Abstract
BACKGROUND
Male pattern baldness and prostate cancer may share common pathophysiological mechanisms in terms of advancing age, heritability, and endogenous hormones. Results from previous epidemiologic studies are inconsistent. Therefore, we investigated the association of prostate cancer risk with male pattern baldness at age 30 years, age 45 years, and baseline (median age=60.5 years) in the VITamins And Lifestyle (VITAL) cohort study.
METHODS
We included 32,583 men who were 50–76 years and without prior cancer diagnosis (excluding non-melanoma skin cancer) at the start of follow-up. First primary incident prostate cancers were ascertained via linkage to the western Washington Surveillance, Epidemiology, and End Results (SEER) program. Hazard ratios (HRs) and 95% confidence intervals (95%CIs) were estimated using Cox proportional-hazards regressions with adjustment for potential confounders.
RESULTS
During follow-up (median=9 years), 2,306 incident prostate cancers were diagnosed. Male pattern baldness at age 30 years, age 45 years, and baseline were not significantly associated with overall or subtypes of prostate cancer.
CONCLUSION
This study did not provide support for the hypothesis that male pattern baldness may be a marker for subsequent prostate cancer. Previous evidence indicates that a distinct class of frontal with vertex balding may be associated with increased prostate cancer risk, but all such balding classes were captured as a single exposure category by the VITAL cohort questionnaire.
Keywords: male pattern baldness, prostate cancer, androgen, cohort study
INTRODUCTION
In the U.S., approximately one in every six men will be diagnosed with prostate cancer during his lifetime, and about one man in 36 will die from prostate cancer [1]. Only four risk factors for prostate cancer have been established—advancing age, black race, family history of the malignancy, and certain genetic polymorphisms [2,3]. Current screening tests for prostate cancer leads to diagnosis of a substantial number of slow-growing, localized prostate cancers that will never impose a life-threatening risk to patients. Therefore, additional research to improve our understanding of the etiology of prostate cancer, particularly aggressive or lethal malignancies, is needed.
Male pattern baldness is a common progressive hair-loss process in a well-defined pattern. The prevalence of extensive baldness (Norwood-Hamilton stage IV or greater) in healthy white men increases with advancing age, with estimated rates of 22% at ages 20–29 years, 44% at 40–49 years and 74% at 50–79 years [4]. Male pattern baldness and prostate cancer seem to share common pathophysiological mechanisms—aging, heritability and endogenous hormones [5,6]. For example, heritable factors contribute to 42% of prostate cancer risk [5] and 81% of male pattern baldness [6]. With regard to endogenous hormones, both hair follicles and the prostate gland are androgen-responsive. Men with baldness seem to have higher circulating androgens than those without [7,8]. Bald scalp has been characterized by higher levels of dihydrotestosterone (DHT) [9] and elevated expression of androgen receptors (ARs) [10-12]. Finasteride, a type II 5α-reductase inhibitor, has been showed in clinical trials to slow hair loss and stimulate new hair growth [13]. Similarly, pre-pubertally castrated men and male pseudohermaphrodites with deficit type II 5α-reductase have not been observed to develop prostate cancer, although such studies typically have limited sample sizes [14-16]. Genetic polymorphisms in SRD5A2 [17], CYP17 [18] and HSD3B [19] androgen metabolism genes, as well as genomic regulatory elements binding to AR [20,21], have also been found to be associated with prostate cancer risk. Although associations of circulating androgen concentrations with prostate cancer risk are inconclusive in epidemiologic studies [22-26], the majority of such studies have been limited by assessment of a narrow range of single sex steroid hormones as well as quantitation using a single blood sample typically drawn after middle-age. Given that male pattern baldness is clinically observable decades earlier than prostate cancer, and that early-life sex steroid hormone levels may be more etiologically relevant for the devolvement of prostate cancer [27], male pattern baldness may serve as a potential non-invasive phenotypic attribute of long-term hormonal homeostasis.
Results from prior epidemiologic studies of associations between male pattern baldness and prostate cancer are inconclusive [8,28-42]. A majority of published studies have used a case-control study design, which relative to cohort studies, may heighten measurement errors due to increased recall time to age-specific hair-loss patterns as well as differential misclassification of exposure due to treatment-associated hair loss. In addition, many of these studies have had a small or moderate number of cases for analysis, typically less than 400. A recent meta-analysis of seven case-control studies [28] and two cohort studies [34,36] showed a positive association between male pattern baldness and prostate cancer risk. However, this meta-analysis and the individual cohort study did not present subtype-specific analyses by aggressive prostate cancer, nor did they assess male pattern baldness at multiple ages with sufficient statistical power. Our previous analysis of male pattern baldness at age 45 years in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial found a positive association between frontal with moderate vertex balding and aggressive prostate cancer risk (HR=1.39, 95%CI=1.07, 1.80; In press), but male pattern baldness at younger ages was not assessed.
To overcome the noted shortfalls, and attempt to replicate the prior association we obtained in our analysis of the PLCO Cancer Screening Trial, we assessed the relationship between male pattern baldness at age 30 years, age 45 years, and baseline in relation to the risks of overall and subtypes of prostate cancer in the VITamins And Lifestyle (VITAL) cohort study.
MATERIALS AND METHODS
Analytic cohort
The analytic cohort was drawn from male participants in the VITAL cohort study. A detailed description of the VITAL cohort study has been published previously [43]. In brief, the VITAL cohort study was designed to investigate associations between supplement use and cancer risk among residents aged 50 to 76 years in the 13-county area in western Washington State covered by the Surveillance, Epidemiology, and End Results (SEER) program cancer registry. This study was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
During 2000 to 2002, the baseline questionnaire was mailed to 195,465 men using a purchased commercial mailing list, with a reminder postcard sent two weeks later. Of these, 37,382 (19.1%) were returned and deemed eligible for the VITAL cohort study. For this analysis, we excluded men who reported a history of any cancer except non-melanoma skin cancer [NMSC] (n=4,659) at baseline, men who did not report whether they had a history of cancer at baseline (n=128), and men who only had prostate cancer on death certificates (n=12) without an available date of diagnosis during follow-up. This resulted in a total of 32,583 men for analysis.
Exposure ascertainment
Men completed a 24-page self-administered baseline questionnaire covering demographics, diet, risk factors, medical history, and family history of cancer. For hair-loss patterns, men were asked to select among three sets of pictures (Figure 1) that best described their hair-loss at age 30 years, age 45 years, and baseline. These three sets of pictures reflected different degrees of hair-loss: 1) little or no hair loss (Norwood-Hamilton stage I and II); 2) loss at forehead only (Hamilton-Norwood stage IIa, III, IIIa, and IVa); 3) loss at top of head and forehead (Norwood-Hamilton stage III-vertex, IV, V, Va, VI, and VII ) [44]. The test-retest reliability of self-reported hair-loss patterns was respectively estimated to be 0.74, 0.71 and 0.81 for age 30 years, age 45 years, and at baseline [44]. The inter-rater agreement between self-report and interviewer’s assessment for hair-loss patterns at baseline was 0.47 [44].
FIGURE 1.
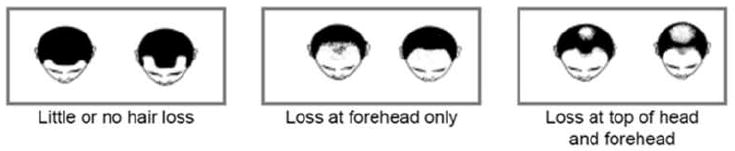
Diagrams Used to Ascertain Male Pattern Baldness at Age 30 years, Age 45 years, and Baseline in the Baseline Questionnaire of VITAL Cohort Study.
Outcome ascertainment
We ascertained first primary incident cancers by annual linkage to the western Washington SEER program, as well as grade, stage, and other tumor characteristics. The cancer registry ascertained cases through all area hospitals, state death certificates, and offices of pathologists, oncologists, and radiotherapists. The linkage of the VITAL cohort to SEER information is based on number of matches of personal identifiers (e.g., name, social security number, date of birth). Linkage is automatic for matches with high concordance, and is supplemented by visual inspections or follow-up phone calls for uncertain matches. Deaths occurring in the cohort were ascertained by linkage to the Washington state death file, using a similar linkage procedure as used for linkage to SEER.
Statistical analysis
Pearson chi-square tests were used to compare categorical characteristics by male pattern baldness and by case status. Spearman rank correlations were used to assess the correlation of male pattern baldness between the three time points. Cox proportional-hazards regression models, with age as the time-metric, were used to estimate hazard ratios (HRs) and 95% confidence intervals (95%CIs) of associations between male pattern baldness at three age points and prostate cancer risk. Follow-up of the analytic cohort started from age at baseline and continued until age at event (i.e., incident prostate cancer) or age at time of right-censoring (i.e., withdrawal from the study, diagnosis with non-prostate cancer [excluding NMSC], death, residential relocation out of the catchment area of the SEER cancer registry, last date of follow-up [12/31/2011]), whichever occurred first. For subtype-specific analyses, men who experienced a prostate cancer subtype that was not of interest were right-censored at the age of diagnosis.
To explore the extent to which covariates potentially confounded the relationships between male pattern baldness and prostate cancer risk, we calculated HRs and 95%CIs derived from: 1) Unadjusted model, with age modeled as the underlying time metric; 2) multivariable model, additionally adjusted for race/ethnicity, marital status, Charlson’s comorbidity index, body mass index (BMI, kg/m2) at age 45 years, alcohol use in the year before baseline, smoking status, and baby aspirin use in the past ten years. These covariates were selected for their associations with total prostate cancer and with male pattern baldness at baseline in univariable models (chi-square tests) at the threshold of P-value <0.05; male pattern baldness at baseline was used given an assumed smaller measurement error compared with such at ages 30 and 45 years. We additionally adjusted for covariates that share potential pathophysiological pathway with prostate cancer (i.e., family history of prostate cancer in first-degree relatives, and benign prostatic hyperplasia), and covariates that are related to prostate cancer detection (i.e., impotence in the year before baseline, PSA screening in last two years, and benign prostate biopsy) separately and collectively in the multivariable model.
In addition to the analysis of total incident prostate cancer, we conducted subtype-specific analyses by prostate cancer aggressiveness. Aggressive prostate cancer was defined as Gleason score (from biopsy or prostatectomy) ≥7, or regional/distant metastases (SEER summary staging), or prostate cancer as the underlying cause of death. Subtype-specific analyses by high-grade (Gleason score≥7) and high-stage (regional/distant metastases)/fatal tumors were also preformed separately. In addition , we conducted sensitivity analyses: 1) stratified baseline hazard by age at baseline (<55, 55–59, 60–64, 65–69, ≥70 years), to assess effects of differential recall durations; 2) stratified models by age at baseline (<55, 55–59, 60–64, 65–69, ≥70 years), to assess the possibility of survival bias; 3) restricted to white non-Hispanic men for potential effect-modification by race/ethnicity; and 4) restricted to men who had a PSA test within the two-year period prior to baseline in an attempt to assess the effect of existing disease.
In order to evaluate more refined definitions of aggressive prostate cancer, we conducted sensitivity analyses in two sub-cohorts. Given that American Joint Committee on Cancer (AJCC) stage (TNMs) was only available for prostate cancer diagnosed from 2004 onward, we conducted subtype-specific analyses by prostate cancer aggressiveness during the period of 01/01/2004–12/31/2011 (sub-cohort #1), with aggressive prostate cancer defined as Gleason score ≥7, or AJCC stage ≥ III, or prostate cancer as the underlying cause of death. Given that Gleason patterns (i.e., primary + secondary) were only available for prostate cancer diagnosed during 01/01/2004 to 12/31/2009 (sub-cohort #2), we conducted a second subtype-specific analyses by aggressive prostate cancer, defined as Gleason score ≥ 8 or Gleason Score=4+3, or AJCC stage ≥ III, or prostate cancer as the underlying cause of death.
Male pattern baldness at any of the three time-points was missing for less than 4% of individuals, and any single covariate was missing for less than 9% of individuals. We conducted multiple imputation using baseline data by a sequence of regression models [45] via IVEware [46] in SAS®. Five imputed datasets were created and analyzed individually. HR estimates from each imputed analysis were combined via PROC MIANALYZE in SAS®. The proportional hazards assumption was tested in each imputed dataset by examining interaction terms between individual predictors and age, as well as through visual inspection of log(−log) survival plots. SAS® v.9.3 (Cary, NC, USA) was used for all analyses. P-values were two-sided with 0.05 set as threshold for statistical significance.
RESULTS
Of the 32,583 men in our analytic cohort, hair-loss was reported by 20%, 46%, and 61% men at age 30 years, age 45 years, and baseline, respectively. Correlations with hair-loss at baseline were 0.45 and 0.75 for such at ages 30 and 45 years. During follow-up (median=9 years), 2,306 incident prostate cancers were diagnosed. The median age at prostate cancer diagnosis was 68.6 years (IQR=63.4–73.7). Baseline characteristics of the analytic cohort by case status and by male pattern baldness are presented in Table 1 and Supplemental table 1. The analytic cohort was comprised primarily of non-Hispanic whites (92%). Compared with non-prostate cancer participants, men with prostate cancer were more likely to be older, former smokers, married or cohabiting, have taken baby aspirin in the last ten years, have consumed more alcohol in the last year, and were less likely to be obese or have had other co-morbidities. Furthermore, men with prostate cancer were more likely to have had a family history of prostate cancer, impotence in the last year, at least one PSA test in last two years, a history of benign prostate biopsy and benign prostatic hypoplasia.
TABLE 1. Baseline Characteristics of the Analytic Cohort by Case Status (2000–2011, N=32,583).
| Characteristics | Non-prostate cancer participants N=30,277 |
All incident prostate cancer N=2,306 |
P-valuea |
|---|---|---|---|
| Age at baseline (years) | >0.001 | ||
| <55 | 7,735 (25.5%) | 301 (13.1%) | |
| 55–59 | 7,147 (23.6%) | 476 (20.6%) | |
| 60–64 | 5,576 (18.4%) | 522 (22.6%) | |
| 65–69 | 4,862 (16.1%) | 497 (21.6%) | |
| ≥70 | 4,957 (16.4%) | 510 (22.1%) | |
| Race/ethnicity | 0.105 | ||
| Non-Hispanic white | 27,806 (91.8%) | 2,143 (92.9%) | |
| Other | 2,088 (6.9%) | 139 (6.0%) | |
| Marital status | <0.001 | ||
| Married/cohabiting | 25,534 (84.3%) | 2,024 (87.8%) | |
| Never married | 1,032 (3.4%) | 46 (2.0%) | |
| Separated/divorced/widowed | 3,330 (11.0%) | 215 (9.3%) | |
| Baby aspirin use in the past 10 years | 0.005 | ||
| None | 19,492 (64.4%) | 1,410 (61.1%) | |
| Low (<4 times a week or <4 years) | 4,330 (14.3%) | 361 (15.7%) | |
| High (≥4 days/week and ≥4 years) | 3,924 (13.0%) | 337 (14.6%) | |
| Charlson’s comorbidity index | 0.002 | ||
| 0 | 22,599 (74.6%) | 1,784 (77.4%) | |
| 1 | 5,772 (19.1%) | 412 (17.9%) | |
| ≥2 | 1,899 (6.3%) | 109 (4.7%) | |
| Body mass index at 45 years (BMI, kg/m2) | <0.001 | ||
| <25 | 12,649 (41.8%) | 1,021 (44.3%) | |
| 25–29.99 | 13,139 (43.4%) | 1,016 (44.1%) | |
| ≥30 | 3,544 (11.7%) | 196 (8.5%) | |
| Alcohol use last year (g/day) | 0.001 | ||
| None | 2,718 (9.0%) | 158 (6.9%) | |
| Q1 (≤0.66) | 6,661 (22.0%) | 476 (20.6%) | |
| Q2 (0.67–5.20) | 6,833 (22.6%) | 522 (22.6%) | |
| Q3 (5.21–15.64) | 6,798 (22.5%) | 543 (23.5%) | |
| Q4 (≥15.65) | 6,710 (22.2%) | 566 (24.5%) | |
| Smoking status | 0.001 | ||
| Never smoker | 11,765 (38.9%) | 924 (40.1%) | |
| Current smoker | 2,762 (9.1%) | 159 (6.9%) | |
| Former smoker | 15,255 (50.4%) | 1,195 (51.8%) | |
| Number of first-degree relatives with prostate cancer | <0.001 | ||
| 0 | 26,133 (86.3%) | 1,823 (79.1%) | |
| 1 | 3,518 (11.6%) | 395 (17.1%) | |
| ≥2 | 198 (0.7%) | 59 (2.6%) | |
| Impotence last year | 0.002 | ||
| No | 22,679 (74.9%) | 1,661 (72.0%) | |
| Yes | 7,591 (25.1%) | 644 (27.9%) | |
| PSA test in the last two years | <0.001 | ||
| No | 8,695 (28.7%) | 456 (19.8%) | |
| Yes | 21,211 (70.1%) | 1,824 (79.1%) | |
| Benign prostate biopsy | <0.001 | ||
| No | 27,949 (92.3%) | 1,965 (85.2%) | |
| Yes | 2,328 (7.7%) | 341 (14.8%) | |
| Benign prostatic hyperplasia | <0.001 | ||
| No | 25,688 (84.8%) | 1,780 (77.2%) | |
| Yes | 4,582 (15.1%) | 525 (22.8%) | |
| Male pattern baldness at 30 years | 0.933 | ||
| Little or no loss | 22,992 (75.9%) | 1,757 (76.2%) | |
| Loss at forehead only | 3,936 (13.0%) | 306 (13.3%) | |
| Loss at top of head and forehead | 2,188 (7.2%) | 164 (7.1%) | |
| Male pattern baldness at 45 years | 0.516 | ||
| Little or no loss | 15,442 (51.0%) | 1,165 (50.5%) | |
| Loss at forehead only | 6,696 (22.1%) | 534 (23.2%) | |
| Loss at top of head and forehead | 7,293 (24.1%) | 545 (23.6%) | |
| Male pattern baldness at baseline | 0.167 | ||
| Little or no loss | 10,821 (35.7%) | 788 (34.2%) | |
| Loss at forehead only | 3,198 (10.6%) | 241 (10.5%) | |
| Loss at top of head and forehead | 15,101 (49.9%) | 1,202 (52.1%) |
NOTE: Column percent may not add up to 100% due to missing data.
P-values were calculated from Pearson chi-square tests using non-missing values, compared all incident prostate cancer with non-prostate cancer participants.
As shown in Table 2, male pattern baldness at age 30 years, age 45 years, and baseline were not associated with total incident or subtypes of prostate cancer, each compared with little or no hair loss in unadjusted models. Further adjustment in multivariable models did not substantially alter the HR estimates. Additional inclusion of covariates that may share physiopathologic pathways with prostate cancer, or that are related to prostate cancer detection, did not substantially alter the HR estimates (data not shown). Subtype-specific analyses by high-grade (N=1,159) and high-stage/fatal (N=372) prostate cancer provided similarly null estimates (Supplemental Table 2). Sensitivity analyses with baseline hazard stratified by age at baseline, restricting the cohort to non-Hispanic whites, or restricting the cohort to men who had a PSA test within the two-year period prior to baseline did not substantially alter the HR estimates (data not shown). Sensitivity analysis stratified models by age at baseline suggested no significant interaction between classes of baldness and age at baseline for overall or subtypes of prostate cancer (data not shown). In sensitivity analyses using AJCC staging (sub-cohort #1, 2004–2011; N=30,377, cases=1,771) or Gleason patterns (sub-cohort #1, 2004–2009; N=30,377, cases=1,364) to define prostate cancer sub-groups, results were not materially altered (data not shown).
TABLE 2. Hazard Ratios (HR) and 95% Confidence Intervals (95%CIs) for Associations of Self-reported Male Pattern Baldness at Age 30 years, Age 45 years, and Baseline in Relation to Prostate Cancer Risk in the Analytic Cohort Estimated from Cox Proportional-hazards Regressions Models (2000–2011, N=32,583).
| Male pattern baldness | All incident prostate cancer N=2,306
|
Aggressive prostate cancer a N=1,236
|
Nonaggressive prostate cancer N=1,031
|
|||
|---|---|---|---|---|---|---|
| Unadjusted b | Multivariable c | Unadjusted b | Multivariable c | Unadjusted b | Multivariable c | |
| Age 30 years | ||||||
| Little or no loss | reference | reference | reference | |||
| Loss at forehead only | 1.02 (0.90, 1.15) | 1.02 (0.90, 1.15) | 1.01 (0.85, 1.19) | 1.01 (0.85, 1.19) | 1.03 (0.86, 1.24) | 1.03 (0.86, 1.24) |
| Loss at top of head and forehead | 1.01 (0.86, 1.18) | 1.02 (0.87, 1.20) | 1.03 (0.82, 1.28) | 1.02 (0.82, 1.28) | 1.03 (0.81, 1.30) | 1.05 (0.83, 1.33) |
| Age 45 years | ||||||
| Little or no loss | reference | reference | reference | |||
| Loss at forehead only | 1.02 (0.92, 1.13) | 1.02 (0.92, 1.13) | 1.02 (0.89, 1.17) | 1.02 (0.89, 1.17) | 1.02 (0.88, 1.19) | 1.02 (0.88, 1.19) |
| Loss at top of head and forehead | 1.04 (0.94, 1.15) | 1.05 (0.94, 1.16) | 1.06 (0.92, 1.22) | 1.06 (0.92, 1.21) | 1.04 (0.89, 1.20) | 1.04 (0.90, 1.21) |
| Age at baseline | ||||||
| Little or no loss | reference | reference | reference | |||
| Loss at forehead only | 1.00 (0.86, 1.16) | 1.00 (0.86, 1.16) | 1.06 (0.87, 1.29) | 1.06 (0.87, 1.30) | 0.91 (0.73, 1.14) | 0.91 (0.73, 1.13) |
| Loss at top of head and forehead | 1.01 (0.92, 1.10) | 1.00 (0.92, 1.10) | 1.01 (0.89, 1.14) | 1.01 (0.89, 1.14) | 1.01 (0.88, 1.15) | 1.00 (0.88, 1.14) |
NOTE: Missing values were imputed by sequential regression multiple imputation via IVEware in SAS v.9.3.
Aggressive prostate cancer: Gleason score ≥7, or regional/distant metastases (SEER summary stage), or fatal prostate cancer.
Unadjusted model—modeled age as the underlying time metric.
Multivariable model—adjusted for race/ethnicity (Non-Hispanic white, Hispanic, black, American Indian/Alaska Native, Asian/Pacific Islander, other), marital status (married/cohabiting, never married, separated/divorced/widowed), Charlson’s comorbidity index (0, 1, ≥2), BMI at age 45 years (<25, 25–29.99, ≥30 kg/m2), alcohol use last year (quartiles, g/day), smoking status (never/former/current smoker), and baby aspirin use in the past ten years (none, low, high).
DISCUSSION
In this analysis, male pattern baldness at age 30 years, age 45 years, and baseline were not associated with overall prostate cancer risk or with aggressive prostate cancer, despite assessment of three different definitions for the latter endpoint. The classifications of aggressive and nonaggressive prostate cancer were similar in the analytic cohort and sub-cohort #1, despite use of different staging schemes. However, individuals classified as having aggressive prostate cancer in these two cohorts were approximately 50% more likely to be down-graded to nonaggressive in sub-cohort #2, due to inclusion of Gleason score 7 with primary Gleason pattern 3 in the nonaggressive category. Nevertheless, we found similarly null associations in all three cohorts.
Results from previous epidemiologic studies on the association of male pattern baldness with prostate cancer are inconclusive [8,29-42] due to different study designs, modest sample size, as well as different baldness measurements with respect to baldness classification, time window (age) and examination types (trained observers versus self-report). Despite differences in the granularity of vertex balding, a recent meta-analysis of seven case-control studies reported that any vertex balding was associated with a 25% increased risk with prostate cancer, compared with no balding (odds ratio [OR]=1.25; 95%CI= 1.09-1.44) [28]. Four of such studies used self-reported baldness at younger ages with inconsistent results [29,30,39,41]. However, age-specific baldness in relation to prostate cancer risk was not presented in this meta-analysis due to lack of uniformity in ages assessed. Only one of these prior studies used the same balding classification as our study. This study reported an inverse association between any balding at age 30 years and prostate cancer risk (case/control=1,001/942; OR=0.71, 95%CI=0.56, 0.91) [39]. The inverse relationship may be subject to potential recall and selection bias, as suggested by the authors [39]. A more recent case-control study in U.S. veterans found self-reported any balding at age 30 years was associated with increased risks of overall (case/control=167/312; OR=1.88; 95%CI=1.12, 3.16) and high-grade (Gleason score≥7; OR=2.39; 95%CI=1.12, 5.10) prostate cancer, each compared with no balding [38]; frontal balding only was the pattern driving the latter association. This may be explained by the fact that over 50% of cases were African American men, given similar findings from another case-control study (case/control=318/219) in this racial group that frontal balding was associated with high-grade (Gleason score≥7; OR=2.20; 95% CI= 1.05, 4.61) and high-stage (T3/T4; OR=2.61; 95% CI=1.10, 6.18) prostate cancer risk, while any vertex balding was not [42].
Cohort studies that have assessed male pattern baldness and prostate cancer risk are sparse but have suggested a positive association. The NHANES I follow-up study (NHEFS) reported that any degree of baldness assessed by dermatology residents at baseline was associated with a 50% increased risk of overall prostate cancer [34]. However, the median of baseline age was about 55 years, when baldness was assessed, and therefore baldness at younger age was not evaluated with sufficient statistical power. The Melbourne Collaborative Cohort Study (MCCS) predicted an 81% increased risk for prostate cancer at age 55 years, comparing vertex balding (Norwood-Hamilton stage III vertex –VII) with no balding at age 40 years [36]. This study also suggested that vertex balding at 40 years was associated with an average three-year earlier age at prostate cancer diagnosis [36]. However, male pattern baldness was self-assessed after prostate cancer diagnosis, and men with aggressive prostate cancer were more likely to dropout from follow-up resulting in differentially missing self-reported hair-loss patterns.
Compared with the prior studies that provide some evidence for an association between vertex balding and aggressive prostate cancer, the null associations in this analysis could be due to the fact that frontal and vertex baldness were captured as a single exposure class. For example, in our prior prospective analysis in the PLCO Cancer Screening Trial, we observed a positive and statistically significant association between frontal with moderate vertex baldness and aggressive prostate cancer (HR=1.39, 95%CI=1.07, 1.80; In press), which diminished when we assessed frontal balding and vertex balding as a combined, singular exposure class (HR=1.11, 95%CI=0.92, 1.35). In addition, we used self-reported baldness, the earlier two time-points of which were recalled over a significant period of time. Although similar prevalence of baldness was observed in white men at these two ages [4,47], misclassification of baldness is expected based on the moderate accuracy of the modified Norwood-Hamilton Scale, which may bias results towards the null. Finally, survival bias in the VITAL cohort may have precluded detection of an association; higher stage of male pattern baldness has been associated with earlier ages of prostate cancer diagnosis—as suggested by the MCCS analysis [36]—as well as with severe/fatal coronary heart disease [48,49], yet such men may not have been captured in the VITAL cohort given that median age at baseline was 60.5 years (IQR=55.1–67.4 years).
Other limitations of this study also warrant discussion. Firstly, this cohort is predominantly comprised of non-Hispanic white men. Two prior studies support associations between any degree of balding and higher risk of prostate cancer in African American men with risk estimates being 1.69 –2.10 [34,42]. Secondly, we only have information of Proscar® use in the past two weeks, and this low dosage of finasteride—a type II 5α-reductase inhibitor—may modify the effect of male pattern baldness on prostate cancer risk. However, the reported rate of usage is low (0.6%) in this cohort which likely precluded any major effect in the estimates attained.
CONCLUSION
This prospective analysis allowed us to evaluate male pattern baldness at multiple ages prior to prostate cancer diagnosis, as well as assess various definitions of aggressive and nonaggressive prostate cancer with strong statistical power. We did not find any association between male pattern baldness and prostate cancer risk in this analysis. Given the inconsistency in study results, future analyses should seek to use a standardized measurement tool for male pattern baldness to simultaneously capture age at baldness onset, the rate of hair loss and distinct patterns of baldness. Future analyses should also ensure they can adequately assess risks of aggressive and/or fatal prostate cancer.
Supplementary Material
Baselines Characteristics of the Analytic Cohort by Male Pattern Baldness at Age 30 years, Age 45 years, and Baseline (column %, 2000-2011, N=32,583)
Subtype-specific Sensitivity Analyses by High-grade and High-stage/fatal Prostate Cancer: Hazard Ratios (HR) and 95% Confidence Intervals (95%CIs) for Associations of Self-reported Male Pattern Baldness at Age 30 years, Age 45 years, and Baseline in Relation to Prostate Cancer Risk in the Analytic Cohort Estimated from Cox Proportional-hazards Regressions (2000–2011, N=32,583)
Acknowledgments
The authors thank Dr. Ruth M. Pfeiffer at Division of Cancer Epidemiology and Genetics, National Cancer Institute for her statistical consultation support.
Funding Sources: This research was supported by the Intramural Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health
Footnotes
The authors indicated no potential conflicts of interest.
References
- 1.Howlader N, N A, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review 1975-2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012. based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Frontiers in bioscience : a journal and virtual library. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 3.Al Olama AA, Kote-Jarai Z, Giles GG, Guy M, Morrison J, Severi G, Leongamornlert DA, Tymrakiewicz M, Jhavar S, Saunders E, Hopper JL, Southey MC, Muir KR, English DR, Dearnaley DP, Ardern-Jones AT, Hall AL, O’Brien LT, Wilkinson RA, Sawyer E, Lophatananon A, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper C, Donovan JL, Hamdy FC, Neal DE, Eeles RA, Easton DF On BAUSS, Treatment UPTC. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet. 2009;41(10):1058–1060. doi: 10.1038/ng.452. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton JB. Patterned loss of hair in man; types and incidence. Annals of the New York Academy of Sciences. 1951;53(3):708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. The New England journal of medicine. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 6.Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic basis of male pattern baldness. J Invest Dermatol. 2003;121(6):1561–1564. doi: 10.1111/j.1523-1747.2003.12615.x. [DOI] [PubMed] [Google Scholar]
- 7.Bang HJ, Yang YJ, Lho DS, Lee WY, Sim WY, Chung BC. Comparative studies on level of androgens in hair and plasma with premature male-pattern baldness. J Dermatol Sci. 2004;34(1):11–16. doi: 10.1016/j.jdermsci.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Demark Wahnefried W, Lesko SM, Conaway MR, Robertson CN, Clark RV, Lobaugh B, Mathias BJ, Strigo TS, Paulson DF. Serum androgens: Associations with prostate cancer risk and hair patterning. J Androl. 1997;18(5):495–500. [PubMed] [Google Scholar]
- 9.Schweikert HU, Wilson JD. Regulation of human hair growth by steroid hormones. II. Androstenedione metabolism in isolated hairs. The Journal of clinical endocrinology and metabolism. 1974;39(6):1012–1019. doi: 10.1210/jcem-39-6-1012. [DOI] [PubMed] [Google Scholar]
- 10.Price VH, Menefee E, Sanchez M, Kaufman KD. Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. Journal of the American Academy of Dermatology. 2006;55(1):71–74. doi: 10.1016/j.jaad.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Randall VA, Thornton MJ, Messenger AG. Cultured Dermal Papilla Cells from Androgen-Dependent Human Hair-Follicles (Eg Beard) Contain More Androgen Receptors Than Those from Non-Balding Areas of Scalp. J Endocrinol. 1992;133(1):141–147. doi: 10.1677/joe.0.1330141. [DOI] [PubMed] [Google Scholar]
- 12.Sawaya ME, Honig LS, Hsia SL. Increased Androgen Binding-Capacity in Sebaceous Glands in Scalp of Male-Pattern Baldness. J Invest Dermatol. 1989;92(1):91–95. doi: 10.1111/1523-1747.ep13071290. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss) The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2003;8(1):20–23. doi: 10.1046/j.1523-1747.2003.12167.x. [DOI] [PubMed] [Google Scholar]
- 14.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA: a cancer journal for clinicians. 1972;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 15.Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186(4170):1213–1215. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- 16.Wu CP, Gu FL. The prostate in eunuchs. Progress in clinical and biological research. 1991;370:249–255. [PubMed] [Google Scholar]
- 17.Li X, Huang Y, Fu X, Chen C, Zhang D, Yan L, Xie Y, Mao Y, Li Y. Meta-analysis of three polymorphisms in the steroid-5-alpha-reductase, alpha polypeptide 2 gene (SRD5A2) and risk of prostate cancer. Mutagenesis. 2011;26(3):371–383. doi: 10.1093/mutage/geq103. [DOI] [PubMed] [Google Scholar]
- 18.Wang F, Zou YF, Feng XL, Su H, Huang F. CYP17 gene polymorphisms and prostate cancer risk: a meta-analysis based on 38 independent studies. Prostate. 2011;71(11):1167–1177. doi: 10.1002/pros.21332. [DOI] [PubMed] [Google Scholar]
- 19.Chang BL, Zheng SQL, Hawkins GA, Isaacs SD, Wiley KE, Turner A, Carpten JD, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB, Xu JF. Joint effect of HSD3B1 and HSD3B2 genes is associated with hereditary and sporadic prostate cancer susceptibility. Cancer Res. 2002;62(6):1784–1789. [PubMed] [Google Scholar]
- 20.Jia L, Landan G, Pomerantz M, Jaschek R, Herman P, Reich D, Yan CL, Khalid O, Kantoff P, Oh W, Manak JR, Berman BP, Henderson BE, Frenkel B, Haiman CA, Freedman M, Tanay A, Coetzee GA. Functional Enhancers at the Gene-Poor 8q24 Cancer-Linked Locus. Plos Genet. 2009;5(8) doi: 10.1371/journal.pgen.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu YZ, Zhang Z, Yu HJ, Zheng SL, Isaacs WB, Xu JF, Sun JL. Functional Annotation of Risk Loci Identified Through Genome-Wide Association Studies for Prostate Cancer. Prostate. 2011;71(9):955–963. doi: 10.1002/pros.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Hsing AW, Crawford ED, Andriole GL, Kaaks R, Hayes RB. Endogenous sex hormones and the risk of prostate cancer: a prospective study. International journal of cancer Journal international du cancer. 2008;122(10):2345–2350. doi: 10.1002/ijc.23326. [DOI] [PubMed] [Google Scholar]
- 23.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer I. 2008;100(3):170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gershman B, Shui IM, Stampfer M, Platz EA, Gann PH, Sesso HL, DuPre N, Giovannucci E, Mucci LA. Prediagnostic circulating sex hormones are not associated with mortality for men with prostate cancer. European urology. 2014;65(4):683–689. doi: 10.1016/j.eururo.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierorazio PM, Ferrucci L, Kettermann A, Longo DL, Metter EJ, Carter HB. Serum testosterone is associated with aggressive prostate cancer in older men: results from the Baltimore Longitudinal Study of Aging. BJU international. 2010;105(6):824–829. doi: 10.1111/j.1464-410X.2009.08853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller RL, Gerber L, Moreira DM, Andriole G, Castro-Santamaria R, Freedland SJ. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the Reduction by Dutasteride of Prostate Cancer Events trial. European urology. 2012;62(5):757–764. doi: 10.1016/j.eururo.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Sutcliffe S, Colditz GA. Prostate cancer: is it time to expand the research focus to early-life exposures? Nat Rev Cancer. 2013;13(3):208–518. doi: 10.1038/nrc3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amoretti A, Laydner H, Bergfeld W. Androgenetic alopecia and risk of prostate cancer: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2013 doi: 10.1016/j.jaad.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Cremers RG, Aben KK, Vermeulen SH, den Heijer M, van Oort IM, Kiemeney LA. Androgenic alopecia is not useful as an indicator of men at high risk of prostate cancer. Eur J Cancer. 2010;46(18):3294–3299. doi: 10.1016/j.ejca.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Demark-Wahnefried W, Schildkraut JM, Thompson D, Lesko SM, McIntyre L, Schwingl P, Paulson DF, Robertson CN, Anderson EE, Walther PJ. Early onset baldness and prostate cancer risk. Cancer Epidem Biomar. 2000;9(3):325–328. [PubMed] [Google Scholar]
- 31.Faydaci G, Bilal E, Necmettin P, Fatih T, Asuman O, Ugur K. Baldness, benign prostate hyperplasia, prostate cancer and androgen levels. Aging Male. 2008;11(4):189–192. doi: 10.1080/13685530802400995. [DOI] [PubMed] [Google Scholar]
- 32.Giles GG, Severi G, Sinclair R, English DR, McCredie MRE, Johnson W, Boyle P, Hopper JL. Androgenetic alopecia and prostate cancer: Findings from an Australian case-control study. Cancer Epidem Biomar. 2002;11(6):549–553. [PubMed] [Google Scholar]
- 33.Greenwald P, D A, Kirmss V, Polan AK. Physical and demographic features of men before developing cancer of the prostate. J Natl Cancer Inst. 1974;53(2):341–346. doi: 10.1093/jnci/53.2.341. [DOI] [PubMed] [Google Scholar]
- 34.Hawk E, Breslow RA, Graubard BI. Male pattern baldness and clinical prostate cancer in the epidemiologic follow-up of the first National Health and Nutrition Examination Survey. Cancer Epidem Biomar. 2000;9(5):523–527. [PubMed] [Google Scholar]
- 35.Hsieh CC, Thanos A, Mitropoulos D, Deliveliotis C, Mantzoros CS, Trichopoulos D. Risk factors for prostate cancer: A case-control study in Greece. Int J Cancer. 1999;80(5):699–703. doi: 10.1002/(sici)1097-0215(19990301)80:5<699::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Muller DC, G G, Sinclair R, Hopper JL, English DR, Severi G. Age dependent associations between androgenetic alopecia and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0860. [DOI] [PubMed] [Google Scholar]
- 37.Oishi K, Okada K, Yoshida O, Yamabe H, Ohno Y, Hayes RB, Schroeder FH. Case-Control Study of Prostatic-Cancer in Kyoto, Japan - Demographic and Some Lifestyle Risk-Factors. Prostate. 1989;14(2):117–122. doi: 10.1002/pros.2990140205. [DOI] [PubMed] [Google Scholar]
- 38.Thomas JA, Antonelli JA, Banez LL, Hoyo C, Grant D, Demark-Wahnefried W, Platz EA, Gerber L, Shuler K, Eyoh E, Calloway E, Freedland SJ. Androgenetic alopecia at various ages and prostate cancer risk in an equal-access multiethnic case-control series of veterans. Cancer Cause Control. 2013;24(5):1045–1052. doi: 10.1007/s10552-013-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright JL, Page ST, Lin DW, Stanford JL. Male pattern baldness and prostate cancer risk in a population-based case-control study. Cancer epidemiology. 2010;34(2):131–135. doi: 10.1016/j.canep.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wynder EL MK, Whitmore WF Jr. Epidemiology of cancer of the prostate. Cancer. 1971;28(2):344–360. doi: 10.1002/1097-0142(197108)28:2<344::aid-cncr2820280214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Yassa M, Saliou M, De Rycke Y, Hemery C, Henni M, Bachaud JM, Thiounn N, Cosset JM, Giraud P. Male pattern baldness and the risk of prostate cancer. Ann Oncol. 2011;22(8):1824–1827. doi: 10.1093/annonc/mdq695. [DOI] [PubMed] [Google Scholar]
- 42.Zeigler-Johnson C, Morales KH, Spangler E, Chang BL, Rebbeck TR. Relationship of Early-Onset Baldness to Prostate Cancer in African-American Men. Cancer Epidem Biomar. 2013;22(4):589–596. doi: 10.1158/1055-9965.EPI-12-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White E, Patterson RE, Kristal AR, Thornquist M, King I, Shattuck AL, Evans I, Satia-Abouta J, Littman AJ, Potter JD. VITamins And Lifestyle cohort study: Study design and characteristics of supplement users. Am J Epidemiol. 2004;159(1):83–93. doi: 10.1093/aje/kwh010. [DOI] [PubMed] [Google Scholar]
- 44.Littman AJ, White E. Reliability and validity of self-reported male balding patterns for use in epidemiologic studies. Ann Epidemiol. 2005;15(10):771–772. doi: 10.1016/j.annepidem.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Raghunathan TE, Lepkowski JM, Hoewyk JV, Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27(1):85–95. [Google Scholar]
- 46.Raghunathan TE, Solenberger P, Hoewyk JV. IVEware: Imputation and Variance Estimation Software. Vol. 2013. Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan performs; [Google Scholar]
- 47.DeMuro-Mercon C, Rhodes T, Girman CJ, Vatten L. Male-pattern hair loss in Norwegian men: a community-based study. Dermatology. 2000;200(3):219–222. doi: 10.1159/000018386. [DOI] [PubMed] [Google Scholar]
- 48.Lotufo PA, Chae CU, Ajani UA, Hennekens CH, Manson JE. Male pattern baldness and coronary heart disease: the Physicians’ Health Study. Arch Intern Med. 2000;160(2):165–171. doi: 10.1001/archinte.160.2.165. [DOI] [PubMed] [Google Scholar]
- 49.Yamada T, Hara K, Umematsu H, Kadowaki T. Male pattern baldness and its association with coronary heart disease: a meta-analysis. BMJ open. 2013;3(4) doi: 10.1136/bmjopen-2012-002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baselines Characteristics of the Analytic Cohort by Male Pattern Baldness at Age 30 years, Age 45 years, and Baseline (column %, 2000-2011, N=32,583)
Subtype-specific Sensitivity Analyses by High-grade and High-stage/fatal Prostate Cancer: Hazard Ratios (HR) and 95% Confidence Intervals (95%CIs) for Associations of Self-reported Male Pattern Baldness at Age 30 years, Age 45 years, and Baseline in Relation to Prostate Cancer Risk in the Analytic Cohort Estimated from Cox Proportional-hazards Regressions (2000–2011, N=32,583)


