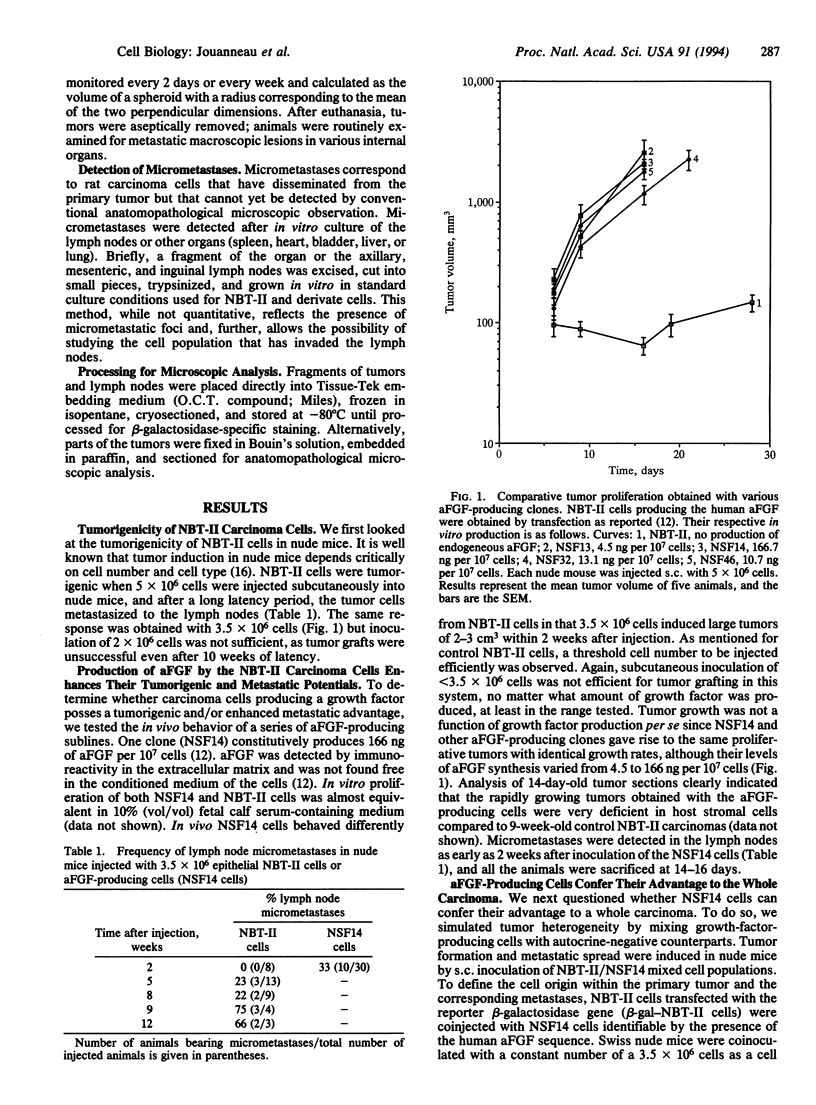
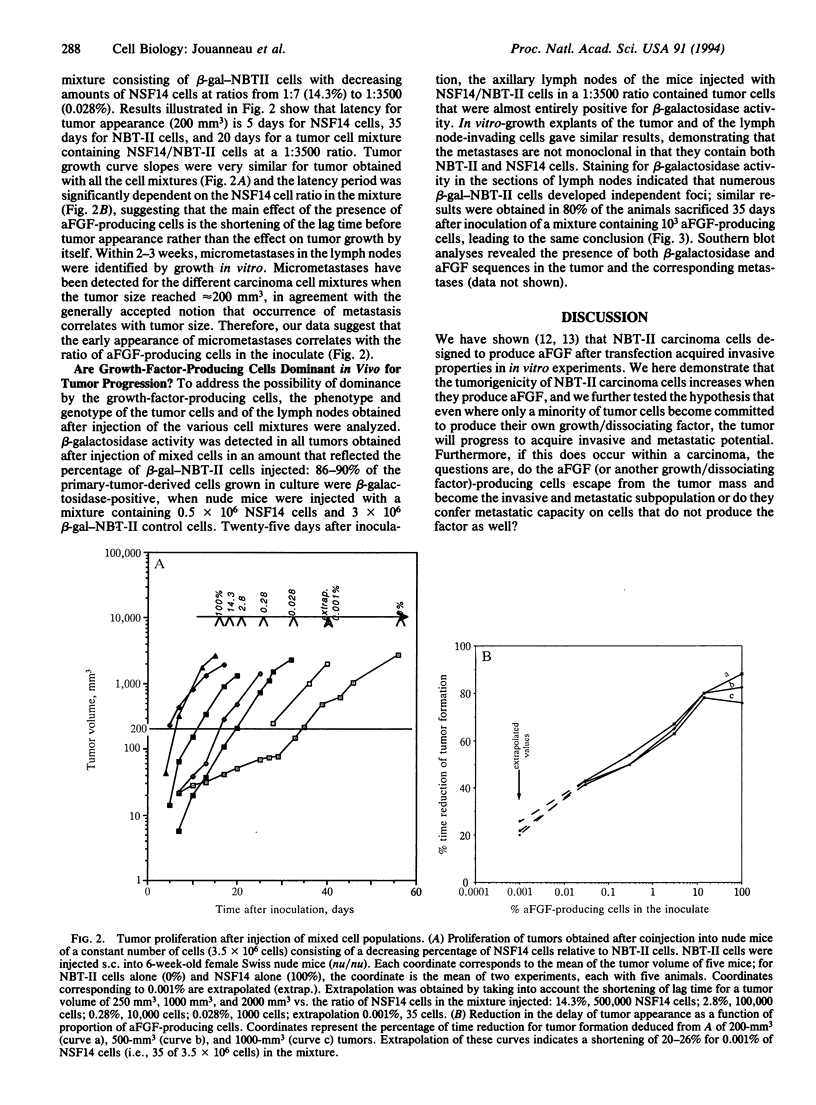
Abstract
It is generally accepted that primary tumors become heterogeneous as a consequence of tumor-cell genetic instability. Clonal dominance has been shown to occur in some experimental models allowing a subpopulation of cells to overgrow the primary heterogeneous tumor and to metastasize. Alternatively, interactions among coexisting tumor subpopulations may contribute to the emergence of a malignant invasive primary solid tumor. We asked the question whether emergence of carcinoma cells producing a growth/dissociating factor within a tumor cell population may be a determinant for tumor progression and for clonal dominance. To mimic such a situation, we have investigated the impact of tumor subpopulation heterogeneity in an in vivo model in which mixtures of carcinoma cells that differ in their ability to produce acidic fibroblast growth factor are injected into nude mice. Our data indicate that a growth-factor-producing cell subpopulation can confer increased tumorigenicity to an entire cell population and subsequently elicit a shorter delay for appearance of metastasis. A community effect via cell interactions may account for a heterogeneous tumor cell population rather than clonal dominance during progression of certain tumor types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Boyer B., Tucker G. C., Vallés A. M., Franke W. W., Thiery J. P. Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol. 1989 Oct;109(4 Pt 1):1495–1509. doi: 10.1083/jcb.109.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps J. L., Chang S. M., Hsu T. C., Freeman M. R., Hong S. J., Zhau H. E., von Eschenbach A. C., Chung L. W. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci U S A. 1990 Jan;87(1):75–79. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. C., Chou C. K., Wong F. H., Chang C. M., Hu C. P. Overexpression of epidermal growth factor and insulin-like growth factor-I receptors and autocrine stimulation in human esophageal carcinoma cells. Cancer Res. 1991 Apr 1;51(7):1898–1903. [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A. Modes of FGF release in vivo and in vitro. Cancer Metastasis Rev. 1990 Nov;9(3):227–238. doi: 10.1007/BF00046362. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990 Jan 3;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Tiller E., Roberts J., Kato K. A community effect in muscle development. Curr Biol. 1993 Jan;3(1):1–11. doi: 10.1016/0960-9822(93)90139-f. [DOI] [PubMed] [Google Scholar]
- Heppner G. H. Tumor heterogeneity. Cancer Res. 1984 Jun;44(6):2259–2265. [PubMed] [Google Scholar]
- Jouanneau J., Gavrilovic J., Caruelle D., Jaye M., Moens G., Caruelle J. P., Thiery J. P. Secreted or nonsecreted forms of acidic fibroblast growth factor produced by transfected epithelial cells influence cell morphology, motility, and invasive potential. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2893–2897. doi: 10.1073/pnas.88.7.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S. Growth dominance of the metastatic cancer cell: cellular and molecular aspects. Adv Cancer Res. 1990;55:87–132. doi: 10.1016/s0065-230x(08)60469-8. [DOI] [PubMed] [Google Scholar]
- Korczak B., Kerbel R. S., Dennis J. W. Autocrine and paracrine regulation of tissue inhibitor of metalloproteinases, transin, and urokinase gene expression in metastatic and nonmetastatic mammary carcinoma cells. Cell Growth Differ. 1991 Jul;2(7):335–341. [PubMed] [Google Scholar]
- Leith J. T., Michelson S., Faulkner L. E., Bliven S. F. Growth properties of artificial heterogeneous human colon tumors. Cancer Res. 1987 Feb 15;47(4):1045–1051. [PubMed] [Google Scholar]
- Miller B. E., Miller F. R., Wilburn D., Heppner G. H. Dominance of a tumor subpopulation line in mixed heterogeneous mouse mammary tumors. Cancer Res. 1988 Oct 15;48(20):5747–5753. [PubMed] [Google Scholar]
- Miller F. R., Heppner G. H. Cellular interactions in metastasis. Cancer Metastasis Rev. 1990 Jul;9(1):21–34. doi: 10.1007/BF00047586. [DOI] [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida H., Hirabayashi N., Hirai T., Iwata T., Saeki S., Toge T. Significance of freshly cultured fibroblasts from different tissues in promoting cancer cell growth. Int J Cancer. 1991 May 30;48(3):423–427. doi: 10.1002/ijc.2910480320. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Dulski K. M., Trosko J. E. Loss of intercellular junctional communication correlates with metastatic potential in mammary adenocarcinoma cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):473–476. doi: 10.1073/pnas.85.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ohmura E., Okada M., Onoda N., Kamiya Y., Murakami H., Tsushima T., Shizume K. Insulin-like growth factor I and transforming growth factor alpha as autocrine growth factors in human pancreatic cancer cell growth. Cancer Res. 1990 Jan 1;50(1):103–107. [PubMed] [Google Scholar]
- Picard O., Rolland Y., Poupon M. F. Fibroblast-dependent tumorigenicity of cells in nude mice: implication for implantation of metastases. Cancer Res. 1986 Jul;46(7):3290–3294. [PubMed] [Google Scholar]
- Pritchett T. R., Wang J. K., Jones P. A. Mesenchymal-epithelial interactions between normal and transformed human bladder cells. Cancer Res. 1989 May 15;49(10):2750–2754. [PubMed] [Google Scholar]
- Sharkey F. E., Fogh J. Considerations in the use of nude mice for cancer research. Cancer Metastasis Rev. 1984;3(4):341–360. doi: 10.1007/BF00051459. [DOI] [PubMed] [Google Scholar]
- Theodorescu D., Cornil I., Sheehan C., Man S., Kerbel R. S. Dominance of metastatically competent cells in primary murine breast neoplasms is necessary for distant metastatic spread. Int J Cancer. 1991 Jan 2;47(1):118–123. doi: 10.1002/ijc.2910470121. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Ito N., Hiasa Y., Kamamoto Y., Makiura S. Tissue culture of urinary bladder tumor induced in a rat by N-butyl-N-(-4-hydroxybutyl)nitrosamine: establishment of cell line, Nara Bladder Tumor II. J Natl Cancer Inst. 1971 Nov;47(5):979–985. [PubMed] [Google Scholar]
- Tucker G. C., Delouvée A., Jouanneau J., Gavrilovic J., Moens G., Vallés A. M., Thiery J. P. Amplification of invasiveness in organotypic cultures after NBT-II rat bladder carcinoma stimulation with in vitro scattering factors. Invasion Metastasis. 1991;11(6):297–309. [PubMed] [Google Scholar]
- Vallés A. M., Boyer B., Badet J., Tucker G. C., Barritault D., Thiery J. P. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1124–1128. doi: 10.1073/pnas.87.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallés A. M., Tucker G. C., Thiery J. P., Boyer B. Alternative patterns of mitogenesis and cell scattering induced by acidic FGF as a function of cell density in a rat bladder carcinoma cell line. Cell Regul. 1990 Dec;1(13):975–988. doi: 10.1091/mbc.1.13.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D. R., Fabra A., Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]