Abstract
BACKGROUND
How platelet (PLT) product characteristics such as dose, source (whole blood-derived (WBD) vs. apheresis), storage duration, and ABO matching status affect the risks of transfusion-related adverse events (TRAEs) is unclear. Similarly, more information is needed to define how recipient characteristics affect the frequency of TRAEs following PLT transfusion.
STUDY DESIGN AND METHODS
In the multicenter Platelet Dose (“PLADO”) study, pediatric and adult hematology-oncology patients with hypoproliferative thrombocytopenia were randomized to receive low-dose (LD), medium-dose (MD), or high-dose (HD) PLT prophylaxis for a pre-transfusion PLT count ≤10,000/μL. All PLT units (apheresis or WBD) were leukoreduced. Post hoc analyses of PLADO data were performed using multi-predictor models.
RESULTS
5034 PLT transfusions to 1102 patients were analyzed. A TRAE occurred with 501 PLT transfusions (10.0%). The most common TRAEs were fever (6.6% of transfusions), allergic/hypersensitivity reactions (1.9%), and sinus tachycardia (1.8%). Patients assigned HD PLTs were more likely than LD or MD patients to experience any TRAE (OR for HD vs. MD 1.50, 95% CI (1.10, 2.05), three-group comparison p=0.02). PLT source and ABO matching status were not significantly related to overall TRAE risk. Compared to a patient’s first PLT transfusion, subsequent PLT transfusions were less likely to have a TRAE reported, primarily due to a lower risk of allergic/hypersensitivity reactions.
CONCLUSION
The most important PLT unit characteristic associated with TRAEs was PLT dose per transfusion. HD PLTs may increase the risk of TRAEs, and LD PLTs may reduce the risk.
Keywords: transfusion reaction, platelets
INTRODUCTION
Prophylactic platelet (PLT) transfusions are routinely used to prevent bleeding in patients with hypoproliferative thrombocytopenia resulting from chemotherapy or hematopoietic stem cell transplantation. The current standard is to administer prophylactic PLT transfusions for a PLT count below 10,000/μL. PLT transfusion has a number of known risks, both infectious and noninfectious. We hypothesized that PLT product characteristics such as dose, source (i.e. whole blood-derived (WBD) versus apheresis), ABO matching, and storage duration as well as recipient characteristics might affect the frequency of adverse events following PLT transfusion. To investigate these issues, we performed a secondary analysis of data collected during the Platelet Dose (PLADO) study.1
The PLADO study1 was a multicenter randomized controlled trial that examined the effects of prophylactic PLT dose on bleeding in hematology-oncology patients with hypoproliferative thrombocytopenia. Patients in the PLADO trial were randomly assigned to one of three study arms: medium-dose (MD), high-dose (HD) or low-dose (LD) PLTs per transfusion for prophylactic transfusions, which were given when the morning PLT count was <10,000/μL. The primary outcome of the PLADO study was the percent of patients with WHO Grade 2 or higher bleeding events.2 As reported,1 this outcome was observed in 69%, 71% and 70% of patients in the MD, LD, and HD groups, respectively (no significant differences between groups). The LD group patients were transfused significantly more often, receiving a median of five PLT transfusions each, versus a median of three PLT transfusions each for both the MD and HD group patients (p<0.001).
We examined how frequently transfusion-related adverse events (TRAEs) were reported in the PLADO study, and whether the risk of TRAEs varied depending on PLT characteristics (dose, source, ABO matching status, and storage duration), number of PLT transfusions received to date, or patient characteristics (gender, age group, and type of transplant or chemotherapy).
MATERIALS AND METHODS
The PLADO study was a multicenter randomized controlled trial conducted by the NHLBI Transfusion Medicine/Hemostasis Clinical Trials Network. The study population was comprised of pediatric and adult patients with hypoproliferative thrombocytopenia secondary to allogeneic or autologous hematopoietic stem cell transplantation (SCT) or chemotherapy for solid or hematologic malignancies. Patients were randomly assigned to one of three different prophylactic PLT dosing strategies. MD PLT transfusions (2.2 × 1011 PLTs/m2 of body surface area) approximated the usual dose per prophylactic PLT transfusion currently administered. HD PLT transfusions (4.4 × 1011 PLTs/m2) represented twice the medium dose, while LD PLT transfusions (1.1 × 1011 PLTs/m2) represented half the medium dose. Randomization was stratified according to four treatment strata (allogeneic hematopoietic SCT, autologous or syngeneic SCT, chemotherapy for solid tumor, or chemotherapy for hematologic cancer), and balanced within each hospital.3
For each patient, the data coordinating center communicated to the blood bank the assigned PLT dose and the ± 25% allowable range but not the patient’s study group. The patient, patient’s physician, clinical staff and research staff were not informed of the assigned study group. The patient’s physician could change the prophylactic transfusion trigger or dose based on clinical indications, with a return to study parameters as soon as possible. PLTs could be given at any time to treat active bleeding, or in association with an invasive procedure. Patients diagnosed with PLT refractoriness could be switched to HLA-matched PLTs. HLA-matched PLT units were transfused in their entirety to avoid PLT wastage, independent of the patient’s study dose assignment.
PLT products were either apheresis or pooled WBD PLT concentrates prepared by the PLT-rich plasma (PRP) method. PLTs were stored under standard conditions (20–24°C with continuous agitation) for up to 5 days, except for a brief interval in which the FDA permitted 7-day storage for apheresis PLTs only. Apheresis PLTs were pre-storage leukoreduced, while WBD PLTs were post-storage leukoreduced prior to transfusion. PLT ABO selection was based on local practice; ABO-identical products were generally selected when possible. PLT ABO matching categories were defined as follows:
ABO identical – PLT donor and recipient have the same ABO red cell antigens and plasma antibodies;
ABO minor mismatch – donor’s plasma ABO antibodies are incompatible with recipient’s red cell ABO antigens (e.g. O PLT donor, A recipient);
ABO major mismatch – donor’s red cell ABO antigens are incompatible with recipient’s plasma ABO antibodies (e.g. A PLT donor, O recipient).
Patients were continued on study until 30 days after the first PLT transfusion; or until they had a 10-day period without a PLT transfusion; or until hospital discharge, death, or withdrawal from the study — whichever came first.
Adverse events
Information was collected prospectively on all serious adverse events that occurred while patients were on study, and on specific TRAEs. TRAEs for which data were collected included: allergic/hypersensitivity reaction, sinus bradycardia, sinus tachycardia, hypertension, hypotension, dyspnea, hypoxia, wheezing, cough, hemolysis, rigors/chills, fever, and infection. Grading of TRAEs was based on the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.4 (See Supplemental Table S1.) Multiple TRAEs, with the same or different CTCAE grades, could be reported for the same transfusion. TRAEs were to be reported if they occurred during or within 4 hours after each transfusion, whether or not the clinical or transfusion service staff considered the event to be caused by the transfusion. No data were collected regarding premedication.
Statistical analysis
PLT transfusions were excluded from the analysis of TRAEs if they had one or more of the following characteristics:
Had missing data on TRAEs (n=4)
Included both apheresis and WBD PLTs in the same transfusion (n=49)
Included any unit with missing data on ABO matching status or units with different ABO matching statuses in the same transfusion (n=259)
Included any units stored 6 or 7 days prior to transfusion, as this storage duration was extremely rare (n=33)
Included any units with missing data on storage duration or any units from different storage duration categories (0 – 2 days, 3 days, 4 days, or 5 days) (n=1298)
Included any volume-reduced units (n=593)
Were given to any of the 41 PLADO subjects who received any HLA-selected units (n=836, of which 314 included HLA-selected units)
Had a TRAE period (start of transfusion to 4 hours post-transfusion) overlapping the TRAE period of any other platelet, granulocyte, or RBC transfusion (n=2037).
Some transfusions had more than one reason for exclusion.
Analyses were carried out for three composite outcomes: any TRAE vs. none, any TRAE of Grade 2 or higher vs. no TRAE of Grade 2 or higher, and any TRAE of Grade 3 or higher vs. no TRAE of Grade 3 or higher. Analyses were also carried out for each specific type of TRAE that occurred in at least 50 (1%) of the transfusions included in the analysis.
For each of these outcomes, a multi-predictor model was fit using a generalized linear model.5 The model included randomized dose group; PLT source; ABO matching status; storage duration (0–2 days, 3 days, 4 days, or 5 days); transfusion number (1st, 2nd, 3rd, 4th, 5th, 6th – 10th, or later PLT transfusion while on study); stratum (autologous/syngeneic SCT, allogeneic SCT, chemotherapy for either solid tumor or hematologic malignancy); age group (0–17 years, 18–64 years, 65 or more years); and gender, plus an interaction term between dose and source, and also took into account the possible correlation of outcomes between different transfusions given to the same subject. If the interaction p-value was > 0.05 the interaction term was dropped from the model.
Some TRAEs may be related to the number of donors contributing to the transfusion. Other TRAEs may be related to the total volume of the transfusion per m2 BSA, the overall infusion rate per m2 BSA for the entire transfusion episode, or the average transfused volume per donor. Generalized linear models were used to compare these characteristics between randomized dose groups, taking into account within-person correlation. Additional generalized linear models were fit for each TRAE outcome to determine if the relationship between platelet dose and the TRAE outcome was due to differences in either number of donors or overall infusion rate.
Because these were hypothesis-generating, exploratory analyses, no adjustment was made for the number of comparisons performed.
RESULTS
There were 8158 PLT transfusions administered to the 1231 patients who received at least one PLT transfusion but no HLA-selected PLTs. After exclusions for missing data or other reasons (Methods), 5034 PLT transfusions to 1102 patients were included in the TRAE analysis. Characteristics of these transfusions are shown in Table 1.
Table 1.
Individual characteristics of the platelet transfusions.
| Platelet or patient characteristic | N (%) |
|---|---|
| Randomized Treatment Group | |
| Low Dose | 2267 (45) |
| Medium Dose | 1668 (33) |
| High Dose | 1099 (22) |
| PLT Source | |
| Apheresis | 3700 (74) |
| WBD | 1334 (26) |
| PLT Storage Duration | |
| 0–2 Days | 406 (8) |
| 3 Days | 1119 (22) |
| 4 Days | 1730 (34) |
| 5 Days | 1779 (35) |
| ABO Matching Status | |
| Identical | 3213 (64) |
| Major Mismatch | 1412 (28) |
| Minor Mismatch | 409 (8) |
| Transfusion Number | |
| 1st | 822 (16) |
| 2nd | 680 (14) |
| 3rd | 565 (11) |
| 4th | 438 (9) |
| 5th | 340 (7) |
| 6th – 10th | 1027 (20) |
| 11th or later | 1162 (23) |
| Recipient Gender | |
| Male | 3109 (62) |
| Female | 1925 (38) |
| Recipient Age Group | |
| 0 – 17 years | 1210 (24) |
| 18 – 64 years | 3351 (67) |
| 65+ years | 473 (9) |
| Recipient Treatment Category | |
| Allogeneic SCT | 2639 (52) |
| Autologous/Syngeneic SCT | 1100 (22) |
| Chemotherapy without SCT | 1295 (26) |
As expected, the total volume of each transfusion per m2 BSA differed by randomized dose group (median 79 mL/m2 for transfusions given to patients in the LD group, 147 mL/m2 in the MD group, and 269 mL/m2 in the HD group, p<0.0001). For a “usual size” patient of 1.7 m2 BSA, these median volumes correspond to 134 mL per transfusion for LD, 250 mL for MD and 457 mL for HD. The transfusion rate for the entire transfusion episode, in mL/minute/m2 BSA, also differed by randomized dose group, with the rate in the LD group significantly lower than rates in the other two groups (medians 2.4, 3.0, and 3.6 for the LD, MD, and HD groups respectively, p<0.001 for the three-group comparison).
For apheresis PLT transfusions, 97% of transfusions in the LD and MD groups were single apheresis units, while only 43% of HD transfusions were single units. The median of the average volume transfused per individual unit was 141 mL for LD, 252 mL for MD, and 259 mL for HD PLTs. For WBD transfusions, the median number of individual donors per transfusion episode was 3 for LD, 5 for MD, and 10 for HD, and the median transfused volume per individual donor was 52 mL in all three groups.
Frequency of transfusion-related adverse events (TRAEs)
There were 310 transfusions (6.2%) associated with a maximum TRAE grade of 1; 150 transfusions (3.0%) with a maximum grade of 2; and 41 transfusions (0.8%) with a maximum grade of 3. No transfusions in the analysis dataset had a Grade 4 TRAE. The number and percentage of PLT transfusions associated with each specific type of TRAE are shown in Figure 1. The most common TRAEs were fever (occurring in 6.6% of transfusions), followed by allergic/hypersensitivity reaction (1.9%), sinus tachycardia (1.8%), and rigors/chills (1.1%).
Fig. 1. Frequency of Transfusion-Related Adverse Events (TRAEs).
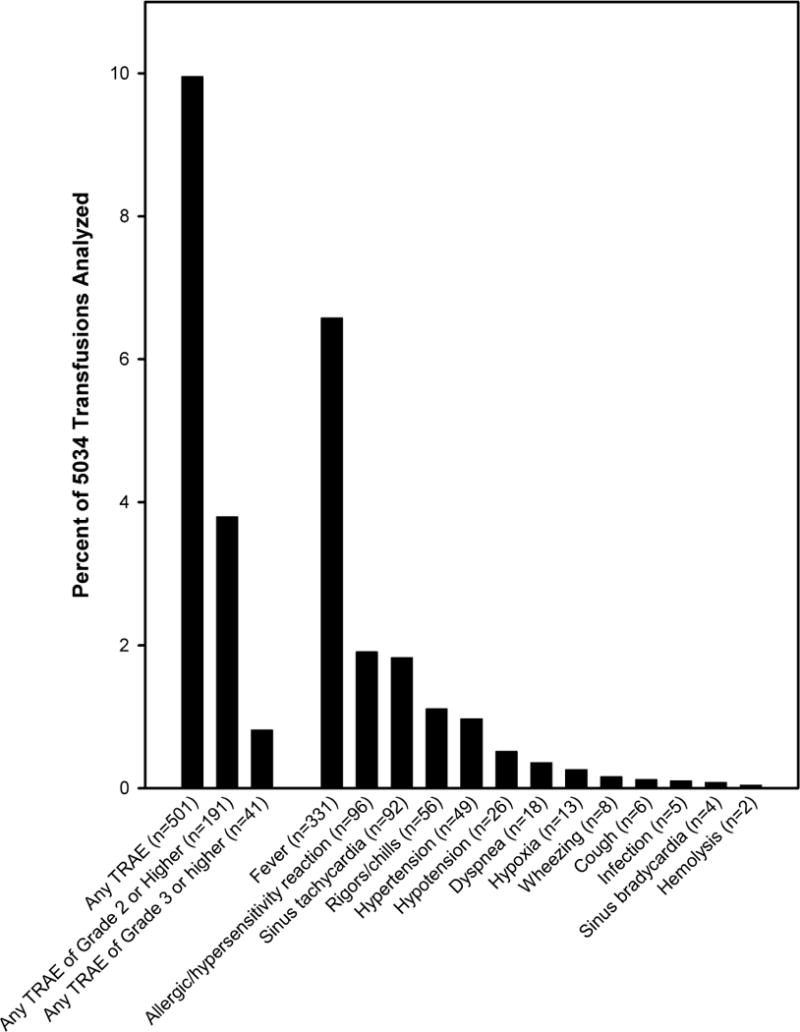
Although seven PLT transfusions in the PLADO study were associated with a Grade 4 TRAE, all seven met one or more of the exclusion criteria for the TRAE analysis.
Relationships between PLT product and recipient characteristics and the occurrence of any TRAE during or within 4 hours after transfusion were evaluated in a multi-predictor model (Figure 2). Platelet dose assignment (low, medium, or high) was a significant predictor of whether any TRAE was associated with the transfusion (p=0.02 for the three-group comparison). LD and MD transfusions had similar risk, but HD transfusions were associated with a higher risk of a TRAE. PLT source, PLT storage duration, and ABO matching status were not significantly related to the risk of any TRAE occurring. The risk of a TRAE tended to decline with later transfusions (p=0.02 for the seven-group comparison), and the comparison with initial PLT transfusions reached statistical significance for the categories of 6th – 10th transfusions and 11th and later transfusions. This trend was not due to patients who experienced any TRAE receiving fewer PLT transfusions than patients without TRAEs. The number of PLT transfusions was higher among patients with any TRAE than in patients with none (median 7 transfusions versus 4, p<0.0001). Autologous SCT recipients were at lowest risk and chemotherapy patients at highest risk of a TRAE occurring, but this did not reach statistical significance (p=0.06 for the three-group comparison).
Fig. 2. Multi-predictor logistic regression model for any TRAE vs. no TRAE.
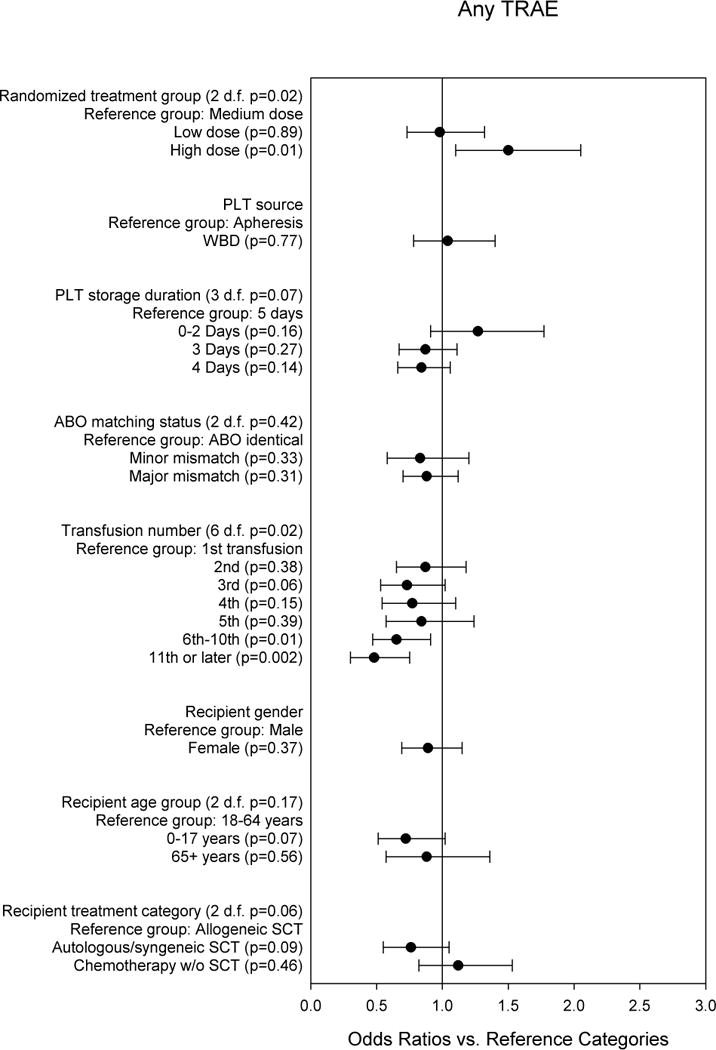
The odds ratios are adjusted for all other variables in the model, and for within-person correlation.
Figure 3 shows the multi-predictor model for the composite outcome of whether at least one TRAE of Grade 2 or higher occurred during or within 4 hours after the transfusion. Transfusions in the LD group were least likely to have a TRAE Grade 2 or higher, whereas transfusions in the HD group were most likely to have this outcome. However, the overall comparison of the three dose groups was not statistically significant (p=0.17). PLT source, storage duration, and ABO matching were not significantly associated with Grade 2 or higher TRAEs. Risk of a TRAE Grade 2 or higher was generally lower for transfusions after the first, although the overall comparison was not statistically significant (p=0.15). Recipient gender, age group, and treatment category were not significantly associated with Grade 2 or higher TRAEs. None of the product or recipient variables in the multi-predictor model was significantly associated with occurrence of a Grade 3 or higher TRAE (data not shown).
Fig. 3. Multi-predictor logistic regression model for any TRAE of Grade 2 or higher vs. no TRAE of Grade 2 or higher.
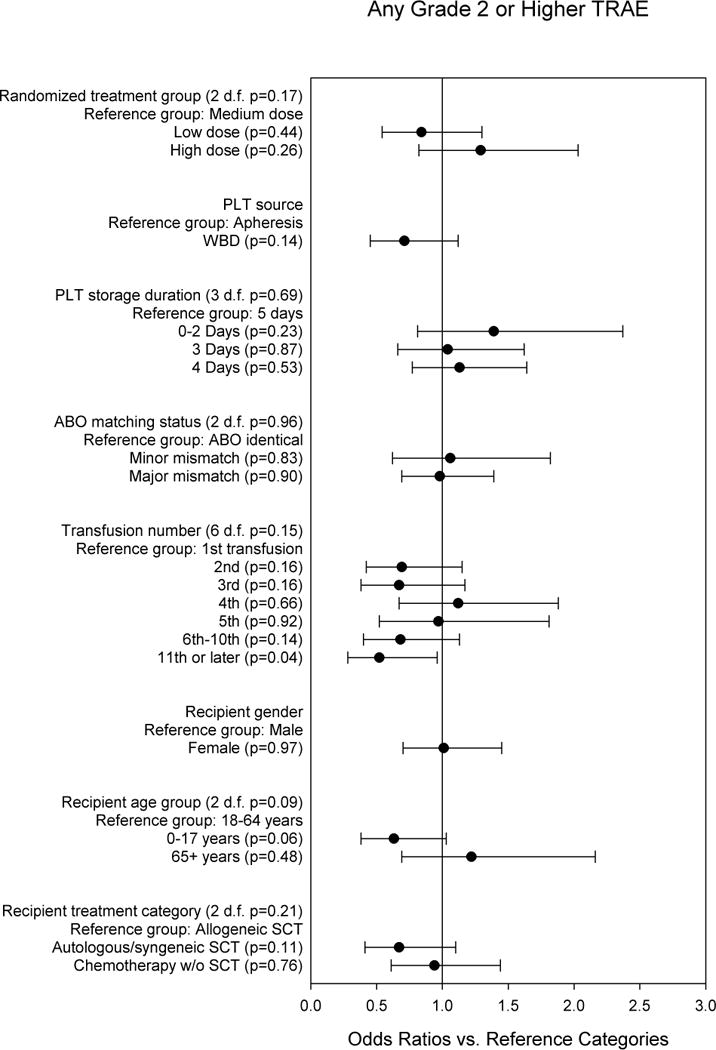
The odds ratios are adjusted for all other variables in the model, and for within-person correlation.
Febrile TRAEs
Figure 4 shows the multi-predictor model for the most common TRAE, fever. PLT dose was a significant predictor of fever, with transfusions in the HD group having a significantly greater risk of fever than transfusions in the MD group. ABO matching status was also a significant predictor, with minor mismatches associated with lower risk.
Fig. 4. Multi-predictor logistic regression model for any fever TRAE vs. no fever TRAE.
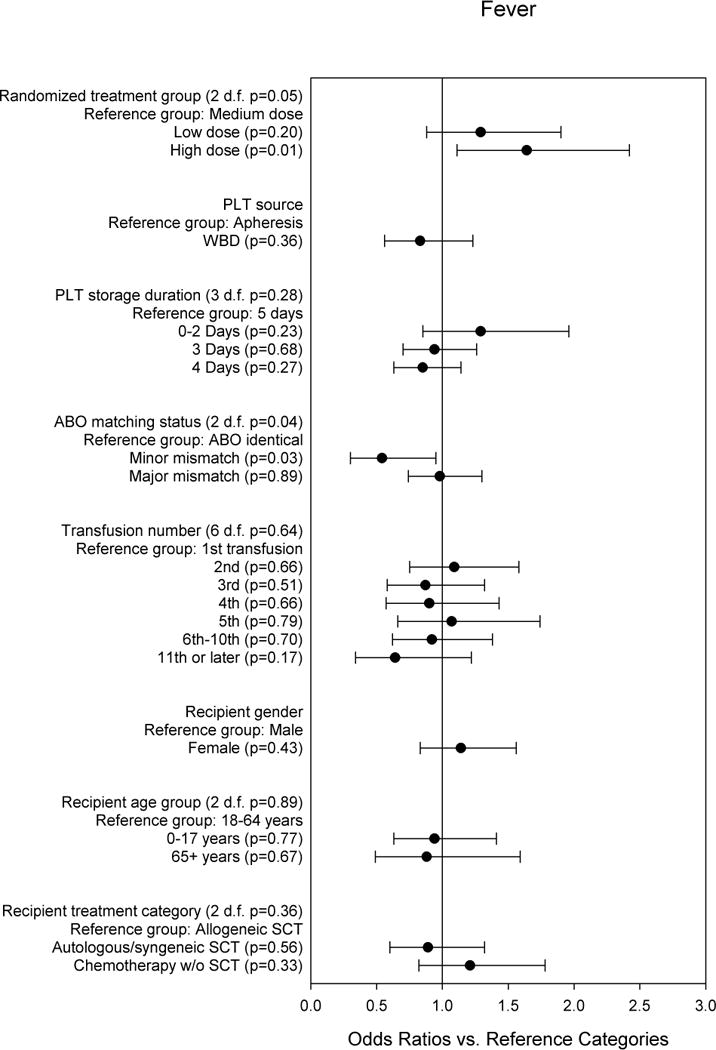
The odds ratios are adjusted for all other variables in the model, and for within-person correlation.
Allergic TRAEs
Figure 5 shows the multi-predictor model for allergic/hypersensitivity TRAEs. There was a significant interaction between PLT dose and PLT source (p=0.04). Among transfusions of apheresis PLTS, LD transfusions had the lowest risk, and MD transfusions had the greatest risk. Among transfusions of WBD platelets, the MD group had the lowest risk, and the HD group had significantly higher risk than the MD group. The risk of allergic/hypersensitivity TRAE of a MD WBD PLT transfusion was significantly lower than that of a MD apheresis PLT transfusion (OR 0.19, 95% CI 0.05–0.76, p=0.02). The risk was also lower for LD WBD PLT transfusions versus LD apheresis PLT transfusions although this was not statistically significant (OR 0.59, 95% CI 0.22–1.59, p=0.30). The observed risk was higher for HD WBD PLT transfusions versus HD apheresis PLT transfusions, but this also was not statistically significant (OR 1.94, 95% CI 0.78–4.82, p=0.15). The risk of an allergic/hypersensitivity reaction tended to decrease with subsequent transfusions (7-group p<0.001, Figure 5). This trend was not due to patients who experienced any allergic/hypersensitivity reaction receiving fewer PLT transfusions than patients who did not experience such a reaction. The number of transfusions was similar among patients with any allergic/hypersensitivity event and those with no such event (median 5 versus 5, p=0.20). Children ages 0–17 years were much less likely than adults to have allergic TRAEs (OR 0.20, 95% CI 0.08–0.51, p=0.001).
Fig. 5. Multi-predictor logistic regression model for any allergic/hypersensitivity TRAE vs. no allergic/hypersensitivity TRAE.
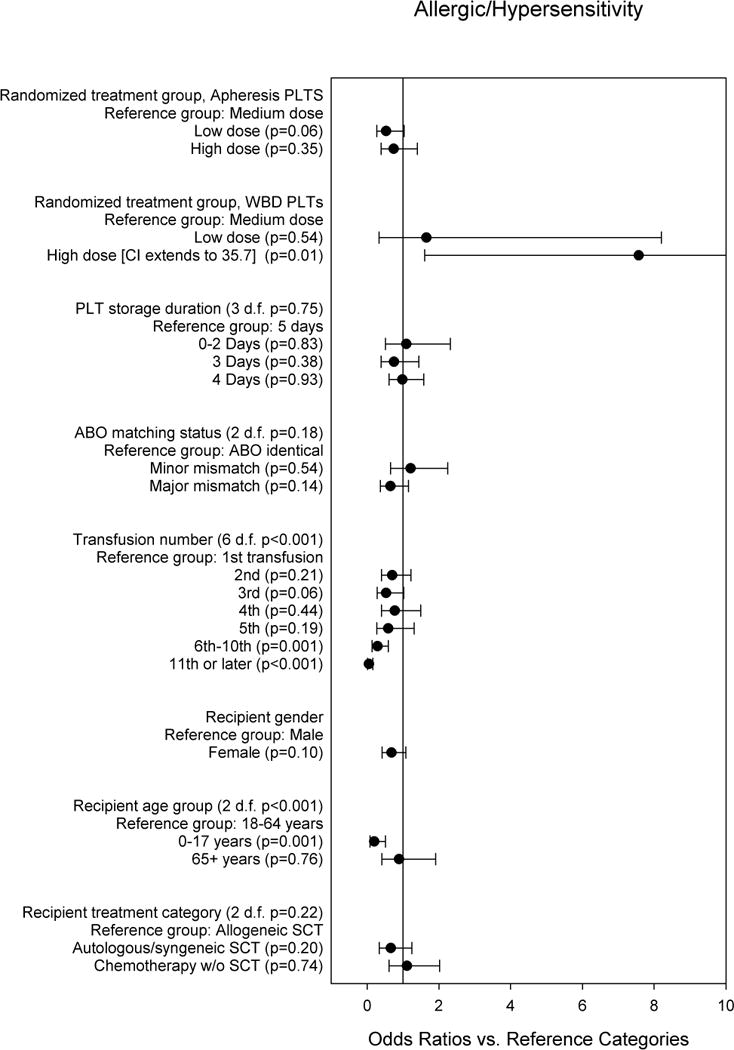
The odds ratios are adjusted for all other variables in the model, and for within-person correlation.
Other TRAEs
Sinus tachycardia was the third most common TRAE reported in the PLADO study, but it was not usually observed in isolation. Among the 92 transfusions with sinus tachycardia, one or more additional TRAEs were reported in 80 (87.0%). Fever occurred in 74 (80.4%) cases of transfusion-related sinus tachycardia. Other TRAEs often associated with sinus tachycardia included: allergic/hypersensitivity reactions (8.7%); rigors/chills (8.7%); dyspnea (6.5%); hypertension (6.5%); hypoxia (6.5%); and hypotension (5.4%). Sinus tachycardia was not significantly associated with any PLT unit characteristics. However, children aged 0–17 years were at much higher risk of sinus tachycardia than adults (OR 5.29, 95% CI 2.86–9.76; three-group p=0.001).
In the multi-predictor model for chills/rigors, the risk appeared to differ between randomized dose groups (OR for the LD vs. MD group: 0.66, 95% CI 0.32–1.36; OR for HD vs. MD: 1.95, 95% CI 0.98–3.88), (three-group p=0.03)). Children aged 0–17 years were at lower risk than adults for chills/rigors (OR compared to 18–64 year group 0.13, 95% CI 0.03–0.50; three-group p<0.001).
Other than those noted above, no other significant associations between PLT unit or recipient characteristics and TRAEs were identified in the multi-predictor models. When number of donors was added to each of the models, it was at least borderline significant for Any TRAE (OR for each additional donor 1.17, p<0.001), Grade 2 or Higher TRAE (OR 1.15, p=0.07), and Fever (OR 1.14, p=0.01). For all three outcomes, the ORs for the dose effects were closer to 1.00 after adjusting for number of donors. For all composite and individual TRAE outcomes analyzed, infusion rate was not statistically significant when added to the multi-predictor model, nor did adding this covariate have a major effect on the relationship between transfusion dose and TRAE outcomes.
DISCUSSION
In this analysis, we assessed whether various characteristics of PLT products and recipients were associated with the risk of TRAEs. PLT dose per transfusion was the most important PLT unit characteristic associated with TRAEs in the PLADO study. PLT dose was a significant predictor of any TRAE versus no TRAE, and of fever and chills/rigors. Although not reaching statistical significance, increasing dose was also associated with increased risk for TRAEs of Grade 2 or higher. An increase in the number of donors per transfusion seems to explain at least some of the association between increased dose and increased risk. For any TRAE, Grade 2 or higher TRAE, and fever, the number of donors had a significant or borderline significant relationship to the outcome, and adding this variable to the model reduced the odds ratio for HD v. MD. Some of the increased risk of HD PLTs may be related to the increased volume of plasma infused per transfusion. In a classic study by Heddle et al6 reported in 1994, plasma and cellular components of non-leukoreduced pooled PLT concentrates were transfused separately and in random order to thrombocytopenic recipients. Most of the febrile nonhemolytic reactions followed transfusion of the plasma fraction, and a strong correlation was seen between the reactions and plasma concentrations of interleukin-1β and -6.
There was some indication that dose was also related to allergic/hypersensitivity reactions, although the pattern of results varied depending on whether the units were apheresis or WBD. This analysis did not provide clear data on the question of whether allergic reactions are more likely to occur with apheresis PLTs versus WBD PLTs.7 LD and MD apheresis PLTS were more likely to be associated with allergic/hypersensitivity TRAEs than WBD PLTS in the respective dose groups. In contrast, HD apheresis PLTs were associated with lower allergic/hypersensitivity TRAE risk than HD WBD PLTS.
The incidence of any TRAE versus none tended to be higher for the initial transfusion to a given patient, and decreased with later transfusions. This relationship appeared to be primarily driven by the association between transfusion number and allergic/hypersensitivity TRAEs. It is possible that this pattern is due to an increased use of premedication among patients with reactions to a previous transfusion. Premedication strategies were not specified in the PLADO protocol, and data were not collected on whether, or which, premedications were administered, so this hypothesis cannot be addressed directly. However, most studies performed to date have failed to demonstrate that premedication, typically with acetaminophen and diphenhydramine, is effective in preventing febrile or allergic transfusion reactions.8–11 In addition, the risk of fevers stayed fairly constant as the number of PLT transfusions rose, making the premedication hypothesis less likely, unless premedication to prevent allergic reactions is more efficacious than premedication to prevent fever. A second possibility is that recurrent exposure to donor PLTs caused recipients to become less likely to experience allergic reactions.12 Experiments performed in volunteers during the 1940s did in fact show a “desensitization” effect on repeat exposure to reconstituted donor serum.13 These two hypotheses are not mutually exclusive.
A minority of the PLT transfusions in PLADO were ABO mismatched, either major (28%) or minor (8%). ABO identical PLT transfusions are well established to provide a better increment than ABO major-mismatched PLT transfusions.14,15 However, the effect of PLT ABO matching on transfusion reaction risks has been less clear.16 In this analysis, ABO matching status was not significantly associated with any of the TRAE outcomes except fever. Minor ABO mismatched PLTs were associated with a lower risk of fever; we speculate that this was a chance association.
Some patient characteristics were associated with TRAE outcomes. Compared to allogeneic HSCT recipients, autologous HSCT recipients were somewhat less likely to have any TRAE, while patients receiving chemotherapy were slightly more likely to have any TRAE. Sinus tachycardia was more common in children than adults. However, allergic reactions and rigors/chills were much less common in children than adults. The published data are conflicting regarding the relative rates of allergic transfusion reactions in children versus adults.17, 18 We speculate that children may be less likely than adults to clearly report symptoms of allergic reactions or other types of reactions.
This study had a number of important limitations. While all PLADO data were collected prospectively, the analysis of TRAEs was a post hoc secondary analysis. Only the dose of prophylactic PLT transfusions was randomized in PLADO, and the results obtained for characteristics other than PLT dose may have been affected by confounding factors that were not included in the models. The primary outcome of the PLADO study was clinical bleeding, and PLADO was not specifically powered to look at TRAE end points, most of which were fairly rare. Some or all of the statistically significant findings may be due to chance. A large number of comparisons were performed, and because this was an exploratory analysis statistical adjustments for multiple comparisons were not made. All patients in the PLADO study were hematology-oncology patients; therefore the results obtained may not be applicable to other patient groups. Presumably, not all of the TRAEs reported in this paper represented true transfusion reactions. For example, in the hematologic malignancy patient population studied, intercurrent infections occur frequently. Many of the fevers classified as TRAEs were probably caused by underlying disease rather than transfused leukoreduced PLTs. Data were not collected on whether the caregivers considered each TRAE to be a true transfusion reaction, or whether there were other more plausible explanations for the event.
Considerable uncertainty exists around the true incidence of adverse reactions caused by PLT transfusions. Febrile non-hemolytic transfusion reactions, for example, have been reported to occur with anywhere from 0.09% to over 27% of PLT transfusions, an extraordinarily wide range.8 Many factors potentially contribute to the variability in published adverse event rates, including prospective vs. retrospective data collection, disparities in reporting, variation in the PLT products transfused (WBD v. apheresis; leukoreduced v. non-leukoreduced, etc.), and differences among the recipient populations studied. Rigorously conducted prospective trials in transfusion medicine provide the potential advantage of capturing more detailed and consistent reporting of TRAEs than is commonly done as part of standard care. Additionally, clinical trial data may allow comparisons to be adjusted for more potentially confounding variables than data from retrospective observational studies. For the current analysis, we were able to leverage the large volume of data systematically collected during the PLADO trial to examine the effects of both product and recipient factors on the risks of various adverse consequences of PLT transfusion. However, even large clinical trials such as PLADO include fewer transfusions than many hemovigilance studies, resulting in lower statistical power, especially for less common outcomes.
The PLADO study showed that PLT dose per transfusion had no impact on whether patients experienced any Grade 2 or higher bleeding, or on the time that it took for Grade 2 or higher bleeding to develop. Additional post hoc analyses of PLADO data demonstrated that PLT source, storage duration, and ABO matching status were not significantly related to clinical bleeding outcomes. These factors were, however, significantly associated with post-transfusion PLT count increments in transfusion recipients. In this secondary analysis, we determined that LD PLT transfusions carried a lower overall TRAE risk than HD PLT transfusions. The TRAE risk of LD transfusions was similar to, and possibly lower than, that of MD PLT transfusions, which approximate current standard care (one apheresis PLT unit or a pool of 4 – 6 WBD units for a typical adult dose). We believe that this analysis further supports the concept that low-dose PLTs are a safe and effective strategy for PLT prophylaxis.
Supplementary Material
Acknowledgments
The authors would like to thank the Transfusion Medicine/Hemostasis Clinical Trials Network investigators, study coordinators, research staff, and patients who participated in this study. R.M.K., S.F.A., D.J.T., R.G.S., P.N., and S.J.S. participated in trial design and conducted the clinical trial; R.M.K., D.J.T., R.G.S., P.N., and S.J.S. collected data; R.M.K., S.F.A., D.J.T., R.G.S., P.N. and S.J.S. analyzed and interpreted the data; S.F.A. and S.G. performed the statistical analyses; and all authors gave critical review and final approval of the manuscript.
Support: Supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health to the Data Coordinating Center at New England Research Institutes (HL072268), Case Western Reserve University (HL072033), Children’s Hospital Boston (HL072291), Cornell University (HL072196), Duke University (HL072291), Emory University (HL072248), Johns Hopkins University (HL072191), Massachusetts General Hospital (HL072299), Puget Sound Blood Center (HL072305), Tulane (HL072274), University of Iowa (HL072028), University of Maryland (HL072359), University of Minnesota (HL072027), University of North Carolina (HL072355), University of Oklahoma (HL072283), University of Pennsylvania (HL072346), University of Pittsburgh (HL072331), and the Blood Center of Wisconsin (HL072290).
Footnotes
Conflicts of interest: Darrell J. Triulzi is on the medical advisory board for Fenwal Fresenius Kabi. Paul Ness is a consultant for TerumoBCT, Lakewood, CO. The remaining authors declare no conflicts of interest.
Reprints will not be available from the author.
References
- 1.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 4.Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Available from http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 5.Lindstrom MJ, Bates DM. Newton-Raphson and EM Algorithms for Linear Mixed Effects Models for Repeated Measures Data. JASA. 1988;83:1014–21. [Google Scholar]
- 6.Heddle NM, Klama L, Singer J, Richards C, Fedak P, Walker I, Kelton JG. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625–8. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 7.Xiao W, Tormey CA, Capetillo A, Maitta RW. Allergic transfusion reactions to platelets are more commonly associated with prepooled than apheresis components. Vox Sang. 2013;105:334–40. doi: 10.1111/vox.12063. [DOI] [PubMed] [Google Scholar]
- 8.Geiger TL, Howard SC. Acetaminophen and diphenhydramine premedication for allergic and febrile nonhemolytic transfusion reactions: good prophylaxis or bad practice? Transfus Med Rev. 2007;21:1–12. doi: 10.1016/j.tmrv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48:2285–91. doi: 10.1111/j.1537-2995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 10.Marti-Carvajal AJ, Sola I, Gonzalez LE, Leon de Gonzalez G, Rodriguez-Malagon N. Pharmacological interventions for the prevention of allergic and febrile non-haemolytic transfusion reactions. Cochrane Database Syst Rev. 2010:CD007539. doi: 10.1002/14651858.CD007539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47:1089–96. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 12.Savage W, Tobian AA, Ness PM, Kaufman RM. Desensitization in allergic transfusion reactions: evidence from the Trial to Reduce Alloimmunization to Platelets. Transfusion. 2014;54:496–8. doi: 10.1111/trf.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maunsell K. Desensitization in Allergic Recipients after Serum Transfusions. Br Med J. 1944;2:236–9. doi: 10.1136/bmj.2.4363.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julmy F, Ammann RA, Taleghani BM, Fontana S, Hirt A, Leibundgut K. Transfusion efficacy of ABO major-mismatched platelets (PLTs) in children is inferior to that of ABO-identical PLTs. Transfusion. 2009;49:21–33. doi: 10.1111/j.1537-2995.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 15.Triulzi DJ, Assmann SF, Strauss RG, Ness PM, Hess JR, Kaufman RM, Granger S, Slichter SJ. The impact of platelet transfusion characteristics on posttransfusion platelet increments and clinical bleeding in patients with hypoproliferative thrombocytopenia. Blood. 2012;119:5553–62. doi: 10.1182/blood-2011-11-393165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shehata N, Tinmouth A, Naglie G, Freedman J, Wilson K. ABO-identical versus nonidentical platelet transfusion: a systematic review. Transfusion. 2009;49:2442–53. doi: 10.1111/j.1537-2995.2009.02273.x. [DOI] [PubMed] [Google Scholar]
- 17.Savage WJ, Tobian AA, Fuller AK, Wood RA, King KE, Ness PM. Allergic transfusion reactions to platelets are associated more with recipient and donor factors than with product attributes. Transfusion. 2011;51:1716–22. doi: 10.1111/j.1537-2995.2010.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage WJ HR, Tobian AAR, Milne GL, Kaufman RM, Savage JH, Borge D, Ness P. Defining risk factors and presentations of allergic reactions to platelet transfusion. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.03.030. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


