Abstract
Background
The Drug-Induced Liver Injury Network (DILIN) studies hepatotoxicity due to conventional medications as well as herbals and dietary supplements (HDS).
Rationale
To characterize hepatotoxicity and its outcomes from HDS versus medications, patients with hepatotoxicity attributed to medications or HDS were enrolled prospectively between 2004 and 2013. The study took place among eight US referral centers that are part of the DILIN. Consecutive patients with liver injury referred to a DILIN center were eligible. The final sample comprised 130 (15.5%) of all subjects enrolled (839) who were judged to have experienced liver injury due to HDS. Hepatotoxicity due to HDS was evaluated by expert opinion. Demographic and clinical characteristics and outcome assessments including death and liver transplantation were ascertained. Cases were stratified and compared according to the type of agent implicated in liver injury; 45 had injury due to bodybuilding HDS, 85 due to non-bodybuilding HDS, and 709 due to medications.
Main Results
Liver injury due to HDS increased from 7% to 20% (p < 0.001) during the study period. Bodybuilding HDS caused prolonged jaundice (median 91 days) in young men but did not result in any fatalities or liver transplantation. The remaining HDS cases presented as hepatocellular injury, predominantly in middle-aged women and more frequently led to death or transplantation compared to injury from medications (13% vs. 3%, p < 0.05).
Conclusions
The proportion of liver injury cases attributed to HDS in DILIN has increased significantly. Liver injury from non-bodybuilding HDS is more severe than from bodybuilding HDS or medications, as evidenced by differences in unfavorable outcomes; death and transplantation.
Keywords: hepatotoxicity, herbs, causality assessment, transplantation
Approximately half the US adult population consumes herbals and dietary supplements (HDS),(1,2) with recent reports showing their use to be increasing.(3,4) Supplement users are more commonly women, non-Hispanic whites, over age 40, and have higher levels of education than non-users.(4-7) NHANES III data indicate that multivitamins and minerals are the most common supplements used, followed by calcium and fish oils.(5) However, the range of HDS is far broader and includes numerous commercial products.
Although dietary supplements are perceived as safe (8), the current regulatory framework established by the Dietary Supplement Health and Education Act of 1994 (9) requires less evidence of safety prior to marketing as assessed by the Food and Drug Administration (FDA) than is required for pharmaceuticals. The FDA and other regulatory bodies can take action against a manufacturer only if there is proven adulteration or injury from its supplement. Recent cases of life-threatening hepatotoxicity from the dietary supplement OxyElite Pro (10) underscore the potential adverse consequences of this oversight process.
The Drug-Induced Liver Injury Network (DILIN), supported by the National Institutes of Diabetes and Digestive and Kidney Diseases, was established in 2003 to identify, enroll, and characterize cases of drug-induced liver injury attributable to medications (excluding acetaminophen) and HDS (ClinicalTrials.gov Identifier:NCT00345930).(11) The original DILIN report identified HDS as the second most common cause for liver injury. (12) Since that report, many more cases have been accrued by the DILIN. Thus, we examined the burden and characteristics of liver injury attributable to HDS in the DILIN, and compared this injury with that due to conventional medications.
Methods
Study Design
The DILIN investigators (see Appendix 1) prospectively enrolled consecutive cases of suspected non-acetaminophen hepatotoxicity. Enrollees were asked to sign written informed consent prior to enrollment. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by each institution's review committee.
Inclusion Criteria And Patient Ascertainment Procedures
Patients had to be at least 2 years of age at enrollment and suspected of having experienced drug-induced liver injury within the preceding six months.(11) Inclusion criteria were jaundice (total bilirubin ≥ 2.5 mg/dL) or coagulopathy (INR > 1.5) with any elevations in alanine or aspartate aminotransferase (ALT or AST) or alkaline phosphatase (Alk P) levels, respectively; or, absent jaundice or coagulopathy, elevations of ALT or AST above 5 times the upper limit of normal (ULN) or Alk P above 2 times ULN on two consecutive measurements at least 24 hours apart. For patients with documented hepatic biochemical test abnormalities prior to the onset of hepatotoxicity, the ALT or AST must have been above 5 times the baseline value, or Alk P above 2 times the baseline value, on two consecutive measurements. Injury onset was the date when inclusion criteria were met.
Patients were evaluated for the differential diagnostic possibilities of non-drug liver diseases. This included testing for serologic markers of viral and autoimmune hepatitis and for metabolic and inherited blood markers, including serum ceruloplasmin, iron studies (serum iron, TIBC, ferritin), and alpha-1-antitrypsin level; hepatic imaging was also required. Liver biopsies were assessed when available for diagnostic and causality assessment purposes. Patients underwent physical examinations by physician investigators and were queried using standard data collection procedures on the chronological use of all drugs and HDS, as well as on co-morbid conditions and alcohol use. Exclusion criteria included liver injury caused by acetaminophen, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis or other chronic biliary tract disease. Also excluded were patients who had undergone liver or allogeneic bone marrow transplantation prior to injury onset. The presence of chronic hepatitis B or C or of HIV infection were not reasons for exclusion from enrollment or adjudication.
Causality Assessment and Outcomes
As described previously, a standardized protocol was used to assess the relationship between the use of a medication or HDS and liver injury. (11) The first task was to assess whether liver injury was likely to be a result of hepatotoxicity through review of diagnostic information by the clinical investigator responsible for the case and two additional investigators, and then whether the medication or HDS might have been responsible. In the case of medications, if more than one had been used and hepatotoxicity appeared likely, each was scored independently for the likelihood of causality relative to the other medications consumed. Causality was graded by the three investigators; consensus was achieved by means of discussion. When the three did not reach consensus, there was detailed consideration of the case by the full DILIN Committee on Causality Assessment, a larger group of experienced hepatologists, drawn from all 8 clinical centers, the data coordinating center (Duke University), and the NIDDK. The final scores were definite (>95% likelihood), highly likely (75-95%), probable (50-74%), possible (25-49%) or unlikely (<25%). Compared with conventional medications, adjudicating HDS was more complex because several products may have been used simultaneously, most containing multiple ingredients. Accordingly, HDS taken by any patient were grouped together and adjudicated as a single agent, even if several were taken concurrently.
Analysis of the cases was confined to those in which causality assessment was graded as probable, highly likely or definite. If both medications and HDS were implicated, HDS was selected as the culprit only if all adjudication criteria indicated it to be a more likely cause for injury than the medication(s). Outcomes from liver injury events were assessed as liver-related death or liver transplantation occurring at any time after onset of liver injury. Rates of hospitalization were compared among the groups. Additionally, a severity score (DILIN Severity Score) was assigned as one of 5 levels as previously described; mild, moderate, moderate-hospitalized, severe, and fatal/transplant (11). A binary outcome of severe vs not severe was created for analysis by combining the severe and fatal/transplant cases into the severe category, as shown in table 2.
Table 2. Outcomes.
| Outcome | Liver Injury Due to Bodybuilding HDS n=45 | Liver Injury Due to Non-bodybuilding HDS n=85 | Liver Injury Due to Conventional Medications n=709 | p-value |
|---|---|---|---|---|
| Hospitalization | 32(71%) | 58(68%) | 414(58%) | .069 |
| Liver transplantation at any time after onset on injury | 0 (0%) | 11 (13%) | 24 (3%) | <.001 |
| Death at any time after onset of injury | 0 (0%) | 3 (4%) | 50 (7%) | 0.095 |
| Severe/Fatal per DILIN severity score | 6 (13%) | 30 (35%) | 181 (26%) | 0.02 |
Liver Injury Patterns
The “R” ratio, by convention, describes the pattern of liver injury as hepatocellular, cholestatic, or mixed. Specifically, the R value is calculated from the ratio of serum ALT to the serum Alk P, both expressed as multiples of the ULN. (13) The ratio was calculated using laboratory values at the onset of injury.
Implicated HDS and Categorization of Patients
Two authors (VN, JS) divided the patients with liver injury due to HDS into two broad categories: those with injury from bodybuilding HDS and those with injury from non-bodybuilding HDS. The rationale for this separation was that bodybuilding products accounted for the largest subgroup among those with hepatotoxicity from HDS with certain prima facie distinguishing features (e.g. predominantly men, prolonged jaundice, eventual recovery), whereas non-bodybuilding HDS produced injury that varied widely, as did the clinical features among the subjects. Classification into bodybuilding or non-body-building HDS product type was based on review of product label and internet marketing information.
Data from three groups were compared: patients with hepatotoxicity from bodybuilding HDS, non-bodybuilding HDS, and conventional medications. To avoid overlap among groups, patients were excluded if they had used both bodybuilding and non-bodybuilding HDS together, or if both a medication and HDS were implicated and thought to be equally likely to have caused the injury.
Statistical Analysis
Continuous data were summarized with median values and interquartile ranges. Categorical data were summarized with frequency and percentage. Kruskal-Wallis test and Fisher exact test were used to compare the groups for continuous data and categorical data, respectively. Time to event analysis was used to compare course of liver injury (days from peak enzyme value to 50% of its peak value) between the groups where median and interquartile times were estimated. Cochran–Armitage test for linear trend was carried out to investigate temporal trends in liver injury. Multivariate logistic regression models were carried out for dichotomous outcome of liver transplant and DILIN severity score to determine the adjusted group effects after adjusting for clinical and demographical variables that were different between the groups. Model selections were carried out based on stepwise, backward and forward procedures as well as manual selection based on clinical input. The final models were used for reporting. A p-value of 0.05 or less was considered as statistically significant. All statistical analyses were carried out by Statistical Analysis Software (SAS) version 9.3 and were performed by one author (H Barnhart). All authors contributed to interpretation of the data.
Results
Liver Injury Cases
As of March 2013, 1219 patients with liver injury from medications, HDS, or both were enrolled; 1035 completed causality assessment and were eligible for inclusion in this study (Figure 1). Among these, 847 (82%) were adjudicated as probable, highly likely or definite; two were excluded because both medications and HDS were assessed as equally likely to be the cause for hepatotoxicity, and six were excluded because both bodybuilding and non-bodybuilding HDS were implicated. Among the remaining 839 patients included in the final analysis, 709 (85%) had liver injury from medications, and 130 (15.5%), injury from HDS. The 130 patients with liver injury from HDS consisted of 45 (35%) who had taken bodybuilding HDS and 85 (65%) who had taken non-bodybuilding HDS.
Figure 1.
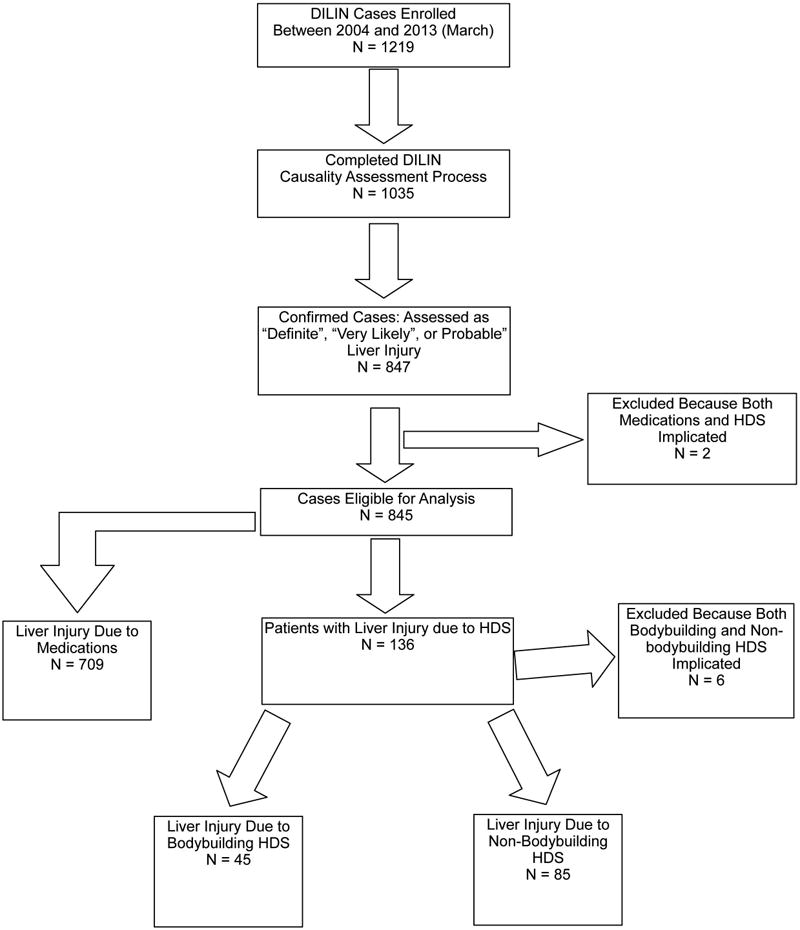
Case Enrollment, 2004 – 2013. The selection of cases for this report is shown. There were 1219 patients who were enrolled into the DILIN during the period from 2004 to March, 2013. Only the 847 patients who completed the causality assessment process and who were confirmed as having liver injury from a medication or HDS, that is with a definite, very likely, or probable causal association between the agent and liver injury, were eligible for this analysis. Of this group, 8 were excluded; 2 because they had injury that could have resulted from either medications or HDS and 6 because they had consumed both bodybuilding and non-bodybuilding HDS. There were remaining 709 patients in whom medications were the cause for injury, and 130 in which HDS were the cause, 45 due to bodybuilding HDS and 85 due to non-bodybuilding HDS.
Demographic Characteristics
Demographic characteristics are shown in Table 1. Patients with liver injury attributed to bodybuilding HDS were younger compared to those with injury from non-bodybuilding HDS and medications (median age 31 vs. 47 vs. 52 years, respectively, p<0.001) and were exclusively male (100% vs. 35% vs. 37%, respectively, P<0.001). Liver injury from non-bodybuilding HDS involved non-Hispanic Whites and non-Hispanic Blacks less frequently (p =0.002), and Hispanic/Latinos more frequently (p < 0.001) than did injury attributed to either bodybuilding HDS or medications.
Table 1. Demographic Characteristics of the Subject Population.
| Characteristic | Total n=839 | Liver Injury Due to Bodybuilding HDS n=45 | Liver Injury Due to Non-bodybuilding HDS n=85 | Liver Injury Due to Conventional Medications n=709 | p-value |
|---|---|---|---|---|---|
| Age, median (25th, 75th) | 50 (37, 61) | 31 (26, 37) | 47 (38, 61) | 52 (39, 62) | <.001 |
| Gender | <.001 | ||||
| Male | 337 (40%) | 45 (100%) | 30 (35%) | 262 (37%) | |
| Female | 502 (60%) | 0 (0%) | 55 (65%) | 447 (63%) | |
| Race | |||||
| Non-Hispanic White | 657 (78%) | 37 (82%) | 57 (68%) | 563 (80%) | 0.002 |
| Non-Hispanic Black | 97 (12%) | 5 (11%) | 7 (8%) | 85 (12%) | |
| Hispanic | 87 (10%) | 6 (13%) | 20 (24%) | 61 (9%) | <.001 |
Temporal Trends in Liver Injury
The proportion of patients with liver injury from HDS in the DILIN registry increased during the study at a greater rate than that of injury ascribed to conventional medications. Specifically, 7% of DILIN cases were attributed to HDS during the first two years of the registry compared to 20% ten years later (p = 0.0007) (Figure 2); the increase involved both bodybuilding HDS (from 2% in 2004-5 to 8% in 2010-12, p = 0.007) and non-bodybuilding HDS (from 5% in 2004-5 to 12% in 2010-12, p = 0.05). Liver injury cases were grouped by two year enrollment cohorts, although patients enrolled during 2012 were included with the final cohort due to the small number that had completed the causality assessment process prior to analysis. The increased rate of liver injury from non-bodybuilding HDS resulted mainly from a disproportionate increase in cases enrolled at two metropolitan centers, Los Angeles, CA, and Philadelphia, PA. Of the total 213 cases in 2010-12, these two centers had 19 (23%) non-bodybuilding cases out of the 82 confirmed cases as compared to 18 (8%) non-bodybuilding cases out of 232 confirmed cases in the other 6 centers (p <0.001).
Figure 2.
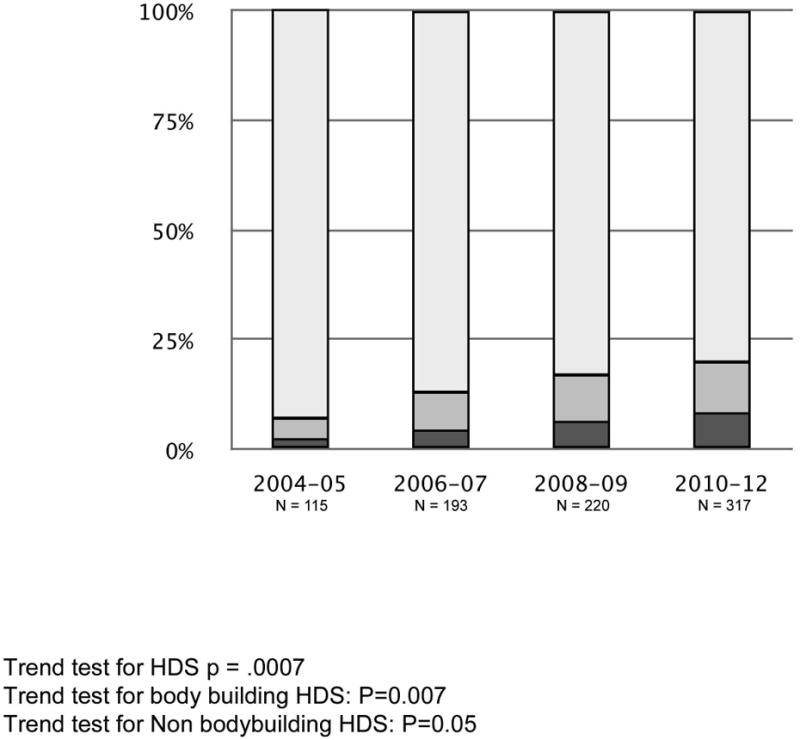
Temporal Trends in DILIN Enrollment.
Liver Injury Outcomes
As shown in Table 2, liver transplantation was required more frequently among patients with injury from non-bodybuilding HDS than with hepatotoxicity from conventional medications (13% vs. 3%, respectively P< 0.001). This difference remained statistically significant (p=0.001) after adjusting for clinical and demographical variables that were different among the groups in a multivariate logistic regression analysis. The considered covariates were age, sex, race, Hispanic ethnicity, weight, history of allergy, alcohol consumption, history of diabetes mellitus, history of neurological disease, history of heart disease, history of renal disease, history of pulmonary disease, history of gastrointestinal disease, history of malignancy, history of congestive heart failure, and any comorbid condition. Only race and weight remained statistically significant in addition to group assignment (ie; bodybuilding, non-bodybuilding, or conventional medication injury group) in the final logistic regression model. No patients with hepatotoxicity attributed to bodybuilding HDS died as a result or required liver transplantation. Hospitalization rates did not differ among the groups. These observations remained unchanged even after excluding from the analyses those patients with preexisting comorbid conditions (data not shown).
A total of 13 patients underwent liver transplantation or died in the non-bodybuilding HDS group (one patient had liver transplant and then died). Their mean age was 56 years (range 27 to 73), All 13 were female, and 9 (69%) were white (Table 3). Not surprisingly, patients with more severe hepatocellular injury (R value > 5) progressed to liver transplantation more quickly than did those with cholestatic/mixed liver injury [median (range) days from onset to death/transplant was 28 (2-77) vs. 234 (61-263) in cholestatic/mixed group, p=0.004. Two out of three patients who died had cholestatic/mixed injury. Two of the three deaths were attributed to the liver injury and the remaining death occurred as a result of an endoscopic procedural complication.
Table 3. Cases of Death or Liver Transplant Resulting from Liver Injury Due to Non-Body-building HDS.
| Case | Age | Gender | Race/Ethnicity | Product Name | Main Marketed Purpose for Use | Clinical Pattern (R value) | Death or Transplant | Days from DILI onset to death/transplant |
|---|---|---|---|---|---|---|---|---|
| 1 | 49 | Female | White | UP YOUR GAS – ENERGY BLASTER | Energy Booster | Hepatocellular (10) | Transplant | 77 |
| 2 | 45 | Female | White | CVS SPECTRAVITE PERFORMANCE | Multivitamin | Hepatocellular (20) | Transplant | 16 |
| 3 | 34 | Male | White | VASOPRO EPHEDRINE | Weight Loss | Hepatocellular (63) | Transplant | 2 |
| 4 | 64 | Male | White | CHINESE HERBAL VIAGRA | Sexual Performance | Cholestatic (1) | Transplant | 234 |
| 5 | 27 | Female | Latino | Slimquick RIPPED FUEL “EXTREME” EPHEDRA FREE |
Weight Loss | Hepatocellular (30) | Transplant | 13 |
| 6 | 66 | Male | Asian | Chinese Herbs | Unknown | Hepatocellular (30) | Transplant | 5 |
| 7 | 58 | Female | White | ULTRA VITALITY MULTIVITAMIN AND MINERAL DRINK | Multivitamin | Cholestatic (<1) | Transplant | 252 |
| 8 | 62 | Male | White | Swanson DHEA Swanson Eurycoma Longifolia Swanson Oyster Extract |
Sexual Performance | Hepatocellular (37) | Transplant and Death | 33 |
| 9 | 71 | Male | White | Complete Natural Products-Gallbladder Complete Native American Nutritionals Ph Rescue |
Miscellaneous | Cholestatic (<1) | Death | 61 |
| 10 | 73 | Female | Asian | Chinese Herbals | Chinese Herbals | Hepatocellular (8) | Transplant | 57 |
| 11 | 64 | Male | White | Unknown Herbal Tablet from Thailand | Unknown | Cholestatic (1.2) | Transplant | 234 |
| 12 | 56 | Male | Multiracia l | Bhumianl Kichurna Haridra Khand Kamdudla Ras Mahamanjisthadi Kwath Tab Arogyavardhini Tab Tagaradi Vati |
Miscellaneous | Mixed (2.7) | Death | 124 |
| 13 | 62 | Female | White | Dual Action Cleanse – Colon Clear Formula Multicleanse Formula – Cleansing Complex with Herbs/Fibers Dual Action Cleanse – Total Body Purifier |
Miscellaneous | Cholestatic (<1) | Transplant | 263 |
The HDS liver injury group was found to have a significantly higher proportion of severe cases, based on the DILIN severity score, than the conventional medications liver injury group (p=0.02). The adjusted group difference in severity score among the three groups remained statistically significant (P=0.04 for body-building HDS vs. conventional medications and p=0.007 for non-bodybuilding HDS vs. conventional medications) after adjusting for baseline clinical and demographical variables that were considered for the DILIN severity score model. Only age, alcohol consumption, history of renal disease and history of congestive failure remained statically significant in addition to group indicators in the final logistic regression model.
Clinical Characteristics
Patients with hepatotoxicity from bodybuilding HDS were heavier but without significant differences in body mass index compared to other groups, presumably because all were males with a greater muscle mass (Table 4) and weight was significantly different between the groups after adjusting for gender (P=0.6) They also had distinctive clinical symptoms in that all were jaundiced (p < 0.001) and most (84%) had pruritus (p < 0.001).
Table 4. Clinical and Laboratory Data.
| Characteristic | Liver Injury Due to Bodybuilding HDS n=45 | Liver Injury Due to Non-bodybuilding HDS n=85 | Liver Injury due to Conventional Medications n=709 | p-value |
| Weight (kg) | <.001 | |||
| Median (25th, 75th) | 86.4 (78.7, 98.1) | 72.7 (62.8, 83.6) | 74.1 (62.4, 89.4) | |
| BMI | 0.954 | |||
| Median (25th, 75th) | 26.4 (24.3, 29.5) | 6.2 (23.1, 30.2) | 26.2 (22.9, 30.4) | |
| Symptoms | ||||
| Jaundice | 45 (100%) | 66 (78%) | 482 (68%) | <.001 |
| Nausea | 27 (60%) | 56 (66%) | 420 (59%) | 0.522 |
| Pruritus | 38 (84%) | 41 (48%) | 373 (53%) | <.001 |
| Fever | 7 (16%) | 17 (20%) | 208 (29%) | 0.033 |
| Abdominal Pain | 26 (58%) | 44 (52%) | 293 (41%) | 0.024 |
| Rash | 11 (24%) | 18 (21%) | 190 (27%) | 0.553 |
| Any co-morbid medical condition* | 9 (21%) | 45 (53%) | 492(69%) | <.001 |
| Diabetes | 0 | 19 (22%) | 192 (27%) | <.001 |
| Neurological Disease | 3 (7%) | 6 (7%) | 125 (18%) | 0.007 |
| Heart Disease | 1 (2%) | 9 (11%) | 150 (21%) | <.001 |
| Pulmonary Disease | 3 (7%) | 9 (11%) | 142 (20%) | 0.009 |
| Gastrointestinal Disease | 4 (9%) | 24 (28%) | 252 (36%) | <.001 |
| Any Alcohol Use | 34 (79%) | 45 (54%) | 338 (48%) | <.001 |
| Liver Enzymes at Onset | ||||
| ALT (U/L), Median (25th, 75th) | 173 (124, 376) | 1019 (360, 1695) | 505 (249, 965) | <.001 |
| AST (U/L), Mean (SD) | 82 (65, 118) | 815 (323, 1437) | 319 (167, 852) | <.001 |
| AlkPhos (U/L), Mean (SD) | 116 (92, 133) | 212 (153, 283) | 222 (142, 269) | <.001 |
| Total Bili (mg/dL), Mean (SD) | 9.8 (7.8, 13.0) | 7.5 (3.0, 13.0) | 4.3 (1.1, 8.0) | <.001 |
| Clinical pattern at onset ** | 0.012 | |||
| Cholestatic | 12 (28%) | 10 (13%) | 164 (25%) | |
| Mixed | 13 (30%) | 13 (17%) | 150 (23%) | |
| Hepatocellular | 18 (42%) | 56 (71%) | 351 (53%) | |
| Days from start of medication or HDS to signs or DILI onset, median (25th, 75th) | 43.5 (25.5, 74.5) | 30.0 (11.0, 59.0) | 26.0 (11.0, 79.0) | 0.157 |
| Course of Injury, median | ||||
| ALT*** (25th,75th) | 28 (11,115) | 14 (6, 26) | 13 (7, 25) | <.0002 |
| AST*** (25th,75th) | 52 (12,135) | 11 (4, 25) | 10 (5, 21) | <.001 |
| AlkPhos*** (25th,75th) | 126 (42,229) | 60 (23,179) | 43 (21,154) | 0.035 |
| Total Bili**** (25th,75th) | 91 (54,173.) | 44 (27, 92) | 35 (15, 66) | <.001 |
Co-morbid medication conditions included endocrine, infectious, psychiatric, neurological, cardiac, renal, pulmonary, gastrointestinal/hepatic, malignant, autoimmune diseases.
Cholestatic was defined as an R value < 2; mixed as R value 2 to 5; and hepatocellular as R value > 5.
Median number of days for the liver test to fall to 50% of its peak value based on time to event analysis.
Median days from peak to < 2.5 mg/dL.
As shown in table 4, co-morbid conditions were less common among patients with injury from bodybuilding HDS compared to the other two groups; 21 % vs. 53% vs. 69% respectively (p<0.001). Not surprisingly, diabetes and neurological, heart, pulmonary, and gastrointestinal disease were more common among the patients with conventional medication-associated liver injury (p <0.001, 0.007, <0.001, 0.009, and <0.001, respectively). Conversely, alcohol use was more frequent in the bodybuilding group than in the non-bodybuilding or medication groups; 79% vs. 54% vs. 48%, respectively (p < 0.001).
At presentation, patients with injury from bodybuilding HDS had the lowest median values for serum ALT (173 U/L), AST (82), and Alk P (116 U/L), but the highest total bilirubin levels (9.8 mg/dL) (p < 0.001 for all liver tests). In contrast, patients with injury from non-bodybuilding HDS had the highest mean ALT (1019 U/L) and AST (815 U/L) values and intermediate mean Alk P (212 U/L) and bilirubin levels (7.5 mg/dL). Patients with medication-induced injury had intermediate ALT and AST elevations (505 and 319, respectively), the highest Alk P and lowest bilirubin levels.
The pattern of liver injury in those using bodybuilding HDS resembled that of bland cholestasis with strikingly elevated total serum bilirubin levels and only modest increases in the ALT, AST, and Alk P values, yet the R value at study entry classified 42% of them as having a hepatocellular pattern of injury. On the other hand, most patients with injury due to non-bodybuilding HDS and medications had R values [>5] indicative of hepatocellular injury.
Course Of Liver Injury
Latency, defined as the number of days between start of the agent and onset of injury, was not significantly different among the three groups (Table 4), although there was substantial variability. In contrast, patients with hepatotoxicity from bodybuilding HDS had a more protracted course of liver injury (assessed as the median number of days to achieve a 50% reduction from the peak ALT and AST abnormalities and from the peak total bilirubin level to less than 2.5 mg/dL), than did the other two groups. Patients with liver injury from bodybuilding HDS were jaundiced for a median of 91 days, compared to 44 and 35 days, respectively, for the non-bodybuilding HDS and medication groups (p< .001).
Supplements Implicated In Liver Injury
The majority of patients used numerous HDS products, most of which contained multiple ingredients, including vitamins, minerals and botanical extracts. Thus, the 130 patients with liver injury from bodybuilding and non-bodybuilding HDS reported that they had taken a total of 217 products. Among these 217 products, 175 (81%; 59 bodybuilding HDS and 116 non-bodybuilding HDS) had identifiable ingredients. Only 7 (12%) of the 59 bodybuilding HDS and 25 (22%) of the 116 non-bodybuilding HDS were labeled as having a single component, while 6 (10%) bodybuilding and 15 (13%) non-bodybuilding products had more than 20 ingredients. The list of implicated HDS products is shown in Supplementary Table 1.
Discussion
Contrary to widespread belief, this study demonstrates that HDS products are not always safe. Indeed, our data suggest that, relative to conventional medication-induced hepatotoxicity, liver injury from HDS not only occurs but may be increasing in frequency over time in the populations surrounding the DILIN centers and, probably, in the USA as a whole. The study also shows that bodybuilding HDS are the most commonly implicated class of products. Most importantly, we found that non-bodybuilding HDS can cause liver injury that is more severe than conventional medications, as reflected in a higher transplantation rate. This finding was independent of co-morbid conditions.
Regarding non-bodybuilding HDS, despite their heterogeneity, the typical pattern of liver injury was hepatocellular, similar to acute viral hepatitis. This injury occurred most often in women. Clearly, the evidence of acute necro-inflammatory liver injury, reflected in the high ALT and AST levels and the R value, identifies a greater degree of hepatocyte injury, predisposing to more serious outcomes.
Data from other countries have also noted the occurrence of HDS-related liver injury, ranging from 2% (14) to 16% of all identified cases of hepatotoxicity, (15) but such reports have not reported a temporal trend. Our observation of the rising burden of hepatotoxicity attributed to HDS in the DILIN coincides with their increasing use in the US. In 1990, 34% of U.S. adults used some form of alternative therapies, 2.5% being herbals or dietary supplements.(16) By 1997, the frequency had increased to 42%, 12% using herbals. The NHANES II survey showed a 35% prevalence of supplement use between 1976 and 1980,(17) rising to 52% in the 1999-2000 survey.(4) Between 1988 and 1994, the increased use was not gender-specific, with rates in men increasing from 30% to 42% and in women, from 42% to 55%.(18) As noted, recent data show that approximately half of U.S. adults use dietary supplements. (1,2)
The increased use of HDS is also reflected in commerce. An estimated $27 billion was spent by consumers for all herbal products in 1997. (17) This figure rose to $33.9 billion in 2007. (19) Additionally, reports from the American Botanical Council showed that sales of herbals increased from 1999 to 2011. (20) These data, allied with our findings, suggest that the incidence of hepatotoxicity from HDS is increasing and is likely to continue to increase. However, the DILIN is not a population-based study and our data may reflect geographical variations in usage patterns,
Our analysis revealed bodybuilding products to be the most common cause for liver injury among those using HDS products, eliciting a distinctive clinical picture of prolonged jaundice in young men with non-fatal outcomes. Despite the prolonged jaundice and only modest increases in ALT or AST values, the initial R values suggested hepatocellular injury in a substantial proportion. This may reflect a shortcoming of the R value determination or of the threshold of > 5 as defining hepatocellular injury, or there may indeed be early hepatocellular injury from bodybuilding HDS. In fact, a recent report of liver injury resulting from the product N.O.-XPLODE, ostensibly a bodybuilding (muscle enhancing) product, showed that one third of patients affected had hepatocellular patterns of injury. (21) A planned comparison of the R value to the histological findings may further clarify this point. Another important consideration is that there is no standard nomenclature or classification schema for HDS; therefore, the process of grouping various HDS by their intended effect may be flawed, as it does not take into account ingredients and their potential mechanisms of action or injury.
There are numerous reports of liver injury from bodybuilding products, some shown or suspected to contain anabolic steroids.(22-24) The similarity in the pattern of injury in the bodybuilding group in this study with those reported previously suggest that there may be a common susceptibility factor or that the products may contain 17-alkyl substituted (anabolic) steroids, which are well known to cause this injury pattern.(25) Alternatively, host susceptibility factors, such as drug-or ingredient-specific genetic determinants of drug disposition may account for the injury.(26-28)
Assessing potential HDS hepatotoxicity presents unique challenges. The numerous products that frequently contain multiple ingredients, often with unclear chemical descriptors and variable common names, can confound pinpointing the specific toxic agent. Furthermore, some products may seem quite innocuous, such as multivitamins, making it difficult to conceive of any toxic potential. There are many reports of contamination of herbals with microbials (29,30), pharmaceuticals (31-33), mycotoxins (34), and heavy metals.(35-38) Also, unidentified interactions with medications used concomitantly may be responsible for toxicity, yet are difficult to establish. Although the causality assessment process gave us confidence that our cases, in fact, represented bona fide hepatotoxicity from HDS, any one of these factors could have been present.
Our findings underscore our still rudimentary understanding of liver injury from HDS, and create a mandate for further research into their safety. Although we demonstrate that numerous HDS products have the capacity to cause liver injury and such injury is more likely to result in transplantation than injury from conventional medications, identifying the specific ingredient responsible for the injury or perhaps even permissive host factors, remains a daunting challenge. The most effective approach to identify culprit agents would require a painstaking separation of products into their component ingredients, followed by in-vitro and in-vivo toxicological evaluation. Arguably, the cost of such an extensive approach would be prohibitive to most funding agencies. Alternatively, an effort to list every identifiable ingredient in all implicated HDS products and confining toxicological analysis to those ingredients that appear frequently among such products might represent a more focused and practical approach. Large registries will be critical in continuing to amass products for this purpose.
As noted, the DILIN is not a population based study, and although there was an increasing proportion of disease attributable to HDS during the study, it cannot be concluded that the problem is actually on the rise in the US. Therefore, population-based studies to investigate the incidence of liver injury will inform several avenues of future investigation and regulation. Notwithstanding the need to accurately determine the incidence of drug and dietary supplement induced liver injury in the U.S., a better understanding of the impact of the problem on the population will permit proportionate allocation of resources toward research. All stakeholders, including the dietary supplement industry, regulatory agencies, health care providers and consumers must take note of these findings if a culture of safety for HDS use is to be established.
Supplementary Material
Acknowledgments
Financial Support: The Drug Induced Liver Injury Network (DILIN) is a cooperative network funded by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK). See DILIN website https://dilin.dcri.duke.edu/publications-1 for a complete listing of funding sources, sites, investigators, co-investigators, coordinators, and staff.
Footnotes
Trial Registration: The Drug Induced Liver Injury Network (DILIN),ClinicalTrials.gov Identifier: NCT00345930, https://dilin.dcri.duke.edu/
Contributor Information
Huiman Barnhart, Email: huiman.barnhart@duke.edu.
Herbert L. Bonkovsky, Email: Herbert.Bonkovsky@carolinashealthcare.org.
Timothy Davern, Email: davernt@sutterhealth.org.
Robert J. Fontana, Email: rfontana@med.umich.edu.
Lafaine Grant, Email: lafaine.grant@utsouthwestern.edu.
K. Rajender Reddy, Email: rajender.reddy@uphs.upenn.edu.
Leonard B. Seeff, Email: mahler68@hotmail.com.
Jose Serrano, Email: SerranoJ@extra.niddk.nih.gov.
Averell H. Sherker, Email: averell.sherker@nih.gov.
Andrew Stolz, Email: astolz@usc.edu.
Jayant Talwalkar, Email: Talwalkar.Jayant@mayo.edu.
Maricruz Vega, Email: VegaMari@einstein.edu.
Raj Vuppalanchi, Email: rvuppala@iu.edu.
References
- 1.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141(2):261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picciano MF, Dwyer JT, Radimer KL, et al. Dietary supplement use among infants, children, and adolescents in the United States, 1999-2002. Arch Pediatr Adolesc Med. 2007;161(10):978–985. doi: 10.1001/archpedi.161.10.978. [DOI] [PubMed] [Google Scholar]
- 3.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 6.Foote JA, Murphy SP, Wilkens LR, Hankin JH, Henderson BE, Kolonel LN. Factors associated with dietary supplement use among healthy adults of five ethnicities: the Multiethnic Cohort Study. Am J Epidemiol. 2003;157(10):888–897. doi: 10.1093/aje/kwg072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block G, Jensen CD, Norkus EP, et al. Usage patterns, health, and nutritional status of long-term multiple dietary supplement users: a cross-sectional study. Nutr J. 2007;6:30. doi: 10.1186/1475-2891-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder FJ, Dundas ML, Kirkpatrick C, Neill KS. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. Journal of Nutrition for the Elderly. 2009;28:81–95. doi: 10.1080/01639360802634043. [DOI] [PubMed] [Google Scholar]
- 9.Dietary Supplements. Food and Drug Administration; [Accessed 07/5/2013]. http://www.fda.gov/Food/DietarySupplements/default.htm. [Google Scholar]
- 10.FDA Investigates Acute Hepatitis Illnesses Potentially Linked to Products Labeled OxyElite Pro. Food and Drug Administration; [Accessed on 11/16/2013]. http://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm370849.htm. [Google Scholar]
- 11.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Rationale, design and conduct of the Drug Induced Liver Injury Network prospective study. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings; application to drug-induced liver injuries. J ClinEpidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 14.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury; an analysis of 461 incidences submitted to the Spanish registry over a 10 year period. Gastroenterology. 2005;129:512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Bjornsson E, Bergman OM, Bjornsson HK, Kyaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. NEJM. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national study. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 18.Ervin RB, Wright JD, Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988-1994. Vital Health Stat 11. 1999:i–iii. 1–14. [PubMed] [Google Scholar]
- 19.Nahin RL, Barnes PM, Stussman BJ, Bloom B. National health statistics reports. 18. Hyattsville, MD: National Center for Health Statistics; 2009. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. [PubMed] [Google Scholar]
- 20.Lindstrom A, Ooyen C, Lynch ME, Blumenthal M. Herb supplement sales increase 5.5% in 2012: herbal supplement sales rise for the 9th consecutive year: turmeric sales jump 40% in herbal channel. HerbalGram. 2013;99:60–65. [Google Scholar]
- 21.Martin DJ, Partridge BJ, Shields W. Hepatotoxicity associated with the dietary supplement N.O.-XPLODE. Ann Int Med. 2013;159(7):503–504. doi: 10.7326/0003-4819-159-7-201310010-00019. [DOI] [PubMed] [Google Scholar]
- 22.Ishak KG. Hepatic lesions caused by anabolic and contraceptive steroids. Semin Liver Dis. 1981;1:116–28. doi: 10.1055/s-2008-1040724. [DOI] [PubMed] [Google Scholar]
- 23.Singh V, Rudraraju M, Carey EJ, et al. Severe hepatotoxicity caused by a Methasteron-containing performance-enhancing supplement. J Clin Gastroenterol. 2009;43:287. doi: 10.1097/MCG.0b013e31815a5796. [DOI] [PubMed] [Google Scholar]
- 24.Timchec-Hariri A, Balali-Mood M, Aryan E, Sadeghi M, Riahi-Zanjani B. Toxic hepatitis in a group of 20 male body-builders taking dietary supplements. Food ChemToixicol. 2012;50:3826–32. doi: 10.1016/j.fct.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Buttner A, Thieme D. Side effects of anabolic androgenic steroids: pathological findings and structure-activity relationships. Handb Exp Pharmacol. 2010;195:459–84. doi: 10.1007/978-3-540-79088-4_19. [DOI] [PubMed] [Google Scholar]
- 26.Urban TJ, Shen Y, Stolz A, et al. Limited contribution of common genetic variants to risk for liver injury due to a variety of drugs. Pharmacogenetics and Genomics. 2012;22:784–795. doi: 10.1097/FPC.0b013e3283589a76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucena MI, Molokhia M, Shen Y, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 29.Kneifel W, Czech E, Kopp B. Microbial contamination of medicinal plants. Planta Med. 2000;68:5–15. doi: 10.1055/s-2002-20060. [DOI] [PubMed] [Google Scholar]
- 30.Stickel F, Droz S, Patsenker E, Bogli-Stuber K, Aebi B, Leib SL. Severe hepatotoxicity following ingestion of Herbalife contaminated with Bacillus subtilis. J Hepatol. 2009;50:111–7. doi: 10.1016/j.jhep.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Miller GM, Streipp R. A study of western pharmaceuticals contained within samples of Chinese herbal/patent medicines collected from New York City's Chinatown. Leg Med (Tokyo) 2007;9:258–64. doi: 10.1016/j.legalmed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Guns ES, Goldenberg SL, Brown PN. Mass spectral analysis of PC-SPES confirms the presence of diethylstilbestrol. Can J Urol. 2002;9:1684–8. [PubMed] [Google Scholar]
- 33.Oh WK, Small EJ. Complementary and alternative therapies in prostate cancer. SeminOncol. 2002;29:575–84. doi: 10.1053/sonc.2002.50007. [DOI] [PubMed] [Google Scholar]
- 34.Gray SL, Lackey BR, Tate PL, Riley MB, Camper ND. Mycotoxins in root extracts of American and Asian ginseng bind estrogen receptors alpha and beta. Exp Biol Med. 2004;229:560–8. doi: 10.1177/153537020422900615. [DOI] [PubMed] [Google Scholar]
- 35.Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the internet. JAMA. 2008;300:915–23. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong MK, Tan P, Wee YC. Heavy metals in some Chinese herbal plants. Biol Trace Elem. 1993;36:135–42. doi: 10.1007/BF02783172. [DOI] [PubMed] [Google Scholar]
- 37.Koh HL, Woo SO. Chinese propriety medicine in Singapore: regulatory control of toxic heavy metals and undeclared drugs. Drug Saf. 2000;23:351–62. doi: 10.2165/00002018-200023050-00001. [DOI] [PubMed] [Google Scholar]
- 38.Au AM, Ko R, Boo FO, Hsu R, Perez G, Yang Z. Screening methods for drugs and heavy metals in Chinese patent medicines. Bull Environ ContamToxicol. 2000;65 doi: 10.1007/s0012800102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


