Abstract
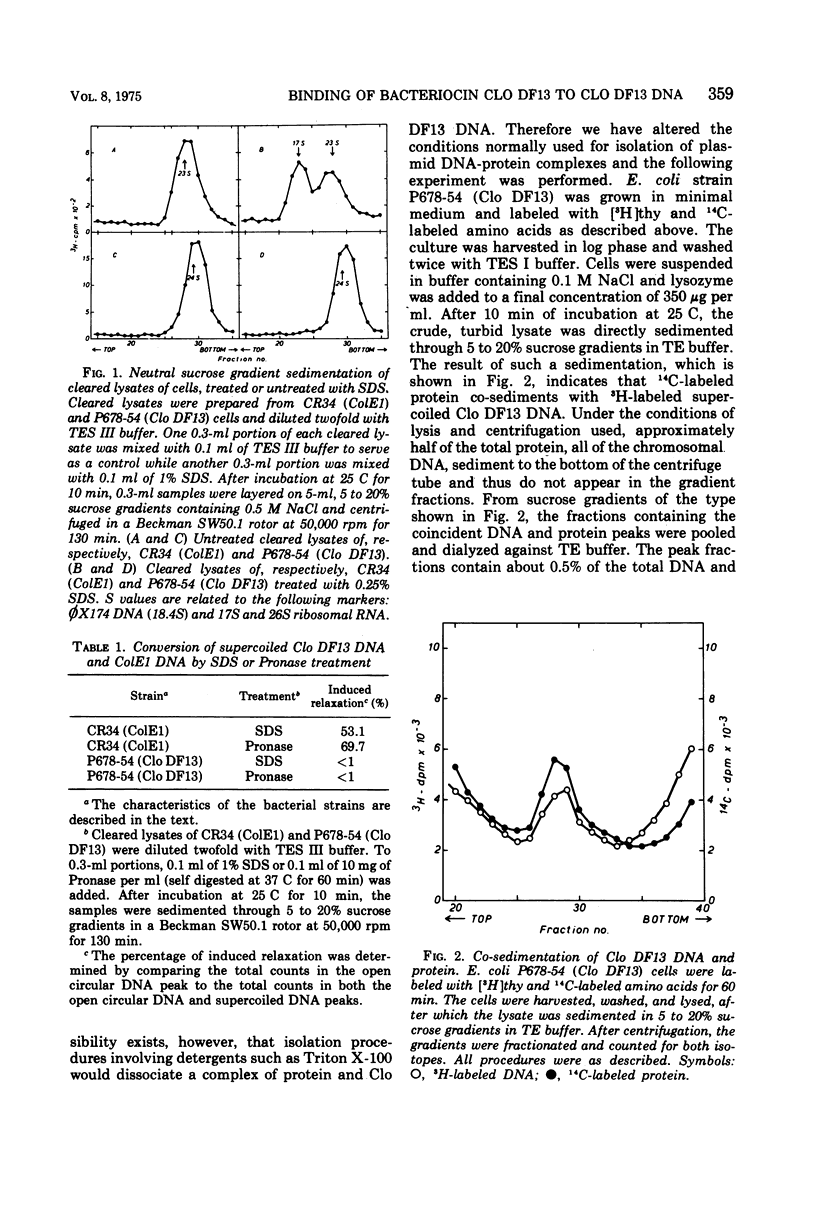
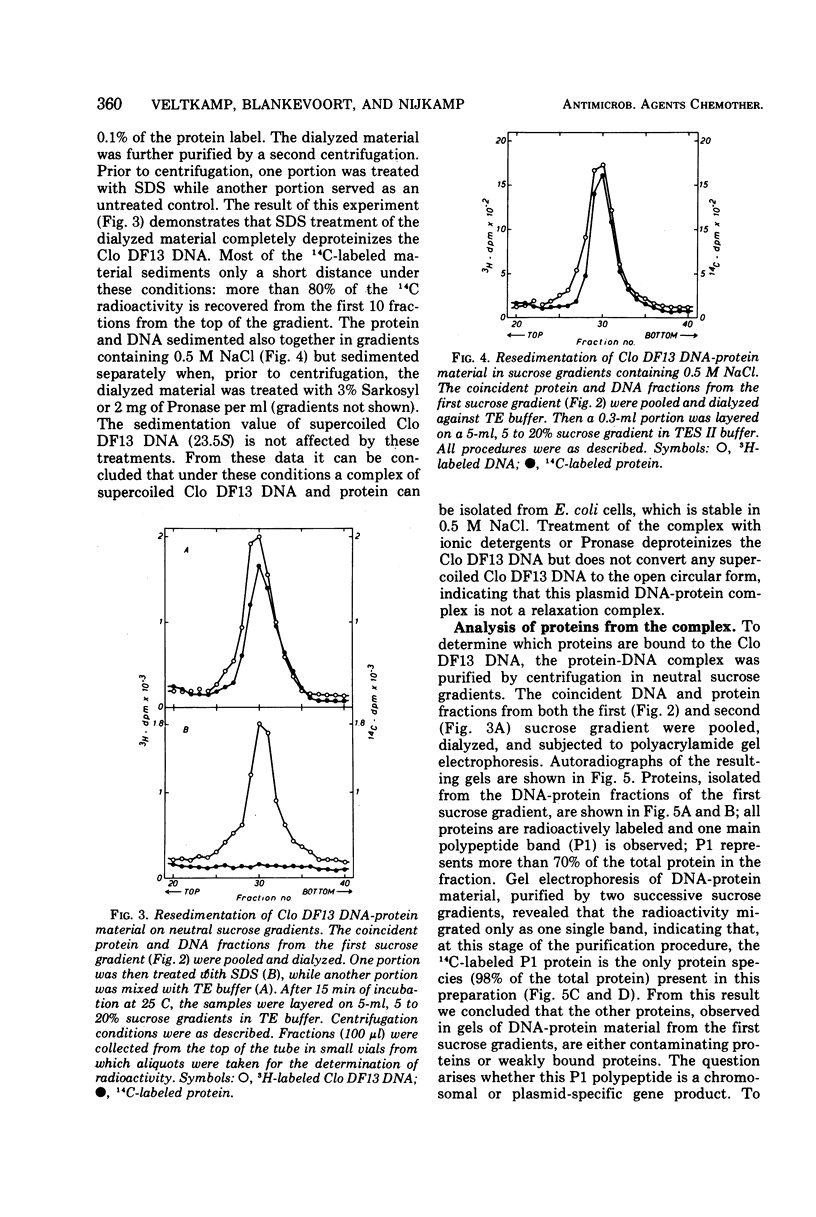
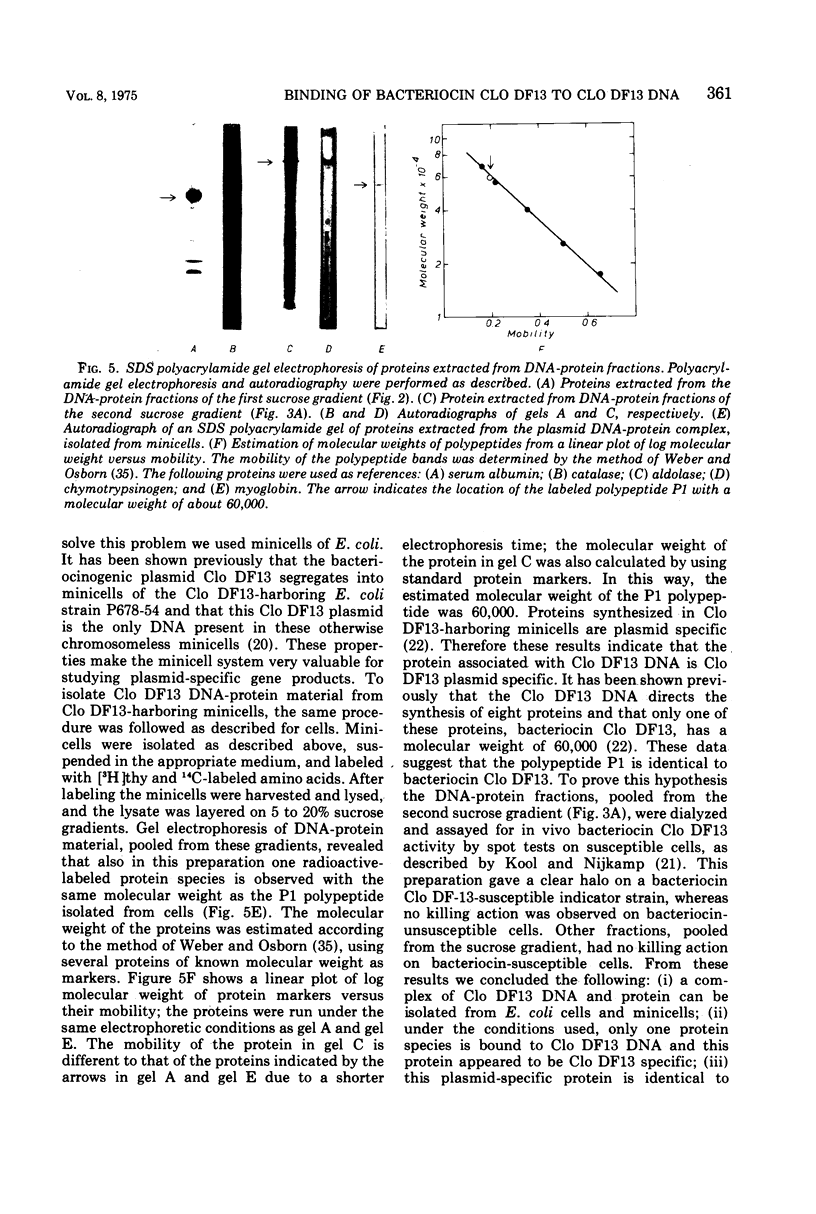
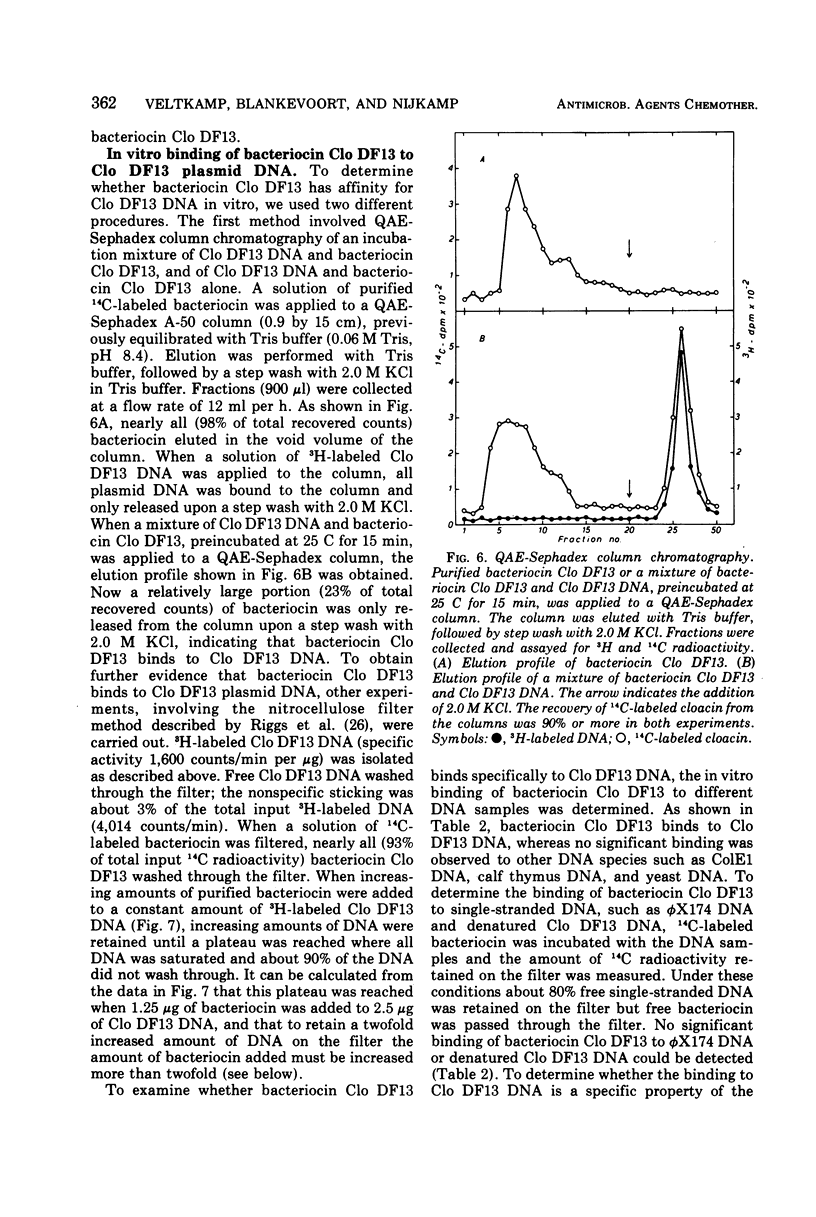
The bacteriocinogenic plasmid Clo DF13 is present in Escherichia coli to the extent of 10 copies per cell. A complex of Clo DF13 plasmid deoxyribonucleic acid (DNA) and protein can be isolated from cells. Treatment of the complex with ionic detergents or proteases dissociates the complex but does not convert any supercoiled Clo DF13 DNA to the open circular form, indicating that this complex is not a relaxation complex. The complex is stable in 0.5 M NaCl and contains one polypeptide species. The protein, present in the complex, appeared to be bacteriocin Clo DF13 for the following reasons: (i) the protein is de novo synthesized in Clo DF13-harboring minicells, indicating that this protein is Clo DF13 specific; (ii) this protein shows bacteriocinogenic activity on a bacteriocin Clo DF13-susceptible indicator strain; (iii) this protein has the same molecular weight (60,000) as bacteriocin Clo DF13. DNA-protein binding experiments, involving QAE-Sephadex column chromatography and nitrocellulose membrane filters, demonstrate that bacteriocin Clo DF13 has also affinity in vitro for Clo DF13 DNA. Membrane filter binding experiments revealed that bacteriocin Clo DF13 does not interact with other DNA species, such as ColE1 DNA, yeast DNA, calf thymus DNA, φX174 DNA, and also not with denatured Clo DF13 DNA. In addition no binding to Clo DF13 DNA of a related bacteriocin, colicin E3, could be detected. These results indicate that the binding of bacteriocin Clo DF 13 to double-stranded Clo DF13 DNA is very specific.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. E. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Commun. 1970 Oct 9;41(1):150–156. doi: 10.1016/0006-291x(70)90481-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Evidence for the existence of the colicinogenic factors E2 and E3 as supercoiled circular DNA-protein relaxation complexes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):608–613. doi: 10.1016/0006-291x(70)90947-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkacz B. W., Sherratt D. J. Segregation kinetics of colicinogenic factor col E1 from a bacterial population temperature sensitive for DNA polymerase I. Mol Gen Genet. 1973;121(1):71–75. doi: 10.1007/BF00353694. [DOI] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Replication of the minicircular DNA of E. coli 15 is dependent on DNA polymerase I but independent of DNA polymerase 3. Biochem Biophys Res Commun. 1972 Oct 17;49(2):591–600. doi: 10.1016/0006-291x(72)90452-4. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Grindley N. D., Anderson E. S. DNA--protein complexes of delta-mediated transfer systems. Biochim Biophys Acta. 1972 Dec 6;287(2):355–360. doi: 10.1016/0005-2787(72)90385-1. [DOI] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Helinski D. R. F 1 sex factor of Escherichia coli. Size and purification in the form of a strand-specific relaxation complex of supercoiled deoxyribonucleic acid and protein. Biochemistry. 1971 Dec 21;10(26):4975–4980. doi: 10.1021/bi00802a022. [DOI] [PubMed] [Google Scholar]
- Kool A. J., Borstlap A. J., Nijkamp H. J. Bacteriocinogenic Clo DF13 minicells of Escherichia coli synthesize a protein that accounts for immunity to bacteriocin Clo DF16: action of the immunity protein in vivo and in vitro. Antimicrob Agents Chemother. 1975 Jul;8(1):76–85. doi: 10.1128/aac.8.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Nijkamp H. J. Isolation and characterization of a copy mutant of the bacteriocinogenic plasmid Clo DF13. J Bacteriol. 1974 Nov;120(2):569–578. doi: 10.1128/jb.120.2.569-578.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Pols C., Nijkamp H. J. Bacteriocinogenic Clo DF13 minicells of Escherichia coli synthesize a protein that accounts for immunity to bacteriocin Clo DF13: purification and characterization of the immunity protein. Antimicrob Agents Chemother. 1975 Jul;8(1):67–75. doi: 10.1128/aac.8.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Pranger M., Nijkamp H. J. Proteins synthesized by a non-induced bacteriocinogenic factor in minicells of Escherichia coli. Mol Gen Genet. 1972;115(4):314–323. doi: 10.1007/BF00333170. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Miklos G. L., Helinski D. R. Properties of the relaxation complexes of supercoiled deoxyribonucleic acid and protein of the R plasmids R64, R28K, and R6K. J Bacteriol. 1974 Oct;120(1):545–548. doi: 10.1128/jb.120.1.545-548.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S. On the assay, isolation and characterization of the lac repressor. J Mol Biol. 1968 Jul 14;34(2):361–364. doi: 10.1016/0022-2836(68)90260-x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Tieze G. A. Bacteriocin production by members of the genus Klebsiella. Antonie Van Leeuwenhoek. 1966;32(2):171–182. doi: 10.1007/BF02097457. [DOI] [PubMed] [Google Scholar]
- Tieze G. A., Stouthamer A. H., Jansz H. S., Zandberg J., van Bruggen E. F. A bacteriocinogenic factor of Enterobacter cloacae. Mol Gen Genet. 1969;106(1):48–65. [PubMed] [Google Scholar]
- Tsai R. L., Green H. Studies on a mammalian cell protein (P8) with affinity for DNA in vitro. J Mol Biol. 1973 Feb 19;73(3):307–316. doi: 10.1016/0022-2836(73)90344-6. [DOI] [PubMed] [Google Scholar]
- Veltkamp E., Barendsen W., Nijkamp H. J. Influence of protein and ribonucleic acid synthesis on the replication of the bacteriocinogenic factor Clo DF13 in Escherichia coli cells and minicells. J Bacteriol. 1974 Apr;118(1):165–174. doi: 10.1128/jb.118.1.165-174.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp E., Nijkamp H. J. Replication of the bacteriocinogenic plasmid Clo DF13 in thermosensitive Escherichia coli mutants defective in initiation or elongation of deoxyribonucleic acid replication. J Bacteriol. 1974 Dec;120(3):1227–1237. doi: 10.1128/jb.120.3.1227-1237.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp E., Nijkamp H. J. The role of DNA polymerase I, II and 3 in the replication of the bacteriocinogenic factor Clo DF 13. Mol Gen Genet. 1973 Sep 27;125(4):329–340. doi: 10.1007/BF00276588. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yarus M., Berg P. Recognition of tRNA by aminoacyl tRNA synthetases. J Mol Biol. 1967 Sep 28;28(3):479–490. doi: 10.1016/s0022-2836(67)80098-6. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Klaasen-Boor P. Purification and characterization of the cloacin DF13 immunity protein. FEBS Lett. 1974 Apr 1;40(2):293–296. doi: 10.1016/0014-5793(74)80247-4. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]



