Abstract
Signal pathways that reduce the levels of tyrosine phosphorylated STAT3 (pSTAT3) allow late retinal progenitors to exit the cell cycle and enter a terminal differentiation pathway into rod photoreceptors. In the mouse retina we previously identified PKC-β1 and -γ isoforms as essential components of a key signal pathway and IGF-1 as a major extrinsic factor regulating rod formation. In this manuscript we demonstrate that PKC decreases phosphotyrosine but not phosphoserine on STAT3 in neonatal mouse retinas. Neither IGF-1 nor PMA induced a significant change in the levels of STAT3, or in the levels of the key proteins regulating STAT3 degradation, SOCS3 and PIAS3. Treatment of neonatal mouse retinal explants with sodium orthovanadate inhibited the PKC-mediated reduction in pSTAT3, indicating a role for a phosphatase. Addition of the PTEN inhibitor bpV(phen) to explant cultures treated with IGF-1 or PMA had no effect on the reduction in pSTAT3 levels, but the effect of both IGF-1 and PMA was blocked by a concentration of the inhibitor NSC87877 that is selective for the phosphatases Shp1 and Shp2. Inhibition of Shp1/2 phosphatases was also sufficient to abolish the IGF1-mediated induction of rod photoreceptor differentiation in the retina explants cultures. We conclude that one or both of these phosphatases are key components regulating the formation of rod photoreceptors in mouse retina.
Keywords: rod photoreceptor, IGF1, STAT3, PTEN, Shp1, Shp2
Introduction
Retinal development is a tightly regulated process in which the coordination of proliferation and cell differentiation results in the ordered formation of the different cell types that populate the retina. In the mouse about 20% of rods exit mitosis between E16 and E20, although the majority of rod photoreceptors are born soon after birth with 95% formed by PN5. (Young, 1985; Livesey and Cepko, 2001; Rapaport et al., 2004).
The cytokines CNTF and LIF are able to completely block rod differentiation during the late embryonic and early postnatal periods (Neophytou et al., 1997; Ezzeddine et al., 1997). This action has been shown to be due entirely to their activation of STAT3 since sustained activation of STAT3 by virally encoded, constitutively-active STAT3 completely blocked rod photoreceptor formation and, conversely, a virally encoded dominant-negative form of STAT3 allowed formation of rods even in the presence of CNTF (Zhang et al 2004). Similar conclusions were reached following experiments using pharmacological inhibitors of STAT3 (Rhee et al., 2004). These results have led to the suggestion that STAT3 serves as a gatekeeper between the transition from progenitor proliferation and commitment to rod differentiation (Zhang et al,. 2004; Rhee et al., 2004).
Several growth factors can promote rod formation in neonatal retinas and in a survey of some of these we found that the most robust effect was induced by IGF-1. We subsequently demonstrated that the action of IGF-1 required the PKC β1 and γ isoforms (Pinzon-Guzman et al., 2011). Stimulation of PKCs with either IGF-1 or PMA increased the number of rods whereas blocking PKC β1 and γ activities resulted in the absence of rod formation. Both IGF-1 and PMA led to decreased levels of tyrosine phosphorylated STAT3, an effect that could be blocked by PKC inhibitors. From these results we suggested that IGF-1 regulated the production of rods in part by reducing the levels of tyrosine phosphorylated STAT3 (Pinzon-Guzman et al., 2011).
Decreases in STAT3 activity can be achieved by altering the levels of STAT3 protein or by changing its phosphorylation state. Two known regulators of STAT3 protein levels are SOCS3 and PIAS3. SOCS3 is a member of a family of intracellular proteins that function as E3 ubiquitin ligases and mediate the degradation of proteins that are associated with these proteins through their N-terminal regions (Kubo et al., 2003, Naka et al., 2005). The protein inhibitor of activated STAT3 (PIAS3) is an E3 SUMO ligase that can decrease STAT3-dependent transcription by blocking STAT3–DNA binding in the nucleus, and have other effects that can modulate photoreceptor development (Chung et al., 1997; Jackson, 2001; Shuai and Liu, 2003; Onishi et al., 2009). Several phosphatases can act on tyrosine phosphorylated STAT3. Among these are the phosphatase and tensin homologue (PTEN), protein-tyrosine phosphatase 1B (PTP1B), and the SH2-containing phosphatases 1 and 2 (Shp1 and Shp2) (Sun and Steinberg 2001; Yasukawa et al., 2000; Shuai and Liu, 2003).
In this study we demonstrate that, during retinal development, IGF1-induced activation of PKC results in reduction of tyrosine phosphorylated STAT3 through actions of a phosphatase. Selective paharmacological inhibitors indicate that the critical enzymes are Shp1 or Shp2, or both, but not either PTEN or PTP1B.
Materials and Methods
Reagents
Polyclonal antibodies against STAT3 C20 (Santa Cruz Biotechnology, Santa Cruz, CA), pSTAT3 (Y705) (Cell Signaling Technology, Inc., Beverly, MA) SOCS3 (Abcam,), PIAS3 (Cell signaling Technology, Inc, Beverly, MA) and β-actin (Sigma-Aldrich, St. Louis, IL), were employed in these experiments. Ret-P1 monoclonal antibody recognizes an epitope on the N-terminus of opsin of rod photoreceptors (Barnstable, 1980; Hicks and Barnstable, 1987). Recombinant LIF was purchased from Millipore (Billerica, MA), IGF1 mouse recombinant from Sigma (St Louis, MO), PMA from LC Laboratories (Woburn, MA), PTEN inhibitor (bpV(phen) from Calbiochem (Darmstadt, Germany), Protein tyrosine phosphatase inhibitor (NSC87877) from Tocris bioscience (Bristol, United Kingdom).
Animals
C57Bl/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All experiments were approved by the Animal Care and Use Committee of Pennsylvania State University College of Medicine. Mice were housed on a 12-hr/12-hr light/dark cycle with ad libitum access to rodent chow. P1 retinas from animals of either sex were used for all experiments. Most of the litters were born on E20, which was considered equivalent to postnatal day 1 (PN1).
Retina isolation and culture
Whole retinas were isolated from pups at post natal day 1 (PN1) as previously described (Zhang et al., 2004; Pinzon-Guzman et al., 2011). Briefly, the sclera and most of the retinal pigmented epithelium (RPE) layer were removed and the retinas cultured in UltraCulture™ (Cambrex Bio Science, Rockland, ME) serum-free medium supplemented with gentamycin (10 μg/ml). For biochemical experiments, retinas were pre-incubated for 5 hrs and then for 30 min in the test compound, unless otherwise stated. For detection of rod photoreceptors, retinas were cultured individually in 1ml of media in a 24-well culture dish at 37°C in a 5% CO2, balance air, atmosphere. Medium was changed every other day by replacing 0.5ml with fresh medium.
Western blot
Whole cell extracts were prepared and Western blot assays performed as described previously (Asao and Fu, 2000; Zhang et al., 2004; Pinzon-Guzman et al., 2011). 25 to 30 μg of lysate were separated by SDS-polyacrylamide gel electrophoresis and transferred to Immun-BlotTM polyvinylidene difluoride membrane (Biorad Hercules, CA). Following incubation with primary and secondary antibodies, immunoreactive bands were visualized using SuperSignalR Chemiluminescent Substrate (Thermo Scientific Rockford, IL). Quantitative image analysis was performed using Image J 1.29 (downloaded from http://rsb.info.nih.gov/ij/).
Histology and immunocytochemistry
Whole eyes and explanted retinas were fixed with 4% paraformaldehyde in PBS for 24 hr at 4°C. After three washes with PBS, fixed tissues were dehydrated through a series of graded ethanols and embedded in paraffin. All samples for one experiment were placed in the same blocks and sectioned for immunohistochemistry. Antigen retrieval was performed as previously described (Zhang et al., 2003, 2004). Following single or double labeling with fluorescent dye-conjugated secondary antibodies (Jackson Immuno-Research Laboratory, West Grove, PA), sections were imaged using an Olympus Fluoview FV1000 confocal microscope (Olympus Center Valley, PA). For each set of experiments, acquisition parameters for each antibody were maintained constant. Cell counts were obtained by averaging the number of rods present in three individual histological cross sections of each retina. At least three different retinas were studied per treatment focusing on the central areas of the retina.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism software. Student’s t test (two-tailed, unpaired) was used to compare two groups and one-way ANOVA (with Newman–Keuls post test) was used to compare more than two groups.
Results
Activation of PKC does not change levels of STAT3 serine phosphorylation or total STAT3 protein
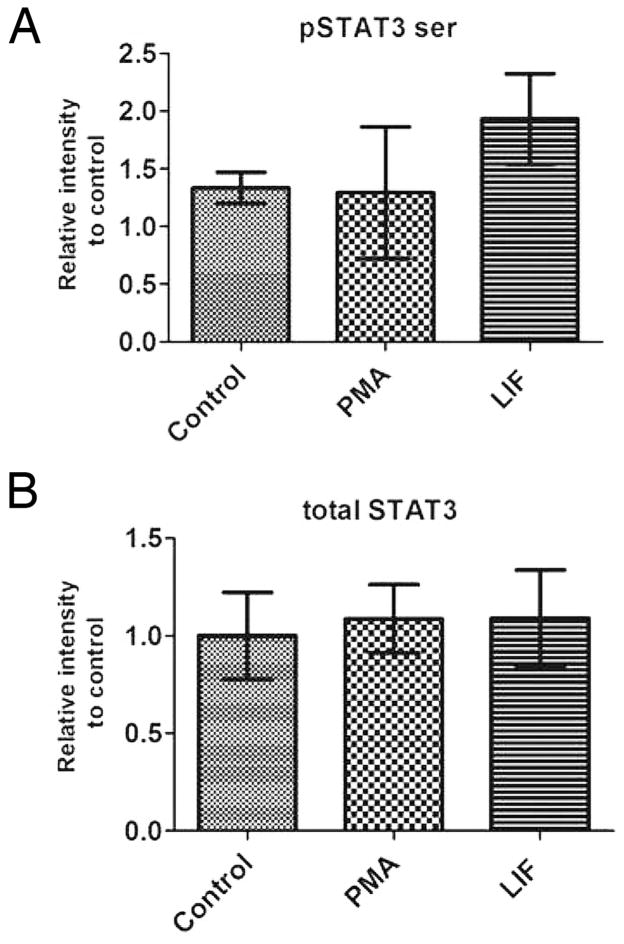
We have previously demonstrated that activation of PKCγ and PKCβ1 with either PMA or IGF1 led to a significant reduction of STAT3 tyrosine phosphorylation during retinal development (Pinzon-Guzman et al 2011). STAT3 activity can also be modulated by serine phosphorylation but after treating retinas with either PMA or the known STAT3 activator LIF we found no significant difference in Ser727 phosphorylation from control values (Fig 1A). Within the time course of these experiments there was also no significant change in the level of STAT3 protein (Fig 1B).
Figure 1.
Retinas treated with PMA or LIF do not change their levels of serine phosphorylated STAT3 or total STAT3 protein. (A) The amount of Ser727 phosphorylated STAT3 and (B) The total amount of STAT3 found in PN1 retinal explants cultured for five hours in ultraculture medium followed by thirty minutes treatment with 100nM PMA or 20ng/mL LIF. For each panel n = 3.
PKCs do not regulate STAT3 through changes in SOCS3 or PIAS3 protein levels
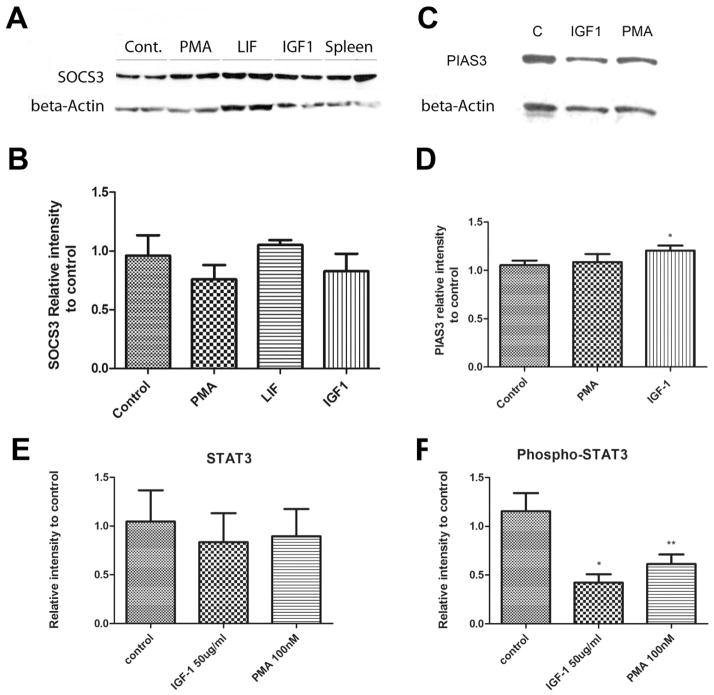
Downregulation of STAT3 can occur by ubiquitination or sumoylation mediated by the proteins SOCS3 and PIAS3 respectively. Each of these proteins can be upregulated by stimuli that downregulate STAT3. Over the time course of these experiments the levels of SOCS3 were not significantly altered by either PMA or IGF-1 (Fig 2A & B). Similarly PMA had no effect on the levels of PIAS3 and IGF1 gave a barely significant increase (Fig 2C & D). In these experiments the treatments did not significantly alter STAT3 levels (Fig 2E) but did reduce pSTAT3 levels (Fig 2F) (P<0.05 and P<0.005 for IGF1 and PMA respectively, n=5). In addition, the western blots of STAT3 protein under these conditions showed a clear single band rather than the multiple bands seen under conditions of ubiquitination. From these results we suggest that, at least in the short term, PKCs do not reduce STAT3 activity by the action of either SOCS3 or PIAS3.
Figure 2. PMA and LIF reduce pSTAT3 levels but have no effect on SOCS3, PIAS3 or STAT3 levels.
(A) Western blot showing SOCS3 protein levels found in PN1 retinas after thirty minute treatment with 100nM PMA, 20ng/mL LIF or 50ng/mL IGF1. (B) Average amount of total SOCS3 protein found in three separate PN1 retinas treated as in A (n=3). (C) Western blot showing PIAS3 levels after 30 min treatment with 50 ng/ml IGF1 or 100 nM PMA. (D) Average amount of total PIAS3 protein found in PN1 retinas treated as in C (n=5). (E & F) the retinas in C and D showed no change in total STAT3 levels (E) but significant reductions in pSTAT3 level (F). * P<0.05, ** P<0.005 vs control.
Activation of PKCs reduce the levels of pSTAT3 through the activation of a phosphatase
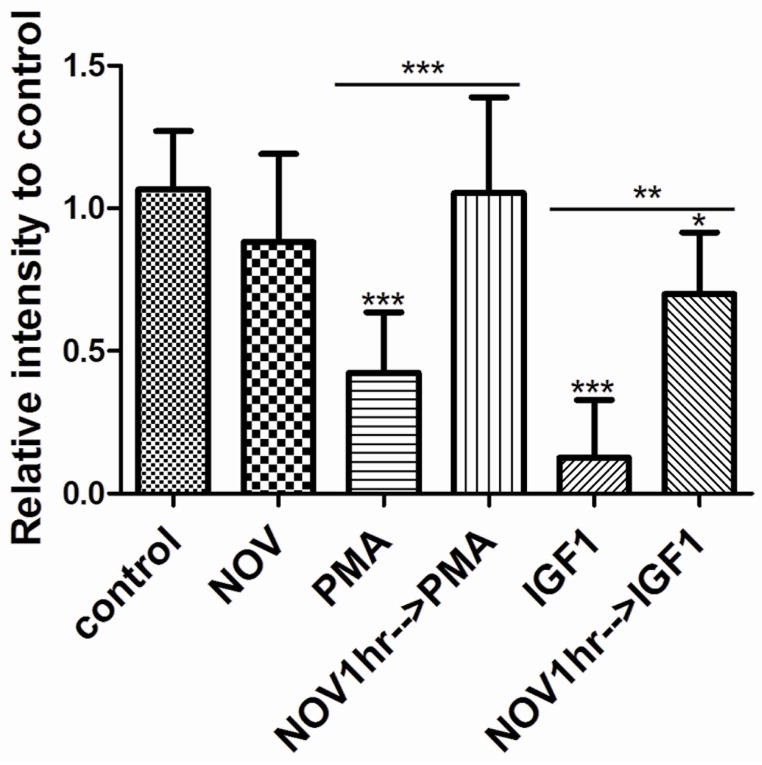
To test the potential role of a phosphatase in regulating STAT3 activity we first used the general phosphatase inhibitor sodium orthovanadate (NOV). We pre-incubated P1 retinal explants for one hour with 100uM of NOV before adding PMA or IGF1 (Fig 3). As expected from our previous studies, retinal explants treated with PMA had pSTAT3 levels reduced to one half compared to controls (0.42 ± 0.07 n=8 P<0.0001) and explants treated with IGF1 decreased pSTAT3 to almost undetectable levels (0.20 ± 0.12 n=6 P<0.0001). One hour pre-incubation with NOV completely blocked the PMA inhibition of STAT3 phosphorylation (1.104 ± 0.1132 n=8 P=0.79). NOV also substantially increased the pSTAT3 levels in the presence of IGF1, although not quite to control levels (0.6999 ± 0.1245 n=3 P<0.05). Therefore we concluded that PKC reduced tyrosine phosphorylation of STAT3 by recruiting a phosphatase.
Figure 3. Activation of PKC inhibits tyrosine phosphorylation of STAT3 through modulation of phosphatase activity.
Amount of tyrosine phosphorylated STAT3 found in P1 retinas after 30 minutes treatment with 100nM PMA, 50ng/mL IGF1, or one hour pre-incubation with 100μM NOV (Sodium Orthovanadate) before addition of IGF1 or PMA. * P<0.05, ** P<0.005, *** P<0.001, n=3. Values are relative to control unless indicated by horizontal bars.
PKCs do not regulate pSTAT3 through the activation of PTEN
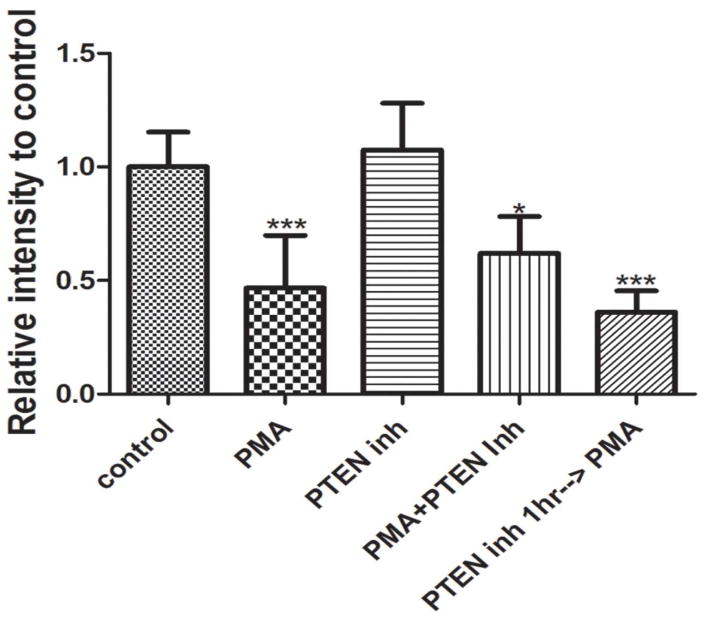
The major substrate of PTEN is phosphatidylinositol (3,4,5)-trisphosphate (PIP3), but it has also been shown to remove phosphate from tyrosine 705 of STAT3. To test the possible involvement of PTEN in regulating STAT3 activity we used the PTEN inhibitor bpV(phen) at a concentration of 50nM, above the IC50 for PTEN (IC50 = 38nM) but well below levels that inhibit other phosphatases. We cultured P1 retinas for thirty minutes in the presence of PMA, with or without bpV(phen). Addition of PTEN inhibitor alone did not change the phosphorylation of STAT3 (1.074 ± 0.078, P=0.48, n=7) compared to control, whereas PMA treatment resulted in a significant decrease (Fig 4). When PMA and bpV(phen) were added together, or when explants were preincubated in bpV(phen) for one hour, tyrosine pSTAT3 levels were still reduced to similar levels as PMA alone (0.61 ± 0.09, P<0.05, n=3 and 0.36 ± 0.054, P<0.001, n=3 respectively) (Fig 4). Therefore, we concluded that PTEN is not required for the PKC-dependent inhibition of STAT3 activity during retinal development.
Figure 4.
Amount of tyrosine phosphorylated STAT3 found in P1 retinas after 30 min treatment with 100nM PMA in the presence of absence of 50nM of bpV(phen), or one hour pre-incubation with 50nM of bpV(phen) before addition of PMA. PMA decreased pSTAT3 by over fifty percent (0.42 ± 0.07, P<0.001, n=8). bpV(phen) was not able to reverse the action of PMA. * P<0.05, *** P<0.001
PKCs can regulate pSTAT3 levels through Shp1 or Shp2
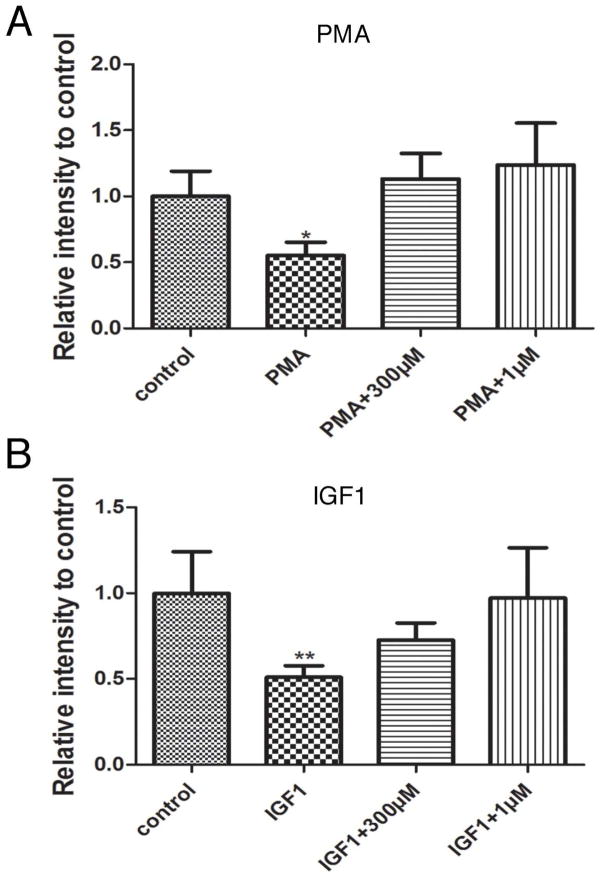
Other phosphatases that have been linked to STAT3 de-activation in a number of tissues include protein tyrosine phosphatases Shp1, Shp2, and PTP1B. NSC87877 is an inhibitor of a group of tyrosine phosphatases. Its IC50 values are 0.318, 0.355, 1.691, 7.745, 65.617, 84.473 and 150μM for Shp2, Shp1, PTP1B, HePTP, DEP1, CD45 and LAR respectively. To test the actions of this inhibitor on pSTAT3 levels, we cultured P1 retinas with PMA in the presence or absence NSC87877. We used two concentrations of NSC87877; 300μM to inhibit all seven phosphatases, and 1 μM to selectively inhibit Shp1 and Shp2. The reduction of STAT3 phosphorylation by PMS was blocked in retinas treated with NSC87877 at either concentration (Fig 5A). We repeated the experiments by treating P1 retinal explants with 50ng/mL IGF1 with or without the phosphatase inhibitor at 1μM or 300μM (Fig 5B). IGF1 treatment reduced the pSTAT3 levels by about fifty percent (0.5 ± 0.03, P<0.01, n=4) but this action was completely blocked by 1μM NSC87877. IGF1 in the presence of 300 μM of the phosphatase inhibitor gave a small, but not statistically significant difference in pSTAT3 levels compared to control. These results strongly suggest that shp1 or shp2 is responsible for IGF1/PMA induced reduction of tyrosine phosphorylated STAT3.
Figure 5.
(A) Amount of tyrosine phosphorylated STAT3 found in WT P1 retinas after 30 minutes treatment with 100nM PMA, 100nM PMA and 300μM NSC87877 and 100nM PMA and 1μM NSC87877. (B) Amount of tyrosine phosphorylated STAT3 found in WT P1 retinas after 30 minutes treatment with 50ng/mL IGF1, 50ng/mL IGF1 and 300μM NSC87877 and 50ng/mL IGF1 and 1μM NSC87877 *P<0.5, **P<0.05, ***P<0.005 vs control.
Inhibition of Shp1 or Shp2 during IGF1 treatment of retinal explants prevents IGF1-induced promotion of rod photoreceptor development
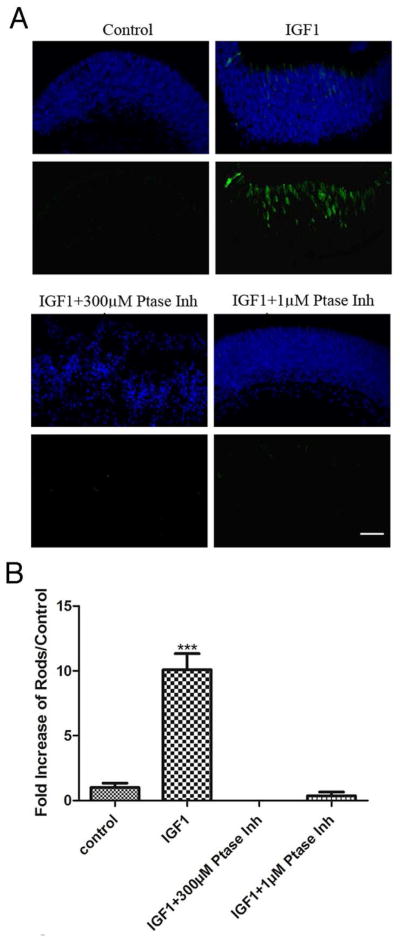
To determine whether Shp1 or Shp2 activity was a necessary component of IGF1-induced rod photoreceptor development, we tested the effect of NSC87877 in PN1 retinal explants cultured for four days in the presence of IGF1 with or without NSC87877 at 1μM or 300 μM. IGF1 treatment resulted in a ten-fold increase in the number of rods at the end of the culture (10.09 ± 0.71, P<0.0005, n=3) and the tissue had a normal cytoarchitecture (Fig 6). However, when retinas were cultured in the presence of IGF1 and 1μM of phosphatase inhibitor, the number of rods was not significantly different to control retinas (Fig 6). Over this time period retinas cultured with IGF1 and 300 μM of NSC87877 had a disrupted cytoarchitecture, suggesting that this concentration of phosphatase inhibitor was toxic to the tissue. These results further support the idea that Shp1, or Shp2, is a key member of the IGF1-induced pathway promoting rod photoreceptor differentiation.
Figure 6. Inhibition of Shp1/2 during IGF1 treatment of retinal explants prevents IGF1-induced promotion of rod photoreceptor development.
(A) Immunofluorescence detection of opsin (green) overlaid with nuclear counterstain (Hoechst, blue) of four day P1 retinal explants. P1 retinas were cultured from WT mice for four days in the presence of 50ng/mL IGF1, or (B) Numbers of rods present on P1 retinas after four days of culture in the presence of 50ng/mL IGF1, 50ng/mL IGF1and 300μM NSC87877 and 50ng/mL IGF1 and 1μM NSC87877. Counts were obtained by averaging the number of rods present in three individual histological cross sections of each retina. At least three different retinas were studied per treatment focusing on the central areas of the retina. ***P<0.005. Scale bar in A, 40μm.
DISCUSSION
Continuous activation of STAT3 completely blocks differentiation of rod photoreceptors in the neonatal mouse retina by a mechanism consistent with maintaining the progenitor pool and preventing the cells from exiting cell cycle and continuing their differentiation process (Zhang et al., 2004, Rhee et al., 2004). There is also extensive evidence that STAT3 plays a similar role at many stages of mammalian development. For example, it is a key transcription factor regulating mouse embryonic stem cell self-renewal (Xie et al., 2009). At later stages, in the embryonic neocortex, STAT3 maintains neural precursor cells by acting on the notch pathway through controlling expression of delta-like ligand 1 (Yoshimatsu et al., 2006). During embryonic stages STAT3 is activated by a variety of pathways including gp130 receptors for ligands such as CNTF and LIF (Fig 7). Although we, and others, have reported a variety of pathways by which STAT3 can be activated, there is less information about how it is inactivated in developing tissues such as the retina (Zhang et al., 2003, 2004, 2005; Hertle et al., 2008).
Several mechanisms by which STAT3 can be inactivated have been described. One well-characterized mechanism is the degradation of STAT3 protein initiated by the proteins SOCS3 and PIAS3 (Yoshimura, 2009; Yagil et al., 2010). SOCS3 is expressed in the outer retina and is required after P0 to shut down the residual STAT3 activation and allow rhodopsin expression and rod photoreceptor cell terminal differentiation (Ozawa et al., 2007). PIAS proteins have SUMO (small ubiquitin-related modifier) E3 ligase activity and PIAS3 has various functions, including regulating the level, and thus the activity, of STAT3. We have previously shown that as rod photoreceptors differentiate they downregulate STAT3 and both SOCS3 and PIAS3 may play a role in this (Zhang et al., 2003). In the present study we found that IGF1 and PMA were able to reduce the level of pSTAT3 without any significant change in the level of STAT3 protein. This suggests that over the time course of these experiments IGF1 and PKC regulates STAT3 activity by another route.
The other major class of STAT3 inactivators is tyrosine phosphatases, and the ability of the non-specific phosphatase inhibitor orthovanadate to block the actions of PMA and IGF-1 indicates a role for a phosphatase downstream of PKC activation in the pathway regulating rod photoreceptor development. Although the full range of phosphatases that can act on pSTAT3 is not known, four have been well documented, PTEN, PTP1B and SHP-1/2 (Sun and Steinberg 2001; Yasukawa et al., 2000; Shuai and Liu, 2003). By the use of selective inhibitors we suggest that either Shp1 or Shp2 are responsible.
In previous studies of nervous system development, Shp2 signaling acted as a critical switch in biasing cortical precursor cells and neural stem cells toward a neuronal fate rather than glial cell (Gauthier et al., 2007, Ke et al., 2007). Moreover, in retina, Shp2 signaling is required for the patterning of the neural retina by promoting progenitor differentiation (Cai et al 2010). These results are consistent with our hypothesis that by removing the tyrosine phosphorylation of STAT3, the progenitor cell is allowed to exit mitosis and begin their differentiation process.
Interactions between PKC and Shp2 have been demonstrated in vitro (Zhao et al., 1994). Later, interactions between SHP2 and PKC isoforms α, β1, β2, and η, in intact cells were shown to result in phosphorylation of Shp2 at serine residues 576 and 591 (Strack et al., 2002). In both cases it appears that phosphorylation does not alter the intrinsic activity of the phosphatase but may alter its ability to associate with various adapter proteins. Inhibition of PKC mediated phosphorylation of Shp-2 blocked its association with Frs2 and Gab1 adaptor proteins and prevented FGF2-mediated activation of Ras in chondrocytes (Krejci et al., 2007). Whether similar adapter proteins are involved in regulating the action of Shp-1/2 on STAT3 has yet to be determined.
Our previous studies described a pathway from IGF1 through PKC-β1 and PKC-γ that resulted in decreased activity of STAT3 and promotion of rod photoreceptor formation (Pinzon-Guzman, et al., 2011). While it is clear that IGF1 can stimulate receptors other than the IGF-1R, our previous results indicate that if there are other pathways involved, they converge at the level of PKC activation. We now suggest that the link between PKC activation and STAT3 inactivation may be through Shp1 or Shp2 phosphatase. Since inhibiting these phosphatases was sufficient to block the action of IGF-1 over a period of 4 days in retinal explant cultures, dephosphorylation of STAT3 is clearly a necessary step in this pathway. We have previously described downregulation of STAT3 protein as rod photoreceptors differentiate suggesting that mechanisms subsequent to dephosphorylation continue the process of eliminating STAT3 action in these cells.
Many of the molecules described in this manuscript and in our previous work are also present in adult mouse retinal cells, including rod photoreceptors (Kinkl et al., 2001; Pinzon-Guzman et al., 2011). Many have been associated with survival pathways and so it is likely that the function of these molecules changes from regulation of development to homeostasis and/or survival. Further studies are needed to determine whether this change in function is associated with any change in, for example, IGF-1R subunits, or any other downstream molecule. It is also very likely that the genes regulated by STAT3 differ between the developing retina and the adult due to the different spectrum of transcription factors and epigenetic marks present in the cell nuclei.
Other factors have been shown to influence rod formation in the developing retina, but their relationship to the pathway we have described is not yet known (Levine et al., 2000; Hunter et al., 2004; Volpert et al., 2009). Given the powerful action of STAT3 as a gatekeeper controlling the transition from progenitor to postmitotic cell, it is tempting to speculate that many of these pathways converge at the point of regulating STAT3 levels or activity. Our continuing elucidation of one key pathway regulating rod photoreceptor formation will allow us to gain insight into the genetic and epigenetic mechanisms governing this complex transition. In addition, STAT3 is present in may parts of the developing nervous system so that the pathways we define in the formation of rod photoreceptors may well serve as models for the development of many types of neuron.
Acknowledgments
This work was supported by NIH grant EY 013865 and by the Macula Vision Research Foundation
References
- Asao H, Fu XY. Interferon-gamma has dual potentials in inhibiting or promoting cell proliferation. J Biol Chem. 2000;275:867–874. doi: 10.1074/jbc.275.2.867. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980;286:231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Cai Z, Feng GS, Zhang X. Temporal requirement of the protein tyrosine phosphatase Shp2 in establishing the neuronal fate in early retinal development. J Neurosci. 2010;30:4110–4119. doi: 10.1523/JNEUROSCI.4364-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- Ezzeddine ZD, Yang X, DeChiara T, Yancopoulos G, Cepko CL. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124:1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–262. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle D, Schleichert M, Steup A, Kirsch M, Hofmann HD. Regulation of cytokine signaling components in developing rat retina correlates with transient inhibition of rod differentiation by CNTF. Cell Tissue Res. 2008;334:7–16. doi: 10.1007/s00441-008-0651-3. [DOI] [PubMed] [Google Scholar]
- Hicks D, Barnstable CJ. Different rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J Histochem Cytochem. 1987;35:1317–1328. doi: 10.1177/35.11.3655327. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Zhang M, Ferguson JW, Koch M, Brunken WJ. The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci. 2004;27:477–488. doi: 10.1016/j.mcn.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PK. A new RING for SUMO: wrestling transcriptional responses into nuclear bodies with PIAS family E3 SUMO ligases. Genes Dev. 2001;15:3053–3058. doi: 10.1101/gad.955501. [DOI] [PubMed] [Google Scholar]
- Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng GS. Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol Cell Biol. 2007;27:6706–6717. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkl N, Sahel J, Hicks D. Alternate FGF2-ERK1/2 signaling pathways in retinal photoreceptor and glial cells in vitro. J Biol Chem. 2001;276:43871–43878. doi: 10.1074/jbc.M105256200. [DOI] [PubMed] [Google Scholar]
- Krejci P, Masri B, Salazar L, Farrington-Rock C, Prats H, Thompson LM, Wilcox WR. Bisindolylmaleimide I suppresses fibroblast growth factor-mediated activation of Erk MAP kinase in chondrocytes by preventing Shp2 association with the Frs2 and Gab1 adaptor proteins. J Biol Chem. 2007;282:2929–2936. doi: 10.1074/jbc.M606144200. [DOI] [PubMed] [Google Scholar]
- Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Levine EM, Fuhrmann S, Reh TA. Soluble factors and the development of rod photoreceptors. Cell Mol Life Sci. 2000;57:224–234. doi: 10.1007/PL00000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: Lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Naka T, Fujimoto M, Tsutsui H, Yoshimura A. Negative regulation of cytokine and TLR signalings by SOCS and others. Adv Immunol. 2005;87:61–122. doi: 10.1016/S0065-2776(05)87003-8. [DOI] [PubMed] [Google Scholar]
- Neophytou C, Vernallis AB, Smith A, Raff MC. Müller-cell-derived leukaemia inhibitory factor arrests rod photoreceptor differentiation at a postmitotic pre-rod stage of development. Development. 1997;124:2345–2354. doi: 10.1242/dev.124.12.2345. [DOI] [PubMed] [Google Scholar]
- Onishi A, Peng GH, Hsu C, Alexis U, Chen S, Blackshaw S. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron. 2009;61:234–246. doi: 10.1016/j.neuron.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, Nakao K, Shimazaki T, Shimmura S, Kurihara T, Ishida S, Yoshimura A, Tsubota K, Okano H. SOCS3 is required to temporally fine-tune photoreceptor cell differentiation. Dev Biol. 2007;303:591–600. doi: 10.1016/j.ydbio.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Pinzon-Guzman C, Zhan SS, Barnstable CJ. Specific protein kinase C isoforms are required for rod photoreceptor differentiation. J Neurosci. 2011;31:18606–18617. doi: 10.1523/JNEUROSCI.2578-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasamura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Rhee KD, Goureau O, Chen S, Yang XJ. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J Neuroscience. 2004;24:9779–9788. doi: 10.1523/JNEUROSCI.1785-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–11. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- Strack V, Krützfeldt J, Kellerer M, Ullrich A, Lammers R, Häring HU. The Protein-tyrosine-phosphatase SHP2 is phosphorylated on serine residues 576 and 591 by protein kinase C isoforms alpha, beta 1, beta 2, and eta. Biochemistry. 2002;41:603–608. doi: 10.1021/bi011327v. [DOI] [PubMed] [Google Scholar]
- Sun S, Steinberg BM. PTEN is a negative regulator of STAT3 activation in human papillomavirus-infected cells. J Gen Virol. 2002;83(Pt 7):1651–8. doi: 10.1099/0022-1317-83-7-1651. [DOI] [PubMed] [Google Scholar]
- Volpert KN, Tombran-Tink J, Barnstable C, Layer PG. PEDF and GDNF are key regulators of photoreceptor development and retinal neurogenesis in reaggregates from chick embryonic retina. J Ocul Biol Dis Infor. 2009;2:1–11. doi: 10.1007/s12177-009-9014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Chan KS, Cao F, Huang M, Li Z, Lee A, Weissman IL, Wu JC. Imaging of STAT3 signaling pathway during mouse embryonic stem cell differentiation. Stem Cells Dev. 2009;18(2):205–14. doi: 10.1089/scd.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil Z, Nechushtan H, Kay G, Yang CM, Kemeny DM, Razin E. The enigma of the role of protein inhibitor of activated STAT3 (PIAS3) in the immune response. Trends Immunol. 2010;31:199–204. doi: 10.1016/j.it.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu T, Kawaguchi D, Oishi K, Takeda K, Akira S, Masuyama N, Gotoh Y. Non-cell-autonomous action of STAT3 in maintenance of neural precursor cells in the mouse neocortex. Development. 2006;133(13):2553–63. doi: 10.1242/dev.02419. [DOI] [PubMed] [Google Scholar]
- Yoshimura A. Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J Med. 2009;58:73–83. doi: 10.2302/kjm.58.73. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell proliferation during postnatal development of the retina in the mouse. Brain Res. 1985;353:229–39. doi: 10.1016/0165-3806(85)90211-1. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Wei JY, Li C, Barnstable CJ, Fu XY. Expression and activation of STAT proteins during mouse retina development. Exp Eye Res. 2003;76:421–431. doi: 10.1016/s0014-4835(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Wei J, Qin H, Zhang L, Xie B, Hui P, Deisseroth A, Barnstable CJ, Fu XY. STAT3-mediated signaling in the determination of rod photoreceptor cell fate in mouse retina. Invest Ophthalmol Vis Sci. 2004;45:2407–12. doi: 10.1167/iovs.04-0003. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Liu MG, Kano A, Zhang C, Fu XY, Barnstable CJ. STAT3 activation in response to growth factors or cytokines participates in retina precursor proliferation. Exp Eye Res. 2005;81:103–115. doi: 10.1016/j.exer.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Shen SH, Fischer EH. Phorbol ester-induced expression, phosphorylation, and translocation of protein-tyrosine-phosphatase 1C in HL-60 cells. Proc Natl Acad Sci U S A. 1994;91:5007–5011. doi: 10.1073/pnas.91.11.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]