Abstract
The relationships between telomerase and telomeres represent attractive targets for new anti-cancer agents. Here, we report that the nucleoside analogue 6-thio-2’-deoxyguanosine (6-thio-dG) is recognized by telomerase and is incorporated into de novo synthesized telomeres. This results in modified telomeres, leading to telomere dysfunction, but only in cells expressing telomerase. 6-thio-dG, but not 6-thioguanine, induces telomere dysfunction in telomerase positive human cancer cells and hTERT expressing human fibroblasts, but not in telomerase negative cells. Treatment with 6-thio-dG resulted in rapid cell death for the vast majority of the cancer cell lines tested, whereas normal human fibroblasts and human colonic epithelial cells were largely unaffected. In A549 lung cancer cell-based mouse xenograft studies, 6-thio-dG caused a decrease of the tumor growth rate, superior to that observed with 6-thioguanine treatment. Additionally, 6-thio-dG increased telomere dysfunction in tumor cells in vivo. These results indicate that 6-thio-dG may provide a new telomere-addressed telomerase-dependent anti-cancer approach.
Keywords: cancer, telomere shortening, telomere induced foci, 6-thioguanine
Introduction
Telomeres, which are found at the end of eukaryotic linear chromosomes, are essential for chromosome maintenance and genomic stability (1). Mammalian telomeres are composed of repetitive d-(TTAGGG) sequences and telomere-specific “shelterin” complex proteins, that protect the chromosome ends from being recognized as DNA damage and preventing end-to-end chromosomal fusions (2). The shelterin proteins, (TRF1, TRF2, POT1, TIN2, TPP1 and Rap1), form a protective complex that is present at telomeres throughout the cell cycle (3). Due to the “end replication problem”, oxidative damage and other replication associated end processing events, telomeres progressively shorten with each round of DNA replication in normal somatic cells (4). The ribonucleoprotein enzyme complex, termed telomerase, counteracts telomere shortening by adding hexameric telomeric DNA (TTAGGG) repeats to the end of linear chromosomes in cancer cells but only partially counteracts progressive telomere shortening in some normal human proliferative stem-like cells. Telomerase has two main functional components: a protein component hTERT (human telomerase reverse transcriptase) and a functional RNA component hTR or hTERC (contains the telomerase template sequences, which facilitates the correct synthesis of TTAGGG repeats). While most normal somatic human cells do not have telomerase activity, it is almost universally detected in ~ 85–90% of primary human cancers (5, 6). It is widely accepted that progressive telomere shortening in normal cells leads to replicative senescence that provides an initial barrier against tumor progression (7, 8). Many previous studies have focused on targeting telomerase activity in cancer cells (9, 10), with the rationale being that telomerase addressed therapies might have low general toxicity, high tumor specificity and reduced side effects as compared to other therapeutic approaches (11).
One of the leading telomerase inhibitors GRN163L (Imetelstat sodium) is a 13-mer thio-phosphoramidate oligonucleotide with the following sequence: 5′-TAGGGTTAGACAA-3′. The compound is complementary to the template region of the telomerase RNA subunit, hTR, and it is a highly potent, direct and competitive telomerase inhibitor (12). Since Imetelstat treatment causes progressive telomere shortening due to telomerase inhibition (9, 13–15), it has been stated that it generally requires relatively prolonged treatment periods to induce therapeutically relevant tumor reduction effects (15). During this treatment period, most tumor cells will continue to grow until already short telomeres become even shorter, and the cells eventually undergo apoptosis or growth arrest. Therefore, Imetelstat treatment without any adjuvant therapy may be limited in its utility as a broad-spectrum anti-tumor therapy due to the prolonged lag period required to have effects. In addition, the development of Imetelstat, an oligonucleotide-based telomerase inhibitor, has been put on hold for solid tumors due to hematological and hepatotoxic dose limited side effects (16, 17). Thus, developing additional telomerase-dependent therapeutic approaches are not only timely but are critically needed to target cancer cell telomeres via novel mechanisms.
Dysfunctional telomeres are associated with DNA damage response factors such as 53BP1, gamma-H2AX, Rad17, ATM and Mre11 (18). When the shelterin protein TRF2 is compromised, telomeres become dysfunctional and display DNA damage signals that can be detected using immunofluorescence imaging techniques. These telomere associated DNA damage signals are referred to as Telomere dysfunction-Induced Foci (TIFs). TIFs can be visualized by co-localization of telomeres with DNA damage response factors. Critically short telomeres, or impaired telomere protective proteins in the shelterin complex can lead to “uncapped” telomere structures, which in turn can induce rapid senescence, apoptosis and/or chromosome end fusions (18–20).
Thiopurines, such as 6-thioguanine and 6-mercaptopurine are currently used as anti-inflammatory, anticancer (for leukemia) and immunosuppressive agents in clinical practice (21). Thiopurine metabolism is complex and involves both activation and inactivation reactions (22). In activation reactions, 6-thioguanine is converted to 6-thioguanosine monophosphate by the hypoxanthine guanine phosphoribosyl transferase (HPRT) enzyme. Then, 6-thioguanosine monophosphate is further metabolized to 6-thio-2’-deoxyguanosine 5’-triphosphate by kinases and RNA reductases, which eventually may be incorporated into DNA strands during DNA replication. DNA-incorporated 6-thioguanine may also generate reactive oxygen species (21, 23), which may cause additional damage to DNA, proteins and other cellular macromolecules, and thus block cellular replication (21). Although the thiopurines are in clinical use for the treatment of some types of leukemia, their utility for solid tumor treatment has been limited in part due to increased toxicities and the development of other therapies.
We reasoned that it may be possible to utilize telomerase by itself as a key functional intermediary for anti-cancer effects, and by doing this, to decrease general non-specific thiopurine toxicity by using 6-thioguanine containing prodrugs (23). Since telomerase has a high affinity for guanine-bases containing 2’-deoxyguanosine 5’-triphosphate, and also for DNA substrates with –GGG motifs at the 3’–terminus (such as the repetitive TTAGGG repeats in telomeres), we designed an analogue of 6-thioguanine that would be preferentially recognized by telomerase, become incorporated into de novo synthesized telomeres by telomerase, and lead to a relatively rapid uncapping of telomeres, resulting in TIF formation and cancer cell growth arrest or death. This may be described as a telomerase-mediated telomere-poisoning approach. Others have suggested that telomerase may recognize 6-thio-2’-deoxyguanosine 5’-triphosphate, and this molecule may be incorporated into oligonucleotide primer extension products in cell free biochemical assays (24), but this observation has never been experimentally tested in vitro or in vivo in cancer cells or other telomerase-positive cells. We hypothesized that a key nucleoside precursor of 6-thio-2’-deoxyguanosine 5’-triphosphate, 6-thio-2’deoxyguanosine, may be less toxic and rapidly converted to the 6-thio-2’deoxyguanosine 5’-triphosphate in cells. Thus, in cells expressing telomerase, 6-thio-2’deoxyguanosine 5’-triphosphate should be incorporated into de novo extended telomeric products, leading to TIF formation. This would make the telomeres structurally and functionally different from native telomeres, since some guanine bases within -GGG- telomeric repeats will be replaced by 6-thio groups. These guanine-base modified telomeres, with 6-thio-groups replacing 6-oxygen counterparts, while being synthesized by telomerase, would result in alteration of the overall chemistry, structure and function of the shelterin complex, (such as G-quadruplex forming properties and protein recognition) (25), leading to their recognition as telomeric DNA damage signals, but almost exclusively in cells expressing telomerase.
In this study, we evaluated 6-thio-2’-deoxyguanosine (6-thio-dG) to determine its therapeutic effects and also general toxicity in cancer and normal cells in vitro and in vivo. The activity and telomere-targeting properties of 6-thio-dG were compared with 6-thioguanine. Our results provide an experimental rational for a potentially new cancer treatment approach based on the administration of 6-thio-dG. The approach represents a different paradigm for treatment of telomerase positive human cancers, irrespective of their telomere length, based on a dual mechanism of action; acute cytotoxicity derived from 6-thio-dG anti-metabolic properties and its incorporation into genomic DNA, accompanied by telomeric DNA incorporation, modification and telomere “uncapping” reducing the “lag” time for efficacy. The chemical structures of 6-thioguanine and 6-thio-2’-deoxyguanosine are shown in Fig. 1A.
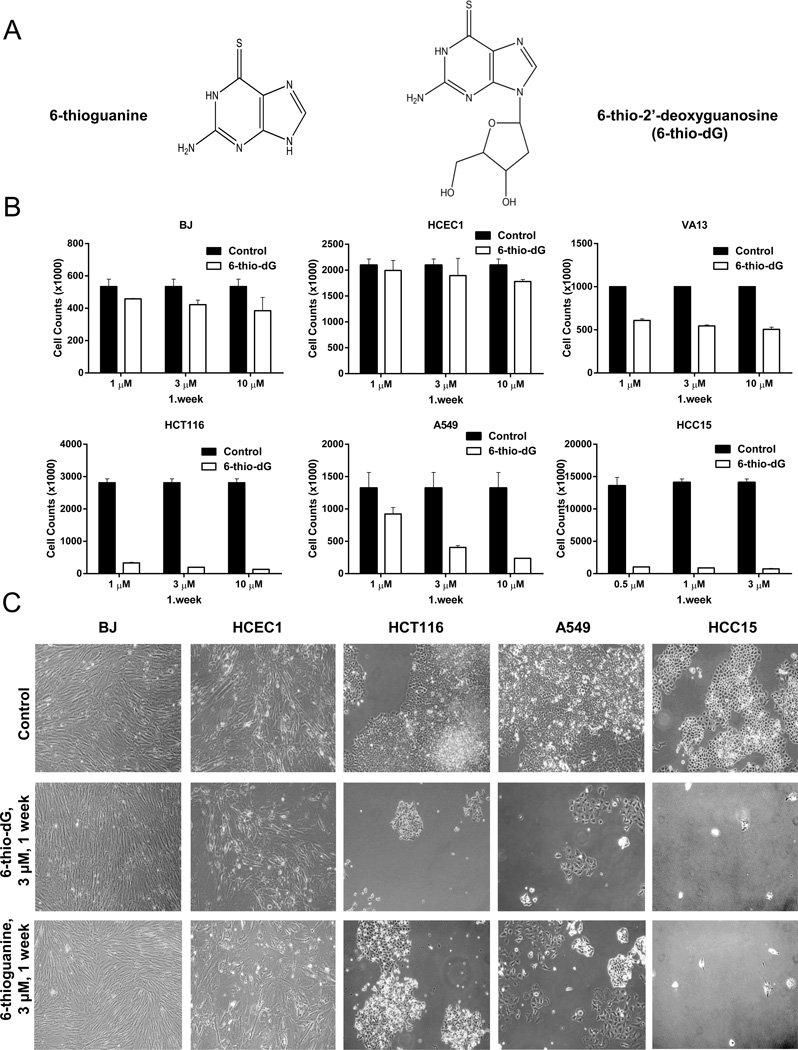
Figure 1.
(1A) The chemical structures of 6-thioguanine and 6-thio-2’deoxyguanosine (6-thio-dG). (1B) Cell counts of BJ (normal human fibroblasts), HCEC1 (human diploid colonic epithelial cells), VA13 (telomerase negative, ALT cells), HCT116, A549, and HCC15 telomerase positive cells treated with 6-thio-dG (0.5–10 µM) for 1 week. (Control; DMSO treated), (SDs from two independent experiments). (1C) The morphology of BJ, HCEC1, HCT116, A549, and HCC15 cells treated with 6-thio-dG (3µM) and 6-thioguanine (3µM) for 1 week (treatment every three days).
Results
Growth inhibition kinetics of colon cancer, non-small cell lung cancer, ALT cells, and normal human fibroblasts and colonic epithelial cells
HCT116 colon, A549 lung cancer, along with an additional panel of non-small cell lung cancer cell lines (H2882, HCC2429, HCC827, HCC15, H2087, HCC4017, HCC515, H2009), normal human BJ fibroblasts, colonic epithelial cells (HCEC), and ALT cancer cells (telomerase negative cell lines) were treated with 0.5–10µM 6-thio-dG and 6-thioguanine every three days over a one week period. Treatment with 6-thio-dG and 6-thioguanine resulted in death of the vast majority of HCT116, A549, H2882, HCC2429, HCC827, HCC15, H2087, HCC4017, HCC515, and H2009 cells (in the sub-micromolar to low-micromolar range), whereas normal BJ fibroblasts, HCEC1 and ALT cells were less affected (higher micromolar range). The growth inhibition results of representative images of HCT116, A549, HCC15, BJ fibroblasts, HCEC1 and VA13 (ALT) and morphology of HCT116, A549, HCC15, BJ fibroblasts and HCEC1 following 1 week treatment are shown in Fig. 1B and C, respectively.
Effects of 6-thio-dG and 6-thioguanine on cell viability
In order to investigate the effect of 6-thio-dG and 6-thioguanine, we seeded HCT116 cells, 9 NSCLC-derived cell lines, also normal BJ fibroblast cells and HCEC1 in 96-well plates, then treated the cells with different concentrations of 6-thio-dG or 6-thioguanine every three days. Following one week of treatment (using ten different doses of the compounds with serial dilutions), the cell viability was determined by the CellTiterGlo luminescent cell viability assay. We found that both 6-thio-dG and 6-thioguanine inhibited cell viability in a dose dependent manner. The IC50 values of all cells are summarized in Supplemental Table 1 and Supplementary Fig. S1. We observed that the cancer cells were highly sensitive to 6-thio-dG (observed IC50 values are between 0.7–2.9µM, depending on the specific cell type), and 6-thioguanine (IC50 values are between 0.7–3.5µM), whereas BJ cells and HCEC1 were mostly resistant to both drugs in terms of cell viability (Supplementary Fig. S1). In addition, these cell lines had a variety of telomere lengths but cell killing was independent of initial telomere sizes (Supplemental Table 1).
6-thio-dG treatment results in telomere dysfunction
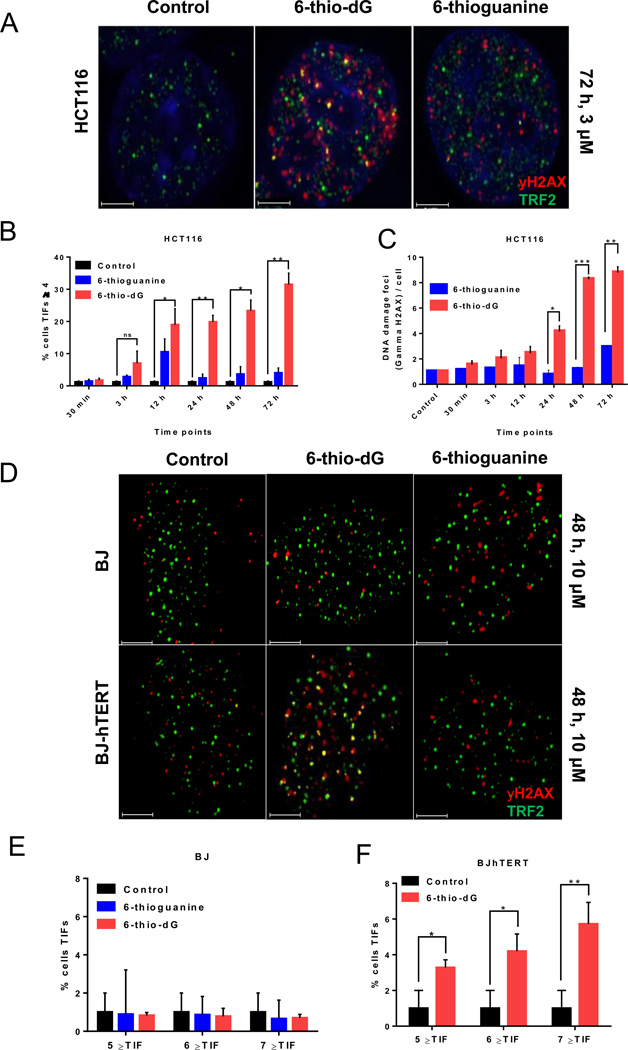
In order to observe whether 6-thio-dG and 6-thioguanine treatment lead to telomere de-protection, HCT116 cells were seeded in chamber slides. Following cell attachment, 6-thio-dG (3µM) and 6-thioguanine (3µM) were either not added (0 time point) to fresh medium or added for various time points of 30 minutes, 3 hours, 12 hours, 24 hours, 48 hours, 72 hours and the cells were then fixed. To test if 6-thio-dG and 6-thioguanine cause telomere dysfunction, interphase TIF (Telomere dysfunctional Induced Foci) analysis was conducted. Using combination of gamma-H2AX and TRF2 co-localization by immuno-staining, we were able to identify and distinguish between non-telomeric genomic DNA damage and telomere-specific damage signals. 6-thio-dG treatment induces a 7.8-fold increase in telomeric DNA damage as compared to 6-thioguanine after 72 hours (Fig 2A, 2B). Since telomeric DNA only represents about 1/6,000th of the total genomic DNA, any TIFs above background, would be considered highly significant. In addition to the increase in telomere damage by 6-thio-dG, there was also an overall modest increase in genomic DNA damage as compared to 6-thioguanine (Fig. 2C). In contrast, telomerase negative normal BJ cells had less genomic and no detectable telomeric damage signals under similar treatment conditions (Fig. 2D, 2E). To test the initial hypothesis that DNA damage induced by 6-thio-dG at telomeres is actually telomerase dependent, we established a hTERT expressing/telomerase activity positive BJ cell line. Telomeric DNA damage signals in this hTERT positive BJ [BJ-hTERT(+)[ cells were compared to that in normal BJ cells [BJ-hTERT(−)], both treated with 6-thio-dG (10µM) or 6-thioguanine (10µM). Following 48 hours treatment, TIF analysis confirmed that in interphase cells gamma-H2AX and TRF2 co-localize in 3.3% (TIF≥5), 4.2% (TIF≥6) or 5.7% (TIF≥7) of cells following 6-thio-dG treatment in BJ hTERT(+) cells, compared to background in telomerase activity negative BJ cells and 6 thioguanine BJ hTERT(+) (Fig. 2D–F).
Figure 2.
DNA damage (gamma-H2AX) and telomere damage induced foci (TIFs). (2A) 6-thio-dG induced telomeric localization of gamma-H2AX in HCT116 cells (with 3µM treatment), but not 6-thioguanine (with 3µM treatment). (2B) TIF index (percentage of TIF positive cells) of HCT116 cells treated with 6-thio-dG (3µM) or 6-thioguanine (3µM). Cells with four or more gamma-H2AX foci co-localizing with TRF2 were scored as TIF positive by Imaris software (n=55 for HCT116, SDs from two independent experiments). *P<0.05, **P=0.006 (compared with vehicle control), ns, not significant differences in the unpaired Student t test. (Control; untreated). (2C) DNA damage foci per cell. HCT116 cells treated with 6-thio-dG (3µM) and 6-thioguanine (3µM) (n=55, SDs from two independent experiments). **P=0.003, ***P=0.0005, *P=0.0141 (6-thio-dG:6-thioguanine), ns, not significant differences in the unpaired Student t test. (Control; DMSO treated). (2D–F) Representative images (2D) and quantitative TIF analysis following 6-thio-dG (10µM) and 6-thioguanine (10µM) treatment in BJ-hTERT- (2E) and for 6-thio-dG in BJ-hTERT+ cells (2F) are shown. 6-thio-dG induced telomeric localization of gamma-H2AX in BJ-hTERT+ cells, but not in BJ-hTERT- cells. 6-thioguanine did not significantly induce telomeric localization of gamma-H2AX in BJ-hTERT(+) and BJ-hTERT(−) cells [n=85 for control, n=83 for 6-thio-dG BJ-hTERT- and n=81 for 6-thioguanine treated BJ-hTERT(−) experiments, SDs are from two independent experiments for BJ-hTERT(−) and three independent experiments for BJ-hTERT(+) cells]. Images were obtained by DeltaVision and then deconvoluted by Autoquant X3. DNA was stained with DAPI (blue). Red dots show DNA damage (gamma-H2AX), green dots show TRF2 and yellow dots show TIF (DNA damage co-localizing with telomeres) in merged images. *P=0.02, **P=0.064 (control:6-thio-dG) in the unpaired Student t test.
Treatment with 6-thio-dG, but not 6-thioguanine, results in progressive telomere shortening in cancer cells
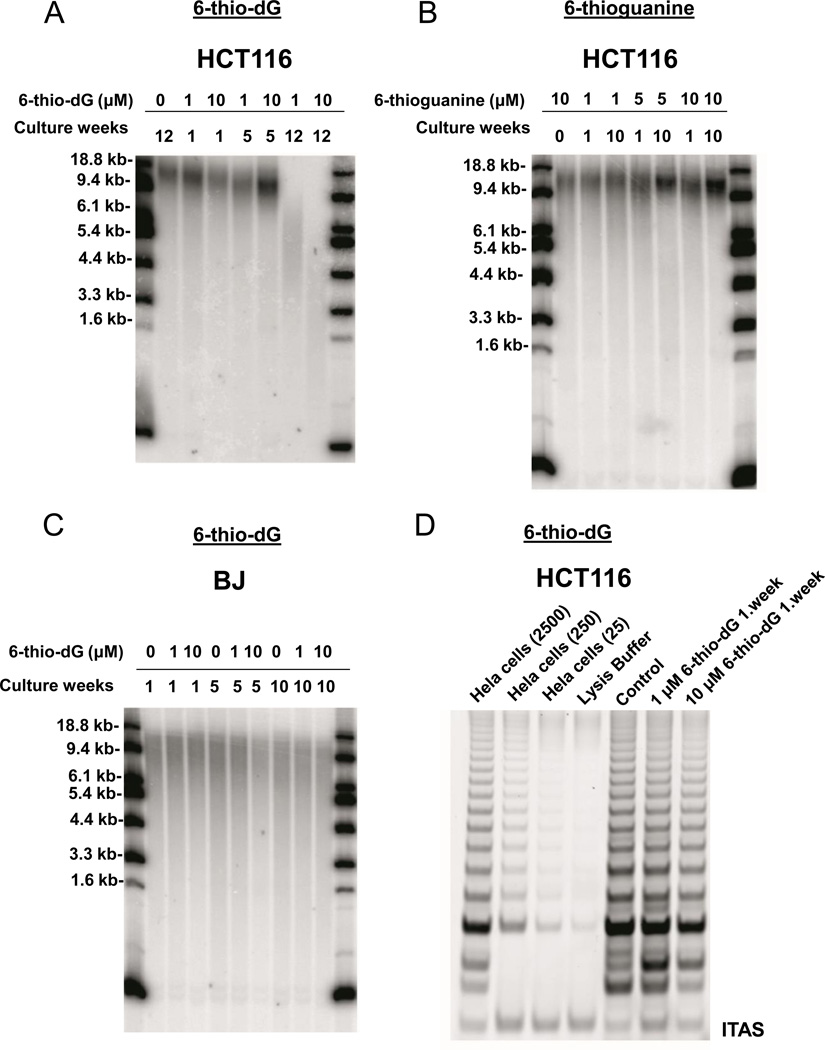
To determine if administration of 6-thio-dG leads to progressive telomere shortening, in addition to the observed acute TIF induction, telomere lengths of the treated cells were evaluated by the TRF (terminal restriction fragment) assay. Colon cancer derived HCT116 cells and normal BJ fibroblasts were treated with either 1µM or 10µM 6-thio-dG for 1 to 12 weeks. In addition, HCT116 cells were also treated with either 1µM or 10µM 6-thioguanine for 1–10 weeks to determine whether it has effects on telomere length maintenance. We found that telomere shortening was detectable as early as in one week in surviving cells, with more dramatic telomeric shortening after 12 weeks of continuous treatment with 6-thio-dG (Fig. 3A). However, administration of 6-thioguanine did not result in any significant effect on telomere length of HCT116 cells (Fig. 3B). This suggests that intracellular metabolic pathways of 6-thio-dG and 6-thioguanine are different, and that 6-thio-dG may be much more readily converted in to the corresponding 5’-triphosphate, which is being recognized by telomerase and incorporated into telomeres. In addition, BJ-hTERT(−) fibroblast cells treated for 10 weeks with 6-thio-dG (Fig. 3C), or 6-thioguanine (data not shown) did not show enhanced telomere shortening, as compared to untreated control cells. When telomerase activity of HCT116 cells treated with 6-thio-dG was evaluated (by the in vitro TRAP assay), no inhibition of telomerase activity was observed for 6-thio-dG (Fig. 3D) or 6-thioguanine (data not shown). This indicates that 6-thio-dG does not directly inhibit the telomerase holoenzyme, but causes progressive telomere shortening in cells that are not immediately killed by 6-thio-dG via a mechanism independent from telomerase inhibition in HCT116 cells.
Figure 3.
TRF (Terminal Restriction Fragment) and TRAP (Telomeric Repeat Amplification Protocol) analysis. (3A, B, C) HCT116 and BJ cells treated with 6-thio-dG or 6-thioguanine. Cells were treated with 1–10µM 6-thio-dG or 6-thioguanine every 3 days for the indicated numbers of weeks. Each week samples were collected for TRF analysis at 1×106 cells/sample. (3D) TRAP analysis of HCT116 cells treated with 6-thio-dG. Cells were treated with 1 and 10µM 6-thio-dG every 3 days for 1 week. The samples were collected for TRAP analysis at 1×105 cells/sample. TRF lengths are expressed as kilobase pairs. (Control; untreated).
Determination of 6-thio-dG general toxicity in vivo
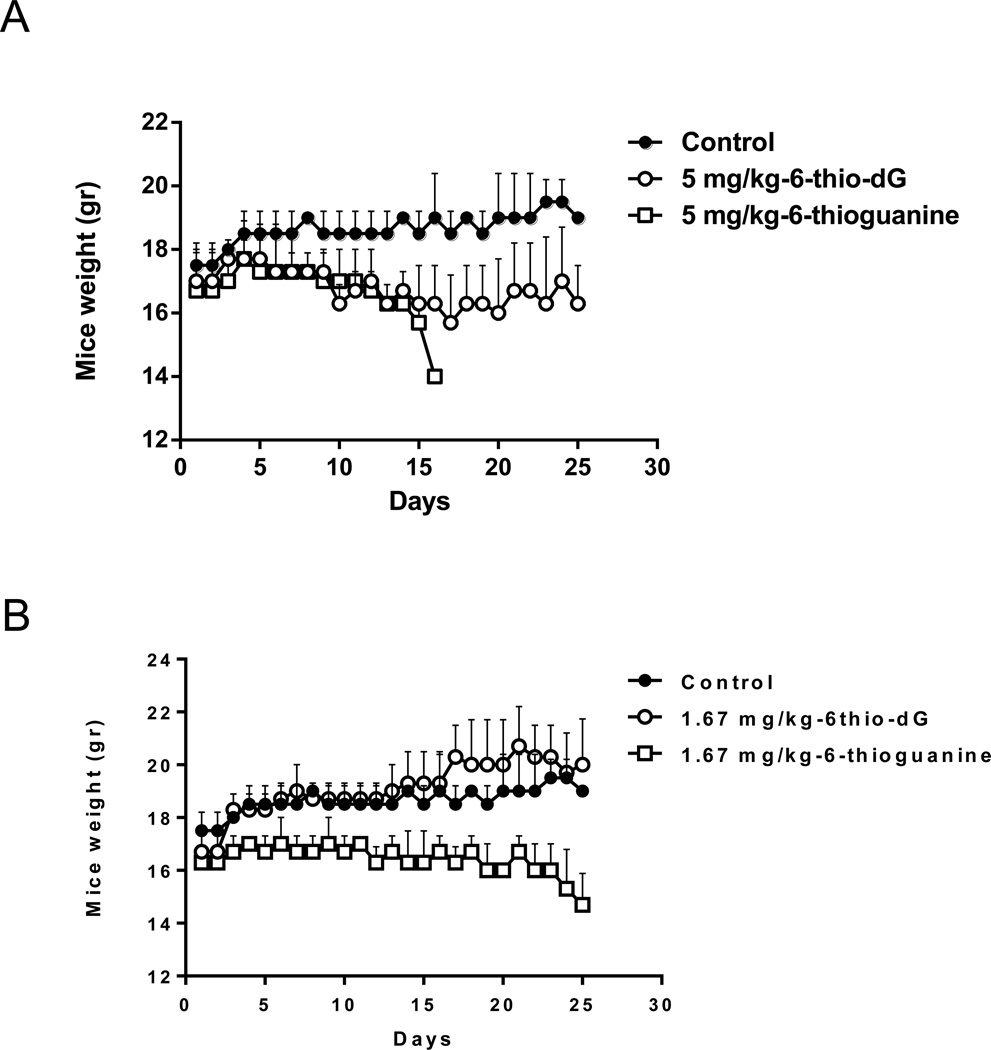
In order to determine an appropriate drug concentration for in vivo efficacy studies, we initiated experiments with 129S2 wild type female mice using different drug concentrations to determine the general toxicity of 6-thio-dG. It was reported that 6-thioguanine doses above 3mg/kg are toxic for mice (26). Based on this report, we chose the highest dose as 5mg/kg (Fig. 4A), and the lowest dose as 1.67mg/kg (Fig 4B), for daily intraperitoneal injections into mice, for 25 days. Following 17 days of treatment, three mice treated with 5mg/kg of 6-thioguanine died. The mice treated with 1.67mg/kg 6-thioguanine did not gain weight and started to lose weight by 24 days of treatment. In contrast, 5mg/kg of 6-thio-dG treatment did not cause any mice deaths, and also the average animal weight was stable over the 25 days course of treatment. Moreover, we did not observe any animal deaths or weight loss in the lower dose group (1.67mg/kg) of 6-thio-dG treated mice (Fig. 4 A , B). Based on these in vivo dose-defining results, we initiated mice xenograft efficacy experiments with 2mg/kg 6-thio-dG dose.
Figure 4.
The wild type mice weight following 6-thio-dG and 6-thioguanine treatment. (4A) 5mg/kg 6-thio-dG (compared with DMSO/PBS or 5mg/kg 6-thioguanine), (4B) 1.67mg/kg 6-thio-dG (compared with DMSO/PBS or 1.67mg/kg 6-thioguanine) was injected i.p. into the 129S female wild type mice for 25 days. The mice weight (gram: gr) were scaled every day (n=3).
Evaluation of 6-thio-dG effect on hematological counts, liver and kidney function tests and organ histological analysis
To determine if there are toxicity difference between 6-thio-dG and 6-thioguanine beyond weight loss, wildtype female (129S2) mice were injected intraperitoneally every two days at a dose of 2mg/kg 6-thio-dG and 6-thioguanine for a total of 14 days. Two days after the last injection, mice were sacrificed and whole blood was collected via cardiac draw. Red blood cell (RBC), white blood cell counts (WBC), hemoglobin (HGB), lymphocytes, hematocrit (HCT), monocytes, neutrophils, and basophils were evaluated. In addition, alanine transaminase (ALT)/aspartate transaminase (AST) for liver function, creatinine (CREA)/urea nitrogen (BUN) for kidney function were evaluated in serum. 6-thio-dG and 6-thioguanine did not cause any hematologic toxic effects on ALT, BUN, CREA levels except minor neutropenia (Supplementary Fig S2). Interestingly, while 6-thio-dG did not alter AST levels, 6-thioguanine (the already approved compound) caused a slight increase of AST enzyme levels, which is one of the markers to show toxic effect on liver function, yet this increase was not statistically significant. Additionally, we evaluated the histopathology of the liver, kidney, spleen and colon and did not observe any toxic effect with 6-thio-dG treatment compared to control (Supplementary Fig. S3). We did observe some necrosis in the liver after 6-thioguanine treatment (data not shown).
6-thio-dG treatment decreases the tumorigenicity of A549 cells in vivo
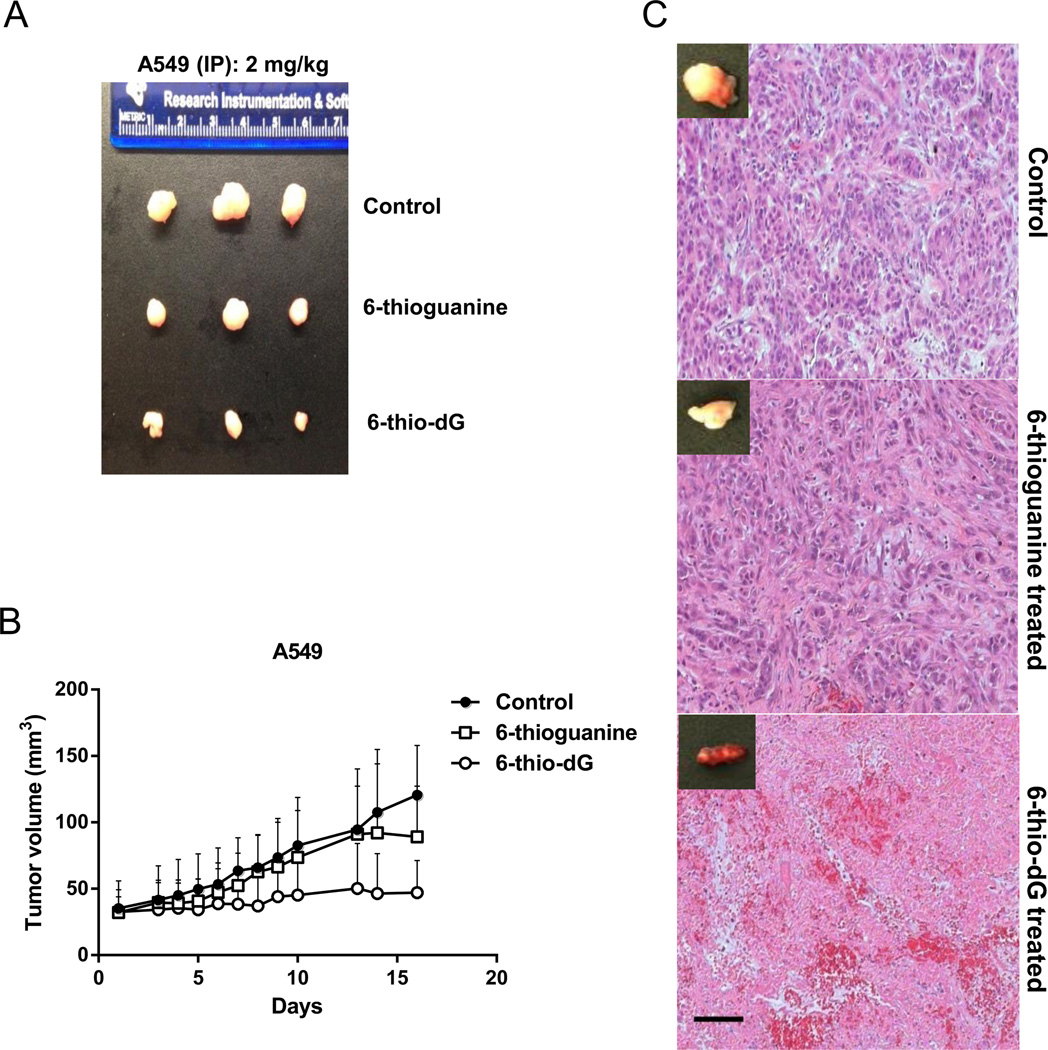
In order to assess anticancer activity of 6-thio-dG and 6-thioguanine in vivo, we used A549 lung cancer cell line-derived xenograft murine model. During the in vivo treatment phase of the experiments, 2mg/kg of 6-thio-dG or 6-thioguanine, (approximately 7µM/kg or 12µM/kg, respectively), were injected intraperitoneally every other day. Tumors were first established in mice and then treatments were initiated. We observed a complete prevention of progressive tumor growth by administration of 6-thio-dG, as compared to controls or 6-thioguanine treated animal groups (Fig. 5A). We also evaluated Ki67 staining in these tumor samples treated with 6-thio-dG and 6-thioguanine compared to controls. Ki67 is a biomarker that correlates with proliferation levels. We observed that 6-thio-dG treatment caused decrease in Ki67 staining compared to 6-thioguanine and controls, which may in part explain the difference of tumor growth rate using similar doses of 6-thio-dG and 6-thioguanine (Supplementary Fig. S4). To test whether local injection into the tumor was more effective in comparison with IP injection, we injected intratumorally (IT) 2.5mg/kg of 6-thio-dG or 6-thioguanine daily for 17 days. 6-thio-dG tumor growth rate showed even more dramatic decreases compared to 6-thioguanine or untreated controls (Fig. 5B). Importantly, residual small tumors from the 6-thio-dG treated mice were generally highly vascularized with infiltrating inflammatory cells (Fig. 5C).
Figure 5.
Intraperitoneally or intratumorally injection of 6-thio-dG or 6-thioguanine (5A) 2mg/kg 6-thio-dG or 6-thioguanine injected to the A549-derived tumor for 17 days every two days, intraperitoneally. Representative images of isolated tumors are shown. (5B) The tumor volume (mm3) after 2.5mg/kg 6-thio-dG or 6-thioguanine injection intratumoral (IT) every day for 17 days. Tumor size was measured by calipers and recorded either every day or every two days. Tumor volumes were calculated by taking length to be the longest diameter across the tumor and width to be the corresponding perpendicular diameter, using the following formula: (length × width2) mm2× 0.5. (5C) The images of with hematoxylin and eosin at the end of intratumoral injection (IT). (Control: DMSO/PBS). Note the increased inflammatory and RBCs infiltrating in the 6-thio-dG residual tumor mass at sacrifice (17 days). Magnifications 10×, scale bar: 100-µm.
6-thio-dG mediated tumor growth rate reduction correlates with telomere dysfunction in vivo
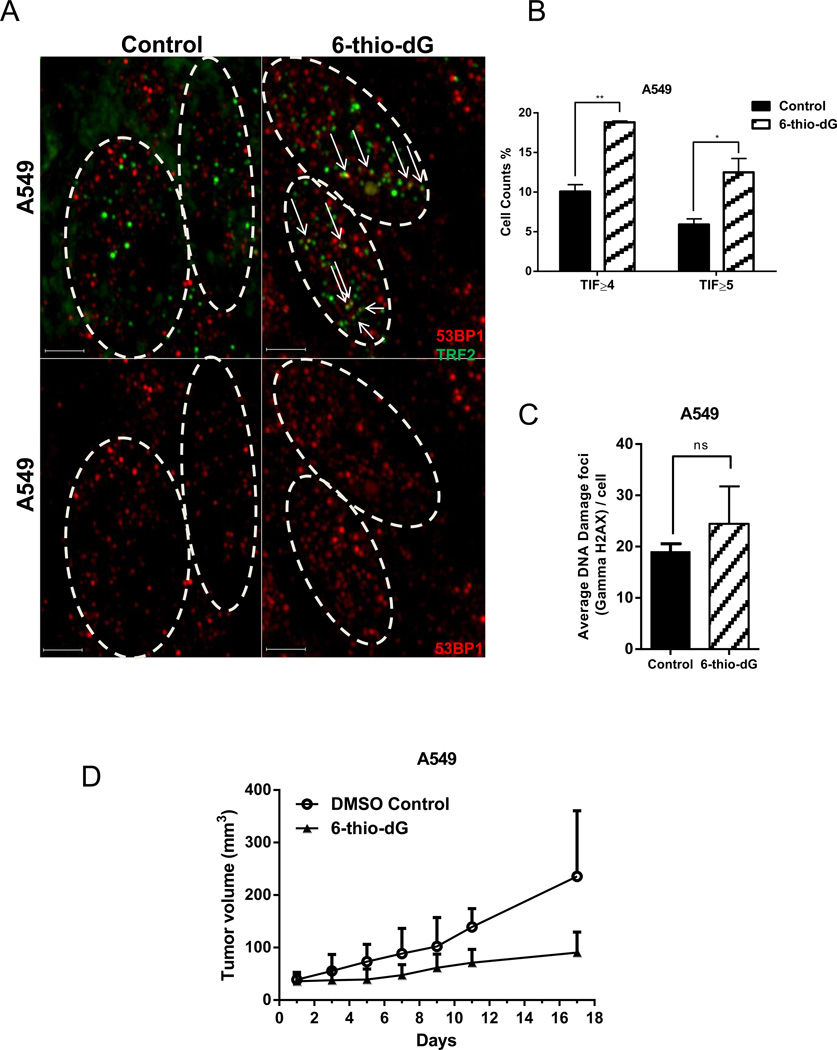
To test whether 6-thio-dG causes telomere dysfunction in tumor cells, 2mg/kg 6-thio-dG was injected intraperitoneally every other day for 17 days. Tumor cells were then scored for TIFs. 6-thio-dG caused a significant ~2-fold induction in TIFs compare to control (when counting positive cells as those that have TIF≥4 and TIF≥5), demonstrating that 6-thio-dG alters the telomere structure and function in A549-derived tumors in vivo (Fig. 6A and 6B). Additionally, there was no significant difference in ongoing general DNA damage between 6-thio-dG treated group and control tumor cells (Fig. 6C). Overall, we observe reduction of tumor growth rate by 6-thio-dG (Fig. 6D) correlates with telomere dysfunction in vivo.
Figure 6.
Binding of 53BP1 on uncapped telomeres. (6A) 6-thio-dG induced telomeric localization of 53BP1 in A549-derived tumor cells (representative data). Images were obtained by DeltaVision and then deconvoluted by Autoquant X3. Red dots show DNA damage (53BP1), green dots show telomeres and yellow dots show TIF (DNA damage on telomeres) in merged images. Scale bars: 3-µm (6B) TIF index (percentage of TIF positive cells) of A549-derived tumor cells treated with 6-thio-dG. Cells with ≥4 or ≥5 53BP1 foci co-localizing with telomere were scored as TIF positive by Imaris software (n=150 for control, n=200 for 6-thio-dG treatment). SDs from two independent experiments). **P=0.0051, *P<0.05 (compared with control), in the unpaired Student t test. (6C) DNA damage foci per cell. A549-derived tumor cells treated with 6-thio-dG (n=150 for control, n=200 for 6-thio-dG treatment, SDs from two independent experiments). ns, not significant differences in the unpaired Student t test. (control versus 6-thio-dG), (Control; DMSO treated). (6D) The tumor volume (mm3) of A549-derived tumor with 6-thio-dG administration. 2mg/kg 6-thio-dG or DMSO/PBS injected to the A549-derived tumor for 17 days every two days, intraperitoneally.
Discussion
Purine analogues, such as 6-thioguanine and 6-mercaptopurine, are chemotherapeutic agents that can be converted into nucleotide analogues in vivo. Clinically used nucleoside base analogues are nucleoside mono-, di-, and tri- phosphate pro-drug forms and also antimetabolite agents. One of the main problems with these compounds is their relatively high toxicity towards normal non-cancerous cells. Additionally, the therapeutic effectiveness towards the majority of solid tumors is relatively low (27). Thus, 6-thioguanine is not widely used in current cancer treatment protocols, mainly due to the low drug efficacy and dose-limiting toxic side effects (28). In addition, patients with Crohn’s disease or leukemia being treated with 6-thioguanine can develop hepatotoxicity such as liver veno-occlusive disease. Thus, slight increases in AST levels from the mice treated with 6-thioguanine but not 6-thio-dG, may reflect different toxicity profiles and partially explain the increased efficacy of 6-thio-dG over 6-thioguanine in the present experiments. The enzyme hypoxanthine guanine phosphoribosyl transferase (HGPRT) produces 6-thio-2’-ribo-guanosine 5’-monophosphate from 6-thioguanine both in vitro and in vivo. Following this conversion, 6-thio-2’-deoxy-guanosine 5’-triphosphate (6-thio-dGTP) is formed by a series of transformations, catalyzed by kinases and RNA nucleotide reductases. 6-thio-dGTP is then incorporated into de novo synthesized DNA, resulting in DNA damage, followed by cell cycle arrest and apoptosis. Cytotoxicity of 6-thio-dGTP is specific for the S-phase of the cell cycle (29). Moreover, substitution of 2’-deoxyguanosine by 6-thio-2’-deoxyguanosine in G-rich oligodeoxyribonucleotides inhibits the formation of G-tetrad structures in DNA (30). These studies suggest that the 5’-triphosphate form of 6-thio-2’-deoxyguanosine may be able to interact with telomerase, be incorporated into the telomeres, and lead to disruption of telomere structure and function much more effectively compared to 6-thioguanine.
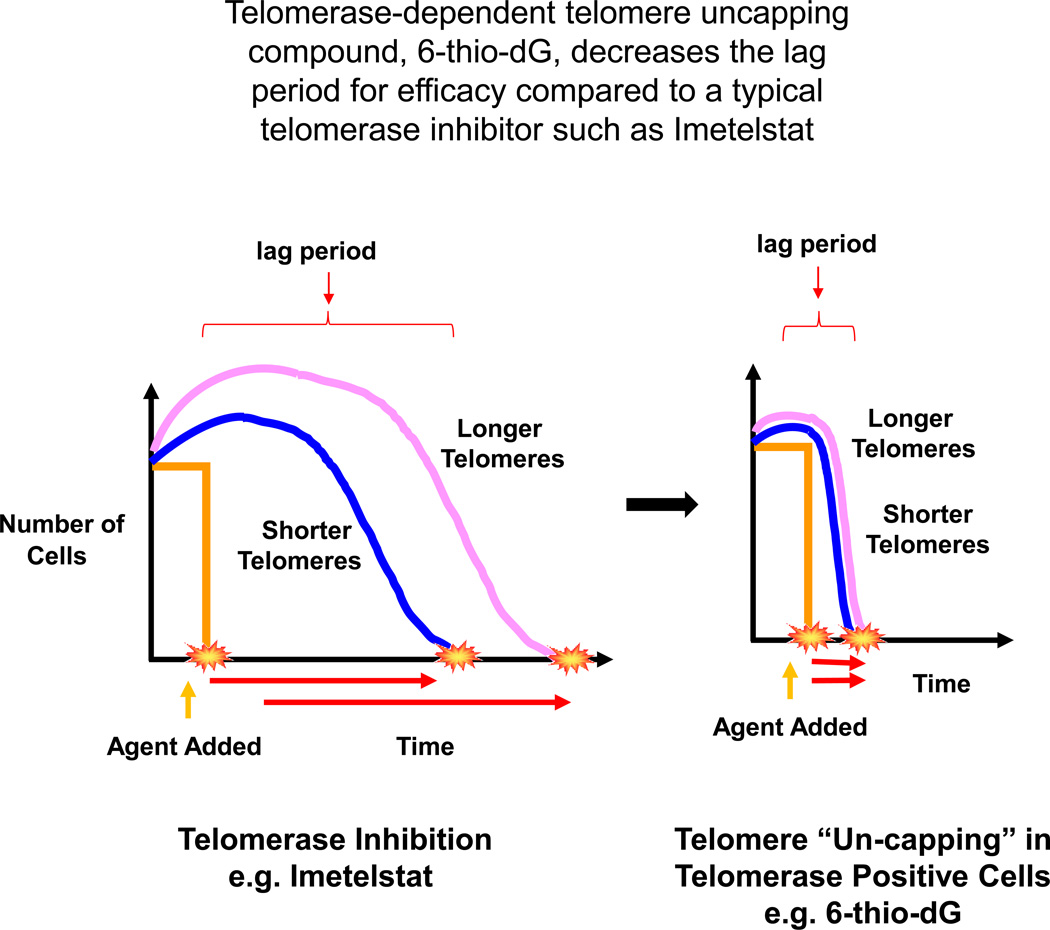
Most of the single agent anticancer therapies have not been very effective in clinical practice yet, largely due to the development of drug resistance, leading to cancer recurrences and metastasis. One of the main limitations for potential clinical success of Imetelstat, a potent telomerase inhibitor, is variable telomere lengths in the tumor cells of the treated patients. This limitation requires a relatively prolonged treatment regimen, resulting in hematologic side-effects. In human clinical trials combining Imetelstat with other chemotherapeutic drugs for solid tumors have been terminated primarily due to the observed toxicities in patients (16, 17). Thus, initial tumor telomere length variability and a substantial “lag period” are the main limitations for telomerase inhibitor-based therapy (Figure 7, left side). Therefore, additional new approaches need to be evaluated, which aim to increase an overall therapeutic efficacy, accompanied by fewer side effects. In the current study, we have focused on the heterocyclic guanine base modified nucleoside analogue −6-thio-2’-deoxyguanosine. In contrast to other telomerase inhibitor based therapies, 6-thio-2’-deoxyguanosine is not a direct telomerase inhibitor per se, but a precursor of the telomerase substrate 6-thio-dGTP. We demonstrated in the current studies that administration of this molecule, both in vitro and in vivo, significantly shortened the lag period (Fig. 7, right side) by causing telomere uncapping, leading to cancer cell death independent of the initial tumor telomere length.
Figure 7.
The efficacy of telomerase inhibition based therapies, such as imetelstat, depends on the tumor telomere length at the beginning of treatment. The tumors with longer telomeres require a longer treatment period (longer lag period) to have therapeutic effects compare to tumors with shorter telomeres (7-left side). Tumor telomeres (with shorter or longer) start gradually shortening with telomerase inhibition targeted agents. This leads to delay of effect (e.g. tumor shrinkage) and potentially generates adverse effects (such as increased toxicities) due to the long treatment period. However, adding a telomerase mediated telomere uncapping agent such as 6-thio-2’deoxyguanosine (6-thio-dG) results in a much quick effect that reduces the lag period irrespective of tumor initial telomere length (7-right side).
In the present study, we found that 6-thio-dGTP, which is formed in cells from 6-thio-dG, is efficiently recognized by telomerase and is incorporated into telomeres. This guanine base-modified nucleoside incorporation led to the marked increased in telomere dysfunction induced foci (TIFs) in HCT116 and BJ hTERT(+) cells, but not in telomerase silent normal BJ hTERT(−) cells, indicating that this effect is mostly telomerase dependent. The incorporation of 6-thio-dG into telomeres and also into genomic DNA shows that the compound may have a bi-functional mode of action, targeting both telomeric and genomic DNA, in addition to acting as a potential nucleoside anti-metabolite. Importantly, at therapeutically relevant doses we did not observe any significant toxicity in normal BJ or HCEC1 cells for 6-thio-dG, demonstrating good selectivity of 6-thio-dG towards telomerase positive cancer cells. We also observed progressive telomere shortening with 6-thio-dG in the remaining or surviving cells not immediately killed by drug treatment, which was not based on telomerase inhibition as demonstrated by in vitro TRAP assay. In addition to the in vitro studies, in vivo studies with wild type mice and in mouse xenografts demonstrated less toxicity (lack of weight loss, no changes in hematological, renal or liver functions except minor neutropenia) and more efficacies with 6-thio-dG in comparison with control or 6-thioguanine treated mice. We believe that these toxicity differences on weight loss may be because of the differences in structure of 6-thioguanine (a heterocyclic base) and 6–thio-dG (a nucleoside). While 6-thioguanine participates in multiple intracellular biochemical and metabolic processes including purine nucleoside biochemical synthesis in cells (21), 6-thio-dG may participates in fewer biochemical and metabolic processes. Also differences in bio-distribution, relative solubility (i.e. 6-thio-dG is more hydrophilic compared to 6-thioguanine), plasma protein binding and major organ accumulation in vivo may also contribute to the observed toxicity differences.
Previous studies show that telomere dysfunction is a hallmark of senescence and in some instances can lead to apoptotic cell death (18, 31). Telomere dysfunction induced senescence or apoptosis can limit tumor growth in animal models (32, 33). DNA replication stress induced telomere dysfunction can prevent progression of cancer in precancerous lesions and limit growth rate in cancers (34). In the present studies we demonstrate that dysfunctional telomeres (TIFs) in 6-thio-dG treated tumor cells (A549-derived) were significantly increased compared with control tumor cells in vivo (A549-derived). This suggests that 6-thio-dG can be incorporated into telomeres (~1/6000th of the genome) during replication and consequently arrest tumor growth in response to telomere dysfunction induced checkpoints. Interestingly, we did not observe any significant difference between control and 6-thio-dG treated A549-derived tumor cells in vivo on general DNA damage, whereas 6-thio-dG increased general DNA damage in HCT116 cells compare to control in vitro. This demonstrates that some cancer cells have ongoing DNA damage signaling in vivo that is being repaired but that 6-thio-dG induced telomere DNA damage that may not be easily repaired.
While effects on telomerase positive stem cells is a potential concern for advancing 6-thio-dG to human clinical trials, pilot in vivo toxicity testing at effective doses did not reveal any significant hematological, renal, or gastrointestinal system rate limiting side effects. While there is likely to be some effects of 6-thio-dG on transient amplifying telomerase positive cells, quiescent stem cells are known to be telomerase silent and unlikely to be affected by 6-thio-dG. Other potential side effects will be further addressed in broader in depth toxicology testing as this molecule is advanced through formal pre-clinical IND-enabling studies. Thus, since 6-thio-dG will be most likely used in clinical trials over a much shorter time period compared to Imetelstat, any side effects observed may be transient. In conclusion, this study provides the first experimental evidence for the bi-functional mechanism of action for the nucleoside analogue 6-thio-dG, targeting both genomic and telomeric DNA and rapid cancer cell death irrespective of the initial cancer telomere length in vitro. Moreover, administration of 6-thio-dG has a profound effect in reducing the tumor growth rate following telomere dysfunction in mouse xenograft cancer models.
These findings provide an initial scientific rationale for the development of new clinical cancer treatment approaches based on targeting telomeres in telomerase positive cancer cells. In the minimal residual disease setting, where cancer relapse is often a predicted outcome, modified nucleoside molecules, such as 6-thio-dG, may prevent or delay the recurrence of the disease. In addition, in the maintenance setting, along with other chemotherapeutic agents, 6-thio-dG may be more efficacious, while offering lower overall toxicity.
Methods
Cell lines
The panel of authenicated non-small lung cancer cell lines (H2882, HCC2429, HCC827, HCC15, H2087, HCC4017, HCC515, H2009) were provided by John D. Minna at the University of Texas Southwestern Medical Center. HCT116 human colon, A549 human non-small cell lung cancer (NSCLC), the panel of additional non-small cell lung cancer cell lines (H2882, HCC2429, HCC827, HCC15, H2087, HCC4017, HCC515, H2009) and BJ human fibroblasts were grown in a Medium X (DMEM:199, 4:1, Hyclone, Logan, UT) supplemented with 10% cosmic calf serum (Hyclone) without antibiotic. HCEC1 cells were cultured in medium consisting of medium X supplemented with 2% cosmic calf serum, and insulin. BJ and HCEC1 cells were cultured at 37°C in low oxygen (2–5%). BJ cells were immortalized by transfection of a retroviral hTERT-TK-hygromycin cassette. Successful hTERT-hygromycin expression was confirmed in clones by testing for hygromycin resistance and the presence of telomerase activity. All NSCLC lines listed above and used in these studies were obtained from the Hamon Cancer Center Collection (University of Texas Southwestern Medical Center, Dallas, TX) and have been DNA fingerprinted using the PowerPlex 1.2 kit (Promega) and are found mycoplasma free using the e-Myco kit (Boca Scientific). All cells were compared to the complete database in our own collection and that of the ATCC. The HCT116 cell line was directly obtained from the ATCC but not re-authenicated by the authors. The BJ and HCEC1 cells were each shown to contain a normal diploid karyotype and found to be mycoplasma free but beyond that not authenticated by the authors.
Drug preparation
6-thio-dG (Metkinen Oy, Kuopio, Finland) and 6-thioguanine (Sigma, St Louis, MO) was dissolved in DMSO/water (1:2) to prepare 50 mM or 10 mM stock solutions, which were kept frozen at –20°C. Once in vitro experiments were conducted, a 1mM final concentration was prepared in serum free medium and added at varying amounts for cell treatments. For mouse in vivo studies, drugs were prepared in a 5% DMSO solution.
Cell Viability Assay
HCT116 (0.5 × 103 cells/well), A549 (0.6 × 103 cells/well), and H2882, HCC2429, HCC827, HCC15, H2087, HCC4017, HCC515, H2009 (1.5 × 103 cells/well), BJ and HCEC1 cells (2 × 103 cells/well) were plated in growth media in 96 well plates. Cells were incubated for 1 week and treated with varying concentrations of 6-thio-dG and 6-thioguanine or DMSO every three days. The 96 well plates were analyzed according manufacturer’s directions for the CellTiterGlo luminescent cell viability assay (Promega, Madison, WI) to obtain IC50 values. The IC50 is defined as the concentration of drug at which 50% of the cells are inhibited by the drug. Sigmoidal dose-response curves (GraphPad Prism, La Jolla, CA) were used to calculate IC50 values. All samples were analyzed in triplicate and SDs are from two independent experiments.
Long-term cell culture studies
For long-term cellular experiments, HCT116 (1,000 cells/cm2) and BJ (10,000 cells/cm2) cells were treated with 6-thio-dG (1, 3, 10µM) containing medium every three days. The cells were counted and replated approximately every week for 10–12 weeks. Additionally, HCT116 cells (1,000 cells/cm2) were fed with 6-thioguanine (1, 3, 10µM) every three days, cells were counted weekly, and 1×106 cells collected for TRF (Telomere Restriction Fragment) analysis and the remainder replated or cryopreserved.
Telomerase activity assay
Telomerase activity was measured by the TRAP assay (Telomeric Repeat Amplification Protocol) (35). Briefly, HCT116 cells were treated with 1 or 10µM 6-thio-dG for 1 week. 1×105 cells were then collected and lysed with ice-cold NP-40 lysis buffer (10mM Tris–HCl pH 8.0, 1.0mM MgCl2, 1mM EDTA, 1% NP-40, 0.25mM sodium deoxycholate, 10% glycerol, 150mM NaCl, 5mM β-mercaptoethanol) for 30 minutes. One microliter cellular lysate was used for each reaction. Hela cells were used as a positive control and lysis buffer was used as a negative control. Samples were prepared and then the telomerase extension products were amplified using PCR (95°C for 5 minutes to inactivate telomerase, then 95°C for 30 seconds, 52°C for 30 seconds, 72°C for 30 seconds; 24 cycles). Samples were run on a 10% non-denaturating acrylamide gel and visualized using a Typhoon PhosphorImager scanner system (Molecular Dynamics, GE Healthcare, Piscataway, NJ) that is capable of reading Cy5 fluorescence.
Telomere length assay (TRF, Terminal Restriction Fragment)
1×106 cells were collected and washed with PBS. DNA was isolated using the manufacturer’s instructions (Qiagen, Valencia, CA). 2.5µg DNA was digested with six different restriction enzymes (HhaI, HinfI, MspI, HaeIII, RsaI, AluI) (New England Bio, Ipswich, MA) and incubated at 37°C overnight. Digested DNA was separated on a 0.7% agarose gel overnight at 70 V. The terminal restriction fragment (TRF) gel was placed in denaturating solution for 20 minutes (0.5M NaOH, 1.5M NaCl, pH 13.2) and dried on Whatman 3MM paper under vacuum for 3 hours at 56°C. The gel was then placed for 15 minutes in neutralization buffer (1.5M NaCl, 0.5M Tris-HCl, pH 8.0) and then probed with a radiolabeled telomeric probe (C-rich) for 16 hours at 42°C in 5× SSC buffer, 5×Denhardt’s solution, 10mMl/L Na2HPO4, and 1mM/L Na2H2P207. The gel was washed once with 2× SSC, 0.1% SDS, twice with 0.5× SSC, 0.1% SDS and then twice with 0.5× SSC, 1% SDS at room temperature for 15 minutes. Gels were exposed to a PhosphorImager screen overnight and analyzed using a Typhoon PhosphorImager scanner system (Molecular Dynamics). ImageQuant and graphpad prism were used to determine average telomere length of cells.
Telomere dysfunction Induced Foci (TIF) assay
The TIF assay is based on the co-localization detection of DNA damage by an antibody against broken double stranded DNA, such as gamma-H2AX, and telomeres using an antibody against the telomeric shelterin protein TRF2. Briefly, HCT116 cells were plated in 4-well chamber slides and after the cells attached to the surface, either 3 µM 6-thio-dG or 3 µM 6-thioguanine was added to the medium at different time points (0, 30 minutes, 3 hours, 12 hours, 24 hours, 48 hours, 72 hours). BJ hTERT(−) and BJ hTERT(+) cells were plated in 4-well chamber slides and after the cells attached to the surface, either 10µM 6-thio-dG or 10µM 6-thioguanine was added to the medium for 48 hours. Slides were rinsed once with PBS and fixed in 4% paraformaldehyde in PBS for 10 minutes. Then, cells were washed twice with PBS and permeabilized in 0.5% Nonidet-P40 in PBS, blocked with 0.5% Bovine Serum Albumin (BSA) and 0.2% fish gelatin in PBS for 30 min. Gamma-H2AX (mouse) (Millipore, Billerica, MA) was diluted 1:1000 and TRF2 (rabbit) (Abcam, Cambridge, MA) was diluted 1:200 in blocking solution and this primary Ab mixture was incubated on cells for 2 hours. Following three washes with PBST (1× PBS in 0.1% Triton) and 3 washes with PBS, cells were incubated with Alexaflour 488 conjugated goat anti rabbit (1:500) (Invitrogen, Grand Island, NY) and Alexaflour 568 conjugated goat anti mouse (1:500) (Invitrogen) for 40 minutes, then washed six times with PBS. After drying, the slides were mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were captured with Deltavision wide-field microscope using the 60× objective, then deconvoluted using Autoquant X3. TIFs were quantified using Imaris software.
Drug toxicity animal experiments
All procedures and experiments involving mice were approved by University of Texas Southwestern (UTSW) Institutional Animal Care and Use Committee (IACUC) and conducted as per institutional guidelines. Wildtype female 129S2 mice were used to determine a 6-thio-dG drug toxicity curve. Mice were randomly divided to control, 6-thio-dG and 6-thioguanine treatment groups. Animals were injected intraperitoneally every day at a dose 5mg/kg or 1.67mg/kg in 100 µL DMSO/PBS mixture per mouse. Animals were weighed and observed every day for 25 days (n=3).
Histology and serum analysis
Wildtype female 129S2 mice were used for histology and serum analysis. Mice were randomly divided into control, 6-thio-dG and 6-thioguanine treatment groups. Animals were injected intraperitoneally every two days at a dose 2mg/kg in 100 µL DMSO/PBS mixture per mouse for 14 days and sacrificed two days after the last injection. After euthanasia by CO2, a panel of tissues and organs were fixed in 10% neutral buffered formalin. Fixed tissues were embedded in paraffin, sectioned at 5 µM thickness, stained with hematoxylin and eosin. The following tissues were examined: liver, kidney, spleen and colon. Images were captured with Axiovision software v4.6.3 on an Axioskop 2 plus microscope mounted with AxioCamHR color camera (Carl Zeiss Microscopy) using Plan-APOCHROM 20× objectives, scale bars: 100 µM. For hematological analysis, whole blood was collected via cardiac draw in Sarstedt 100µl K3E EDTA tubes and immediately analyzed for red (RBC) and white (WBC) blood cell counts, hemoglobin (HGB), hematocrit (HCT), and differential WBC lymphocytes, monocytes, neutrophil, basophil using automated IDEXX ProCyte DX Hematology analyzer (IDEXX Laboratories, Inc., Westbrook, ME, USA). All biochemical serum evaluations used to investigate organ functions were performed at the same time to minimize analytical variability, and determined on a Vitros 250 Analyzer (Ortho Clinical Diagnostics, Johnson & Johnson Co, Rochester, NY). We evaluated ae liver panel: aspartate transaminase (AST), alanine transaminase (ALT), and a kidney panel: creatinine (CREA), and urea nitrogen (BUN).
Establishment of xenograft models
A subcutaneous xenograft mouse model of the human A549 NSCLC was used to evaluate the effects of 6-thio-dG and 6-thioguanine treatment in vivo. Athymic NCR nu/nu female mice (~6 weeks old) were used (National Cancer Institute, Bethesda, MD). A total of 2.5 × 106 A549 cells were inoculated subcutaneously into the left and right dorsal flanks of the nude mice in 100 µL phosphate buffered saline (PBS). When tumors reached ~40mm3 average volume, mice were randomly divided into control, 6-thio-dG and 6-thioguanine treatment groups (3 animals in each group). Animals were injected intraperitoneally every two days for 17 days at a dose of 2mg/kg in 100µL DMSO/PBS mixture per mouse. In addition, different animals were injected intratumorally every day for 16 days at a dose of 2.5mg/kg in 50µL DMSO/PBS mixture per mouse. Tumor size was measured by calipers and recorded either every day or every two days. Tumor volumes were calculated by taking length to be the longest diameter across the tumor and width to be the corresponding perpendicular diameter, using the following formula: (length × width2) mm2 × 0.5. No animal died during the experimental period. The animals were sacrificed by CO2 inhalation 24 hours after the last dose of treatment. The tumors were resected, fixed with 4% paraformaldehyde and paraffin-embedded for sectioning and immunohistochemistry staining.
Ki67 proliferation assay
Tissues were processed, paraffin-embedded, and cut into 5-µm sections. Sections were deparaffinized, rehydrated, and then antigens retrieved with citrate buffer (10mM/L sodium citrate, pH 6.0; 0.05% Tween 20) in a GE (model 1540WW002) microwave oven at power 5 (20 min). Endogenous peroxidase, biotin, and proteins were sequentially blocked with solutions of 3% hydrogen peroxide (Sigma-Aldrich), the Avidin/Biotin Blocking Kit (Vector Laboratories), and 10% bovine serum albumin (Vector Laboratories). Ki67 primary antibody (1:1000) was diluted in 1× PBS and then sections were incubated 30 min at room temperature. After washing with PBS, secondary antibody and ABC reagent were applied using the VECTASTAIN ABC Kit (Vector Laboratories) following the manufacturer's protocol. Tissue sections were then incubated with ImmPACT DAB peroxidase substrate (Vector Laboratories), counterstained with hematoxylin, and then dried overnight before coverslip mounting. Images were captured with Axiovision software v4.6.3 on an Axioskop 2 plus microscope with an AxioCamHR color camera (Carl Zeiss Microscopy) using Plan-APOCHROM 20×, 40× and 63× objectives, scale bars: 100-µm.
ImmunoFISH
Tissue sections were processed for TIF analysis by fluorescence in situ hybridization (FISH)
as described previously (34). Briefly, to deparaffinize 5-µm tissue sections, xylene (2×5 minutes), 100% ethanol (2×2 minutes), 95% ethanol (1×2 minutes), 75% ethanol (1×2 minutes), 50% ethanol (1×2 minutes) were sequentially used and then washed with tap water (2×3 minutes). Deparaffinized tissue sections were incubated in sodium citrate buffer (10mM Na-citrate, 0.05% Tween 20, pH=6.0) at microwave (power 5) for 20 minutes to retrieve antigens. After tissue sections cooled down, they were rinsed with 1× PBS for 5 minutes and then dehydrated in 95% ethanol for 3 minutes. After air-drying, denaturation was conducted with hybridization buffer containing FITC-conjugated telomere sequence (TTAGGG)3-specific peptide nucleic acid (PNA) probe (PNA Bio, Thousand Oaks, CA), 70% formamide, 12mM Tris-HCl pH=8.0, 0.5mM KCl, 1mM MgCl2, 0.08% Triton X-100 and 0.25% BSA for 5 minutes at 80°C, followed by 2 hours incubation in the same buffer at 37°C. Slides were washed sequentially with 70% formamide (Ambion, Life Technologies, Grand Island, NY) / 0.6 × SSC (Invitrogen) (3 × 15 minutes), 2 × SSC (1 × 15 minutes), PBS (1 × 5 minutes), PBST (PBS + 0.1 % Tween 20; 1 × 5 minutes) and incubated with blocking buffer (4% BSA in PBST) for 30 minutes. Sections were incubated with primary polyclonal anti-53BP1 antibody (1:500) (Novus Bio, Littleton, CO) in blocking buffer at room temperature for 1 hour. Following 2 × 5 minutes washes with PBST, tissue sections incubated with Alexaflour 568 conjugated goat anti Rabbit (1:500) (Invitrogen) in blocking buffer at room temperature for 1 hour. Sections were washed sequentially with PBST (3 × 5 minutes) and PBS (1 × 5 minutes). The slides were mounted with Vectashield mounting medium with DAPI. Images were captured with a Deltavision wide-field microscope using a 100× objective, then deconvoluted using Autoquant X3. TIFs were quantified using Imaris software.
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded tissue was used for immunohistochemical analyses. Sections were cut to 5-µm, deparaffinized and stained with haematoxylin I and eosin Y (Fisher Scientific, Pittsburgh, PA).
Statistical analysis
The observed data are presented as mean values ± Standard Deviation (SD). SDs were shown only above the line to make the graphs more clear. Comparisons of different groups for statistical significance were analyzed using a two-tailed, unpaired Student t test. P value of 0.05 or less was considered significant. Statistical analyses were performed using GraphPad Prism software version 6.01.
Supplementary Material
Statement of significance.
Telomerase is an almost universal oncology target, yet there are few telomerase-directed therapies in human clinical trials. In the present study, we demonstrate a small molecule telomerase substrate approach that induces telomerase-mediated targeted “telomere un-capping”, but only in telomerase positive cancer cells with minimal effects in normal telomerase negative cells.
Acknowledgments
Financial Support: I. Mender was supported by Scientific and Technological Research Council of Turkey (TUBITAK). These studies were supported in part by NCI SPORE P50CA70907, the Simmons Cancer Center Support Grant 5P30 CA142543 and support from the Southland Financial Corporation Distinguished Chair in Geriatric Research (J.W.S. and W.E.W.). This work was performed in space constructed with support from National Institute of Health grant C06 RR30414.
Footnotes
The authors declare that there are no conflicts of interest.
Author contributions
Conception and design: jws, im, sg
Development of methodology: jws, im
Acquisition of data: im, zdg
Analysis and interpretation data: jws, im, sg, ww, zdg
Writing, review and/or revision of the manuscript: jws, im, sg, ww, zdg
Administrative, technical, or material support:
Study supervision: jws and sg
References
- 1.Naito T, Matsuura A, Ishikawa F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nature genetics. 1998;20:203–206. doi: 10.1038/2517. [DOI] [PubMed] [Google Scholar]
- 2.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 3.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & development. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 4.Watson JD. Origin of concatemeric T7 DNA. Nature: New biology. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 5.Shay JW, Wright WE. Telomerase activity in human cancer. Current opinion in oncology. 1996;8:66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Shay JW, Wright WE. Telomeres and telomerase: implications for cancer and aging. Radiation research. 2001;155:188–193. doi: 10.1667/0033-7587(2001)155[0188:tatifc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Shay JW, Wright WE, Werbin H. Defining the molecular mechanisms of human cell immortalization. Biochimica et biophysica acta. 1991;1072:1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- 8.Wright WE, Shay JW. Time, telomeres and tumours: is cellular senescence more than an anticancer mechanism? Trends in cell biology. 1995;5:293–297. doi: 10.1016/s0962-8924(00)89044-3. [DOI] [PubMed] [Google Scholar]
- 9.Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer research. 2005;65:7866–7873. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- 10.Bechter OE, Zou Y, Walker W, Wright WE, Shay JW. Telomeric recombination in mismatch repair deficient human colon cancer cells after telomerase inhibition. Cancer research. 2004;64:3444–3451. doi: 10.1158/0008-5472.CAN-04-0323. [DOI] [PubMed] [Google Scholar]
- 11.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Seminars in cancer biology. 2011;21:349–353. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gryaznov SM, Jackson S, Dikmen G, Harley C, Herbert BS, Wright WE, et al. Oligonucleotide conjugate GRN163L targeting human telomerase as potential anticancer and antimetastatic agent. Nucleosides, nucleotides & nucleic acids. 2007;26:1577–1579. doi: 10.1080/15257770701547271. [DOI] [PubMed] [Google Scholar]
- 13.Djojosubroto MW, Chin AC, Go N, Schaetzlein S, Manns MP, Gryaznov S, et al. Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology. 2005;42:1127–1136. doi: 10.1002/hep.20822. [DOI] [PubMed] [Google Scholar]
- 14.Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, et al. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:3184–3192. doi: 10.1158/1078-0432.CCR-05-2760. [DOI] [PubMed] [Google Scholar]
- 15.Gellert GC, Dikmen ZG, Wright WE, Gryaznov S, Shay JW. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast cancer research and treatment. 2006;96:73–81. doi: 10.1007/s10549-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 16.Williams SC. No end in sight for telomerase-targeted cancer drugs. Nature medicine. 2013;19:6. doi: 10.1038/nm0113-6. [DOI] [PubMed] [Google Scholar]
- 17.Thompson PA, Drissi R, Muscal JA, Panditharatna E, Fouladi M, Ingle AM, et al. A phase I trial of imetelstat in children with refractory or recurrent solid tumors: a Children's Oncology Group Phase I Consortium Study (ADVL1112) Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6578–6584. doi: 10.1158/1078-0432.CCR-13-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Current biology : CB. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 19.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 20.Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- 21.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nature reviews Cancer. 2008;8:24–36. doi: 10.1038/nrc2292. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Weinshilboum R. Thiopurine S-methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene. 2006;25:1629–1638. doi: 10.1038/sj.onc.1209372. [DOI] [PubMed] [Google Scholar]
- 23.Gunnarsdottir S, Elfarra AA. Distinct tissue distribution of metabolites of the novel glutathione-activated thiopurine prodrugs cis-6-(2-acetylvinylthio)purine and trans-6-(2-acetylvinylthio)guanine and 6-thioguanine in the mouse. Drug metabolism and disposition: the biological fate of chemicals. 2003;31:718–726. doi: 10.1124/dmd.31.6.718. [DOI] [PubMed] [Google Scholar]
- 24.Tendian SW, Parker WB. Interaction of deoxyguanosine nucleotide analogs with human telomerase. Molecular pharmacology. 2000;57:695–699. doi: 10.1124/mol.57.4.695. [DOI] [PubMed] [Google Scholar]
- 25.Stefl R, Spackova N, Berger I, Koca J, Sponer J. Molecular dynamics of DNA quadruplex molecules containing inosine, 6-thioguanine and 6-thiopurine. Biophysical journal. 2001;80:455–468. doi: 10.1016/S0006-3495(01)76028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Issaeva N, Thomas HD, Djureinovic T, Jaspers JE, Stoimenov I, Kyle S, et al. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer research. 2010;70:6268–6276. doi: 10.1158/0008-5472.CAN-09-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang B, Testa JR, Kruger WD. Increasing the therapeutic index of 5-fluorouracil and 6-thioguanine by targeting loss of MTAP in tumor cells. Cancer biology & therapy. 2012;13:1082–1090. doi: 10.4161/cbt.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brem R, Karran P. Oxidation-mediated DNA cross-linking contributes to the toxicity of 6-thioguanine in human cells. Cancer research. 2012;72:4787–4795. doi: 10.1158/0008-5472.CAN-12-1278. [DOI] [PubMed] [Google Scholar]
- 29.Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. European journal of clinical pharmacology. 2008;64:753–767. doi: 10.1007/s00228-008-0478-6. [DOI] [PubMed] [Google Scholar]
- 30.Rao TS, Durland RH, Seth DM, Myrick MA, Bodepudi V, Revankar GR. Incorporation of 2'-deoxy-6-thioguanosine into G-rich oligodeoxyribonucleotides inhibits G-tetrad formation and facilitates triplex formation. Biochemistry. 1995;34:765–772. doi: 10.1021/bi00003a009. [DOI] [PubMed] [Google Scholar]
- 31.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends in cell biology. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 32.Cosme-Blanco W, Shen MF, Lazar AJ, Pathak S, Lozano G, Multani AS, et al. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO reports. 2007;8:497–503. doi: 10.1038/sj.embor.7400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer cell. 2007;11:461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suram A, Kaplunov J, Patel PL, Ruan H, Cerutti A, Boccardi V, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. The EMBO journal. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. European journal of cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.