Abstract
The employment of high-throughput next-generation sequencing techniques in multiple tumor types during the last few years has identified NTRK1, 2, and 3 gene rearrangements encoding novel oncogenic fusions in 19 different tumor types to date. These recent developments have led us to revisit an old oncogene, Trk, (originally identified as OncD), which encodes the TPM3-NTRK1 gene fusion and was one of the first transforming chromosomal rearrangements identified 32 years ago. However, no drug has yet been approved by the US FDA for cancers harboring this oncogene. This review will discuss the biology of the Trk family of receptors, their role in human cancer, the types of oncogenic alterations, and drugs that are currently in development for this family of oncogene targets.
Introduction
The identification of dominant oncogenic mutations, and our ability to specifically inhibit these genetic abnormalities with targeted inhibitors has altered the therapeutic approach for many cancer patients, particularly those with non-small cell lung cancer (NSCLC). Activating point mutations, in-frame insertions/deletions, gene amplification, and gene rearrangements can serve as predictive biomarkers for oncogene-targeted therapies and thus help select patients that have a high likelihood of benefiting from a particular therapy. There are currently two well-established paradigms of this targeted therapy approach in NSCLC, both of which highlight the potential success of this strategy for other oncogene targets. The treatment of epidermal growth factor receptor (EGFR) mutation positive NSCLC patients (comprising approximately 18% of lung adenocarcinomas) and anaplastic lymphoma kinase, ALK, gene rearrangement positive NSCLC patients (encompassing approximately 5% of lung adenocarcinomas), respond significantly better to the targeted therapies erlotinib and crizotinib, respectively, compared with the standard-of-care chemotherapy (1). EGFR mutation positive patients who are treated with an EGFR tyrosine kinase inhibitor (TKI) have an objective response rate (ORR) of about 70%, and a progression free survival (PFS) time of approximately 10 months, both of which are superior to chemotherapy (2). ALK gene rearrangement positive patients showed a response rate of approximately 65%, and a PFS of approximately 8 months when treated with crizotinib, also superior to chemotherapy (3).
The paradigm of cancer treatment is shifting towards precision oncology. In this model, patients are selected for therapy using predicted biomarkers, such as oncogenic mutations, rather than using empiric chemotherapy. Many of the actionable or potentially actionable oncogenes that represent molecular subtypes in NSCLC involve genomic rearrangements with genes encoding receptor tyrosine kinases (RTKs) such as ALK, ROS1, RET, and most recently NTRK1 (4–7). The unprecedented improvement in patient outcomes with oncogene-targeted therapies suggest that even rare oncogenes, such as ROS1 gene rearrangements (which occur at a frequency of ~1–2%) should be investigated as therapeutic targets, as this molecular subset represents approximately 2,500 patients in the U.S. each year (8, 9). Indeed a recent study of crizotinib in ROS1+ NSCLC patients highlights the ability to successfully accrue rare oncogene subtypes (10). The study of these low frequency oncogenes not only applies to NSCLC, but is also directly relevant to the treatment of numerous other cancer types: ALK, ROS1, RET, and NTRK1 gene rearrangements have also been observed in other malignancies, expanding the relevance of this work to colorectal cancer, thyroid cancer, cholangiocarcinoma, glioblastoma, inflammatory myofibroblastic tumors (IMT), ovarian cancer, bladder cancer, sarcomas, and others (11–17). Indeed, isolated reports show the success of targeting oncogenes across multiple tumor types (15, 18). It was estimated in 2007 that gene fusions were reported in approximately 20% of all cancers accounting for a significant proportion of cancer morbidity and mortality (19). The emergence of high-throughput genomics technologies and programmatic sequencing efforts such as the NCI/NHGRI Cancer Genome Atlas Network and the Sanger Cancer Genome Project have generated the molecular profiles of numerous cancers, and this emergent technology has enabled the identification of many additional gene fusions that are putative oncogenes and predicted to be conserved as drivers across breast, glioblastoma, lung, colorectal cancer, and others tumors (16, 17, 20–22). This article describes the emergence of an increasingly described class of potential oncogene targets in cancer, the Trk family of kinases.
Trk Family Biology
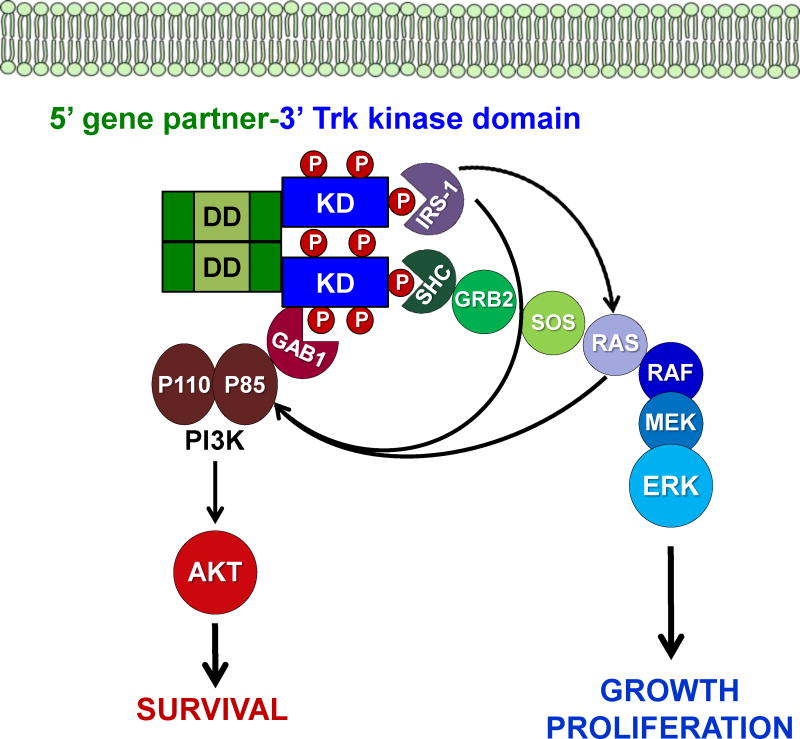
The NTRK1 gene encodes the TrkA receptor tyrosine kinase, the TRK proto-oncogene, which is a member of the Trk (tropomyosin-receptor kinase) family of RTKs that includes TrkB (encoded by NTRK2) and TrkC (encoded by NTRK3) proteins (12, 23). TrkA, B, and C play important roles in nervous system development through their regulation of cell proliferation, differentiation, apoptosis, and survival of neurons in both the central and peripheral nervous systems. The Trk receptors are expressed abundantly in the nervous system, as well as many other non-neuronal cell types and tissues, including monocytes, the lung, bone, and pancreatic beta cells (24). TrkA, B, and C are most frequently activated by their primary ligands nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3 (NT-3), respectively (25, 26). However, overexpression studies in cell lines suggest the possibility of promiscuity among these neurotrophins ligands and all 3 receptors that may be cell-type and neurotrophin concentration dependent (23). Additional studies have shown NT-3 may activate TrkA and TrkB, and NT-4/5 may activate TrkB (27). The binding of each of these ligands to its cognate receptor, such as binding of NGF to TrkA, induces receptor homodimerization and transphosphorylation of five critical tyrosine (Y) residues (Y496, Y676, Y680, Y681, and Y791). Y496 and Y791 serve as phosphorylation-dependent binding sites for various adaptor proteins that contain SH2 or PTB domains, primarily SHC1, PLCγ, and GAB1, but others include FRS2, GRB2, IRS-1, IRS-2, SH2B (12, 28–30) (Fig. 1). Evidence from several studies points to activation of the PI3K signaling pathway from RAS or GAB1, although it may also be activated from other mechanisms (29, 31–33). Once activated, the three wild-type Trk family members most frequently signal through several downstream signaling pathways including SHC/RAS/MAPK, PI3K/AKT, or PLC-γ/PKC, depending on which docking protein(s) bind to the critical phosphorylated tyrosines Y496, and Y791 (34). Activation of these signaling cascades results in transcriptional and other cell programs that mediate cellular proliferation, synaptic plasticity, neurite outgrowth and repair, prevention or repair of neurodegeneration, sensory neuron maintenance, or apoptosis (12, 34–37).
Figure 1. Trk fusion signaling.
Schematic showing common signaling mechanisms for an example of a cytoplasmic (non-membrane bound) chimeric Trk gene fusion are shown. Gene fusions are constitutively activated, or phosphorylated, often as a result of dimerization mediated by sequences in the 5′ gene. SH2 and PTB domain containing adaptors compete for binding at specific tyrosine residues, which most frequently results in propagation of the downstream signaling pathways shown.
It is expected most Trk fusions would employ many or all of the same downstream signaling cascades as the full-length receptors given the preservation of the kinase domain and the critical tyrosine docking sites, however this is a relatively unexplored area compared to decades of detailed studies on the signaling mechanisms employed by the full-length receptors, particularly in the rat pheochromocytoma cell line PC12 (Fig. 1). The ETV6-NTRK3 fusion might be an exception, because it lacks the critical Y845 docking site for the preferential adaptor SHC1 due to the location of the breakpoint in the fusion and evidence points to the use of an alternate adaptor, IRS-1 (38). Cell-type context and differential subcellular localization of fusions might alter the signaling program of the oncogenic fusion kinases.
Studies of TrkA fusions in thyroid cancer have revealed the Trk oncogenes (Trk, Trk-T1-T3) are capable of binding a number of different adaptor molecules, similar to full-length TrkA, but predominantly engaged in signaling through the RAS/RAF/MAPK pathway (39–41) (Fig. 1). The STAT3 signaling pathway was identified for playing a role in NIH-3T3 transformation by TRK oncogenes (42). Interestingly, the constitutive signaling induced by Trk oncogenes has also been shown to result in neuronal differentiation of PC12 cells (43). It was also elegantly demonstrated that the Trk oncogenes are capable of transforming the more relevant in vivo model of cellular transformation, thyroid epithelial cells, not just NIH-3T3 fibroblasts, a more commonly utilized model system for studies of oncogenic transformation (44). Similarly, in vivo transformation of mammary epithelial was shown using the ETV6-NTRK3 fusion, clearly demonstrating the potency of these oncogenes in multiple model systems (45). Studies of TrkA fusion signaling in endogenous colorectal (KM12) and lung cancer (CUTO-3) have been conducted recently by our lab. The TPM3-NTRK1, MPRIP-NTRK1, and CD74-NTRK1 fusions seem to signal predominantly through the SHC/RAS/MAPK pathway in endogenous colorectal and lung cancer cell lines, but can engage PI3K/AKT or STAT3 signaling in certain cell-types (4). Studies of the ETV6-NTRK3 fusion have demonstrated oncogenic signaling is engaged through IRS-1, but due to the limited availability of cell lines expressing the ETV6-NTRK3, most studies were conducted using a cDNA of the fusion expressed in various cell-lines, such as fibroblasts, resulting predominantly in activation of the RAS-MAPK signaling pathway, but also PI3K/AKT, often simultaneously (38) (Fig. 1). The potential for simultaneous, dual activation of multiple downstream pathways may result in a potent oncogene, as this enables the activation of both pro-proliferative and anti-apoptotic pathways (46). Novel studies by the same group have also demonstrated a critical role for upstream RTK signaling, through the Insulin-like Growth Factor Receptor (IGF1R) in ETV6-NTRK3 fusion driven tumorigenesis (38). Studies in mice in select NTRK1 and NTRK3 fusions have suggested these fusions likely play an important, early role in tumor progression (45, 47). Although P75NTR can modulate the activity and signaling of the full length Trk receptors, its interaction with oncogenic Trk fusions have not been studied to date (23).
Trk Family in Cancer
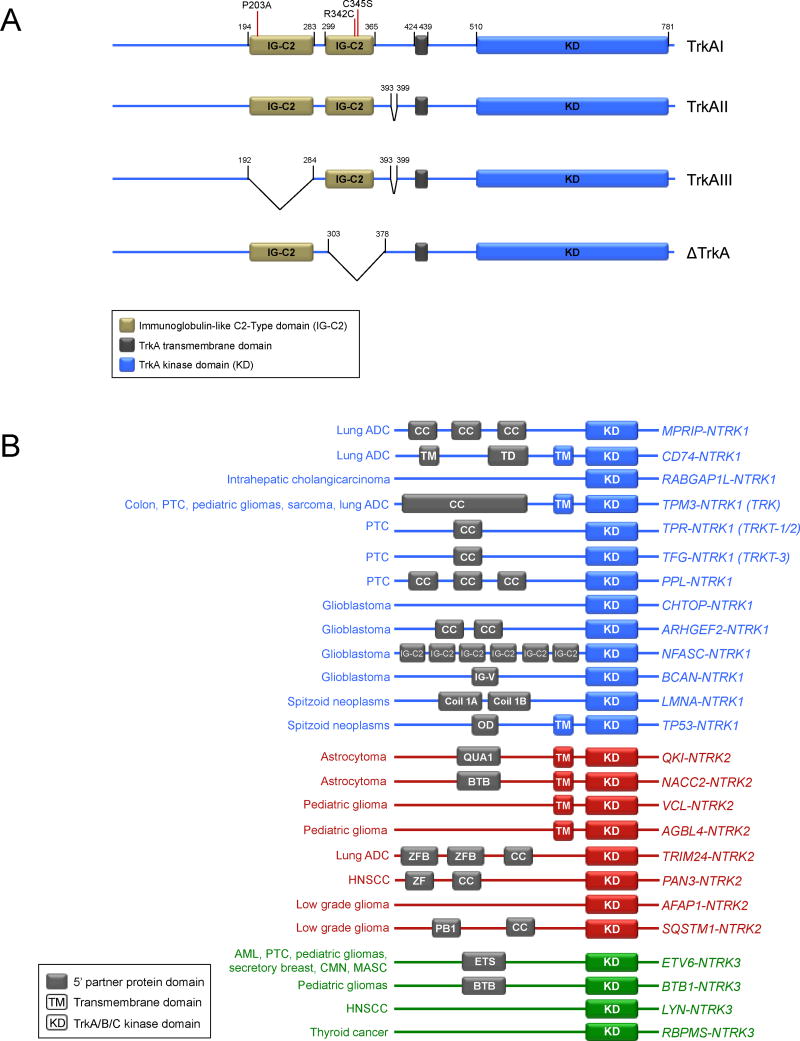
Mutations in Trk family members have been reported in numerous malignancies, including ovarian cancer, colorectal cancer, melanoma, and lung cancer, but among TrkA alterations other than gene fusions, only an in-frame deletion of NTRK1 (ΔTrkA) in acute myeloid leukemia (AML) and a splice variant of NTRK1 (TrkAIII) in neuroblastoma have been functionally characterized as oncogenic to date (48–54) (Fig. 2A). The deletion in ΔTrkA results in the loss of 75 amino acids in the extra-cellular domain of TrkA, removes four glycosylation sites adjacent to the transmembrane domain, and transforms both fibroblasts and epithelial cells (53). The TrkAIII splice variant, which was identified in a neuroblastoma cell line, results in the loss of exons 6, 7, and 9, and the corresponding loss of the extracellular domain IG-C2 (Ig-like C2-type 1) as well as multiple glycosylation sites (51). TrkAIII is constitutively active (ligand-independent), and its expression is promoted by hypoxic conditions (51). Although not yet identified in human tumors samples to date, mutations in the extra-cellular domain of TrkA, P203A and C345S, have both been characterized as transforming (55, 56). These studies may point to regions of interest where mutations have been identified in human tumors; for example, a relative cluster of mutations occur in NTRK1 at the R342 position in close proximity to the C345 site identified by mutagenesis (57). The most common mechanism of oncogenic activation of TrkA is through genomic rearrangement, and the creation of a gene fusion (12). Interestingly, all of these different mechanisms of oncogenic activation of TrkA (gene rearrangements, deletion, and splice variant) contain the loss of some of the extracellular domain of TrkA. The loss of these common sequences suggests the presence of critical regulatory domain(s) in the extracellular domain of TrkA (and potentially B and C) that when lost, results in constitutive activation of the kinase domain and thus its oncogenic capacity and is supported by mutagenesis studies where Ig-like domains in the extracellular region of TrkA were deleted (58).
Figure 2. TrkA oncogenic variants and Trk gene fusion partners.
A. Schematic of TrkA isoforms, deletions, and mutations are shown. Mutations are shown in the TrkAI isoform. Amino acid position numbers are shown in black. B. Schematic showing the known NTRK1 (blue), NTRK2 (red), and NTRK3 (green) fusions and the tumor types in which they have been identified. It is important to note that not all of these gene fusions have yet been characterized functionally, but each one occurred in-frame with an intact Trk kinase domain and are thus potentially oncogenic. Known 5′ dimerization domains are shown in grey, and 3′ domains shown in blue (NTRK1), red (NTRK2), or green (NTRK3). No protein domains are shown for fusions that lack a reported breakpoint. Fusion proteins are not drawn to scale.
Autocrine and paracrine signaling by Trk receptors have been implicated to be pro-tumorigenic in several different tumor types. An autocrine loop involving TrkA and NGF is associated with pro-tumorigenic activity in both breast and prostate carcinoma; similarly, TrkB and BDNF have been shown to play a pro-tumorigenic role in several malignancies, including both breast and prostate cancers (59, 60). TrkB signaling has also been shown to promote anoikis resistance and induce metastatic programs in numerous cancers (61). Expression of TrkA and TrkC wild-type receptors are associated with a positive prognosis in neuroblastoma patients (excluding expression of the splice variant TrkAIII), while TrkB expression is correlated with a poorer prognosis (62, 63).
Trk family oncogenic fusions
The typical gene structure for an oncogenic fusion is that the 3′ region of a proto-oncogene (encoding the kinase-domain) is juxtaposed to 5′ sequences from an unrelated gene via an intra- or interchromosomal rearrangement. The resultant novel oncogene is both aberrantly expressed and has constitutive activation of the kinase domain. In 1982, the same year that the BCR and ABL genes were implicated in the first oncogenic translocation on the Philadelphia chromosome in chronic myelogenous leukemia (CML), the first NTRK1 gene fusion was identified in a colon cancer sample and contained sequences from TPM3 (non-muscle tropomyosin) (64, 65). The incidence and therapeutic potential of TPM3-NTRK1 in colorectal cancer was recently revisited after 32 years by Isacchi and colleagues, reaffirming that this NTRK1 fusion is indeed a recurrent, albeit infrequent, oncogene in colon cancer (22). Each of the identified colorectal cases harboring NTRK1 fusions identified thus far express the TPM3-NTRK1 oncogene, suggesting a preference for TPM3 as the partner gene in this particular tissue, similar to EML4 with ALK in lung cancer (4, 11, 16, 22, 64). Additionally, TrkC and very recently TrkB have also been shown to form oncogenic chimeras in multiple tumor types (66, 67) (Fig. 2B). The ETV6-NTRK3 fusion has been identified as the dominant oncogene in several malignancies, including secretory breast carcinoma, mammary analogue secretory carcinoma (MASC) of the salivary gland, congenital fibrosarcoma, congenital mesoblastic nephroma, acute myeloid leukemia, and radiation-associated papillary thyroid cancer (45, 67–74). ETV6-NTRK3 fusions can vary slightly with regard to the breakpoint in different cancer types, but always retain the SAM dimerization domain from ETV6 and the kinase domain of TRKC. Chromosomal rearrangements have been observed between NTRK1 and TFG, TPM3, or TPR in papillary thyroid carcinoma (PTC), the most common malignancy of the thyroid (12). Interestingly, many of these activating 5′ gene fusion partners are promiscuous amongst various kinase fusion classes (12, 16). While each of the in-frame NTRK1 and NTRK3 fusions identified thus far fit the paradigm and contain a 5′ gene partner with a dimerization domain, at least two of the NTRK2 partners do not (Fig. 2B). 5′ gene partners often contain one or more dimerization domains, such as the stereotypical coiled-coil domain(s), and the corresponding constitutive tyrosine kinase activity that occurs results in uninterrupted downstream signaling messages for the cell to proliferate aberrantly and survive (12). Oncogenic gene rearrangements involving ROS1 may be an exception to this paradigm, as many of the 5′ gene partners have no known dimerization domains, but have still been shown to possess transforming properties (16). One can speculate based on this that activation of ROS1 may only require loss of 5′ sequences that act as an autoinhibitory signal in the full-length RTK. A similar mechanism may be worth investigating in Trk family fusions. VCL-NTRK2 and AGBL4–NTRK2 have not been functionally characterized for transforming properties, but may be activated without a 5′ dimerization domain through the loss of a regulatory domain or by a different unknown mechanism, similar to many ROS1 fusions.
NGS identification of NTRK1, 2, and 3 fusions
In the last year, many next generation sequencing (NGS) efforts, including programmatic, disease-oriented whole genome and/or transcriptome projects and also targeted clinical NGS platforms have resulted in the identification of NTRK family fusions in numerous tumor types. NTRK1 fusions were recently identified in lung adenocarcinoma, intrahepatic cholangiocarcinoma, spitzoid neoplasms, glioblastoma, and pontine glioma (4, 75–79). These findings were further validated in lung adenocarcinoma, as well as the discovery of novel fusions in papillary thyroid cancer, and glioblastoma using a novel, targeted technique known as anchored multiplex PCR (80). It is also important to note that while Trk fusions were not detected initially by the TCGA, revisiting these data in multiple tumor types with a different bioinformatics algorithm resulted in the identification of multiple new oncogenic fusions (9, 17). The first evidence of gene fusions involving the NTRK2 gene came in pilocytic astrocytoma and very soon afterwards in pontine glioma (66, 74, 75). New tumor types with NTRK3 fusions were also identified, including both PTC, pontine glioma, and Philadelphia chromosome like acute lymphoblastic leukemia (Ph-ALL) (68, 75, 81). Each of the three NTRK family genes can rearrange with multiple 5′ gene partners (Fig. 2). A recent study, mentioned briefly above, revisited TCGA data from 20 different cancers with a more efficient computational pipeline for the detection gene fusions, and was able to identify Trk fusions in 8 additional tumor types (17) (Table 1).
Table 1. Oncogenic Trk fusions are found across multiple tumor types.
The frequency of NTRK1 (blue), NTRK2 (red), and NTRK3 (green) gene fusions indicating the tumor type and the detection method that was employed in each study. Only positive studies are listed and thus the actual prevalence may be lower than reported.
| Oncogene | Cancer | Frequency | Detection method(s) |
|---|---|---|---|
| NTRK1 gene fusions | Lung adenocarcinoma | 3/91 = 3.3% | Targeted NGS (FMI), FISH4 |
| NTRK1 gene fusions | Intrahepatic cholangiocarcinoma | 1/28 = 3.6% | Targeted NGS (FMI)77 |
| NTRK1 gene fusions | Colorectal cancer | 3 isolated reports 1/66= 1.5% |
cDNA library, FISH, PCR4, 11, 63 PCR, IHC22 |
| NTRK1 gene fusions | Papillary thyroid cancer | 28/228 = 12.3% | PCR12 |
| NTRK1 gene fusions | Spitzoid neoplasms | 23/140 = 16.4% | Targeted NGS (FMI), FISH, IHC78 |
| NTRK1 gene fusions | Glioblastoma | 2/185 = 1.1% 4/162 = 2.5% 1/157 |
NGS75 NGS/PCR76 |
| NTRK1 gene fusions | Sarcoma (TCGA) | 1/103=1% | RNA-SEQ17 |
| NTRK2 gene fusions | Astrocytoma | 3/96 = 3.1% | NGS65 |
| NTRK2 gene fusions | Lung adenocarcinoma (TCGA) | 1/513=0.2% | RNA-SEQ17 |
| NTRK2 gene fusions | Head and neck squamous cell carcinoma (TCGA) | 1/411=0.2% | RNA-SEQ17 |
| NTRK2 gene fusions | Brain lower grade glioma (TCGA) | 2/461=0.4% | RNA-SEQ17 |
| NTRK3 gene fusions | Secretory breast carcinoma | 12/13 = 92% | FISH, PCR44 |
| NTRK3 gene fusions | Mammary analogue secretory carcinoma | 15/15 = 100% | FISH69 |
| NTRK3 gene fusions | Papillary thyroid cancer | 9/62 = 14.5%* 7/243= 2.9%+ |
RNA-SEQ67, 73 |
| NTRK3 gene fusions | Acute myeloid leukemia | 2 case reports | PCR, FISH68, 72 |
| NTRK3 gene fusions | Congenital mesoblastic nephroma | 5/6 = 83% | PCR and FISH66 |
| NTRK3 gene fusions | Congenital fibrosarcomas | 10/11 = 91% 5/5 = 100% |
PCR71 PCR and FISH66 |
| NTRK3 gene fusions | Ph-like Acute Lymphoblastic Leukemia | 1/154=0.7% | NGS79 |
| NTRK3 gene fusions | Colon adenocarcinoma (TCGA) | 2/286=0.7% | RNA-SEQ17 |
| NTRK3 gene fusions | Thyroid Carcinoma (TCGA) | 7/498=1.5% | RNA-SEQ17 |
| NTRK3 gene fusions | Skin Cutaneous Melanoma (TCGA) | 1/374=0.3% | RNA-SEQ17 |
| NTRK3 gene fusions | Head and neck squamous cell carcinoma (TCGA) | 1/411=0.2% | RNA-SEQ17 |
| NTRK1/NTRK2/NTRK3 | Pediatric gliomas | 8/112 = 7.1% | NGS74 |
post-Chernobyl
sporadic
A unique biological aspect of the ETV6-NTRK3 fusion is that it was the first oncogenic gene fusion to be identified in numerous different cancer tumor tissues. In each of those different tumor types, all of which are relatively rare malignancies, ETV6-NTRK3 is the dominant oncogene. For example 100% of mammary analogous secretory carcinoma of salivary glands (MASC) and 93% of secretory breast cancers harbor ETV6-NTRK3 fusions (45, 70). This observation is similar to CML where BCR-ABL is found in the vast majority of cases. However, in most tumors where Trk fusions are identified, they represent only a small proportion of patients (Table 1). Collectively, the Trk family represents a sizeable number of cases distributed across multiple tumor types.
Trk inhibitors
Given the long history of oncogenic Trk alterations, one might ask why it has taken so long to develop drugs for this target in cancer. Several reasons likely contribute to the slow development of this target including the lack of selective inhibitors and the relative difficulty in screening large tumor cohorts when this oncogene was first identified in the early 1980s. Similar to Trk, oncogenic ALK gene rearrangements were found to be important in cancer long before the first ALK inhibitor was FDA approved. The first ALK gene rearrangement was identified in anaplastic large cell lymphoma in 1994, but no ALK-targeted therapies were developed in this disease until many years later. The critical moment for ALK inhibitor development came in 2007 with the discovery of ALK gene rearrangements in NSCLC (5). The corresponding FDA approval of crizotinib for ALK+ metastatic NSCLC was exceedingly fast taking only 4 years from the time of first identification of ALK in this patient population(5). This rapid approval highlights the successful strategy of precision oncology by matching targeted therapies with biomarker-selected patients. Neurotrophins and Trk receptors, particularly TrkA, have been pursued in the past as drug targets for the treatment of chronic pain (35), and a few studies have pursued the Trk family as an therapeutic target in cancer, (60, 82, 83). High levels of homology between TrkA, B, and C within the intracellular kinase domains have resulted in the synthesis of small molecule inhibitors that target all three Trk family members (pan-Trk inhibitors).
Clinical trials of Trk inhibitors will need to investigate potential side-effects that may arise from inhibition of the full length Trk receptors in normal tissues. Loss of normal regulation of TrkA, B or C receptor activity can result in numerous human diseases. Trk receptors are known for mediating pain sensation and can play a role in chronic pain (35, 84). TrkA loss-of-function mutations are seen in class IV hereditary sensory and autonomic neuropathies (HSAN), such as the genetic disorder congenital insensitivity to pain with anhidrosis (CIPA) (36, 84). Loss-of-function mutations in TrkB result in energy imbalances, the loss of appetite control and obesity, and neuronal defects such as memory impairment (37). Similarly, loss of BDNF expression in the cerebellum of the mutant stargazer mouse is associated with a severe ataxia phenotype (85) and TrkB homozygous mutant mice have severe neurologic deficits (37), suggesting a critical role for the BDNF/TrkB signaling axis in normal neurologic development. However, it remains unclear if inhibition of the full-length TRK receptors will produce symptoms that mimic developmental loss of signaling is this receptor family. A more detailed evaluation of Trk receptors in non-cancer related diseases is beyond the scope of this review, but can be found in other reviews (86, 87). Interestingly, one of the potential beneficial side effects of targeting Trk receptors in cancer might be a decrease in pain sensation, a frequent symptom among cancer patients. Conversely, it will important to monitor for potential neurologic side effects in clinical trials given the expression pattern of the Trk family of receptors. Currently, little data exists on the toxicities of the more selective Trk inhibitors. A phase I study of PHA-848125AC with significant blood-brain penetration produced dose-limiting (grade 3) ataxia and tremors, but as this drug inhibits cyclin-dependent kinases in addition to TrkA, it is unclear which drug target was responsible for these side effects (88). One of the largest reported studies of a drug with TrkA inhibition was a randomized study of lestaurtinib in FLT3 mutant AML (89). Only one neurologic adverse event was noted, a death due to cerebellar toxicity, however given the multiple kinase targets of this drug, no conclusions can be drawn about the relationship of this toxicity to TrkA inhibition. Monoclonal antibodies against TrkA or NGF have been developed for the treatment of pain (35); however, antibodies to TrkA or other Trk family members would not be effective against Trk fusions as the extracellular domains for these fusions are routinely lost in the gene rearrangement.
Currently, several TKIs with activity against the Trk family are being explored in clinical trials (Table 2). Two patients with identified NTRK1 fusions were recently treated with inhibitors that demonstrated the potential clinical benefit of targeting this family of oncogenes. The first was a lung cancer patient harboring the MPRIP-NTRK1 fusion, who was treated off-label with the multi-kinase inhibitor crizotinib (4). Crizotinib has only modest activity against TrkA and only produced a proportionally transient, minor radiographic response emphasizing the need for more potent Trk inhibitors in the clinic. More recently, a colorectal cancer patient with a TPM3-NTRK1 gene fusions was treated in the phase I portion of a clinical trial investigating an interrupted dosing schedule of RXDX-101 (Ignyta) (90). RXDX-101 is a pan-Trk inhibitor that also has activity against two other gene fusion targets, ALK and ROS1. The TrkA+ (TPM3-NTRK1) patient experienced a partial response and provides the first evidence of clinical activity of a Trk inhibitor in a patient with an oncogenic Trk alteration (90). A phase I/II study entitled STARTRK-1 of RXDX-101 is currently accruing patients with Trk alterations (NCT02097810). LOXO-101 (Loxo Oncology) is a selective pan-TRK inhibitor that has no significant activity outside of the Trk family and is currently being investigated in a phase Ia/Ib trial across multiple tumor types (NCT02122913). TSR-011 (Tesaro) is a TrkA and ALK inhibitor that is currently in phase I study (NCT02048488). PLX-7486 (Plexxikon) is a pan-TRK inhibitor that also targets Fms. It is currently being investigated as monotherapy or in combination with nab-paclitaxel in pancreatic cancer, but there are plans to explore activity in patients with oncogenic Trk alterations in the future (NCT01804530). DCC-701 (Deciphera), XL-184 (Exelexis), and MGCD516 (Mirati) multi-kinase inhibitors all have trials are all also currently open for several different molecular sub-types, including Trks. Lestaurtinib (CEP-701; Cephalon) showed promising pre-clinical activity in NTRK1 fusion models, but its clinical development remains unclear (4).
Table 2. Trk inhibitors under development.
A chart listing the Trk inhibitors that are currently in clinical trials, additional non-Trk targets, the current stage of clinical development, and the identifier for the relevant clinical trial at clinicaltrials.gov.
| Drug | Stage of Development | Targets | Clinical Trial Identifier |
|---|---|---|---|
| DCC-2701 | Phase 1A/1B in advanced solid tumors | TRKA/B/C, MET, TIE2, VEGFR | NCT02228811 |
| LOXO-101 | Phase Ia/Ib in patients with genetic alterations in TRKA, TRKB, or TRKC | TRKA/B/C | NCT02122913 |
| MGCD516 | Phase 1/1b in patients with advanced NSCLC with genetic alterations in MET, AXL, RET, TRK, DDR2, KDR, PDGFRA or KIT. or HNSCC with alterations in MET | TRK, MET, AXL, RET, DDR2, KDR, PDGFRA, and KIT | NCT02219711 |
| PLX7486 | Phase I as single agent and in combination with gemcitabine & nab-paclitaxel in solid tumors (pancreatic cancer expansion cohort) | TRKA/B/C, Fms | NCT01804530 |
| RXDX-101 | Phase Ia/Iia in patients with genetic alterations in TRKA, TRKB, TRKC, ROS1, and ALK | TRKA/B/C, ALK, ROS1 | NCT02097810 |
| TSR-011 | Phase I/Iia in solid tumors and hematologic malignancies (ALK or TRKA positive) | TRKA, ALK | NCT02048488 |
| XL-184 | Phase II in advanced NSCLC with NTRK, RET, or ROS1+ fusions, or increased MET or AXL activity | TRKA, RET, AXL | NCT01639508 |
Summary
Oncogenic Trk is one of the first oncogenes identified more than 3 decades ago. The Trk oncogenes occur across a broad array of tumor types. Oncogenic fusions involving NTRK1, NTRK2, and NTRK3 and in-frame deletions or splice variants of NTRK1 are likely to be actionable oncogenes based on pre-clinical data. The first clinical evidence of tumor response in a patient with a TrkA fusion suggests that this family of oncogenes will represent a new valid drug target in cancer.
Key concepts.
NTRK1 gene fusions were first identified in colon cancer in 1982, but have since been identified in 8 solid tumor types in recent years; gene fusions involving the highly homologous, NTRK2 and NTRK3 genes have also been identified in 11 different tumor types.
Drugs with activity against the Trk family of receptor tyrosine kinases are currently in development for patients with oncogenic alterations in NTRK1, 2, and 3.
Acknowledgments
Grant Support
This work was supported by the V Foundation for Cancer Research, NIH/NCI P50 CA058187 (University of Colorado Lung Cancer SPORE), NIH/NCI 5K12CA086913, and NIH/NCATS UL1 TR000154 (Colorado CTSI) to RCD.
Footnotes
Disclosures: Consulting from LOXO Oncology, Research grant from Mirati Therapeutics and Consulting and research grant from Pfizer to RCD. Licensed patent to Abbott Molecular to RCD and ATL.
References
- 1.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–80. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 4.Vaishnavi A, Capelletti M, Le AT, Kako S, Butaney M, Ercan D, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19:1469–72. doi: 10.1038/nm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 6.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Ju YS, Lee WC, Shin JY, Lee S, Bleazard T, Won JK, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–45. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 9.Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-Rearranged Non-Small-Cell Lung Cancer. N Engl J Med. 2014 doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743–8. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- 12.Greco A, Miranda C, Pierotti MA. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol Cell Endocrinol. 2010;321:44–9. doi: 10.1016/j.mce.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Perot G, Soubeyran I, Ribeiro A, Bonhomme B, Savagner F, Boutet-Bouzamondo N, et al. Identification of a recurrent STRN/ALK fusion in thyroid carcinomas. PLoS One. 2014;9:e87170. doi: 10.1371/journal.pone.0087170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aisner DL, Nguyen TT, Paskulin DD, Le AT, Haney J, Schulte N, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res. 2014;12:111–8. doi: 10.1158/1541-7786.MCR-13-0479-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory Myofibroblastic Tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014 doi: 10.1158/2159-8290.CD-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–81. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 17.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011;364:775–6. doi: 10.1056/NEJMc1013224. [DOI] [PubMed] [Google Scholar]
- 19.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nature reviews Cancer. 2007;7:233–45. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462:1005–10. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ardini E, Bosotti R, Borgia AL, De Ponti C, Somaschini A, Cammarota R, et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol Oncol. 2014 doi: 10.1016/j.molonc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 24.Coppola V, Barrick CA, Southon EA, Celeste A, Wang K, Chen B, et al. Ablation of TrkA function in the immune system causes B cell abnormalities. Development. 2004;131:5185–95. doi: 10.1242/dev.01383. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–60. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Jing SQ, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–97. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 27.Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–93. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- 28.Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 29.Holgado-Madruga M, Moscatello DK, Emlet DR, Dieterich R, Wong AJ. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci U S A. 1997;94:12419–24. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian X, Riccio A, Zhang Y, Ginty DD. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron. 1998;21:1017–29. doi: 10.1016/s0896-6273(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 32.Ashcroft M, Stephens RM, Hallberg B, Downward J, Kaplan DR. The selective and inducible activation of endogenous PI 3-kinase in PC12 cells results in efficient NGF-mediated survival but defective neurite outgrowth. Oncogene. 1999;18:4586–97. doi: 10.1038/sj.onc.1202814. [DOI] [PubMed] [Google Scholar]
- 33.Baxter RM, Cohen P, Obermeier A, Ullrich A, Downes CP, Doza YN. Phosphotyrosine residues in the nerve-growth-factor receptor (Trk-A). Their role in the activation of inositolphospholipid metabolism and protein kinase cascades in phaeochromocytoma (PC12) cells. Eur J Biochem. 1995;234:84–91. doi: 10.1111/j.1432-1033.1995.084_c.x. [DOI] [PubMed] [Google Scholar]
- 34.Loeb DM, Stephens RM, Copeland T, Kaplan DR, Greene LA. A Trk nerve growth factor (NGF) receptor point mutation affecting interaction with phospholipase C-gamma 1 abolishes NGF-promoted peripherin induction but not neurite outgrowth. J Biol Chem. 1994;269:8901–10. [PubMed] [Google Scholar]
- 35.Siniscalco D, Giordano C, Rossi F, Maione S, de Novellis V. Role of neurotrophins in neuropathic pain. Curr Neuropharmacol. 2011;9:523–9. doi: 10.2174/157015911798376208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13:485–8. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 37.Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–22. [PubMed] [Google Scholar]
- 38.Morrison KB, Tognon CE, Garnett MJ, Deal C, Sorensen PH. ETV6-NTRK3 transformation requires insulin-like growth factor 1 receptor signaling and is associated with constitutive IRS-1 tyrosine phosphorylation. Oncogene. 2002;21:5684–95. doi: 10.1038/sj.onc.1205669. [DOI] [PubMed] [Google Scholar]
- 39.Ranzi V, Meakin SO, Miranda C, Mondellini P, Pierotti MA, Greco A. The signaling adapters fibroblast growth factor receptor substrate 2 and 3 are activated by the thyroid TRK oncoproteins. Endocrinology. 2003;144:922–8. doi: 10.1210/en.2002-221002. [DOI] [PubMed] [Google Scholar]
- 40.Miranda C, Greco A, Miele C, Pierotti MA, Van Obberghen E. IRS-1 and IRS-2 are recruited by TrkA receptor and oncogenic TRK-T1. J Cell Physiol. 2001;186:35–46. doi: 10.1002/1097-4652(200101)186:1<35::AID-JCP1003>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 41.Roccato E, Miranda C, Ranzi V, Gishizki M, Pierotti MA, Greco A. Biological activity of the thyroid TRK-T3 oncogene requires signalling through Shc. Br J Cancer. 2002;87:645–53. doi: 10.1038/sj.bjc.6600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miranda C, Fumagalli T, Anania MC, Vizioli MG, Pagliardini S, Pierotti MA, et al. Role of STAT3 in in vitro transformation triggered by TRK oncogenes. PLoS One. 2010;5:e9446. doi: 10.1371/journal.pone.0009446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greco A, Orlandi R, Mariani C, Miranda C, Borrello MG, Cattaneo A, et al. Expression of TRK-T1 oncogene induces differentiation of PC12 cells. Cell Growth Differ. 1993;4:539–46. [PubMed] [Google Scholar]
- 44.Russell JP, Powell DJ, Cunnane M, Greco A, Portella G, Santoro M, et al. The TRK-T1 fusion protein induces neoplastic transformation of thyroid epithelium. Oncogene. 2000;19:5729–35. doi: 10.1038/sj.onc.1203922. [DOI] [PubMed] [Google Scholar]
- 45.Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–76. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 46.Tognon C, Garnett M, Kenward E, Kay R, Morrison K, Sorensen PH. The chimeric protein tyrosine kinase ETV6-NTRK3 requires both Ras-Erk1/2 and PI3-kinase-Akt signaling for fibroblast transformation. Cancer Res. 2001;61:8909–16. [PubMed] [Google Scholar]
- 47.Edel MJ, Shvarts A, Medema JP, Bernards R. An in vivo functional genetic screen reveals a role for the TRK-T3 oncogene in tumor progression. Oncogene. 2004;23:4959–65. doi: 10.1038/sj.onc.1207667. [DOI] [PubMed] [Google Scholar]
- 48.Marchetti A, Felicioni L, Pelosi G, Del Grammastro M, Fumagalli C, Sciarrotta M, et al. Frequent mutations in the neurotrophic tyrosine receptor kinase gene family in large cell neuroendocrine carcinoma of the lung. Hum Mutat. 2008;29:609–16. doi: 10.1002/humu.20707. [DOI] [PubMed] [Google Scholar]
- 49.Geiger TR, Song JY, Rosado A, Peeper DS. Functional characterization of human cancer-derived TRKB mutations. PLoS One. 2011;6:e16871. doi: 10.1371/journal.pone.0016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomasson MH, Xiang Z, Walgren R, Zhao Y, Kasai Y, Miner T, et al. Somatic mutations and germ line sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood. 2008;111:4797–808. doi: 10.1182/blood-2007-09-113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tacconelli A, Farina AR, Cappabianca L, Desantis G, Tessitore A, Vetuschi A, et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell. 2004;6:347–60. doi: 10.1016/j.ccr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Harada T, Yatabe Y, Takeshita M, Koga T, Yano T, Wang Y, et al. Role and relevance of TrkB mutations and expression in non-small cell lung cancer. Clin Cancer Res. 2011;17:2638–45. doi: 10.1158/1078-0432.CCR-10-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reuther GW, Lambert QT, Caligiuri MA, Der CJ. Identification and characterization of an activating TrkA deletion mutation in acute myeloid leukemia. Mol Cell Biol. 2000;20:8655–66. doi: 10.1128/mcb.20.23.8655-8666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miranda C, Mazzoni M, Sensi M, Pierotti MA, Greco A. Functional characterization of NTRK1 mutations identified in melanoma. Genes Chromosomes Cancer. 2014 doi: 10.1002/gcc.22200. [DOI] [PubMed] [Google Scholar]
- 55.Coulier F, Kumar R, Ernst M, Klein R, Martin-Zanca D, Barbacid M. Human trk oncogenes activated by point mutation, in-frame deletion, and duplication of the tyrosine kinase domain. Mol Cell Biol. 1990;10:4202–10. doi: 10.1128/mcb.10.8.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arevalo JC, Conde B, Hempstead BI, Chao MV, Martin-Zanca D, Perez P. A novel mutation within the extracellular domain of TrkA causes constitutive receptor activation. Oncogene. 2001;20:1229–34. doi: 10.1038/sj.onc.1204215. [DOI] [PubMed] [Google Scholar]
- 57.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arevalo JC, Conde B, Hempstead BL, Chao MV, Martin-Zanca D, Perez P. TrkA immunoglobulin-like ligand binding domains inhibit spontaneous activation of the receptor. Mol Cell Biol. 2000;20:5908–16. doi: 10.1128/mcb.20.16.5908-5916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolle L, Adriaenssens E, El Yazidi-Belkoura I, Le Bourhis X, Nurcombe V, Hondermarck H. Nerve growth factor receptors and signaling in breast cancer. Curr Cancer Drug Targets. 2004;4:463–70. doi: 10.2174/1568009043332853. [DOI] [PubMed] [Google Scholar]
- 60.Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, et al. Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res. 2001;7:2237–45. [PubMed] [Google Scholar]
- 61.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328:847–54. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 63.Tacconelli A, Farina AR, Cappabianca L, Gulino A, Mackay AR. Alternative TrkAIII splicing: a potential regulated tumor-promoting switch and therapeutic target in neuroblastoma. Future Oncol. 2005;1:689–98. doi: 10.2217/14796694.1.5.689. [DOI] [PubMed] [Google Scholar]
- 64.Pulciani S, Santos E, Lauver AV, Long LK, Aaronson SA, Barbacid M. Oncogenes in solid human tumours. Nature. 1982;300:539–42. doi: 10.1038/300539a0. [DOI] [PubMed] [Google Scholar]
- 65.de Klein A, van Kessel AG, Grosveld G, Bartram CR, Hagemeijer A, Bootsma D, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–7. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 66.Jones DT, Hutter B, Jager N, Korshunov A, Kool M, Warnatz HJ, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–32. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubin BP, Chen CJ, Morgan TW, Xiao S, Grier HE, Kozakewich HP, et al. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am J Pathol. 1998;153:1451–8. doi: 10.1016/S0002-9440(10)65732-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120:799–807. doi: 10.1002/cncr.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eguchi M, Eguchi-Ishimae M. Absence of t(12;15) associated ETV6-NTRK3 fusion transcripts in pediatric acute leukemias. Med Pediatr Oncol. 2001;37:417. doi: 10.1002/mpo.1223. [DOI] [PubMed] [Google Scholar]
- 70.Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol. 2013;44:1982–8. doi: 10.1016/j.humpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bourgeois JM, Knezevich SR, Mathers JA, Sorensen PH. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol. 2000;24:937–46. doi: 10.1097/00000478-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–7. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 73.Kralik JM, Kranewitter W, Boesmueller H, Marschon R, Tschurtschenthaler G, Rumpold H, et al. Characterization of a newly identified ETV6-NTRK3 fusion transcript in acute myeloid leukemia. Diagn Pathol. 2011;6:19. doi: 10.1186/1746-1596-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123:4935–44. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–50. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frattini V, Trifonov V, Chan JM, Castano A, Lia M, Abate F, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45:1141–9. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Lee Y, Cho HJ, Lee YE, An J, Cho GH, et al. NTRK1 fusion in glioblastoma multiforme. PLoS One. 2014;9:e91940. doi: 10.1371/journal.pone.0091940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross JS, Wang K, Gay L, Al-Rohil R, Rand JV, Jones DM, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19:235–42. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng Z, Liebers M, Zhelyazkova B, Cao Y, Panditi D, Lynch KD, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014 doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 81.Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371:1005–15. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desmet CJ, Peeper DS. The neurotrophic receptor TrkB: a drug target in anti-cancer therapy? Cell Mol Life Sci. 2006;63:755–9. doi: 10.1007/s00018-005-5490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minturn JE, Evans AE, Villablanca JG, Yanik GA, Park JR, Shusterman S, et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother Pharmacol. 2011;68:1057–65. doi: 10.1007/s00280-011-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–9. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 85.Richardson CA, Leitch B. Phenotype of cerebellar glutamatergic neurons is altered in stargazer mutant mice lacking brain-derived neurotrophic factor mRNA expression. J Comp Neurol. 2005;481:145–59. doi: 10.1002/cne.20386. [DOI] [PubMed] [Google Scholar]
- 86.Rotthier A, Baets J, Timmerman V, Janssens K. Mechanisms of disease in hereditary sensory and autonomic neuropathies. Nat Rev Neurol. 2012;8:73–85. doi: 10.1038/nrneurol.2011.227. [DOI] [PubMed] [Google Scholar]
- 87.Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov. 2013;12:507–25. doi: 10.1038/nrd4024. [DOI] [PubMed] [Google Scholar]
- 88.Weiss GJ, Hidalgo M, Borad MJ, Laheru D, Tibes R, Ramanathan RK, et al. Phase I study of the safety, tolerability and pharmacokinetics of PHA-848125AC, a dual tropomyosin receptor kinase A and cyclin-dependent kinase inhibitor, in patients with advanced solid malignancies. Invest New Drugs. 2012;30:2334–43. doi: 10.1007/s10637-011-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117:3294–301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Braud FG, Lorenzo P, Niger M, Damian S, Bardazza B, Martinetti A, et al. Phase 1 open label, dose escalation study of RXDX101, an oral pan-trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations. J Clin Oncol. 2014;32(5s suppl):abstr 2502. [Google Scholar]