Abstract
Aedes aegypti allatostatin-C (AeaAST-C or PISCF-AST) is a strong and fast reversible inhibitor of juvenile hormone III (JH III) synthesis by the corpora allata (CA) of mosquitoes; however, its mechanism of action remains poorly understood. AeaAST-C showed no inhibitory activity in the presence of any of the intermediate precursors of JH III indicating that the AeaAST-C target is located before the entry of acetyl-CoA in the pathway. Stimulation experiments using different sources of carbon (glucose, pyruvate, acetate and citrate) suggest that AST-C acts after pyruvate is transformed to citrate in the mitochondria. In vitro inhibition of the citrate mitochondrial carrier (CIC) mimicked the effect of AeaAST-C, and was overridden by addition of citrate or acetate. Our results provide compelling evidence that AeaAST-C inhibits JH III synthesis by blocking the CIC carrier that transports citrate from the mitochondria to the cytosol, obstructing the production of cytoplasmic acetyl-CoA that sustains JH III synthesis in the CA of mosquitoes.
Keywords: Allatostatin, mosquito, juvenile hormone, membrane citrate transport
1. Introduction
Allatostatins (AST) are pleiotropic neuropeptides which act as reversible inhibitors of juvenile hormone (JH) synthesis in insects (Stay and Tobe, 2007). JHs are synthesized in the corpora allata (CA), a pair of endocrine glands connected to the brain, and play critical roles as regulators of development and reproduction (Goodman and Cusson, 2012). Three types of ASTs have been described in insects: cockroach ASTs (FGLamides or AST-A), cricket ASTs (W(X)6 amides or AST-B) and Manduca ASTs (PISCFs or AST-C) (Gilbert et al., 2000; Bendena et al., 1999). The mechanism of action of ASTs in the regulation of JH synthesis remains poorly understood (Weaver and Audsley, 2009; Stay and Tobe, 2007); AST-C receptors have been described in two dipteran species, Drosophila melanogaster (DAR-1 and DAR-2) (Kreienkamp et al., 2002) and Aedes aegypti (AeaAST-Cr-A and AeaAST-Cr-B) (Mayoral et al., 2010); they are G-protein-coupled receptors (GPCRs) operating through the cAMP and phosphatidylinositol signaling pathways (Tuteja, 2009).
We have previously described that the biosynthetic activity of the CA in vitro was reversibly inhibited by mosquito brain extracts, as well as by Anopheles gambiae PISCF-AST (Li et al., 2004). Aedes aegypti AST-C (AeaAST-C) was isolated and characterized by MS/MS analysis (Li et al., 2006). AeaAST-C is a 15 amino acid amidated peptide synthesized by neurosecretory cells in the protocerebral lobes of the brain (Hernandez-Martinez et al., 2005). Maximum level of the peptide in the brain of female mosquitoes is detected at 3 days after adult eclosion, correlating with an increased sensitivity of the CA to inhibition by AeaAST-C (Li et al., 2006). Lastly, the AeaAST-C GPCR receptor expression in CA of mosquitoes was reported (Mayoral et al., 2010). In vitro studies have previously shown that AST inhibition is developmentally dependent (Yagi et al., 2005; Tobe et al., 2000; Weaver et al., 1998; Lorenz and Hoffmann, 1995); the sensitivity to inhibition by AeaAST-C in the in vitro assay also fluctuates, revealing that the mosquito CA is most sensitive during periods of low synthetic activity (Li et al., 2006).
JH III synthesis is very dynamic during the gonotrophic cycle of female mosquitoes; and its regulation involves a complex interplay of changes of precursor pools and enzyme levels (Rivera-Perez et al., 2014; Nouzova et al., 2011). JH III is synthesized through the mevalonate pathway (MVAP), which consists of multiple enzymatic steps through which acetyl-CoA is gradually transformed into the 5-carbon compound isopentenyl-pyrophosphate (IPP), and later onto the 15-carbon farnesyl-pyrophosphate (FPP) (Belles and Piulachs, 2005). In the CA of mosquitoes, FPP is sequentially transformed to farnesol (FOL), farnesal (FAL), farnesoic acid (FA), methyl farnesoate (MF) and JH III (Nouzova et al., 2011). Experimental increases in the magnitude of any individual precursor pool generally raise rate of JH III synthesis, suggesting that enzyme concentrations in the CA are often in excess (Rivera-Perez et al., 2014; Nouzova et al., 2011; Feyereisen et al., 1984).
In the present study, we explored the mechanism of action of AeaAST-C in the CA of female A. aegypti mosquitoes. AST receptors transcripts were detected in both CA and corpora cardiaca (CC). In vitro treatment of the adult CA with AeaAST-C resulted in a fast, strong and reversible inhibition of JH III biosynthesis; which was overridden by stimulation with early or late JH III intermediate precursors. The mitochondrial membrane citrate transport protein (CIC) was established as a potential target for AeaAST-C by assessing the contribution of different carbon metabolites as sources of 2C building blocks for the production of JH III. Pharmacological inhibition of CIC mimicked the effect of AeaAST-C, resulting in abolishment of JH III biosynthesis. This inhibitory effect could be superseded by addition of acetate or citrate, reinforcing the idea that the CIC carrier is a main target for AeaAST-C. Altogether our studies provided compelling evidence that AeaAST-C inhibits JH III synthesis by nactivation of the CIC transporter that mobilizes citrate from the mitochondria to the cytosol, obstructing the production of cytoplasmic acetyl-CoA that sustains JH III synthesis in the CA of mosquitoes.
2. Material and Methods
2.1. Insects
A. aegypti of the Rockefeller strain were reared at 28 °C and 80% humidity as previously described (Nouzova et al., 2011). Adult mosquitos were offered a cotton pad soaked in a 20% sucrose solution.
2.2. Chemicals
Custom made peptide Aedes aegypti AST-C (AeaAST-C) (QIRYRQCYFNPISCF) was provided by Biopetide Co. (San Diego, CA), purified by reverse phase liquid chromatography and assessed to be 98% pure by analytical mass spectrometry and amino acid analysis. Stock of aqueous solutions of AeaAST-C were prepared at a concentration of 10−5 M and stored in aliquots at −80 °C. Acetate, citrate, pyruvate, acetyl-CoA (ACoA), mevalonic acid (MVA), mevalonic acid 5-phosphate (MevP), mevalonic acid 5-pyrophosphate (MevPP), farnesal (FAL) and 1,2,3-benzenetricarboxylate (BTC) were purchased from Sigma (St. Louis, MO). Farnesyl diphosphate (FPP), farnesol (FOL), farnesoic acid (FA) and methyl farnesoate (MF) were purchased from Echelon Biosciences (Salt Lake city, UT).
2.3. Dissections of corpora allata complexes
Three days old female mosquitos were cold-anesthetized and dissected in Aedes physiological saline (APS) (MgCl2 2.0 mM; KCl 8.4 mM; NaH2PO4 12.0 mM; Na2HPO4 12.0 mM; NaCl 138.0 mM; Sucrose 42.5 mM; CaCl2 4.0 mM) as previously described (Li et al., 2003). Unless otherwise noted, preparations were of intact corpora allata-corpora cardiaca (CA-CC) complexes connected to the brain and head capsule and are denoted as Br-CA-CC complexes. Two additional preparations of CA complexes for in vitro experiments were used: 1) “denervated” CA-CC complexes, in which the CA-CC were separated from the brain; and 2) “isolated” CA, in which the CA was isolated from both the brain and the CC (Nouzova et al., 2012).
2.4. Quantitative Real-Time PCR (q-PCR)
Total RNA was isolated using RNA-binding glass powder as previously described (Noriega et al., 1993). Contaminating genomic DNA was removed using the DNA-free™ kit (Ambion, Austin, TX). Reverse transcription of RNA was carried out using Qscript (Quanta BioSciences, Gaithersburg, MD), according to the manufacturer’s recommendations. Relative expression of selected genes was quantified by real-time PCR performed in a 7300 Real-Time PCR System using TaqMan® Gene Expression Assays together with TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Reactions were run in triplicate in a 20 μl volume and normalized to the house keeping gene 60S ribosomal protein L32 (rpL32) mRNA expression for each sample. Primer probes sequences for L32 and allatostatin-C receptor A and B genes (AeaAST-CrA and AeaAST-CrB) were previously described (Mayoral et al., 2010). Primer probes for adipokinetic hormone (AKH), crustacean cardioacceleratory peptide (CCAP), juvenile hormone acid methyltransferase (JHAMT) and epoxidase (EPOX) genes were previously described (Nouzova et al., 2012).
2.5. Testing the effect of AeaAST-C on JH III synthesis
We previously described that the inhibitory effect of AeaAST-C is dose-dependent, with a maximum at 10−9 M (Li et al., 2006); therefore all AeaAST-C experiments were performed using a concentration of 10−9 M. Br-CA-CC complexes were dissected and incubated in the presence of AeaAST-C at 32 °C for 2 h in 150 μl of tissue culture media M-199 (Lavallette, NJ, USA) containing 2% Ficoll, 25 mM HEPES (pH 6.5) and methionine (50 μM). Controls were not treated with the peptide. Biosynthesized JH III was labelled with a fluorescent tag and analyzed by reverse phase high performance liquid chromatography coupled to a fluorescent detector (HPLC-FD) as previously described (Rivera-Perez et al., 2012).
2.6. Enzymatic activities
For each assay, 10 glands were dissected, homogenized and analyzed for particular enzymatic activities using specific buffers (Rivera-Perez et al., 2014). The reaction products were analyzed by HPLC-FD.
2.7. Statistical analysis
Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as mean ± S.E.M. Significant differences (p< 0.05) were determined with a one tailed students t-test performed in a pair wise manner or by one-way ANOVA followed by Tukey’s test.
3. Results
3.1. AeaAST-C has a reversible inhibitory effect on JH III synthesis
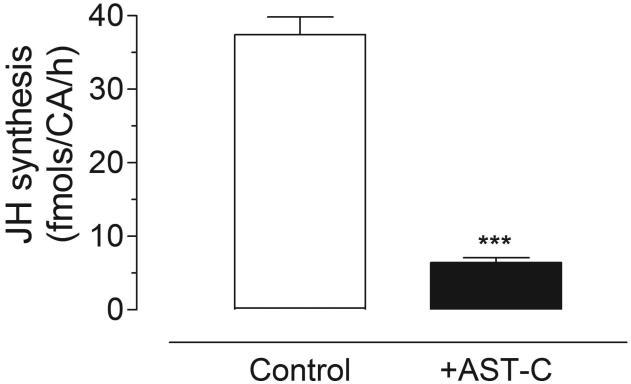
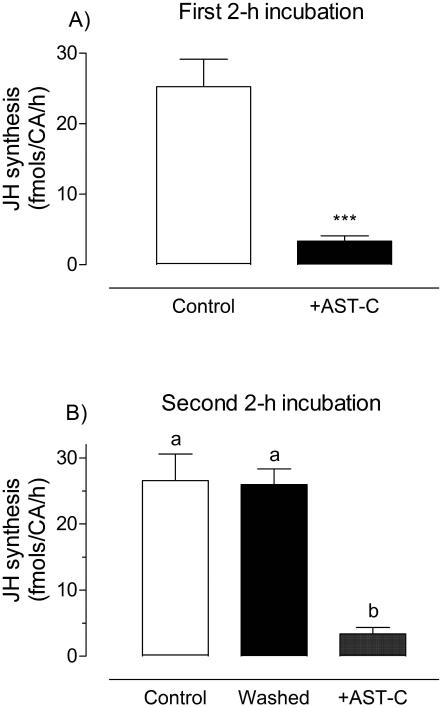
Incubation of Br-CA-CC complexes dissected from 3-day old sugar-fed females with 10−9 M AeaAST-C resulted in over 80% reduction in JH III synthesis (Fig. 1). To test if the effect of AeaAST-C is reversible, Br-CA-CC complexes were first incubated with or without AeaAST-C for a 2 h period. During the first 2 h incubation, JH III released from CA was significantly inhibited in the presence of the synthetic peptide (Fig. 2A). Afterwards, AeaAST-C-treated and control Br-CA-CC complexes were rinsed in fresh medium and incubated for a second 2 h period in medium with or without AeaAST-C. No significant difference in JH III synthesized by control and washed groups was found during the second incubation; only the glands treated for a second time with AeaAST-C showed inhibition, indicating that the modulatory effect of AeaAST-C was reversible (Fig. 2B).
Fig. 1. AeaAST-C inhibits JH biosynthesis.
JH biosynthesis by Br-CA-CC complexes with and without addition of AeaAST-C (10−9 M) was assayed in vitro. Each data point represents the means ± S.E.M. of 35 independent determinations of three Br-CA-CC complexes. Asterisks denote significant differences (unpaired t-test; ***P<0.0001).
Fig. 2. The inhibitory effect of AeaAST-C is reversible.
Br-CA-CC were incubated for two consecutive 2 h periods. A) During the first 2 h, Br-CA-CC complexes were incubated either in the presence or absence of AeaAST-C (10−9 M). After 2 h, glands were rinsed in fresh culture medium. B) During the second 2 h period, control and previously treated glands were incubated in fresh culture medium with and without AeaAST-C. Each data point represents the mean ± S.E.M. of three independent determinations of three Br-CA-CC complexes. Asterisks denote significant differences (unpaired t-test; ***P<0.0001). Different letters above the columns indicate significant differences among treatments (ANOVA P<0.05, with Tukey’s test for multiple comparison).
3.2. AeaAST-C acts directly on the isolated CA
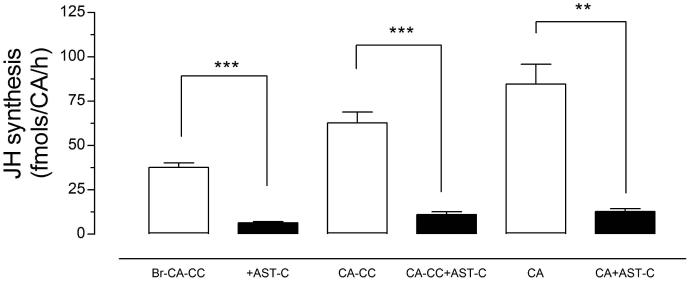
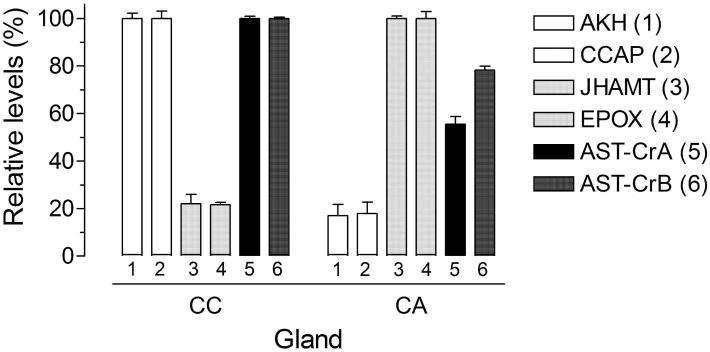
The effect of AeaAST-C on JH III biosynthesis was evaluated on three different CA preparations: 1) Br-CA-CC, 2) “denervated” CA-CC, and 3) isolated CA. Treatment with AeaAST-C produced a significant reduction of JH III synthesis in the three types of preparations (Fig. 3). Separation of the brain and CC produced a 2-fold increase of JH III synthesis; however, addition of AeaAST-C resulted in a significant inhibition of JH III synthesis in isolated CA, suggesting that AeaAST-C acts directly on the CA. A direct effect of AeaAST-C entails the presence of AeaAST-C receptors in the CA; The very low level of expression of AeaAST-C receptors mRNA in the CA made it difficult to use in situ hybridization as the method of choice to investigate the expression of the receptor in the CA and the CC. Alternatively, to investigate the specific expression of the AeaAST-C receptors in the individual components of the CA-CC complexes, we decided to perform a surgical separation of the CA and CC, followed by analysis of expression of specific gene markers for CA and CC in the isolated glands (Fig. 4). CC markers such as adipokinetic hormone (AKH) and crustacean cardioacceleratory peptide (CCAP) (Gäde and Goldsworthy, 2003) had low expression in the CA (about 20%) when compared with transcript levels in the CC. Juvenile hormone biosynthetic enzymes, such as juvenile hormone acid methyltransferase (JHAMT) and methyl farnesoate epoxidase (EPOX), that are known to be expressed in the CA (Ueda et al., 2009), had low expression in the CC (about 20%) when compared with transcripts levels in the CA. These numbers confirm that the surgical separation was successful, with about 20% cross-contamination. Both AeaAST-C receptors were certainly expressed in the CC, but also in the CA, where their expression levels exceeded the levels of CC-specific genes (CCAP and AKH), suggesting that the AeaAST-C receptors are expressed in both glands.
Fig. 3. AeaAST-C acts directly on the CA.
Different types of CA preparations were incubated with AeaAST-C (10−9 M) in vitro, and JH biosynthesis was evaluated. Br-CA-CC (CA-CC connected to the brain), CA-CC (denervated CA-CC) and CA (isolated CA). Each data point represents the means ± S.E.M. of three independent determinations of each preparation. Asterisks denote significant differences (unpaired t-test; ** P ≤ 0.05; *** P ≤ 0.001).
Fig. 4. AeaAST-C receptors A and B are expressed in the CC and CA.
Corpora allata (CA) and corpora cardiaca (CC) were individually dissected from adult female mosquitoes. The expression of the two AeaAST-C receptors (A and B) and four additional marker genes were evaluated in isolated CC and CA. AKH (1): adipokinetic hormone; CCAP (2): crustacean cardioacceleratory peptide; JHAMT (3): juvenile hormone acid methyltransferase; EPOX (4): epoxidase; AeaAST-Cr-A (5): AeaAST-C receptors A; AeaAST-Cr-B (6): AeaAST-C receptors B. Transcripts levels are expressed as percentage of the maximum value found in the two glands. Each q-PCR data point is the mean ± S.E.M. of two independent biological replicates of 20 tissue samples.
3.3. The AeaAST-C target is located before the entry of acetyl-CoA in the JH III pathway
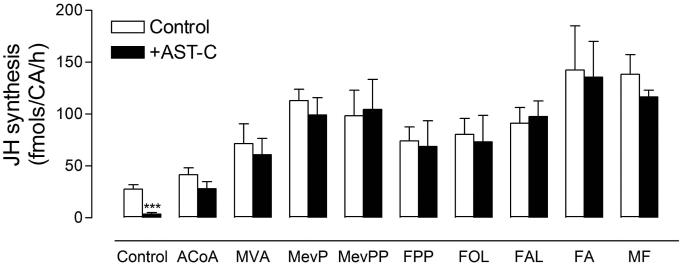
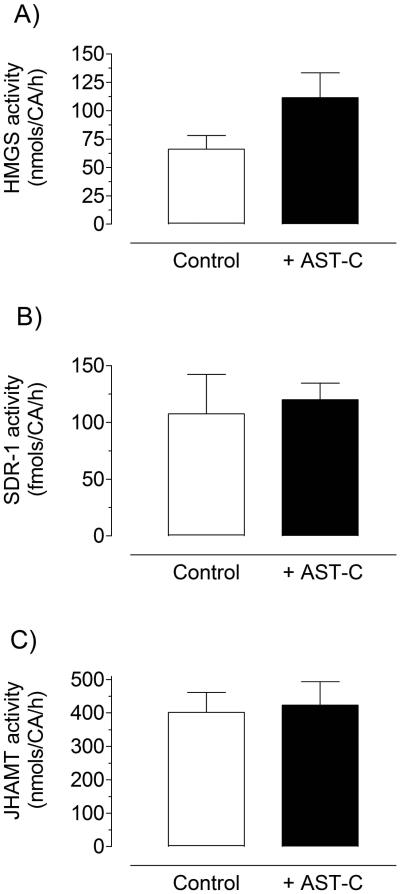
To test if any of the JH biosynthetic enzymes was a target for AeaAST-C inhibition, Br-CA-CC complexes were incubated with and without AeaAST-C (10−9 M) in the presence of nine different JH III precursors (200 μM). The addition of any of the precursors resulted in a significant increase in JH III biosynthesis; and the effect of AeaAST-C on JH III synthesis was overridden by stimulation with each of the nine precursors (Fig. 5); suggesting that AeaAST-C does not affect the activity of any of the JH-biosynthetic enzymes, but prevents the entry of acetyl-CoA into the JH pathway. This was confirmed by incubating the CA with and without AeaAST-C, followed by the analysis of the activity of three enzymes from different sections of the JH biosynthesis pathway (beginning, middle and end): HMG-CoA synthase (HMGS), farnesol oxidase (SDR) and juvenile hormone methyl transferase (JHAMT). The addition of AeaAST-C to the incubation had no effect on the activity of any of the 3 enzymes (Fig. 6).
Fig. 5. Stimulation with precursors overrides the inhibitory effect of AeaAST-C.
The effect of AeaAST-C (10−9 M) on JH biosynthesis was evaluated on Br-CA-CC complexes incubated in culture medium M-199 alone (control) or with the addition of 200 μM of nine different precursors: ACoA (acetyl-CoA), MVA (mevalonic acid), MevP (mevalonic acid 5-phosphate), MevPP (mevalonic acid 5-pyrophosphate), FPP (farnesyl diphosphate), FOL (farnesol), FAL (farnesal), FA (farnesoic acid) and MF (methyl farnesoate). Each data point represents the means ± S.E.M. of 2-4 independent biological replicates of three Br-CA-CC complexes. Asterisks denote significant differences (unpaired t-test; *** P ≤ 0.001).
Fig. 6. AeaAST-C treatment does not modify the activity of the JH biosynthetic enzymes.
Br-CA-CC complexes were incubated for 2 h in the presence or absence of AeaAST-C (10−9 M). After incubation CA-CC were isolated, and the activities of HMG-CoA Synthase (A), SDR-1 (B) and JHAMT (C) were evaluated. Each data point represents the means ± S.E.M. of 2 independent biological replicates of five CA-CC complexes.
3.4. Stimulation with citrate or acetate, but not with glucose or pyruvate overrides AeaAST-C inhibition
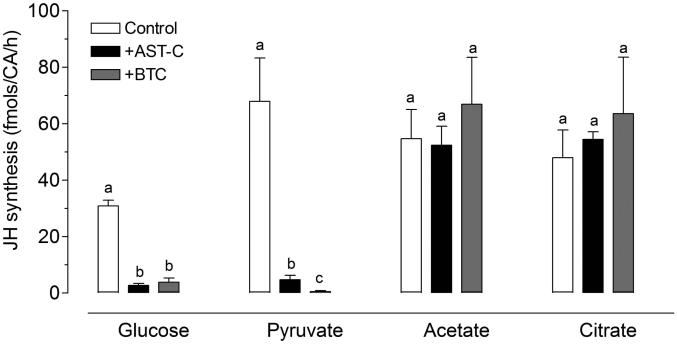
Acetyl-CoA is the basic building block for JH synthesis and it can be generated from different carbon sources (e.g. carbohydrates, lipids and amino acids). To validate that AeaAST-C is affecting the incorporation of acetyl-CoA into the JH pathway, we tested the effect of stimulating the CA with three different carbon sources: 1) glucose that is metabolized by the glycolytic pathway to produce cytosolic pyruvate, which is transferred to the mitochondria and metabolized by the tricarboxylic acid cycle (TCA) to produce citrate that is shuttled out the mitochondria by the membrane citrate transport protein (CIC) (Kaplan and Mayor, 1993). Once in the cytosol, citrate is metabolized to acetyl-CoA by the ATP-citrate lyase. 2) Acetate, which can be converted to acetyl-CoA by the cytosolic enzyme acetyl-CoA synthase, and 3) citrate, which can be metabolized to acetyl-CoA by the ATP-citrate lyase. Pyruvate, an intermediate of the glycolytic pathway was also tested. Because our medium for in vitro CA incubations already contains glucose, a 1:1 molar concentration was used with each of the carbon sources tested (1 mol glucose: 1 mol citrate/acetate/pyruvate). Pyruvate, acetate and citrate were efficiently metabolized by the CA, and addition of any of these precursors resulted in a ~2 fold increase in JH III synthesis; interestingly AeaAST-C was an effective inhibitor of JH III synthesis only when glucose or pyruvate were the available carbon sources (Fig. 7); while addition of citrate or acetate overrode the inhibition, suggesting that AeaAST-C acts downstream of the pyruvate conversion to acetyl-CoA, and implying that ATP-citrate lyase and acetyl-CoA synthase activities were not inhibited by AeaAST-C.
Fig. 7. Addition of citrate or acetate overrides AeaAST-C inhibition of JH biosynthesis.
The effect of AeaAST-C (10−9 M) on JH biosynthesis was evaluated on Br-CA-CC complexes incubated in medium M-199 containing only glucose (5 mM) or with the addition of either pyruvate, acetate or citrate (5 mM). The effect of 1,2,3-benzenetricarboxylate (BTC), an inhibitor of the mitochondrial citrate carrier (CIC), was also tested in the four types of preparations. Each data point represents the means ± S.E.M. of 3-4 independent biological replicates of three Br-CA-CC complexes. Different letters above the columns indicate significant differences among treatments (ANOVA P<0.05, with Tukey’s test for multiple comparison).
3.5. CIC inhibition phenocopies the effect of AeaAST-C
The CIC (a citrate malate antiport) transports citrate from the mitochondria to the cytosol in exchange for the dicarboxylic cytosolic malate. To verify that the activity of the CIC carrier is essential for JH biosynthesis, we used a non-cleavable benzene-tricarboxylate analog (BTC) (2 mM) that suppresses CIC-dependent transport of citrate. Inhibition of CIC activity by BTC resulted in a significant reduction of JH III biosynthesis, phenocopying the AeaAST-C inhibition (Fig. 7). We tested four different carbon sources to assess if the inhibition of the CIC by BTC could be overridden by addition of cytoplasmic acetyl-CoA precursors (Fig. 7). The addition of glucose or pyruvate to a CA incubated with BTC did not revert the inhibition of JH III synthesis; however, addition of citrate or acetate overrode the effect of BTC, resulting in rates of JH III synthesis equivalent to those of control glands. Thus, our results provide evidence that inhibition of the CIC activity by BTC mimics AeaAST-C action; the BTC inhibitory effect was only superseded by addition of acetate or citrate.
4. Discussion
4.1. AeaAST-C is a fast and strong reversible inhibitor of JH III synthesis in adult female mosquitoes
It took almost 40 years from the first demonstration that neuropeptides influence CA activity (Scharrer, 1952) to the identification and functional characterization of the first FGLamide (Woodhead et al., 1989) and PISCF ASTs (Kramer et al., 1991). These two types of allatostatins are associated with unique GPCR receptor families that have vertebrate orthologs; the FGLamide receptors are related to the vertebrate galanin receptors (Birgül et al., 1999), and PISCF receptors show similarity to the somatostatin/opioid receptors (Kreienkamp et al., 2002). Still, more than 60 years after Scharrer’s work was published, the identification of the targets of ASTs actions in the CA, as well as the signaling pathways that connect the binding of ASTs to their membrane receptors with the molecular changes in their targets, are largely unknown (Weaver and Audsley, 2009; Stay and Tobe, 2007).
ASTs effects are very fast and reversible (Li et al., 2004; Bendena et al., 1999). Although depressed, the CA seems to maintain intact its biosynthetic potential, and JH synthesis is rapidly reactivated if ASTs are removed. These features of ASTs action would suggest that activation of gene transcription might not be primarily involved, but instead a fast post-translational regulation such as a reversible phosphorylation of proteins. Sutherland and Feyereisen (1996) reported that D. punctata CA inhibited with FGLamide AST were prevented from using glucose to synthesize JH III, but free to use acetate. In addition FGLamide AST inhibited JH III synthesis without affecting the activities of HMG-CoA synthase or HMG-CoA reductase. The results of these experiments advocated that AST-A targets were either the transport of citrate from mitochondria to cytosol and/or the metabolism of citrate to produce acetyl-CoA (Sutherland and Feyereisen, 1996).
Our studies on a different allatostatin neuropeptide further explored these concepts; by providing an excess of different precursors we ultimately evaluated the effect of AeaAST-C on the activity of all the enzymes of the JH synthesis pathway. AeaAST-C showed no inhibitory activity in the presence of any of the intermediate precursors of the pathway indicating that all the enzymes were fully active and able to process their substrates; this was confirmed by measuring the activity of a few key enzymes in the pathway, which exhibited normal catalytic activities in the presence of AeaAST-C. The fact that all the enzymes were fully functional revealed that in mosquitoes the AeaAST-C target is located before the entry of acetyl-CoA in the JH pathway. Recently, Huang et al. (2014) showed that FGLamide AST inhibits JH III synthesis in D. punctata without modifying transcript levels of enzymes in the JH III biosynthetic pathway. The inhibitory effect was overridden by the addition of acetyl-CoA, mevalonate, diphosphomevalonate or farnesol, confirming that the effect is prior to the entry of precursors into the JH biosynthetic pathway (Huang et al., 2014). Our results exposed that FGLamide and PISCF ASTs may well have similar mechanisms of action. This would be a remarkable example of convergent evolution because both families of peptide have different structures, are ligands for different types of receptors, and are active in groups of insects that diverged hundreds of million years ago.
4.2. Mechanism of action of AeaAST-C on JH synthesis
The biosynthesis of JH starts when two units of acetyl-CoA are condensed by the enzyme acetoacetyl-CoA thiolase; acetyl-CoA is therefore the basic building block for JH synthesis and it can be generated from different carbon sources (e.g. carbohydrates, lipids and amino acids). Glucose is the major carbon source provided in our CA in vitro system; it is metabolized by the glycolytic pathway to produce cytosolic pyruvate, which is transferred to the mitochondria and metabolized to produce citrate that is shuttled out the mitochondria by the membrane citrate transport protein (CIC) (Kaplan and Mayor. 1993). The expression of the CIC gene in CA of D. punctata and A. aegypti has been previously described in transcriptome studies (Noriega et al., 2006). Citrate is a key intermediate in catabolic and anabolic pathways; once in the cytosol, citrate is broken down to acetyl-CoA, providing the immediate carbon source to fuel the synthesis of isoprenoids in the CA (Belles and Piulachs, 2005). Our studies revealed that AeaAST-C affected the incorporation of glucose and pyruvate into the JH pathway, and either acetate or citrate overrode AeaAST-C inhibition and sustained normal JH III synthesis by the CA in mosquitoes. By assessing the contribution of different sources of 2C building blocks for the production of JH, we established the mitochondrial membrane citrate transport protein (CIC) as a potential target for AeaAST-C. Pharmacological inhibition of CIC by BTC mimicked the effect of AeaAST-C, resulting in abolishment of JH III biosynthesis. This inhibitory effect could be superseded by addition of acetate or citrate, reinforcing the idea that the CIC carrier is a main target for AeaAST-C.
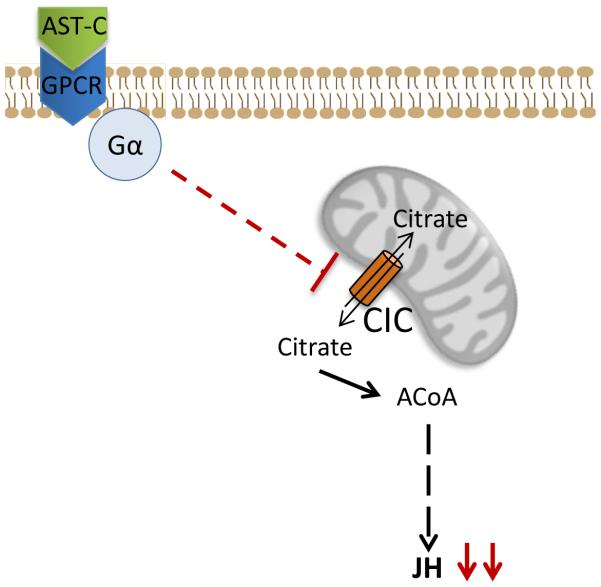
In summary, our results provide compelling evidence that AeaAST-C signaling causes an inactivation of the mitochondrial citrate transporter protein (CIC) and thus blocks transport of citrate to the cytosol. Citrate is the main source of cytoplasmic Acetyl-CoA that sustains synthesis of JH III and its lack causes a fast decrease of JH III. AST-C signaling to the corpora allata is therefore a fast and reversible manner to tune JH III synthesis during female mosquito resting stage according to its variable nutritional status (Fig. 8).
Fig. 8. Schematic representation of the AeaAST-C mechanism of action.
Binding of AeaAST-C to the AST-C receptor results in the inactivation of the CIC carrier, which shuttles the citrate from mitochondria to the cytosol. Decrease of cytoplasmic citrate concentration reduces JH synthesis. GPCR: G-protein coupled receptor; Gα: α subunit; CIC: Mitochondrial citrate transporter. ACoA: acetyl CoA.
Allatostatin-C (AST-C) is a strong and fast reversible inhibitor of JH synthesis in mosquitoes.
AST-C has no effect on the activity of JH biosynthetic enzymes.
The target of AST-C is located before the entry of acetyl-CoA in the pathway.
AST-C blocks the CIC carrier that transports citrate from the mitochondria to the cytosol.
Acknowledgments
This work was supported by NIH Grant No AI 45545 to F.G.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belles X, Martin D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Entomol. 2005;50:181–199. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- Bendena WG, Donly BC, Tobe SS. Allatostatins: a growing family of neuropeptides with structural and functional diversity. Ann. NY. Acad. Sci. 1999;897:311–329. doi: 10.1111/j.1749-6632.1999.tb07902.x. [DOI] [PubMed] [Google Scholar]
- Birgül N, Weise C, Kreienkamp HJ, Richter D. Reverse physiology in Drosophila: identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to mammalian somatostatin/galanin/opioid receptor family. EMBO J. 1999;18:5892–5900. doi: 10.1093/emboj/18.21.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyereisen R, Rueg RP, Tobe SS. Juvenile hormone-III biosynthesis-stoichiometric incorporation of [2-C-14] acetate and effects of exogenous farnesol and farnesoic acid. Insect Biochem. Mol. Biol. 1984;14:657–661. [Google Scholar]
- Gäde G, Goldsworthy GJ. Insect peptide hormones: a selective review of their physiology and potential application for pest control. Pest Manag. Sci. 2003;59:1063–75. doi: 10.1002/ps.755. [DOI] [PubMed] [Google Scholar]
- Gilbert LI, Granger NA, Roe RM. The juvenile hormones: historical facts and speculations on future research directions. Insect Biochem. Mol. Biol. 2000;30:617–644. doi: 10.1016/s0965-1748(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Cusson M. The juvenile hormones. In: Gilbert LI, editor. Insect Endocrinology. Elsevier; 2012. pp. 310–365. [Google Scholar]
- Hernandez-Martinez S, Li Y, Lanz-Mendoza H, Rodriguez MH, Noriega FG. Immunostaining for allatotropin and allatostatin–A and –C in the mosquitoes Aedes aegypti and Anopheles albimanus. Cell Tissue Res. 2005;321:105–113. doi: 10.1007/s00441-005-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Marchal E, Hult EF, Zels S, Vanden Broeck J, Tobe SS. Mode of action of allatostatins in the regulation of juvenile hormone biosynthesis in the cockroach, Diploptera punctata. Insect Biochem. Mol. Biol. 2014;54:61–68. doi: 10.1016/j.ibmb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Kaplan RS, Mayor JA. Structure, function and regulation of the tricaboxylate transport protein from rat liver mitochondria. J. Bioenerg. Biomembr. 1993;25:503–514. doi: 10.1007/BF01108407. [DOI] [PubMed] [Google Scholar]
- Kramer SJ, Toschi A, Miller CA, Kataoka H, Quistad GB, Li JP, Carney RL, Schooley DA. Identification of an allatostatin from the tobacco hornworm Manduca sexta. Proc. Natl. Acad. Sci. USA. 1991;88:9458–62. doi: 10.1073/pnas.88.21.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienkamp HJ, Larusson HJ, Witte I, Roeder T, Birgul N, Honck HH, Harder S, Ellinghausen G, Buck F, Ritcher D. Functional annotation of two orphan G-protein-coupled receptors, Drostar1 and –2, from Drosophila melanogaster and their ligands by reverse pharmacology. J. Biol. Chem. 2002;42:39937–39943. doi: 10.1074/jbc.M206931200. [DOI] [PubMed] [Google Scholar]
- Li YP, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochem. Mol. Biol. 2003;33:1307–1315. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Hernandez-Martinez S, Noriega FG. Inhibition of juvenile hormone biosynthesis in mosquitoes: effect of allatostatic head factors, PISCF- and YXFGL-amide-allatostatins. Reg. Peptides. 2004;118:175–182. doi: 10.1016/j.regpep.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Hernandez-Martinez S, Fernandez F, Mayoral JG, Topalis P, Priestap H, Perez M, Navare A, Noriega FG. Biochemical, molecular, and functional characterization of PISCF- allatostatin, a regulator of juvenile hormone biosynthesis in the mosquito Aedes aegytpi. J. Biol. Chem. 2006;45:34048–34055. doi: 10.1074/jbc.M606341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MW, Hoffmann KH. Allatotropic activity in the subesophageal ganglia of crickets, Gryllus bimaculatus and Achaeta domesticus (Enisfera, Grullidae) J. Insect Physiol. 1995;41:191–196. [Google Scholar]
- Mayoral JG, Nouzova M, Brockhoff A, Goodwin M, Hernandez-Martinez S, Ritcher D, Meyerhof W, Noriega FG. Allatostatin-C receptors in mosquitoes. Peptides. 2010;31:442–450. doi: 10.1016/j.peptides.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A comparison of three methods for isolating RNA from mosquitoes. Insect Mol. Biol. 1993;2:21–24. doi: 10.1111/j.1365-2583.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Ribeiro JMC, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R. Comparative genomics of insect juvenile hormone biosynthesis. Insect Biochem. Mol. Biol. 2006;36:366–374. doi: 10.1016/j.ibmb.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouzova M, Edwards MJ, Mayoral JG, Noriega FG. A coordinated expression of biosynthetic enzyme controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochem. Mol. Biol. 2011;41:660–669. doi: 10.1016/j.ibmb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouzova M, Brochoff A, Mayoral JG, Goodwin M, Meyerhof W, Noriega FG. Functional characterization of an allatotropin receptor expressed in the corpora allata of mosquitoes. Peptides. 2012;34:2001–208. doi: 10.1016/j.peptides.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Noriega FG. A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. PLOS One. 2012;7:e43784. doi: 10.1371/journal.pone.0043784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Lamboglia I, Noriega FG. Metabolomics reveals changes in the mevalonate and juvenile hormone synthesis pathways. Insect Biochem. Mol. Biol. 2014;51:1–9. doi: 10.1016/j.ibmb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharrer B. Neurosecretion. XI. The effects of nerve section on the intercerebralis-cardiacum-allatum system of the insect Leucophaea maderae. Biol. Bull. Woods Hole. 1952;102:261–72. [Google Scholar]
- Stay B, Tobe SS. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu. Rev. Entomol. 2007;52:277–299. doi: 10.1146/annurev.ento.51.110104.151050. [DOI] [PubMed] [Google Scholar]
- Sutherland TD, Feyereisen R. Target of cockroach allatostatin in the pathway of juvenile hormone biosynthesis. Mol. Cell Endocrinol. 1996;120:115–123. doi: 10.1016/0303-7207(96)03825-7. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Zhang JR, Bowser PRF, Donly BC, Bendena WG. Biological activities of the allatostatin family of peptides in the cockroach, Diploptera punctata, and potential interaction with receptors. J. Insect Physiol. 2000;46:231–42. doi: 10.1016/s0022-1910(99)00175-4. [DOI] [PubMed] [Google Scholar]
- Tuteja N. Signaling through G protein coupled receptors. Plant Signal. Behav. 2009;10:942–947. doi: 10.4161/psb.4.10.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Shinoda T, Hiruma K. Spatial expression of the mevalonate enzymes involved in juvenile hormone biosynthesis in the corpora allata in Bombyx mori. J. Insect Physiol. 2009;55:798–980. doi: 10.1016/j.jinsphys.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Weaver RJ, Audsley N. Neuropeptide regulators of juvenile hormone synthesis. Ann. NY. Acad. Sci. 2009;1163:316–329. doi: 10.1111/j.1749-6632.2009.04459.x. [DOI] [PubMed] [Google Scholar]
- Weaver RJ, Edwards JP, Bendena WG, Tobe SS. Structures, functions and occurrences of insect allatostatic peptides. In Recent Advances in Arthropod Endocrinology. In: Coast GM, Webster SG, editors. Soc. Exp. Biol. Seminar Ser. Cambridge University Press; Cambridge: 1998. 65. pp. 3–32. [Google Scholar]
- Woodhead AP, Stay B, Seidel SL, Khan MA, Tobe SS. Primary structure of four allatostatins: neuropeptide inhibitors of juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA. 1989;86:5997–6001. doi: 10.1073/pnas.86.15.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi KJ, Kwok R, Chan KK, Setter RR, Myles TG, Tobe SS, Stay B. Phe-Gly-Leu-amide allatostatin in the termite Reticulitermes flavipes: content in brain and corpus allatum and effect on juvenile hormone synthesis. J. Insect Physiol. 2005;51:357–65. doi: 10.1016/j.jinsphys.2004.12.006. [DOI] [PubMed] [Google Scholar]