Abstract
Purpose
Retrospective studies of preconception health have demonstrated that parents’ health conditions and behaviors can impact a newborn’s birth outcomes and, subsequently, future health status. This study sought to examine the impact of preconception health, measured prospectively, among both mothers and fathers, on two important birth outcomes: birthweight and gestational age.
Methods
Data came from Add Health (the National Longitudinal Study of Adolescent Health), which included interviews with original participants and a subsample of their partners in 2001–02. In 2008, the original respondents again completed an interview for Add Health. For 372 eligible infants born to these couples, birth outcomes (measured in 2008) were regressed on preconception health conditions and behaviors among non-pregnant heterosexual partners (measured in 2001–02).
Results
Mean birthweight was 3399 grams, and mean gestational age was 39 weeks. Birthweight was higher for infants born to mothers with diabetes or high blood pressure, and for mothers who drank alcohol at least once per month, and lower for infants born to fathers with diabetes (p < .05). Infant gestational age was marginally lower for infants born to mothers with higher levels of depression (p < .10), and lower for infants born to fathers with diabetes and with higher levels of fast food consumption (p < .05).
Conclusions
Both maternal and paternal preconception health conditions and behaviors influenced infant birth outcomes. Interventions to promote preconception health should focus on prevention of diabetes and high blood pressure, as well as minimizing consumption of alcohol and fast food.
Keywords: Preconception health, maternal health, birth outcomes, gestational age, birthweight
Preconception health is the health of men and women during their reproductive years, beginning from childhood or pubarche and extending until they conceive a child [1, 2]. The benefits of good preconception health include improved pregnancy and birth outcomes, including reductions in low birthweight or preterm infants [1, 3, 4]. In turn, infants with birthweights and gestational ages in the normal range have reduced risks for adverse health conditions throughout the lifetime, including cardiovascular disease, metabolic diseases, and cancer [5–9]. By investigating how health conditions and behaviors of parents—even before they conceive a child—impact the health of offspring, research in preconception health analyzes processes such as intergenerational transfer of health and lifespan influences on health and wellbeing [2].
Research on preconception health has focused on the influence of maternal health conditions and behaviors on birthweight and gestational age. Studies have shown that offspring birthweight is correlated with maternal obesity [10–13], depression [14, 15], blood pressure [14], substance use [16, 17], and occupational factors [18]. Similarly, gestational age is related to mother’s preconception body mass index [14, 19–21], diabetes [14, 17], blood pressure [20, 22], substance use [20, 22, 23], and occupational factors [18]. Maternal health could impact birth outcomes through a variety of complex mechanisms, including placental functioning [24, 25] and inflammation or stress [26].
In contrast, much less is known about fathers’ preconception contributions to birth outcomes. Some studies have demonstrated that paternal health factors, including obesity, cardiovascular health, and work conditions [18], are associated with birth outcomes, particularly birthweight [27–30]. Human and animal studies demonstrate that mediating mechanisms could include epigenetic processes, such as methylation [31–35] or RNAi-mediated effects [29, 31, 34, 36]. More research is needed to articulate how paternal health is associated with birth outcomes, and whether the process operates independently from or jointly with maternal health [36].
Overall, the empirical literature around preconception health and birth outcomes is not well understood, and researchers are just beginning to explore the effect sizes, timing, and duration of these relationships. Previous studies of the relationship between preconception health and birth outcomes are marked by several limitations. Studies of preconception health often use samples of women preparing to conceive, yet these women are not representative of all individuals who eventually become pregnant, since only half of all pregnancies in the U.S. are planned [1, 37], and they may change their behaviors in anticipation of becoming pregnant. Several studies are based on post-partum reports of preconception health, which are subject to recall bias [37]. Alternatively, studies may focus on prenatal health (i.e., health during pregnancy) instead of preconception health, but relevant factors may change during pregnancy or may affect fetal development even before a woman knows that she is pregnant [37]. Finally, most studies of preconception health focus exclusively on mothers, without reference to paternal influence.
The present study used prospective data from heterosexual couples subsetted from a nationally-representative sample to investigate the relationship of birth outcomes with both maternal and paternal preconception health. We hypothesized that these analyses would demonstrate significant relationships between several parental health conditions and behaviors with offspring birthweight and gestational age.
Methods
Data
Data come from the third and fourth waves of the National Longitudinal Study of Adolescent Health (Add Health). Add Health collected longitudinal data on health, development, and social factors for young people from 1994 through 2008. The design and implementation of this survey is described fully elsewhere [38].
In 1994–1995, a nationally-representative sample of 20,745 adolescents in grades 7–12 completed an in-home questionnaire as part of the first wave of Add Health. In 2001–2002, 15,197 of the original participants completed the third wave of data collection when they were aged 18–26. Wave III also included questionnaires administered to a random sample of 1,507 partners of participants, stratified to include approximately equal numbers of married, cohabitating, and dating partners. To be eligible for inclusion in the partner sample, partners had to be in a current relationship of at least three months with the original participant, of the opposite sex of the original participant, and at least 18 years of age.
Wave IV of Add Health surveyed 15,701 of the original participants in 2008 when they were aged 24–32. Partners were not included in data collection at this wave.
Analytic sample
This study involves analysis of data on a subset of the respondents who participated in data collection for both the Wave III partner survey and the Wave IV survey, including original participants, their partners, and their offspring (n = 372 families). (Note that only original participants completed the Wave IV survey.) From the original sample of 1,507 couples in the Wave III partner survey, participants had to meet several criteria for inclusion in the analytic sample (Figure 1).
Figure 1.
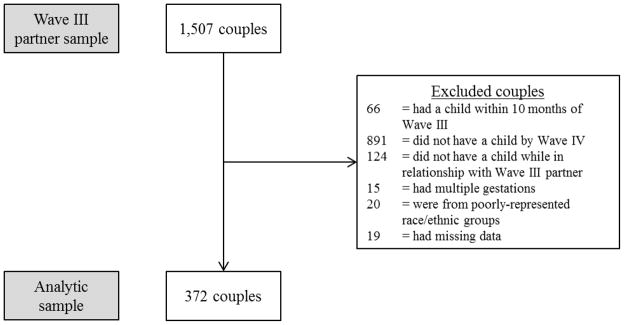
Flow diagram of selection into analytic sample, National Longitudinal Study of Adolescent Health, Waves III–IV, 2001–2008.
To ensure that data from Wave III referred to preconception health, analyses excluded any couples that were expecting a child during the Wave III interview, and any couples that had a child within 10 months after the Wave III interview (n = 66). Additionally, couples whose relationships dissolved without having children or who did not have a child by the Wave IV interview were excluded (n = 891).
To ensure that this analysis only examined the impact of preconception health of the two biological parents, any participant who did not have a birth with the partner interviewed in the Wave III partners’ sample was excluded. Add Health does not ask participants to identify the biological parentage of their children, so parentage was inferred by comparing the estimated month of conception (by subtracting the reported gestational age from the infant’s birth date) to the beginning and end dates of the partnership from the Wave III interview. If the conception month did not fall within the relationship timeframe (as reported at Wave IV), that family was not included in analysis (n = 124). Unfortunately, there was no more direct way to determine parentage available with this dataset.
Among the subsample of participants with live births in the eligible time frame (n = 426), 15 had multiple gestations, and these were excluded from the analysis because singletons have systematically greater birthweights and gestational ages than multiples. Most participants were either non-Hispanic white, non-Hispanic black, or Hispanic, and individuals who identified with any other racial/ethnic category were excluded due to small numbers and concerns about representativeness (n = 20). Finally, any families who did not have complete information on birth outcomes were excluded (n = 19), for a final analytic sample of 372 births. For couples who had more than one infant together between Wave III and Wave IV, only the first birth after Wave III was included in the analysis.
Birth outcomes for the 372 families included in the analytic sample were reported at Wave IV by the original Add Health respondent. Of these respondents, approximately equal numbers were male (52.2%) versus female (47.8%).
Measures
Preconception health was conceptualized as the health conditions and behaviors of individuals prior to conceiving. We selected a suite of conditions and behaviors that included factors demonstrated to correlate with birth outcomes in previous empirical studies, and that were available in the Add Health dataset. Data on preconception sociodemographics, health conditions, and health behaviors of the participants and their partners came from Wave III interviews, and characteristics of the pregnancy and birth outcomes came from Wave IV interviews.
Wave III: Sociodemographic controls
Control variables were parents’ age at birth, in years; race/ethnicity; immigrant status; education level; and socioeconomic status. Race/ethnicity was categorized into three groups: non-Hispanic white, non-Hispanic black, and Hispanic. Immigrant status (yes or no) indicated whether or not the respondent was born in a country outside of the US. Educational level was measured by the highest level of education the participant had completed by Wave III: less than high school, high school degree (including GED), or some college or more. Finally, models included a proxy measure of socioeconomic status indicating receipt of food stamps, housing assistance, or other public welfare funds. These variables have correlated with birth outcomes in previous studies [1] but were not the primary area of investigation for the current analysis.
Wave III: Health conditions
Four preconception health status variables were included: diabetes, high blood pressure, depression, and body mass index (BMI). Participants who reported ever being diagnosed with diabetes and/or taking medication for diabetes were classified as having diabetes [39]. Participants who reported ever being diagnosed with high blood pressure and/or taking medication for high blood pressure were classified as having high blood pressure [39]. Depression was captured with 3 items from the Center for Epidemiological Studies-Depression scale [40–43] that demonstrated measurement invariance across race/ethnic groups in Add Health respondents in a previous study [44]; participants responded to items using a 4-point Likert response scale, and responses were summed to create a depression score (possible range: 0–9). Finally, BMI was calculated using interviewer-measured height and weight and categorized according to standard categories of normal weight, overweight, or obese [45]. Few participants (<1%) were classified as underweight, and these respondents were grouped into the normal weight category.
Wave III: Health behaviors
Six measures of maternal and paternal health behaviors were included in the analysis: 4 measures of substance use, 1 measure of fast food consumption, and 1 measure of physical activity. Frequency of alcohol use was dichotomized to represent drinking once a month or less versus more frequently. A dummy variable indicated whether a participant smoked at least one cigarette per day over the last 30 days. Respondents also reported use of marijuana (yes or no) or other drugs (yes or no) in the last year.
Consumption of fast food was measured by respondents’ report of the number of days per week in which s/he typically eats fast food (e.g., McDonalds, Kentucky Fried Chicken, etc.) (range: 1–7). Physical activity was measured by responses to a series of eight items assessing the number of bouts of physical activity in the last week (observed range: 1–28). A bout of physical activity was defined as an instance in which the participant engaged in a variety of activities, such as bicycling, hiking, roller blading, doing aerobics, playing team sports, participating in individual sports, or walking.
Wave IV: Pregnancy and birth characteristics
Data on pregnancy and birth characteristics came from the Wave IV interview with the original Add Health respondent. Analyses controlled for important characteristics of the pregnancy: whether prenatal care began in the first trimester of pregnancy, or later; sex of the infant; maternal parity at the time of the birth under study (0 previous births or at least one previous birth); and approximate time, in months, between the Wave III interview and conception. Models also controlled for the relationship type at the Wave III interview (i.e., married, cohabitating, or dating).
The two outcome variables also came from the Wave IV questionnaire. The outcomes were offspring gestational age, in weeks, and birthweight, in grams. Offspring gestational age was derived from questions asking respondents how many days and weeks before or after the due date the child was born. These responses were added or subtracted from a typical gestation length of 40 weeks, and rounded to the nearest week. Offspring birthweight was calculated by transforming the reported birthweight in pounds and ounces into grams (i.e., 1 ounce = 28.35 grams).
Data analysis
Descriptive statistics include the distribution of the preconception health variables for parents, as well as separately for mothers and fathers.
We tested the relationships between preconception health and birth outcomes using linear regression models. Analyses accounted for the clustered nature of the data, since the original sampling scheme used schools as the primary sampling units. Data were weighted to account for unequal probability of selection and to provide unbiased estimates of population parameters. Using the sampling weights for the partner sample allows for generalization to the population of young adults who were in grade 7–12 at Wave I (i.e., 1994–1995) and their romantic partners at Wave III (i.e., 2008) [46].
Gestational age and birthweight were regressed on each preconception health condition and behavior, separately, controlling for sociodemographic factors, characteristics of the pregnancy, and Wave III relationship type. All preconception health variables with significant or borderline relationships with birth outcomes (p < .10) were entered into multivariate models, again controlling for sociodemographic factors, characteristics of the pregnancy, and Wave III relationship type. Models including both maternal and paternal measures simultaneously were unstable, likely due to multicollinearity of couples’ preconception health; therefore, these results are not presented here.
Finally, two sensitivity analyses tested the robustness of the results of the main analysis. In the first sensitivity analysis, the regression models were applied only to the subsample of births that occurred within 2 years of the Wave III interview. Restricting the sample in this way increases the salience of the health measures reported at Wave III to the conditions experienced around the time of conception. In the second sensitivity analysis, the preconception health variables were regressed on a third outcome variable, birthweight adjusted for gestational age, which explicitly incorporates the inextricable relationship between these two variables.
The University of North Carolina Non-Biomedical Institutional Review Board approved the study procedures.
Results
Sample characteristics
Characteristics of the entire sample, as well as of mothers and fathers separately, appear in Table 1. Participants were an average of 24.8 years old at the time of the infant’s birth. The majority were non-Hispanic white (69.2%) and not immigrants (96.0%). Few respondents were classified as diabetic (1.9%; n = 10) or having high blood pressure (9.2%; n = 52). Scores on the depression score were relatively low (mean = 1.2). In terms of BMI categories, 36.5% of participants were categorized as normal weight, 28.0% were categorized as overweight, and 35.4% were categorized as obese.
Table 1.
Sample characteristics among 372 mothers and 372 fathers, National Longitudinal Study of Adolescent Health, Wave III, 2001–2002.
| Overall | Mothers | Fathers | ||||
|---|---|---|---|---|---|---|
| n | % (SE) | N | % (SE) | n | % (SE) | |
|
| ||||||
| Control Variables | ||||||
| Age at birth, years, mean | 743 | 24.8 (0.22) | 372 | 24.4 (0.25) | 371 | 25.2 (0.36) |
| Race | ||||||
| Non-Hispanic white | 535 | 69.2% (0.03) | 280 | 71.4% (0.04) | 255 | 66.9% (0.05) |
| Non-Hispanic black | 113 | 15.8% (0.02) | 57 | 17.2% (0.03) | 56 | 14.4% (0.04) |
| Hispanic | 95 | 14.9% (0.02) | 35 | 11.4% (0.04) | 60 | 18.8% (0.04) |
| Education | ||||||
| Less than high school | 119 | 15.9% (0.03) | 48 | 12.1% (0.04) | 71 | 20.2% (0.04) |
| High school diploma | 299 | 40.3% (0.03) | 149 | 40.6% (0.05) | 150 | 40.0% (0.05) |
| At least some college | 325 | 43.7% (0.03) | 175 | 47.2% (0.05) | 150 | 39.8% (0.04) |
| Receipt of welfare, last year | ||||||
| No | 664 | 89.5% (0.02) | 329 | 86.4% (0.04) | 335 | 92.9% (0.02) |
| Yes | 79 | 10.5% (0.02) | 43 | 13.6% (0.04) | 36 | 7.1% (0.02) |
| Immigrant | ||||||
| No | 713 | 96.0% (0.01) | 356 | 95.9% (0.02) | 357 | 96.2% (0.02) |
| Yes | 30 | 4.0% (0.01) | 16 | 4.1% (0.02) | 14 | 3.8% (0.02) |
| Health Conditions | ||||||
| Ever diagnosed with diabetes | ||||||
| No | 733 | 98.1% (0.01) | 364 | 96.7% (0.02) | 379 | 99.4% (0.01) |
| Yes | 10 | 1.9% (0.01) | 8 | 3.3% (0.02) | 2 | 0.6% (0.01) |
| High blood pressure | ||||||
| No | 691 | 90.8% (0.02) | 339 | 87.9% (0.03) | 352 | 94.0% (0.02) |
| Yes | 52 | 9.2% (0.02) | 33 | 12.1% (0.03) | 19 | 6.0% (0.02) |
| Depression scale (range: 0–9) | 729 | 1.2 (0.19) | 371 | 0.8 (0.25) | 355 | 1.5 (0.29) |
| BMI category | ||||||
| Normal weight | 271 | 36.5% (0.03) | 163 | 44.0% (0.05) | 108 | 28.5% (0.04) |
| Overweight | 208 | 28.0% (0.04) | 74 | 19.7% (0.04) | 134 | 37.1% (0.04) |
| Obese | 263 | 35.4% (0.04) | 135 | 36.3% (0.05) | 128 | 34.4% (0.04) |
| Health Behaviors | ||||||
| Alcohol consumption | ||||||
| Once a month or less | 422 | 55.0% (0.04) | 271 | 75.7% (0.06) | 151 | 34.8% (0.06) |
| More often | 321 | 45.0% (0.04) | 101 | 24.2% (0.06) | 220 | 65.2% (0.06) |
| Current smoker | ||||||
| No | 456 | 65.6% (0.03) | 245 | 66.0% (0.05) | 211 | 65.2% (0.05) |
| Yes | 287 | 34.4% (0.03) | 127 | 34.0% (0.05) | 160 | 34.8% (0.05) |
| Marijuana use, last year | ||||||
| No | 553 | 74.7% (0.03) | 293 | 78.9% (0.04) | 260 | 70.0% (0.04) |
| Yes | 190 | 25.3% (0.03) | 79 | 21.1% (0.04) | 111 | 30.0% (0.04) |
| Use of other drugs, last year | ||||||
| No | 689 | 92.9% (0.02) | 355 | 95.5% (0.02) | 334 | 90.1% (0.03) |
| Yes | 54 | 7.1% (0.02) | 17 | 4.5% (0.02) | 37 | 9.9% (0.03) |
| Days/week eating fast food (typical), mean | 743 | 2.6 (0.13) | 372 | 2.5 (0.18) | 371 | 2.7 (0.18) |
| Bouts of physical activity per week, mean | 743 | 4.7 (0.57) | 372 | 3.6 (0.45) | 371 | 5.7 (0.97) |
Note. Frequencies are unweighted; proportions and means are weighted. BMI = body mass index; SE = standard error.
Less than half of respondents consumed alcohol at least once a month (45%) (Table 1). Use of tobacco and marijuana was common, with 34.4% of participants classified as current smokers and 25.3% of participants reporting using marijuana at least once in the last year. On average, respondents ate fast food 2.6 times per week and engaged in 4.7 bouts of physical activity per week.
Birth outcomes and obstetrical factors reported at Wave IV followed expected distributions. Approximately equal percentages of infants were male (54.5%) versus female (45.4%) (Table 2), and about half of families initiated prenatal care in the first trimester of the pregnancy (51.9%). On average, infants were conceived less than two years after the Wave III interview (mean = 18.8 months, standard error [SE] = 1.5), and born approximately nine months later. Most often, infants were born to couples who were married at the Wave III interview (44.6%). The average birthweight was 3398.8 grams (SE = 43.5) and the average gestational age was 39.18 weeks (SE = 0.16).
Table 2.
Relationship of pregnancy characteristics and design variables with birth outcomes for 372 infants, National Longitudinal Study of Adolescent Health, Wave IV, 2008.
| Proportion or Mean (SE) | Birthweight, grams m = 3398.8 (SE = 43.5) | Gestational age, weeks m = 39.18 (SE = 0.16) | |
|---|---|---|---|
|
| |||
| Estimate, 95% CI | Estimate, 95% CI | ||
|
|
|||
| Infant sex | |||
| Boy | 54.5% | (ref) | (ref) |
| Girl | 45.4% | −163.3 (−359.6, 33.1) | 0.34 (−0.38, 1.03) |
| Initiation of prenatal care | |||
| First trimester | 51.9% | (ref) | (ref) |
| Second or third trimester | 48.1% | −96.6 (−290.3, 97.1) | −0.49 (−1.38, 0.40) |
| Parity | |||
| Primiparous | 87.0% | (ref) | (ref) |
| Multiparous | 13.0% | 50.3 (−83.3, 183.8) | −0.40 (−1.04, 0.23) |
| Months between interview and conception | 18.8 (1.5) | 3.0 (−3.8, 9.8) | −0.02 (−0.05, 0.01) |
| Relationship type | |||
| Dating | 28.9% | (ref) | (ref) |
| Cohabitating | 26.6% | 40.2 (−218.9, 299.3) | 0.87 (−0.27, 2.00) |
| Married | 44.6% | 229.2 (−25.8, 484.1) | 0.53 (−0.47, 1.52) |
Note. Proportions and means are weighted. Linear regression models control for maternal and paternal demographic factors: age at birth, race, education, receipt of welfare, and immigration status. SE = standard error; CI = confidence interval.
Offspring birthweight
Offspring birthweight was associated with mothers’ preconception diabetes status, high blood pressure status, and use of alcohol (Table 3). Women who had been diagnosed with diabetes had heavier infants than women who had not been diagnosed with diabetes (difference = 1667.0 grams, 95% CI: 1215.6, 2118.4, p < .01). Women who had been diagnosed with high blood pressure had heavier infants than women who had not been diagnosed with high blood pressure (difference = 393.9 grams, 95% CI: 45.7, 742.2, p = .03). Finally, women who drank alcohol more than once per month had heavier infants than women who drank alcohol once a month or less (difference = 310.1 grams, 95% CI: 88.0, 532.2, p = .01).
Table 3.
Relationship of preconception health conditions and health behaviors with infant birthweight, National Longitudinal Study of Adolescent Health, Waves III–IV, 2001–2008.
| Mothers | Fathers | |||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate | Multivariate | Bivariate | Multivariate | |||||
| Estimate, 95% CI | p | Estimate, 95% CI | p | Estimate, 95% CI | p | Estimate, 95% CI | p | |
|
|
||||||||
| Health Conditions | ||||||||
| Ever diagnosed with diabetes | ||||||||
| No | (ref) | (ref) | (ref) | (ref) | ||||
| Yes | 784.8 (163.3, 1406.2) | 0.01 | 1667.0 (1215.6, 2118.4) | <.01 | −783.9 (−1014.2, − 553.6) | <.01 | −783.9 (−1014.2, − 553.6) | <.01 |
| High blood pressure | ||||||||
| No | (ref) | (ref) | (ref) | -- | ||||
| Yes | 375.8 (120.9, 630.8) | <.01 | 393.9 (45.7, 742.2) | 0.03 | 101.5 (−157.9, 360.9) | 0.44 | -- | |
| Depression scale (range: 0–9) | 30.3 (−8.1, 68.7) | 0.12 | 0 | 33.3 (−84.9, 151.5) | 0.58 | -- | ||
| BMI category | ||||||||
| Normal weight | (ref) | -- | (ref) | -- | ||||
| Overweight | 45.8 (−149.8, 241.5) | 0.32 | -- | 35.6 (−140.0, 211.3) | 0.34 | -- | ||
| Obese | 85.6 (−65.2, 236.4) | 0.13 | -- | 76.8 (−74.6, 228.1) | 0.16 | -- | ||
| Health Behaviors | ||||||||
| Alcohol consumption, last month | ||||||||
| Once a month or less | (ref) | (ref) | (ref) | -- | ||||
| More often | 275.3 (27.4, 523.1) | 0.03 | 310.1 (88.0, 532.2) | 0.01 | −85.9 (−336.2, 164.3) | 0.50 | -- | |
| Current smoker | ||||||||
| No | (ref) | -- | (ref) | -- | ||||
| Yes | −62.8 (−288.1, 162.4) | 0.58 | -- | −219.6 (−537.0, 97.8) | 0.18 | -- | ||
| Marijuana use, last year | ||||||||
| No | (ref) | -- | (ref) | -- | ||||
| Yes | 76.7 (−162.8, 316.1) | 0.53 | -- | 201.9 (−97.6, 501.3) | 0.19 | -- | ||
| Use of other drugs, last year | ||||||||
| No | (ref) | -- | (ref) | -- | ||||
| Yes | −193.0 (−548.2, 162.2) | 0.29 | -- | 390.0 (−216.4, 996.3) | 0.21 | -- | ||
| Days/week eating fast food (typical) | 2.3 (−47.4, 52.0) | 0.93 | -- | −36.0 (−89.8, 17.8) | 0.19 | -- | ||
| Bouts of physical activity per week | 8.4 (−10.5, 27.2) | 0.38 | -- | 1.7 (−13.0, 16.4) | 0.82 | -- | ||
Note. Linear regression models control for maternal and paternal demographic factors (age at birth, race, education, receipt of welfare, and immigration status) as well as pregnancy and design characteristics (infant sex, initiation of prenatal care, parity, time between Wave III interview and conception, and relationship type at Wave III). BMI = body mass index; CI = confidence interval.
In analysis of paternal preconception health, only diabetes status was associated with offspring birthweight (Table 3). Men with diabetes had lighter infants than men who had not been diagnosed with diabetes (difference = −783.9, 95% CI: −1014.2, −553.6, p < .01).
Offspring gestational age
Among maternal health conditions and behaviors, offspring gestational age was only associated with depression (Table 4). Maternal depression levels were negatively correlated with gestational age, but this difference only approached statistical significance (difference = −0.38 weeks for each one-point increase on the depression scale, 95% CI: −0.81, 0.06, p = .09).
Table 4.
Relationship of preconception health conditions and health behaviors with infant gestational age, National Longitudinal Study of Adolescent Health, Waves III–IV, 2001–2008.
| Mothers | Fathers | |||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate | Multivariate | Bivariate | Multivariate | |||||
| Estimate, 95% CI | p | Estimate, 95% CI | p | Estimate, 95% CI | p | Estimate, 95% CI | p | |
|
| ||||||||
| Health Conditions | ||||||||
| Ever diagnosed with diabetes | ||||||||
| No | (ref) | -- | (ref) | (ref) | ||||
| Yes | −0.26 (−2.19, 1.67) | 0.79 | -- | −0.80 (−1.65, 0.05) | 0.06 | −0.86 (−1.57, − 0.14) | 0.02 | |
| High blood pressure | ||||||||
| No | (ref) | -- | (ref) | -- | ||||
| Yes | 0.23 (−0.94, 1.41) | 0.70 | -- | 0.51 (−0.11, 1.13) | 0.10 | -- | ||
| Depression scale (range: 0–9) | −0.38 (−0.81, 0.06) | 0.09 | −0.38 (−0.81, 0.06) | 0.09 | −0.12 (−0.35, 0.11) | 0.30 | -- | |
| BMI category | ||||||||
| Normal weight | (ref) | -- | (ref) | -- | ||||
| Overweight | −.20 (−1.69, 1.30) | 0.40 | -- | −0.19 (−1.30, 0.91) | 0.37 | -- | ||
| Obese | −.32 (−1.43, 0.77) | 0.28 | -- | −0.39 (−1.71, 0.94) | 0.28 | -- | ||
| Health Behaviors | ||||||||
| Alcohol consumption, last month | ||||||||
| Once a month or less | (ref) | -- | (ref) | -- | ||||
| More often | 0.29 (−0.49, 1.07) | 0.47 | -- | −0.10 (−0.96, 0.77) | 0.83 | -- | ||
| Current smoker | ||||||||
| No | (ref) | -- | (ref) | -- | ||||
| Yes | 0.42 (−0.45, 1.29) | 0.34 | -- | −0.31 (−1.20, 0.59) | 0.50 | -- | ||
| Marijuana use, last year | ||||||||
| No | (ref) | -- | (ref) | -- | ||||
| Yes | 1.08 (−0.44, 2.61) | 0.16 | -- | 0.41 (−0.43, 1.25) | 0.34 | -- | ||
| Use of other drugs, last year | ||||||||
| No | (ref) | -- | (ref) | -- | ||||
| Yes | −0.55 (−2.58, 1.47) | 0.59 | -- | −0.31 (−1.99, 1.37) | 0.72 | -- | ||
| Days/week eating fast food (typical) | −0.04 (−0.25, 0.17) | 0.70 | -- | −0.16 (−0.32, − 0.01) | 0.04 | −0.16 (−0.32, − 0.00) | 0.04 | |
| Bouts of physical activity per week | 0.04 (−0.04, 0.12) | 0.33 | -- | 0.02 (−0.04, 0.07) | 0.53 | -- | ||
Note. Models control for maternal and paternal demographic factors (age at birth, race, education, receipt of welfare, and immigration status) as well as pregnancy and design characteristics (infant sex, initiation of prenatal care, parity, time between Wave III interview and conception, and relationship type at Wave III). BMI = body mass index; CI = confidence interval.
Among paternal health conditions and behaviors, offspring gestational age was associated with diabetes status and frequency of eating fast food. Men with diabetes had infants born earlier than men who had not been diagnosed with diabetes (difference = −0.86 weeks, 95% CI: −1.57, −0.14, p = .02) (Table 4). Men who ate fast food more frequently had infants born earlier than men who ate fast food less frequently (difference = −0.16 weeks for each additional day per week during which respondents ate fast food, 95% CI: −0.32, −0.00, p = .04).
Sensitivity analyses
The first sensitivity analysis repeated the regressions of each birth outcome on maternal and paternal health conditions and behaviors for the subsample of births occurring within two years of the Wave III interview (n = 140). The pattern and significance of the results replicated the findings from the full sample (data not shown).
The second sensitivity analysis used birthweight adjusted for gestational age as an outcome measure. This analysis showed a similar pattern of results as the main analysis of birthweight (data not shown).
Discussion
This analysis of 372 mothers, fathers, and infants found that preconception health conditions and behaviors influenced infants’ birth outcomes. Birthweight was associated with mother’s diabetes status, blood pressure, and alcohol use, as well as father’s diabetes status. Gestational age demonstrated a trend toward correlating with mother’s level of depression, and was associated with father’s diabetes status and fast food consumption. This pattern of results was robust to controlling for a variety of sociodemographic and pregnancy factors.
Maternal and paternal diabetes status demonstrated some of the strongest relationships with infant birthweight and gestational age. Interestingly, maternal diabetes was associated with increased birthweight but paternal diabetes was associated with decreased birthweight. These results echo previous findings regarding the influence of maternal [14, 17] and paternal diabetes [14, 17, 30] on birth outcomes. Although the prevalence of diabetes in this sample was low (3.3% in mothers and 0.6% in fathers), nationally, diabetes is becoming more common among young adults [47]; accordingly, diabetes management will become even more important for preconception care.
In addition, maternal high blood pressure status was positively related to infant birthweight. This finding contradicts previous studies demonstrating that high blood pressure prior to conception [14, 48] and during pregnancy [49, 50] is associated with lower birthweight. The discrepancy between previous studies and this one could be due to different sample characteristics or different modes of measurement (i.e., measured blood pressure versus self-report of clinically-elevated blood pressure). It is likely that any hypertension reported in the present study is under control given participants are aware of their condition (i.e., report it and/or report taking antihypertensive medication) and is therefore related to infant birthweight differently than in other studies that measure blood pressure directly. Future studies should continue to investigate how preconception blood pressure is related to birth outcomes.
Health behaviors were less important than health conditions for birth outcomes. Only two relationships with birth outcomes were significant in multivariate analysis, and these relationships were weaker in magnitude than most of the other factors, potentially because of their changeability or their indirect influence on physiologic processes impacting birth outcomes. For example, couples who plan to become pregnant could change their health behaviors in anticipation of pregnancy to be more healthful (i.e., by reducing alcohol and fast food consumption), while changing more stable and fixed health conditions may not be possible. However, additional studies are needed to determine how health behaviors impact birth outcomes and whether and when during the preconception life course such health behaviors matter.
This study had several strengths and limitations. In terms of strengths, the study utilized a prospective longitudinal design, minimizing the effects of recall bias and establishing temporal precedence for the health conditions and behaviors relative to infant birth outcomes. In addition, this study complements the literature on prenatal health by investigating the preconception correlates of birth outcomes. In addition, this study captured both maternal and paternal influences on birth outcomes through the unique design of the couples’ sample of the Add Health survey. The breadth of information gathered in the Add Health survey also allowed for the analysis to control for a variety of sociodemographic and pregnancy factors in order to specify the effects of health conditions and behaviors on birth outcomes.
In terms of limitations, several measurement issues may challenge the study results. First, as described above, paternal parentage was inferred rather than assessed, which likely resulted in some paternal misattribution. Some families may have been included in the analysis in which the adult male was not biologically related to the child; logically, the preconception health of these ‘fathers’ has little consequence on the birth outcomes of their partner’s offspring with another man. Therefore, the relationships between paternal preconception health factors and birth outcomes may be biased, although the direction of this bias is unknown. Second, this analysis uses an estimated time of conception when calculating offspring gestational age. This reflects the larger difficulty in estimating the time of conception [37]. Inaccurate estimations of when conception occurred could decrease the validity of the gestational age measure (as well as the birthweight-adjusted-for-gestational-age construct used as a dependent variable in the second sensitivity analysis), so more studies are needed that measure time of conception more accurately and replicate these analyses. Third, preconception health factors were assessed using self-report, which may be subject to social desirability; however, since participants completed Add Health questionnaires on laptop computers, rather than reporting their answers to interviewers, the likelihood of this bias was minimized. If participants did underreport health conditions and behaviors, estimates of their relationships with birth outcomes may have been biased toward the null. Fourth, (maternal) health behaviors during pregnancy that have direct physiologic consequences on birth outcomes (e.g., tobacco or alcohol consumption) were excluded from the current analysis because only half of mothers (i.e., those who were the original Add Health respondents) were eligible to complete the Wave IV survey. As such, the results presented here are considered preliminary in the absence of data collection procedures designed to capture all variables of interest. Finally, most studies of birth outcomes rely on measures of birthweight and/or gestational age that are either directly assessed or reported by mothers, and little is known about the validity of fathers’ report of birthweight and/or gestational age. In the present study, about half of participants reporting birth outcomes were fathers; to the extent that mothers and fathers respond to these items in systematically different ways, the measures of birthweight and/or gestational age may be biased. However, the direction and influence of this bias is unpredictable without further study.
Another limitation is the modest sample size; because of multiple exclusion criteria, the analytic sample was reduced to 372 mothers, fathers, and infants. This restricted sample allowed for the specific analyses described in this study, but reduced statistical power compared to the overall partners sample (n = 1,507 couples) and the overall Wave III sample (n = 15,197 participants). In addition, the characteristics of the analytic sample in the present study do not necessarily reflect the characteristics of the entire Add Health sample, larger subsets of the Add Health sample, or members of the entire target population. For example, for the analytic sample compared to the overall Wave III sample, levels of fast food consumption were similar (2.6 days per week versus 2.5 days per week, respectively), but levels of educational achievement were different (43.7% versus 53.9% having attended at least some college, respectively) (data not shown). These differences could limit the generalizability of the study results.
Future studies should continue to examine the influence of preconception health on birth outcomes. More research is needed on the timing of the relationship between birth outcomes and preconception health, especially for health behaviors. Understanding when, exactly, health behaviors have the greatest impact on birth outcomes will be important for optimizing preconception health promotion initiatives. In addition, future studies should focus on the independent and joint contributions of both mothers and fathers to birth outcomes.
In summary, preconception health is associated with infant birth outcomes, which in turn, influence health status throughout the lifetime. This study used a sample of 372 mothers, fathers, and infants to analyze how preconception health conditions and behaviors are related to infant birthweight and gestational age. Maternal and paternal diabetes status, maternal high blood pressure, maternal alcohol use, maternal depression, and paternal fast food consumption all correlated with infant birth outcomes. Preconception health promotion initiatives can target these factors in order to improve birth outcomes and, subsequently, health outcomes of future generations.
Acknowledgments
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis.
Footnotes
Ethical standards
All participants provided informed consent before completing questionnaires at each wave of study collection. The University of North Carolina Non-Biomedical Institutional Review Board approved the study procedures and analysis.
Conflict of interest
The authors declare that they have no conflict of interest. Access to the data used in the present analysis is restricted to protect against deductive disclosure, but if the journal requests to review the data, the authors will work with the journal to provide access to the data.
Author contribution:
JL Moss: Protocol/project development; data analysis; manuscript writing
KM Harris: Protocol/project development; data collection or management; manuscript editing
References
- 1.Centers for Disease Control and Prevention (CDC) [Accessed April 20 2013];Preconception care and health care. 2012 http://www.cdc.gov/preconception/overview.html.
- 2.Misra DP, Guyer B, Allston A. Integrated perinatal health framework. A multiple determinants model with a life span approach. Am J Prev Med. 2003 doi: 10.1016/s0749-3797(03)00090-4. S0749379703000904. [DOI] [PubMed] [Google Scholar]
- 3.Ashton DM, Lawrence HC, 3rd, Adams NL, 3rd, Fleischman AR. Surgeon General’s Conference on the Prevention of Preterm Birth. Obstet Gynecol. 2009 doi: 10.1097/AOG.0b013e31819bdba3. [DOI] [PubMed] [Google Scholar]
- 4.Seshadri S, Oakeshott P, Nelson-Piercy C, Chappell LC. Prepregnancy care. BMJ. 2012 doi: 10.1136/bmj.e3467. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993 doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahly DL, Adair LS, Bollen KA. A structural equation model of the developmental origins of blood pressure. Int J Epidemiol. 2009 doi: 10.1093/ije/dyn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson J. The Barker hypothesis. An analysis. Aust N Z J Obstet Gynaecol. 1999 doi: 10.1111/j.1479-828x.1999.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 8.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006 doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 9.Morley R. Fetal origins of adult disease. Semin Fetal Neonatal Med. 2006 doi: 10.1016/j.siny.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Agius R, Savona-Ventura C, Vassallo J. Transgenerational metabolic determinants of fetal birth weight. Exp Clin Endocrinol Diabetes. 2013 doi: 10.1055/s-0033-1345121. [DOI] [PubMed] [Google Scholar]
- 11.Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am. 2009 doi: 10.1016/j.ogc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Strutz KL, Richardson LJ, Hussey JM. Preconception health trajectories and birth weight in a national prospective cohort. J Adolesc Health. 2012 doi: 10.1016/j.jadohealth.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013 doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiely M, El-Mohandes AA, Gantz MG, Chowdhury D, Thornberry JS, El-Khorazaty MN. Understanding the association of biomedical, psychosocial and behavioral risks with adverse pregnancy outcomes among African-Americans in Washington, DC. Matern Child Health J. 2011 doi: 10.1007/s10995-011-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang L, Zhu P, Hao JH, Huang K, Xu SJ, Wang H, Wang L, Tao FB. Pre-pregnancy body mass index moderates the effect of maternal depressive symptoms on small-for-gestational-age infants. Arch Gynecol Obstet. 2013 doi: 10.1007/s00404-013-2720-4. [DOI] [PubMed] [Google Scholar]
- 16.Goetzinger KR, Cahill AG, Macones GA, Odibo AO. The relationship between maternal body mass index and tobacco use on small-for-gestational-age infants. Am J Perinatol. 2012 doi: 10.1055/s-0031-1284224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glinianaia SV, Tennant PW, Bilous RW, Rankin J, Bell R. HbA(1c) and birthweight in women with pre-conception type 1 and type 2 diabetes: a population-based cohort study. Diabetologia. 2012 doi: 10.1007/s00125-012-2721-z. [DOI] [PubMed] [Google Scholar]
- 18.Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review. Occup Environ Med. 2007 doi: 10.1136/oem.2006.026872. oem.2006.026872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jongh BE, Paul DA, Hoffman M, Locke R. Effects of Pre-pregnancy Obesity, Race/Ethnicity and Prematurity. Matern Child Health J. 2013 doi: 10.1007/s10995-013-1296-8. [DOI] [PubMed] [Google Scholar]
- 20.Dekker GA, Lee SY, North RA, McCowan LM, Simpson NA, Roberts CT. Risk factors for preterm birth in an international prospective cohort of nulliparous women. PLoS One. 2012 doi: 10.1371/journal.pone.0039154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogunyemi D, Hullett S, Leeper J, Risk A. Prepregnancy body mass index, weight gain during pregnancy, and perinatal outcome in a rural black population. J Matern Fetal Med. 1998 doi: 10.1002/(SICI)1520-6661(199807/08)7:4<190::AID-MFM5>3.0.CO;2-D. 2-D. [DOI] [PubMed] [Google Scholar]
- 22.Orr ST, Reiter JP, James SA, Orr CA. Maternal health prior to pregnancy and preterm birth among urban, low income black women in Baltimore: the Baltimore Preterm Birth Study. Ethn Dis. 2012 [PubMed] [Google Scholar]
- 23.Mullally A, Cleary BJ, Barry J, Fahey TP, Murphy DJ. Prevalence, predictors and perinatal outcomes of peri-conceptional alcohol exposure--retrospective cohort study in an urban obstetric population in Ireland. BMC Pregnancy Childbirth. 2011 doi: 10.1186/1471-2393-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990 doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne CD, Phillips DI. Fetal origins of adult disease: epidemiology and mechanisms. J Clin Pathol. 2000 doi: 10.1136/jcp.53.11.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care. 2011 doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davey Smith G, Hypponen E, Power C, Lawlor DA. Offspring birth weight and parental mortality: prospective observational study and meta-analysis. Am J Epidemiol. 2007 doi: 10.1093/aje/kwm054. [DOI] [PubMed] [Google Scholar]
- 28.McCowan LM, North RA, Kho EM, Black MA, Chan EH, Dekker GA, Poston L, Taylor RS, Roberts CT. Paternal contribution to small for gestational age babies: a multicenter prospective study. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2010.279. [DOI] [PubMed] [Google Scholar]
- 29.Zaina S, Lund G. Paternal transmission, cardiovascular risk factors and epigenetics. Curr Opin Lipidol. 2012 doi: 10.1097/MOL.0b013e32835918cd. [DOI] [PubMed] [Google Scholar]
- 30.Hillman S, Peebles DM, Williams DJ. Paternal metabolic and cardiovascular risk factors for fetal growth restriction: a case-control study. Diabetes Care. 2013 doi: 10.2337/dc12-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake AJ, Liu L, Kerrigan D, Meehan RR, Seckl JR. Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics. 2011 doi: 10.4161/epi.6.11.17942. [DOI] [PubMed] [Google Scholar]
- 32.Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, Gagne LA, Marsit CJ. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011 doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H, Jaffe AE, Feinberg JI, Tryggvadottir R, Brown S, Montano C, Aryee MJ, Irizarry RA, Herbstman J, Witter FR, Goldman LR, Feinberg AP, Fallin MD. DNA methylation shows genome-wide association of NFIX, RAPGEF2 and MSRB3 with gestational age at birth. Int J Epidemiol. 2012 doi: 10.1093/ije/dyr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, Kurtzberg J, Jirtle RL, Murphy SK, Hoyo C. Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013 doi: 10.1186/1741-7015-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013 doi: 10.1093/humupd/dmt041. [DOI] [PubMed] [Google Scholar]
- 36.Lecomte V, Youngson NA, Maloney CA, Morris MJ. Parental programming: How can we improve study design to discern the molecular mechanisms? Bioessays. 2013 doi: 10.1002/bies.201300051. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox AJ. Fertility and Pregnancy: An Epidemiologic Perspective. Oxford University Press; USA: 2010. [Google Scholar]
- 38.Harris KM. [Accessed December 12 2013];Design features of Add Health. 2011 http://www.cpc.unc.edu/projects/addhealth/data/guides/DesignPaperWIIV.pdf.
- 39.Harris KM. An integrative approach to health. Demography. 2010 doi: 10.1353/dem.0.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 41.Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res. 2004 doi: 10.1016/j.psychres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986 doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 43.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977 doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 44.Perreira KM, Deeb-Sossa N, Harris KM, Bollen KA. What are we measuring? An evaluation of the CES-D across race/ethnicity and immigrant generation. Social Forces. 2005;83:1567–1601. [Google Scholar]
- 45.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998 [PubMed] [Google Scholar]
- 46.Chen P, Tabor J, Chantala K. [Accessed December 12 2013];Guidelines for analyzing Add Health data. 2012 http://www.cpc.unc.edu/projects/addhealth/data/guides/wt-guidelines.pdf.
- 47.Song SH. Emerging type 2 diabetes in young adults. Adv Exp Med Biol. 2012 doi: 10.1007/978-1-4614-5441-0_7. [DOI] [PubMed] [Google Scholar]
- 48.Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011 doi: 10.1097/EDE.0b013e318225c960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steer PJ, Little MP, Kold-Jensen T, Chapple J, Elliott P. Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: prospective study. BMJ. 2004 doi: 10.1136/bmj.38258.566262.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waugh J, Perry IJ, Halligan AW, De Swiet M, Lambert PC, Penny JA, Taylor DJ, Jones DR, Shennan A. Birth weight and 24-hour ambulatory blood pressure in nonproteinuric hypertensive pregnancy. Am J Obstet Gynecol. 2000 doi: 10.1067/mob.2000.106448. [DOI] [PubMed] [Google Scholar]


