (Ref) | 68/1,530 | 0.65 (0.37-1.14) | |
| CARET active intervention b | |||||
| Yes | 43/682 | 1.00 (Ref) | 15/479 | 0.25 (0.08-0.76) | 0.19 |
| No | 111/1,622 | 1.00 (Ref) | 53/1,051 | 0.82 (0.44-1.54) | |
| Total vitamin A intake | |||||
| ≥1,500 μg/d RAE | 10/245 | 1.00 (Ref) | 34/863 | 0.81 (0.26-2.48) | 0.84 |
| <1,500 μg/d RAE | 144/2,059 | 1.00 (Ref) | 34/667 | 0.60 (0.31-1.18) | |
Abbreviations: CI, confidence interval; HR, hazard ratio; p-y, person-years; ref, reference; RAE, Retinol Activity Equivalent.
Adjusted for age, study center, race/ethnicity, education, enrolled as asbestos exposure worker, number of years in high-risk trade, smoking status (for all participants only), smoking pack-years, body mass index, energy intake, total vitamin A intake (except for models stratified by total vitamin A intake), and CARET active intervention (time-dependent covariate with a 3-year extended effect post-intervention; except for models stratified by the CARET active intervention).
Modeled as time-dependent variable with a 3-year extended effect post-intervention.
Wald tests of the cross-product term of total vitamin D intake categories and the CARET active intervention or total vitamin A intake categories (all ordinal variables).
DISCUSSION
In this study population of heavy smokers and/or workers with occupational exposure to asbestos, estimated total vitamin D intake ≥600 versus <200 IU/d was associated with a lower risk for non-small cell lung cancer among former smokers. In addition, inverse associations were observed among participants consuming higher levels of vitamin A from food and supplements or randomized to high-dose retinol+β-carotene supplementation. To our knowledge, this is the first study investigating the role of vitamin A in a supraphysiologic dose in lung cancer prevention in relation to vitamin D.
Vitamin D intake is a significant determinant of vitamin D status.26 Vitamin D supplements, which usually contain a much higher dose of vitamin D compared to foods, can effectively elevate serum 25-hydroxyvitamin D contributions at a rate of 5 nmol/L per 100 IU/day.32 In addition, although photosynthesis of vitamin D in skin after exposure to ultraviolet B is an efficient method to acquire vitamin D, many factors, such as pigmentation, season, latitude, and sedentary lifestyle with little outdoor exposure, can affect the results of vitamin D production.33 Our study was able to consider major determinates of vitamin D photosynthesis, including race and latitude.34 The current Recommended Dietary Allowance (RDA) is 600 IU/day for adults aged 19-70 years and 800 IU/day for those aged 71 years or older living in the U.S. and Canada.26 The vitamin D intake levels in the majority CARET participants did not meet the requirement except for those who used personal supplemental vitamins containing vitamin D. Nevertheless, CARET participants had a similar level of dietary vitamin D intake compared to the participants in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (ATBC), a Finnish study recruiting male heavy smokers (median=205 versus 188 IU/d among controls without lung cancer) although the percentage of using personal supplemental vitamins containing vitamin D was threefold higher in CARET than ATBC (26% versus 7%).10 As to the vitamin D status, serum 25-hydroxyvitmain D concentrations were generally higher in CARET compared to ATBC (interquartile range= 26–51 versus 17–37 nmol/L in darker months, i.e., November to April; 31–65 versus 24–47 nmol/L in sunnier months, i.e., May to October),10 in part due to the lower residential latitude of the CARET participants (6 major cities in the U.S.) than the ATBC participants (Finland) (the serum concentrations in CARET were from a subset of 160 participants without using personal supplemental vitamins; data not shown).
Histological studies using clinical samples suggest that non-small cell lung cancers, including squamous cell carcinoma and adenocarcinoma, have more vitamin D receptors and thus are more responsive to vitamin D, compared with small cell lung cancer.35 Also, vitamin D inhibits lung cancer signaling pathways including mutations in epidermal growth factor receptor, Wnt-β-catenin dysregulation, and K-ras mutation,3, 5, 36 which are associated with non-small cell lung cancer.37 To our knowledge, only one epidemiological study reported the vitamin D-lung cancer association by lung cancer histology. In ATBC, serum 25-hydroxyvitamin D concentrations of approximately ≥40 versus <40 nmol/L were suggestively associated with a reduction in risk for squamous cell carcinoma (OR=0.65, 95% CI=0.42–1.02, 179 cases) and adenocarcinoma (OR=0.68, 95% CI=0.34–1.39, 72 cases), but not small cell lung cancer (OR=1.33, 95% CI=0.72–2.46, 100 cases).10 Both the observations in the ATBC and CARET support that vitamin D status may be more strongly associated with non-small cell lung cancer, compared to small cell lung cancer. Further large-scale investigations are warranted regarding the subtypes of non-small cell lung cancer, as the numbers of cases in squamous cell carcinoma and adenocarcinoma were relatively small in our data.
We and others have observed null associations of vitamin D and lung cancer among current smokers.8-10 Cigarette smoking is associated with lower vitamin D intake38, 39 and serum 25-hydroxyvitamin D concentrations.40, 41 In addition, smoking-produced carcinogen benzo[a]pyrene enhances CYP24A1 activity, which degrades 1,25-dihydroxyvitamin D, the active form of vitamin D.42 Therefore, among current smokers, higher vitamin D intake or vitamin D status may not necessarily lead to a larger biological effect. On the contrary, quitting smoking is associated with increases in both vitamin D intake and vitamin D status to a level that resembles those of never smokers.43,44 Among the non-cases in our study, former smokers consumed approximately 10% more vitamin D compared to current smokers (median total intake= 279 versus 256 IU/d, P=0.13; data not shown). If the vitamin D metabolic functions affected by smoking can be restored after smoking cessation, it is plausible that we are more likely to observe an association of vitamin D intake with lung cancer among former smokers compared to current smokers.
Our observations on effect modification of vitamin A were contrary to our hypothesis. Among nonsmokers or a population with low smoking rate, an inverse association of vitamin D with lung cancer was more likely to be observed among those with no sign of excess vitamin A exposure,8, 9 potentially because excess vitamin A may counteract RXR-VDR functions in this population. However, observed in the current study, high-dose vitamin A may be important for vitamin D's effect on protecting against lung cancer among heavy current or former smokers, maybe because not only RXR-VDR heterodimer requires more 9-cis-retinoid acid in smokers than nonsmokers, but also retinol can reverse tobacco-induced preneoplastic lesions, which commonly exist among smokers.45, 46 Our observations are consistent with the analysis in ATBC that lung cancer risk associated with serum 25-hydroxyvitamin D (quintiles 4–5 versus 1–3) was lower among participants receiving high-dose β-carotene supplements (odds ratio [OR]=0.76, 95% CI=0.46–1.27 for 20 mg β-carotene/day; OR=0.69, 95% CI=0.40–1.17 for 20 mg β-carotene plus 50 mg α-tocopherol/day), compared to that among those receiving placebo (OR=1.34, 95% CI=0.78–2.32).10 It is noteworthy that the effect modification of vitamin A in these analyses was not supported by strong statistical evidence. Also, it is unknown whether the interaction of vitamin A and vitamin D differs by lung cancer histology, as squamous cell carcinomas are more common is smokers, but adenocarcinoma is more common in nonsmokers. Therefore, no clear conclusion can be given from the currently available data regarding the influence of vitamin A on vitamin D in relation to lung cancer and whether the influence may differ between smokers and nonsmokers.
Since the dose of vitamin A was markedly higher in the CARET active intervention compared to total dietary intake, if the vitamin A indeed plays a crucial role in reversing precancerous lesions and supplying 9-cis-retinoid acid to RXR-VDR heterodimer, the largest effect of risk reduction in lung cancer is expected to be in participants receiving the CARET active intervention. This was reflected by the data among former smokers (Table 4), although the statistical evidence for interaction was not significant due to the relative small number of lung cancer in the group. Conversely, the CARET active intervention did not lead to a stronger beneficial association among current smokers. The observation was plausible because CARET current smokers underwent adverse effects that high-dose β-carotene in the active intervention together with cigarette smoke promotes lung carcinogenesis.47 In smokers, β-carotene supplementation should be avoided and studies on high-dose retinol and vitamin D supplementation for lung cancer prevention are warranted.
A major strength of our study was the prospective design, and we were able to estimate lung cancer risk stratified by smoking status and histological subtype. Lung cancer was the primary endpoint of CARET and thus had high completion rate and accuracy. Nevertheless, limitations of this study should be noted. The study observations should be interpreted with caution because several associations in the subgroup and stratified analysis were borderline significant. Large-scale studies are needed to further rule out the possibility that they were chance findings. Validation data for the estimated dietary vitamin D intake were unavailable in CARET, but research suggests that estimated levels of vitamin D from FFQ have a modest to high correlation (r = 0.45–0.89) with actual intake assessed by single or multiple 24-hour recall and/or food record.48-50 Dietary vitamin D intake estimated from CARET FFQ also showed a reasonable agreement with serum 25-hydroxyvitamin D concentrations (r = 0.33 [n=78] in blood drawn in darker months and r = 0.25 [n=82], sunnier months, among a subset of CARET participants without using personal supplemental vitamins; data not shown). In addition, supplemental vitamin D intake was subject to measurement error because the ascertainment of vitamin D potencies from bottle labels was incomplete, and we assumed labeled potencies as daily intake doses. Also, only the baseline assessment was used. These potential measurement errors might have biased the risk estimates toward the null. Lastly, it may not be appropriate to generalize our findings to other populations because CARET participants were heavy smokers and/or had occupational asbestos exposure.
In conclusion, high total vitamin D intake is associated with a lower risk for non-small cell lung cancer among CARET former smokers. Vitamin A intake from diet and supplements may assist vitamin D in preventing lung cancer among smokers.
Novelty & Impact Statements.
Vitamin A (retinol) plays important roles in cell growth, cell differentiation, and the vitamin D signaling axis. We observed inverse associations of estimated vitamin D intake with lung cancer risk among smokers consuming higher levels of vitamin A from food and supplements or receiving high-dose retinol+β-carotene supplementation. To our knowledge, this is the first study investigating the role of vitamin A in supraphysiologic dose in lung cancer prevention in relation to vitamin D.
Acknowledgements
The authors thank Dr. Stephanie Smith-Warner for giving permission to report data on serum 25-hydroxyvitamin D from her ancillary study with CARET and Ms. Rebecca Harbine and Mr. Jeffrey O. Pittman for their assistance in extracting data on vitamin D supplements.
Funding
This work was supported by the National Institutes of Health (grant number U01 CA63673) and the Fred Hutchinson Cancer Research Center, Seattle, WA.
Abbreviations used
- ATBC
the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study
- CARET
the Carotene and Retinol Efficacy Trial
- CI
confidence interval
- FFQ
food frequency questionnaire
- HR
hazard ratio
- RAE
Retinol Activity Equivalent
- RXR
retinoid X receptor
- VDR
vitamin D receptor
Footnotes
financial disclosure:
Authors have no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. Journal of Immunology (Baltimore, Md.: 1950) 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershberger PA, Modzelewski RA, Shurin ZR, et al. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Research. 1999;59:2644–9. [PubMed] [Google Scholar]
- 4.Nakagawa K, Sasaki Y, Kato S, et al. 22-Oxa-1alpha,25-dihydroxyvitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis. 2005;26:1044–54. doi: 10.1093/carcin/bgi049. [DOI] [PubMed] [Google Scholar]
- 5.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 6.Kilkkinen A, Knekt P, Heliövaara M, et al. Vitamin D status and the risk of lung cancer: a cohort study in Finland. Cancer Epidemiol Biomarkers Prev. 2008;17:3274–8. doi: 10.1158/1055-9965.EPI-08-0199. [DOI] [PubMed] [Google Scholar]
- 7.Afzal S, Bojesen SE, Nordestgaard BG. Low Plasma 25-Hydroxyvitamin D and Risk of Tobacco-Related Cancer. Clin Chem. 2013 doi: 10.1373/clinchem.2012.201939. [DOI] [PubMed] [Google Scholar]
- 8.Cheng TY, Neuhouser ML. Serum 25-hydroxyvitamin D, interaction with vitamin A and lung cancer mortality in the U.S. population. Cancer Causes & Control. 2012;23:1557–65. doi: 10.1007/s10552-012-0033-8. [DOI] [PubMed] [Google Scholar]
- 9.Cheng TY, Lacroix AZ, Beresford SA, et al. Vitamin D intake and lung cancer risk in the Women's Health Initiative. Am J Clin Nutr. 2013;98:1002–11. doi: 10.3945/ajcn.112.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein SJ, Yu K, Horst RL, et al. Serum 25-hydroxyvitamin d and risk of lung cancer in male smokers: a nested case-control study. PLoS One. 2011;6:e20796. doi: 10.1371/journal.pone.0020796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 12.Poulain S, Evenou F, Carre MC, et al. Vitamin A/retinoids signalling in the human lung. Lung Cancer. 2009;66:1–7. doi: 10.1016/j.lungcan.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Carlberg C, Bendik I, Wyss A, et al. Two nuclear signaling pathways for vitamin D. Nature. 1993;361:657–60. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- 14.Bettoun DJ, Burris TP, Houck KA, et al. Retinoid X receptor is a nonsilent major contributor to vitamin D receptor-mediated transcriptional activation. Mol Endocrinol. 2003;17:2320–8. doi: 10.1210/me.2003-0148. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Martinez R, Castillo AI, Steinmeyer A, et al. The retinoid X receptor ligand restores defective signalling by the vitamin D receptor. EMBO reports. 2006;7:1030–4. doi: 10.1038/sj.embor.7400776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou YR, Miettinen S, Kagechika H, et al. Retinoic acid via RARalpha inhibits the expression of 24-hydroxylase in human prostate stromal cells. Biochemical and biophysical research communications. 2005;338:1973–81. doi: 10.1016/j.bbrc.2005.10.178. [DOI] [PubMed] [Google Scholar]
- 17.Arnhold T, Tzimas G, Wittfoht W, et al. Identification of 9-cis-retinoic acid, 9,13-di-cis-retinoic acid, and 14-hydroxy-4,14-retro-retinol in human plasma after liver consumption. Life Sci. 1996;59:PL169–77. doi: 10.1016/0024-3205(96)00408-0. [DOI] [PubMed] [Google Scholar]
- 18.Thompson PD, Jurutka PW, Haussler CA, et al. Heterodimeric DNA binding by the vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. Evidence for allosteric receptor interactions. J Biol Chem. 1998;273:8483–91. doi: 10.1074/jbc.273.14.8483. [DOI] [PubMed] [Google Scholar]
- 19.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. The New England Journal of Medicine. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Woods M, Potosky A, et al. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 22.Neuhouser ML, Patterson RE, Thornquist MD, et al. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET). Cancer Epidemiol Biomarkers Prev. 2003;12:350–8. [PubMed] [Google Scholar]
- 23.Physicians’ Desk Reference for Nonprescription Drugs and Dietary Supplements. 14 ed. Medical Economics Data Production, Co; Montvale, NJ: 1993. [Google Scholar]
- 24.Dietary Supplements Labels Database. The National Library of Medicine; 2012. [Google Scholar]
- 25.Institute of Medicine . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press; Washington, D.C.: 2001. [PubMed] [Google Scholar]
- 26.Institute of Medicine . Dietary Reference Intake for Calcium and Vitamin D. The National Academics Press; Washington, DC: 2011. [Google Scholar]
- 27.Therneau TM, Li H. Computing the Cox model for case cohort designs. Lifetime data analysis. 1999;5:99–112. doi: 10.1023/a:1009691327335. [DOI] [PubMed] [Google Scholar]
- 28.Kleinbaum D, Klein M. A Self-Learning Texted. Springer; New York, NY: 2011. Survival Analysis. [Google Scholar]
- 29.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–50. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 30.Mahabir S, Forman MR, Dong YQ, et al. Mineral intake and lung cancer risk in the NIH-American Association of Retired Persons Diet and Health study. Cancer Epidemiol Biomarkers Prev. 2010;19:1976–83. doi: 10.1158/1055-9965.EPI-10-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner DR, Boffetta P, Duell EJ, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the international lung cancer consortium. Am J Epidemiol. 2012;176:573–85. doi: 10.1093/aje/kws151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenna MJ, Murray BF. Vitamin D dose response is underestimated by Endocrine Society's Clinical Practice Guideline. Endocr Connect. 2013;2:87–95. doi: 10.1530/EC-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer . Vitamin D and Cancer. IARC; 2009. [Google Scholar]
- 34.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 35.Menezes RJ, Cheney RT, Husain A, et al. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol Biomarkers Prev. 2008;17:1104–10. doi: 10.1158/1055-9965.EPI-07-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konigshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol. 2010;42:21–31. doi: 10.1165/rcmb.2008-0485TR. [DOI] [PubMed] [Google Scholar]
- 37.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morabia A, Bernstein MS, Antonini S. Smoking, dietary calcium and vitamin D deficiency in women: a population-based study. Eur J Clin Nutr. 2000;54:684–9. doi: 10.1038/sj.ejcn.1601074. [DOI] [PubMed] [Google Scholar]
- 39.Subar AF, Harlan LC, Mattson ME. Food and nutrient intake differences between smokers and non-smokers in the US. Am J Public Health. 1990;80:1323–9. doi: 10.2105/ajph.80.11.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53:920–6. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 41.Jorde R, Saleh F, Figenschau Y, et al. Serum parathyroid hormone (PTH) levels in smokers and non-smokers. The fifth Tromso study. Eur J Endocrinol. 2005;152:39–45. doi: 10.1530/eje.1.01816. [DOI] [PubMed] [Google Scholar]
- 42.Matsunawa M, Amano Y, Endo K, et al. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol Sci. 2009;109:50–8. doi: 10.1093/toxsci/kfp044. [DOI] [PubMed] [Google Scholar]
- 43.Bolton-Smith C, Woodward M, Brown CA, et al. Nutrient intake by duration of ex-smoking in the Scottish Heart Health Study. Br J Nutr. 1993;69:315–32. doi: 10.1079/bjn19930036. [DOI] [PubMed] [Google Scholar]
- 44.Need AG, Kemp A, Giles N, et al. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int. 2002;13:83–8. doi: 10.1007/s198-002-8342-9. [DOI] [PubMed] [Google Scholar]
- 45.Misset JL, Mathe G, Santelli G, et al. Regression of bronchial epidermoid metaplasia in heavy smokers with etretinate treatment. Cancer detection and prevention. 1986;9:167–70. [PubMed] [Google Scholar]
- 46.Gouveia J, Mathe G, Hercend T, et al. Degree of bronchial metaplasia in heavy smokers and its regression after treatment with a retinoid. Lancet. 1982;1:710–2. doi: 10.1016/s0140-6736(82)92623-x. [DOI] [PubMed] [Google Scholar]
- 47.Duffield-Lillico AJ, Begg CB. Reflections on the landmark studies of beta-carotene supplementation. J Natl Cancer Inst. 2004;96:1729–31. doi: 10.1093/jnci/djh344. [DOI] [PubMed] [Google Scholar]
- 48.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Annals of Epidemiology. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 49.Pritchard JM, Seechurn T, Atkinson SA. A food frequency questionnaire for the assessment of calcium, vitamin D and vitamin K: a pilot validation study. Nutrients. 2010;2:805–19. doi: 10.3390/nu2080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L, Wang PP, Roebothan B, et al. Assessing the validity of a self-administered food-frequency questionnaire (FFQ) in the adult population of Newfoundland and Labrador, Canada. Nutr J. 2013;12:49. doi: 10.1186/1475-2891-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]