Abstract
Background
Ethnic disparities in cardiovascular morbidity and mortality are widely documented in the literature. Recently, research has shown that decreased parasympathetic (PNS) cardiac modulation is associated with the established and emerging risk factors for cardiovascular disease (CVD) and stroke. In consideration of the disproportionate CVD risk and disease profile of African Americans (AAs), it is plausible that decreased cardiac PNS functioning may partially explain these disparities. In the present systematic review and meta-analysis, we assess the available evidence for a reliable ethnic difference in tonic vagally-mediated heart rate variability (HRV), an indicator of PNS cardiac modulation.
Methods
A systematic literature search was conducted yielding studies comparing tonic HRV in AAs and European Americans (EAs). Adjusted standardized effect sizes, (Hedges g), were calculated using a mixed effects model with restricted maximum likelihood estimation for 17 studies containing appropriate measures of vagally-mediated HRV.
Results
Meta-analysis results suggest that AAs have greater HRV than EAs (Hedges g = .93, 95% C.I. [.25, 1.62]) even after consideration of several covariates including health status, medication use, and subgroup stratification by gender and age.
Conclusions
These findings suggest that decreased vagally-mediated HRV is not likely to account for the persistent health disparities experienced by AAs with respect to cardiovascular disease risk and burden. These disparities underscore the need for continued research addressing socio-ethnic cardiovascular differences and the biobehavioral mechanisms involved
Keywords: ethnic differences, health disparities, heart rate variability, meta-analysis
Introduction
Ethnic disparities in cardiovascular morbidity and mortality are widely documented in the literature and continue to challenge health delivery efforts (1-3). In particular, African Americans (AAs) evince higher mortality rates from coronary heart disease and stroke (3), as well as the highest prevalence of multiple cardiovascular disease (CVD) risks, including sex- and age-stratified rates of hypertension, diabetes mellitus, elevated cholesterol, physical inactivity, overweight, and current smoking (2, 4). Moreover, racial and social differences in cardiovascular outcomes may be related to lifestyle factors and central obesity (5). With respect to hypertension, frequently cited disparities include an earlier age of onset, greater impairment, and worse prognosis among AAs compared to European Americans (EAs) (6). Similar trends are noted for stroke and diabetes with incidence and mortality rates nearly twice as high for type II diabetes in AAs compared to EAs (7). Given the burden in dollars, lives, and quality of life, research addressing socio-ethnic cardiovascular differences is an essential component in the advancement of efforts (i.e., prevention and intervention) aimed toward attenuating these disparities.
Altered or dysregulated autonomic nervous system (ANS) functioning is one posited mechanism underlying increased CVD risk and outcomes such as hypertension, diabetes and stroke (8). Both cross-sectional and longitudinal studies suggest that autonomic imbalance in the direction of relatively high sympathetic activity and relatively low parasympathetic activity is associated with risk for a wide range of diseases, disorders, and syndromes as well as all-cause mortality (17). According to this view, parasympathetic cardiac (i.e. vagal) activity decreases significantly throughout the lifespan while sympathetic drive increases both centrally (i.e. cardiac) and peripherally (9-11). Importantly, several recent reviews suggest that low vagally-mediated heart rate variability (HRV) — the variation in timing between consecutive heartbeats on the order of milliseconds — is associated with all-cause mortality and increased morbidity from a host of risk factors including total and low-density cholesterol, smoking, obesity, age and positive family history of CVD (8, 13-17). In clinical applications, HRV has been used to predict risk of sudden death after myocardial infarction (19), diabetic neuropathy (20) and hypertension onset (21).
Critically, the mechanisms underlying ethnic disparities in cardiovascular risk are currently not well understood. Given the greater cardiovascular disease burden of AAs and the evidence that decreased vagally-mediated HRV is associated with increased risk, one might expect that a preponderance of research would find that AAs have lower vagally-mediated HRV compared to EAs. However, data from several studies suggests that AAs exhibit higher tonic HRV compared to EAs (22-29). For instance, Liao and colleagues (22) examined 2-minute supine, resting HRV recordings in a sample of 1,984 individuals from the Atherosclerotic Risk in Communities study and found higher levels of high frequency (HF) HRV and lower levels of low frequency (LF) HRV in AAs compared to EAs, adjusting for age and gender. Similarly, Ohira et al. (30) examined 5-minute resting HRV data from 6,652 participants in the Multi-Ethnic Study of Atherosclerosis and found that AAs had higher age- and gender-adjusted HRV compared to white, Hispanic, and Chinese participants. Replications of this effect have been found in postmenopausal women (31), healthy young adults (32, 33), adolescents (34-37), children (38) and infants (39). In addition, results from several twin studies have not only shown higher HRV in AAs (28, 34) but also demonstrated the stability of this pattern longitudinally (29). While large-scale studies have shown greater HRV in AAs, population or sample characteristics such as age, gender, health status, and medication use and study characteristics such as control for potential confounds and explicit testing of ethnic differences require meta-analytic techniques to examine their impact on the observed differences. Moreover, the finding of greater HRV in AAs is not universal and some authors have found lower HRV in AAs (40-43) or no effect of ethnicity (44). As vagally-mediated HRV is generally regarded as “cardio-protective” and high resting levels have been linked to several beneficial health outcomes (8, 17), the presence of higher HRV in AAs would suggest that they should be relatively buffered against cardiovascular risk and disease. That this is not the case requires further investigation.
Thus the purpose of the present systematic review and meta-analysis is to examine the available evidence for ethnic (specifically AA and EA) differences in vagally-mediated HRV, and importantly to assess population- and study-level covariates of this effect. To our knowledge, no prior systematic or meta-analytic reviews of such comparisons have been performed. An examination of the factors that contribute to ethnic differences in resting HRV may have implications for our understanding of differential cardiovascular disease risk as well as for prevention and treatment.
Methods
Literature Search
Cochrane databases were searched to ensure that a similar review had not been previously published. No review to date has been published on the specific topic of race or ethnic differences in HRV. A systematic search of the literature was performed using the electronic databases PubMed and PSYNDEX. For search terms and filters applied see the Appendix. The database search was conducted between November 2009 and September 2011 with an update and final search in June 2014. Data were collected and extracted by three independent reviewers. Where disagreement arose, reviewers consulted until agreement was reached. Retrieved records were initially screened by title plus abstract.
Screening of abstracts
Abstracts of studies that reported an (A) empirical investigation (i.e. excluding reviews, meta-analysis, and single case studies) in (B) healthy humans (excluding animal studies, and studies examining solely in-patient or diseased samples), reporting (C) recordings of HRV in (D) AAs and EAs, were considered potentially eligible for inclusion and retrieved in full-text. No restrictions were made for language, age range, or study design. Healthy samples and samples containing proportions of unhealthy individuals, defined as having some moderate disease risk or burden (e.g., hypertension) were included for review. Samples reporting no or some medication use were also included for review.
Screening of full-texts
Full-texts that did not report at least one resting, vagally-mediated HRV index (see below) in humans, that did not report results by AA and EA ethnicity, that examined solely in-patient or diseased samples, that reported from completely overlapping study samples, or that did not provide sufficient quantitative data were excluded from review. Where longitudinal or pre-post data were reported, only the baseline resting HRV was included to minimize confounding by experimental manipulation and conflation of effect size estimates. Where multiple citations provided data from overlapping samples, only the citation that contained the most information relevant to covariate testing (e.g., stratification by age and gender) was retained. Authors who reported baseline HRV by ethnicity but who did not report sufficient quantitative data (e.g., only a graphical display) were contacted for necessary quantitative information to derive effect size estimates and confidence limits. Furthermore, authors with potential access to data of interest (i.e. reporting a sample including AAs and EAs, and HRV but no analysis of ethnic differences) were contacted. Finally, in addition to the electronic search of databases a hand search was conducted, screening the reference lists of the included studies and various other sources (i.e. Google, Google scholar).
Selection of data
Guidelines from the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [Task Force] (45) were used to define the HRV measurements included for review. Time domain indices are derived directly from the RR interval series and generally measure the variability contained therein; whereas frequency domain measures are derived via spectral analytic techniques (i.e. Fast Fourier Transform (FFT) or Autoregressive (AR) algorithm) applied to the RR interval series with the high frequency (HF) component thought to reflect primarily vagal cardiac modulation. Studies that reported respiratory sinus arrhythmia (RSA), root-mean-square successive RR-interval difference (RMSSD); mean successive difference (MSD); percentage of successive normal to normal intervals that differ by more than 50 milliseconds (pNN50); or any spectral measure in the high frequency (HF) range of 0.15 – 0.14 Hz (natural log transform (lnHF), normalized (HFnu) or absolute power (absHF)) were included for review. In addition, only studies containing short-term measures (i.e., < 25 minutes) of HRV were considered. It is noted that guidelines for the measurement of HRV suggest that spectral analysis of 24-hour HRV (where spectral estimates are calculated over long data epochs that are not likely to be stationary) may not accurately reflect autonomic modulation, which may be better captured by estimates based on shorter data epochs (45). Therefore studies based on these longer estimates or where the data epoch was not clearly stated were excluded. Where citations reported multiple vagally-mediated HRV indices, a hierarchical inclusion was implemented to prevent conflation of effect size estimates as follows: HF power only, next RSA only, next RMSSD only, next MSD only, or else pNN50 only.
Covariates
The exploration of covariates for analysis was of interest in the present review and the following four population level and two study level covariates were documented: age, gender, health status of samples, use of medication, statistical adjustment for baseline confounds, and the implementation of a statistical test of ethnic differences in HRV. Covariates were contrasted as follows: male versus female versus mixed sample, all healthy sample versus some proportion unhealthy, no medication use versus some medication use, statistical adjustment versus no adjustment for potential confounds, and statistical test performed versus no statistical test of ethnic differences in HRV. The age covariate was coded and analyzed as infant (< 2 years), youth (2 to 17 years), adult (18 to 50 years), and older adult (>50 years).
Quantitative Analysis
True effect estimates were computed as adjusted standardized mean differences (Hedges g) and combined with inverse variance weights using a mixed effects model with restricted maximum likelihood estimation (46-48).
The mixed effects approach assumes heterogeneity between studies as do random effects models; however, the total heterogeneity in the mixed effects model also includes residual heterogeneity not accounted for by covariates (49). The aim of a mixed effects model is to estimate the degree of influence of covariates on the point and interval estimates of effect size (49); a mixed effects model was thus the most appropriate for the exploration of covariation of ethnic differences in HRV by age, gender, healthy status, medication use, the implementation of statistical test, and statistical adjustment for baseline confounds. Various estimators for effect size and residual heterogeneity have been frequently used and published, including the Hedges and Olkin (47) as well as DerSimonian and Laird (50) estimators. However, these estimators may yield biased estimation of between-study heterogeneity and narrower effect size interval estimates (46, 51). Restricted maximum likelihood estimation (REML) addresses biased underestimation of residual heterogeneity, is of comparable efficiency, and is generally recommended (52, 53). Effect size and heterogeneity estimates are achieved iteratively through REML and this procedure was used in the present analyses. Each covariate was tested using meta-regression with a single covariate at a time (48), in line with a published meta-analysis of HRV variables (54). Heterogeneity was tested with the standard I2 index (55). Bias was examined using a funnel plot of effect size against standard error for asymmetry as well as Egger's regression test (56). Meta-analytic and meta-regression computations were performed using the metafor package in R (49) in conjunction with OpenMetaAnalyst software (57).
Results
Retrieved Literature
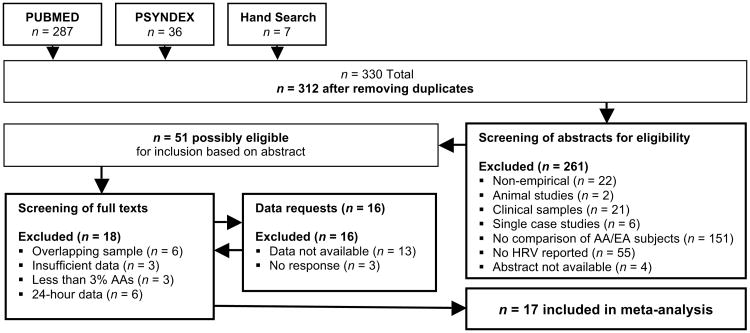
The search yielded 330 citations, of which 312 were screened by abstract and title after duplicate removal. Of these, 255 studies were excluded based on non-human samples (n = 2), clinical samples (n = 21), no HRV reported (n = 55), no comparison between AA and EA ethnicities (n = 146), and other criteria as depicted in Figure 1. The remaining 51 citations were screened by full text. Twenty-three studies were excluded due to overlapping samples (n = 6), insufficient data (n = 9), the inclusion of less than 3% AA sample (n = 3), and the use of spectral estimates on the entire 24-hour EKG series or non-stationary epochs (i.e., not strictly at rest; n = 6). In 16 cases authors of studies were contacted and studies were excluded due to contact with authors who did not respond (n = 3) or who responded but could not provide sufficient information in time (n = 13). The final inclusion for quantitative synthesis comprised 17 studies involving 11,162 participants and k = 27 effect sizes (see Table 1).
Figure 1. Systematic review flow chart.
Table 1.
Summary of included literature.
| Author | Year | n AA | n EA | Gender | Age M (yr) | Age Range | Health Status | Med. Use | HRV Index | Baseline Diff. | Stat. Adjust | Stat. Test | Record Length |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. | 2006 | 57 | 78 | Mixed | 36.9 | 23 to 54 | Healthy | None | logHF | 1,2 | No | Yes | 3min |
| Dorr et al. | 2007 | 21 | 26 | Mixed | n/a | n/a | Healthy | None | RMSSD | 2, 6 | No | Yes | 10min |
| Earnest et al. | 2008 | 102 | 248 | Female | 57.5 | 45 to 75 | J | Some | RMSSD | n/a | No | Yes | 25min |
| Esco et al. | 2010 | 30 | 30 | Male | 22.8 | n/a | Healthy | None | HFnu | 3,6 | No | Yes | 5min |
| Fuller-Rowell et al. | 2013 | 204 | 833 | Mixed | 53.7 | 34 to 83 | K | Some | logHF | 4 | No | Yes | 11min |
| Gutin et al.a | 2005 | 95 | 76 | Female | 16.2 | n/a | Healthy | None | HFnu | 1,2 | No | Yes | 10min |
| Gutin et al.b | 2005 | 64 | 69 | Male | 16.2 | 14 to 18 | Healthy | None | HFnu | 1,2 | No | Yes | 10min |
| Hall et al. | 2013 | 119 | 160 | Female | 51 | 46 to 57 | J, K, M, N | Some | logHF | 1,4,5,6 | Yes | Yes | 2min, night |
| Heffernan et al. | 2009 | 22 | 22 | Male | 23 | n/a | Healthy | None | HFnu | 6 | No | Yes | 15min |
| Hinnant et al. | 2010 | 65 | 113 | Mixed | 8.23 | 8 to 10 | Healthy | None | pvRSA | 4 | No | Yes | 3min |
| Li et al.a | 2009 | 125 | 84 | Female | 23.1 | 15 to 39 | Healthy | None | absHF | 1,2,3 | No | No | 15min |
| Li et al.b | 2009 | 85 | 105 | Male | 23.1 | 15 to 39 | Healthy | None | absHF | 1,2,3 | No | No | 15min |
| Liao et al.a | 1995 | 150 | 423 | Female | n/a | 45 to 54 | K,L,N | Some | logHF | 1,2,3,5,6 | Yes | Yes | 2min |
| Liao et al.b | 1995 | 97 | 351 | Male | n/a | 45 to 54 | K,L,N | Some | logHF | 1,2,3,5,6 | Yes | Yes | 2min |
| Liao et al.c | 1995 | 114 | 359 | Female | n/a | 55 to 64 | K,L,N | Some | logHF | 1,2,3,5,6 | Yes | Yes | 2min |
| Liao et al.d | 1995 | 79 | 411 | Male | n/a | 55 to 64 | K,L,N | Some | logHF | 1,2,3,5,6 | Yes | Yes | 2min |
| Ohira et al. | 2008 | 1843 | 2250 | Mixed | n/a | 45 to 84 | K,M,N,O | Some | RMSSD | 6 | Yes | Yes | 5min |
| Propper et al. | 2008 | 72 | 70 | Mixed | 12 mo. | 12 mo. | Healthy | None | RSA | 6 | No | Yes | 5min |
| Salomon.a | 2005 | 25 | 25 | Female | n/a | 9 to 14 | Healthy | None | MSD | n/a | No | No | 3min |
| Salomon.b | 2005 | 22 | 29 | Male | n/a | 9 to 14 | Healthy | None | MSD | n/a | No | No | 3min |
| Salomon.c | 2005 | 9 | 15 | Female | n/a | 17 to 21 | Healthy | None | MSD | n/a | No | No | 3min |
| Salomon.d | 2005 | 10 | 15 | Male | n/a | 17 to 21 | Healthy | None | MSD | n/a | No | No | 3min |
| Sloan et al.a | 2008 | 421 | 336 | Female | 40 | n/a | K,M,N | Some | absHF | 1,2,3 | Yes | Yes | 10min |
| Sloan et al.b | 2008 | 421 | 336 | Male | 40 | n/a | K,M,N | Some | absHF | 1,2,3 | Yes | Yes | 10min |
| Wang et al.a | 2005 | 74 | 122 | Female | 16 | n/a | Healthy | None | absHF | 2,3,4,6 | Yes | Yes | 4min |
| Wang et al.b | 2005 | 77 | 112 | Male | 16 | n/a | Healthy | None | absHF | 2,3,4,6 | Yes | Yes | 4min |
| Zion et al. | 2003 | 32 | 29 | Male | 22.8 | n/a | Healthy | None | logHF | None | No | Yes | 5min |
Note: The letters a, b,c, or d following author names indicate an independent effect size derived from non-overlapping stratification (e.g., by gender or age). For healthy status: J=overweight/obese; K= hypertension; L=atherosclerosis; M=smoking; N=diabetes; O=high cholesterol. For baseline differences: 1=body mass index; 2=systolic blood pressure; 3=diastolic blood pressure; 4=socio-economic status; 5=smoking; 6=other. HF = high frequency power; absHF = absolute high frequency power; logHF = natural log HF; HFnu = high frequency normalized units; RMSSD = root-mean-square successive RR-interval difference; RSA = respiratory sinus arrhythmia; MSD = mean successive difference; AA = African American; EA = European American; Med.=Medication; Stat.=Statistical.
Study Characteristics
Six of the 17 citations yielded multiple effect size values with HRV stratified independently by age, gender, or both (see Table 1). Of the 27 effect size values, 12 were among men only, ten were among women only, and five involved both sex groups. The most frequent age group reported was among adults (age 18 to 50 years; 40%), followed by youth (age 2 to 17 years; 33%), older adults (> 50 years; 22%), and one study reported ethnic differences in 12-month-old infants (39). The majority of effect sizes involved only healthy samples (63%), while the remainder included participants in their samples with some disease burden or risk factor (e.g., hypertension, diabetes; 37%). These latter effect sizes were predominantly derived from large-scale studies that included both healthy and unhealthy participants (22, 30). The percentage of effect sizes from samples reporting no medication use (63%) or some medication use (37%) was identical to that of health status.
Only reports of resting HRV reflecting short-term, predominantly parasympathetic activity were included for review; the majority of effect sizes were computed from HF power (67%), followed by MSD (15%), RMSSD (11%), and RSA (7%). Notably, a majority of the studies (82%) reported ethnic differences in potentially confounding baseline variables such as systolic blood pressure, body mass index, or socio-economic status, while only one study reported no significant baseline differences (40). In contrast, only a minority of studies (n = 5; 29%) statistically adjusted for such variables when reporting baseline HRV. Lastly, the majority of studies (n = 12; 70%) reported the implementation of some statistical test of ethnic differences in HRV, while the remainder reported baseline differences without statistical testing. Please refer to Table 1 for a summary of study characteristics.
Ethnic Differences in Resting Heart Rate Variability
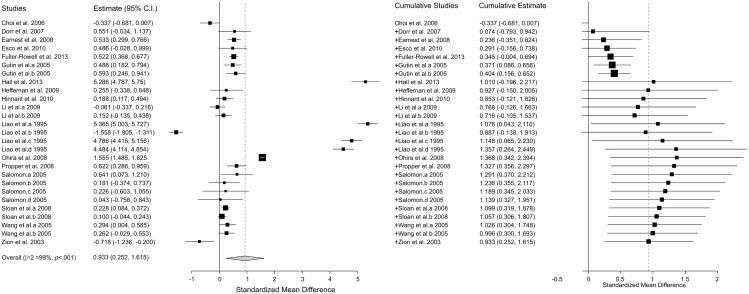
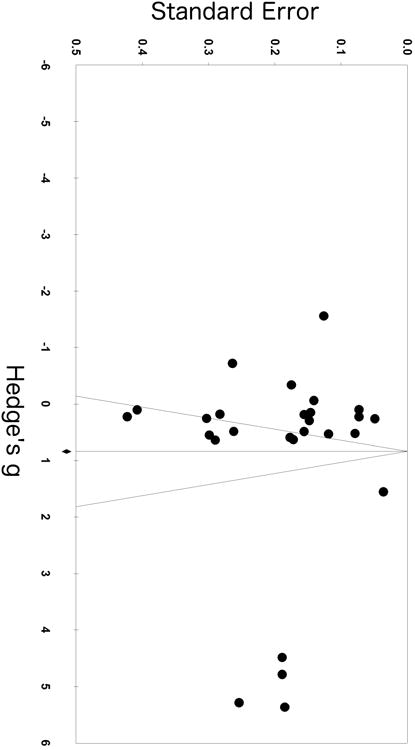
Meta-analyses revealed a sizeable and significant effect of ethnicity on HRV (Hedges' g = 0.93; 95% CI (0.25, 1.62); k = 27) suggesting higher vagal activity in AAs compared to EAs (see Figure 2; positive effect estimates reflect higher HRV in AAs). Two effect-size estimates representing 129 AAs and 380 EAs showed significantly lower HRV in AAs. Twelve effect-size estimates representing 944 AAs and 965 EAs were not significant. Thirteen effect-size estimates representing 3,362 AAs and 5,382 EAs showed higher HRV in AAs (see Figure 2). Significant heterogeneity across all true effects was found (I2 = 99%, p< 0.001), while the Egger's test for publication bias was significant (p = 0.013) and a visual examination of the funnel plot for asymmetry suggested publication or other sources of bias (see Figure 3).
Figure 2.
Forest plot of effect estimates (g) obtained from studies with adequate quantitative data (n = 19). Effects are grouped identically to Table 1 based on gender and recording length with boundaries between groupings indicated by a bold symbol enclosed in parenthesis (i.e. M for males). Bolded triangles represent the combined (i.e. across multiple HRV indices), weighted effect size for each study. Error bars represent 95% confidence intervals. Positive values indicate studies reporting higher HRV in African Americans.
Figure 3. Funnel plot.
Covariate Meta-Regression
Meta-regression coefficients and confidence limits for each tested covariate are reported in Table 2. Significant covariates were age (β = 0.97, p = 0.009) and the statistical adjustment of baseline confounds (β = 1.81, p = 0.003). Specifically, AAs showed significantly higher HRV among youth (g = 0.346; 95% CI (0.220, 0.473); k = 9) and among older adults (g = 2.853; 95% CI (1.072, 4.635); k = 6), and whereas the effect was not significant in adults (g = 0.405; 95% CI (-0.633, 1.444); k = 11) the average effect size was larger than for the youths and the confidence intervals showed significant overlap with the other age groups (see Supplemental Digital Content 1). Too few studies in infants (k = 1) prevent firm conclusions in this age group. Studies that made statistical adjustments for baseline confounds when reporting HRV showed a much stronger effect for higher HRV in AAs (g = 2.08; 95% CI (0.46, 3.69); k = 10) compared to those that did not adjust for confounds (g = 0.27; 95% CI (0.09, 0.45); k = 17). However, both effects were significant and in the same direction of higher HRV for AAs.
Table 2. Meta-regression covariate results.
| Covariate | β | SE β | 95%CI Lower | Upper | p-value |
|---|---|---|---|---|---|
| Age | 0.97 | 0.37 | 0.24 | 1.70 | 0.009 |
| Gender | -0.75 | 0.45 | -1.63 | 0.13 | 0.095 |
| Health Status | 1.89 | 0.61 | 0.70 | 3.07 | 0.002 |
| Medication Use | 1.89 | 0.61 | 0.70 | 3.07 | 0.002 |
| Statistical Adjustment | 1.81 | 0.62 | 0.60 | 3.01 | 0.003 |
| Statistical Test | 0.99 | 0.19 | -0.49 | 2.48 | 0.190 |
Health status and medication use were also significant covariates. The studies included within these subgroups were coincidentally identical and so were their respective parameters (β's = 1.89, p's = 0.002). Studies including some unhealthy populations who used medication showed a larger effect of higher HRV in AAs but with wider confidence limits (g = 2.12; 95% CI (0.53, 3.72); k = 10) compared to studies incorporating only healthy samples that did not report any medication use (g = 0.23; 95% CI (0.06, 0.40); k = 17). Results from partly unhealthy samples may reflect contributions by several of the largest studies included for review (see Table 1). A sensitivity analysis was performed with the two largest studies removed (n > 1000, each) (30, 57) to examine whether this finding was an artifact of large sample size. The effect estimate remained significant and large after removal (g = 2.40; 95% CI (0.43, 4.36); k = 8), although the remaining studies within this subgroup also comprised large samples.
Gender and statistically testing baseline ethnic differences in HRV were not significant covariates in the model (p's = 0.095; 0.190, respectively). Subgroup analysis revealed that female-only samples showed significantly higher HRV in AAs, (g =1.78; 95% CI (0.33, 3.23); k = 10), while the effect was not significant for male or mixed gender samples. However, again the confidence intervals among these groups showed a high degree to overlap. Moreover, two large studies (23, 30) adjusted for gender. When excluding these studies in sensitivity analyses for gender subgroups, the lack of significance remained (data not shown). Similarly, studies that implemented statistical testing showed significantly greater HRV in AAs (g = 1.19; 95% CI (0.30, 2.08); k = 20), while the effect was not significant among studies that did not statistically test baseline differences. Again however the estimates were in the same direction of higher HRV in AAs. Forest plots and meta-regression plots for all covariate subgroups are available in Supplemental Digital Content 1.
Discussion
In the present systematic-review and meta-analysis, we quantitatively synthesized the literature on ethnic differences in resting vagally-mediated heart rate variability (HRV), a marker linked to cardiovascular outcomes, better overall health, and the capacity to adapt to shifting environmental demands. The synthesized literature suggests that African Americans (AAs) show higher resting HRV compared to European Americans (EAs), and this effect of nearly a full standard deviation across all studies (Hedges' g = 0.93; 95% CI (0.25, 1.62); k = 27), though moderated by age, health status, medication use, and study methodology, was consistent across these population and study based covariates. These findings suggest that HRV as a risk factor may not carry the same information value for AAs as it does for EAs. We will return to this point later.
One of the most consistent findings in the HRV literature is that vagally-mediated HRV decreases with increasing age (8, 17). Despite this fact, we found that AAs showed higher HRV than EAs at all age levels from infants to the elderly. Therefore it is unlikely that some age-related factor such as a healthy survivor effect could completely account for our results. Similarly, the effects of medication have been consistently shown to influence HRV levels (60). Again, however, the ethnic difference we report remained robust, whether or not the participants were unhealthy and thus medicated or healthy and un-medicated. In addition, study level covariates such as statistical control of potential confounders and explicit testing of ethnic differences did not alter the finding that AAs showed higher levels of HRV than EAs. Whereas the examined covariates did not alter our findings, it remains possible that other unexamined covariates may have affected the results. Future analyses will be needed to address that possibility.
Given that the covariate analysis did not find a systematic source for the reported ethnic difference, the natural question is what could the source be? In addition to the possible unexamined covariates, our group has investigated two other potential sources for this ethnic difference. One potential source is genetic differences between AAs and EAs. In one study designed to assess the genetic contribution to HRV using behavioral genetics methodology it was shown that AA adolescents had higher resting vagally mediated HRV than similarly aged EAs and that HRV was highly heritable and but there was no evidence for an ethnic difference in heritability estimates (34). A related study (28) found similar results when HRV was assessed during both rest and in response to a psychological stressor. Whereas these studies did not find any differences in heritability of HRV measures it does not completely rule out the possibility of ethnic differences based on genetics but it does suggest that genetics is not an obvious source for this difference.
Another potential source for the observed greater HRV in AAs may be ethnic differences in neural control of the heart. Whereas this is an extremely under investigated area we have recently reported ethnic differences in the association between resting brain perfusion measured using pulsed arterial spin labeling (PASL) and resting HRV (61). There were several differences noted between AAs and EAs. First, greater whole brain perfusion was positively associated with vagally mediated HRV in EAs but was uncorrelated with HRV in AAs. Second, and perhaps most importantly, regions of the anterior cingulate and the medial prefrontal cortex that are associated with emotional regulation and the assessment of threat and safety were negatively associated with HRV in AAs but uncorrelated with HRV in EAs. Specifically, we and others have found that successful emotion regulation is associated with both greater resting HRV as well as HRV increases during emotion regulation including reappraisal and suppression (62,63). In addition, research suggests that AAs often must inhibit or suppress their anger in response to unfair treatment (25). Thus, the greater HRV in AAs may be related to their need to regulate their emotion responses to unfair treatment. This explanation for the present observed ethnic differences in HRV is highly speculative but given the paucity of research in ethnic differences in neural activity it represents a potentially fruitful area for brain-body research into health disparities.
Strengths and Limitations
Our systematic search and meta-analytic synthesis of the literature bears a number of strengths, including quantitative rigor with respect to effect estimates as well as the unique exploration and testing of covariates with meta-regression. However, some limitations warrant mention. First, it should be noted that meta-regression is hypothesis generating and its results are not intended to be causally conclusive. The aims of our covariate analyses were to explore the role of potential population level and study level factors that might account for the observed ethnic differences. Whereas the finding of higher HRV in AAs was consistent and largely independent of these covariates, other potential covariates might still possibly be related to the current results. In addition, the iterative procedures and conclusions drawn from subgroup meta-analyses are limited when too few studies meet criteria for inclusion, for example in the age covariate with only one infant study included. Next, high heterogeneity was present as well as a positive test for bias. The presence of bias in meta-analysis warrants caution in the interpretation of results. However, it should be noted that the source of such bias is not conclusive, and asymmetry of the funnel plot does not accurately predict publication bias (64). For example, funnel plot asymmetry may reflect publication bias, high heterogeneity, methodological variation, and other factors (56). Upon examining the funnel plot, the largest effect sizes were not derived from smaller, imprecise studies but rather from large-scale studies (e.g. 22). This relatively high degree of heterogeneity may reflect the nature of our review question on ethnic differences —one that necessitates quasi-experimental designs that lend to greater heterogeneity across studies. Lastly, our review aims and exclusion criteria were specific to resting, vagally-mediated HRV among mostly healthy populations according to the Task Force guidelines (45). While this enhanced our inclusion consistency, it resulted in stringent exclusion of certain studies. For example, data were excluded from citations that reported spectral estimates on the entire 24-hour EKG series or from long-term recordings within the 24-hour period without specification of whether these data were derived from resting states. The problem of stationarity during long-term recordings is explicitly discussed in the Task Force guidelines and spectral estimates over long epochs are less reliable estimates of cardiac autonomic modulation (45). Some authors have contrarily espoused greater reliability and stability with spectral estimation over the entire 24-hour period and these papers were excluded (e.g., 41). Whereas studies over 24 hours allow for the estimation of circadian variation, this is only true when spectral estimates are derived from shorter, more likely stationary data epochs and the periods over which the shorter estimates are averaged are specified (65). Importantly, night time HRV measures, appropriately estimated, may be the best estimates of cardiac autonomic modulation and studies have shown night time estimates to be more closely associated with risk factors than other time periods (66,67).
Future Directions
The present analyses suggest that low resting HRV may not represent the same risk for disease, disability, and mortality for AAs as it does for EAs. At first, given the strong evidence for low HRV as a risk factor for a wide range of diseases (8) this might seem to question low HRV as a risk factor. However before we reach this conclusion, we must first understand the nature of risk factors. Offord and Kraemer (68) have written informatively about risk factors and note several aspects of risk factors relevant to the present discussion. First, whereas risk factors should have certain characteristics such as preceding the outcome of interest and stratifying individuals in to high and low risk categories, risk factors may not exhibit these characteristics universally. That is, a risk factor may have different value at different points in the disease process or for different populations. This latter situation may be the case for HRV with respect to ethnic differences and health disparities. However, this situation, in which a high score for an ostensibly protective factor is found in an at risk group, is not necessarily uncommon. For example, researchers have noted that despite AAs having higher high-density lipoprotein (HDL) and lower trigylcerides than EAs, AAs still have higher insulin resistance and greater death and disability from cardiovascular disease (69). These paradoxical findings have led some to suggest that a different pattern or different levels of risk factors be adopted for different ethnic groups at least with respect to the metabolic syndrome (69,70). The present results suggest that yet another risk factor may be differentially related in different groups to disease risk. It should be noted that similar differences in the relationship between risk factors and disease risk are widely noted with respect to gender differences (e.g., heart attack symptoms are different for women than for men).
Therefore, other mechanisms for the well-documented health disparities between AAs and EAs may warrant exploration. For example, chronically elevated vascular resistance has been implicated as one marker of heightened sympathetic activity in AAs. Several studies have reported exaggerated vasoconstriction and attenuated vasodilation in response to pharmacologic challenge (71-73) as well as associations of elevated vascular resistance with increased cardiac mass and left ventricle thickness in AAs (71). These and other findings have been recently summarized by Tehzarhada and colleagues (74), in which they note that differences in vascular activity exist between AAs and EAs and that these differences may contribute to the greater cardiovascular disease prevalence in AAs. Relatedly, blood pressure seems to be a much more important risk factor for AAs than for EAs in the metabolic syndrome profile (69,70).
The findings of higher tonic HRV in AAs raise the possibility that AAs may exhibit both higher vagally-mediated HRV and higher vascular resistance. At least one investigation has reported such a pattern: Dorr et al. (25) found that AA males exhibited higher tonic HRV and elevated basal total peripheral resistance compared to EAs. This pattern may represent something of a ‘Cardiovascular Conundrum’ as the potentially cardio-protective effects of HRV may be counteracted by chronically elevated vascular resistance. This could be evidence of impaired vasodilation in response to a vasodilatory stimulus as noted above (71, 74). Thus, in addition to replicating findings of higher HRV in AAs, future research should also consider the mechanisms that contribute to the regulation of both vagal tone and vascular resistance. One candidate is the arterial baroreflex, which works to decrease blood pressure (BP) via an increase in cardiac parasympathetic outflow and inhibition of peripheral sympathetic activity (75). As the short-term (i.e. beat-to-beat) adjustments necessary to maintain, raise or lower arterial BP are largely reflexively governed, ethnic differences in baroreflex sensitivity may account for some portion of the observed between-group variation in tonic HRV.
Presently, few investigations have evaluated ethnic differences in tonic baroreflex sensitivity (BRS). Of these, most have reported no differences in BRS between hypertensive black Africans or African-Caribbeans and White Europeans (76, 77) and in young healthy EAs and AAs (78, 79), respectively. Two investigations have reported lower BRS in AAs (40, 80) while others have indicated that a positive family history of hypertension may be more predictive of BRS than ethnicity (81). As indicated by these inconsistent findings, as well as the heterogeneity of the studies reporting higher tonic HRV in AAs, additional research is needed. This might entail re-examination of existing large-scale and smaller datasets to further evaluate the reliability and magnitude of these differences as well as rigorous new investigations focused on at-risk individuals or those in early stages of hypertension. Thus, prospective studies might be particularly important in ascertaining whether the higher vascular resistance appears first, in which case the higher HRV may represent a type of compensatory response, or whether the higher HRV comes first. It may also be useful to track tonic HRV medically as an added component of routine triage typically conducted in community and private health centers. This approach could be especially fruitful in longitudinal designs and aid in identifying developmental factors as well as the influence of various acute and chronic conditions such as obesity and inflammation, both of which have been linked to decreased HRV (17).
Conclusions
The results of the present meta-analysis suggest that healthy African Americans have greater resting, vagally-mediated heart rate variability compared to European Americans. These differences were found rather consistently across studies that differed in several population and study level covariates. Whereas the present study could not begin to address the potential source for these differences, genetic and neuroimaging studies may yield some insights. Finally, that HRV may represent a differential risk factor for EAs and for AAs adds to a growing list of seemingly paradoxical relationships between risk factor levels and disease burden. The further identification and examination of these paradoxical relationships may begin to increase our understanding of the persistent health disparities that continue to affect our nation's health.
Supplementary Material
Acknowledgments
This research was supported by funding from The Ohio State University Office of Diversity & Inclusion, The Todd Anthony Bell National Resource Center on the African American Male, The Ohio State University Graduate School & The Ohio State University College of Social, Behavioral and Economic Sciences to the first author (L.K.H). L.K.H was supported by National Institute of Aging grant (5T32AG000029-37).
Glossary
- CVD
cardiovascular disease
- ANS
autonomic nervous system
- PNS
parasympathetic nervous system
- SNS
sympathetic nervous system
- HRV
heart rate variability
- RSA
respiratory sinus arrhythmia
- RMSSD
root-mean-square successive RR-interval difference
- MSD
mean successive difference
- pNN50
percentage of successive normal to normal intervals that differ by more than 50 milliseconds
- HF
high frequency power
- lnHF
natural logarithmic transform high frequency power
- HFnu
normalized high frequency power
Appendix
Search strategy: The period of 1948 to 2014 was selected as the publication range for inclusion; PubMed syntax: (black OR African American OR ethnic OR race) AND (cardiac vagal OR HRV OR heart rate variability); active filters: Abstract available, Publication date: From 1948/01/01 to 2014/06/17, humans; Psyndex syntax: (TX HRV OR TX heart rate variability OR TX cardiac vagal) AND (S3 AND S4); active filters: Publication Date: 1948-2014, humans
References
References with an ‘*’ were included in the meta-analysis
- 1.Disparities in premature deaths from heart Disease—50 states and the District of Columbia, 2001. JAMA. 2004;291:1316–7. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Racial/ethnic and socioeconomic disparities in multiple risk factors for heart disease and stroke: United States, 2003. MMWR. 2005;54:113–117. [PubMed] [Google Scholar]
- 3.Keenan NL, Shaw KM. Coronary heart disease and stroke deaths: United States, 2006. MMWR SurveillSumm. 2011;60:S62–66. [PubMed] [Google Scholar]
- 4.Sharma S, Malarcher AM, Giles WH, Myers G. Racial, ethnic, and socioeconomic disparities in the clustering of cardiovascular disease risk factors. EthnDis. 2004;14:43–8. [PubMed] [Google Scholar]
- 5.Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Ann Epidemiol. 2010;20:617–28. doi: 10.1016/j.annepidem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 7.Thayer JF, Friedman BH. A Neurovisceral Integration model of health disparities in aging. In: Anderson NB, Bulato RA, Cohen B, editors. Critical perspectives on racial and ethnic differences in health in late life. Washington, DC: The National Academies Press; 2004. pp. 567–603. 2004. [PubMed] [Google Scholar]
- 8.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens. 2000;13:112S–122S. doi: 10.1016/s0895-7061(00)00228-4. [DOI] [PubMed] [Google Scholar]
- 10.Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. CurrHypertens Rep. 2009;11:199–205. doi: 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J. 1981;102:509–14. doi: 10.1016/0002-8703(81)90739-0. [DOI] [PubMed] [Google Scholar]
- 12.von Euler US. Sympathetic neuro-effectors of the heart. Cardiologia. 1952;21:252–5. doi: 10.1159/000165204. [DOI] [PubMed] [Google Scholar]
- 13.Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: Demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am CollCardiol. 1991;8:464–72. doi: 10.1016/0735-1097(91)90602-6. [DOI] [PubMed] [Google Scholar]
- 14.Saul J. Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. Physiology. 1990;5:32–7. [Google Scholar]
- 15.Pickering TG, Gribben B, Petersen ES, Cunningham DJC, Sleight P. Effects of autonomic blockade on the baroreflex in man at rest and during exercise. CircRes. 1972;30:177–85. doi: 10.1161/01.res.30.2.177. [DOI] [PubMed] [Google Scholar]
- 16.Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A polyvagal theory. Psychophysiology. 1995;32:301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 17.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. BiolPsychol. 2007;74:224–42. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 1970;4:160–7. doi: 10.1093/cvr/4.2.160. [DOI] [PubMed] [Google Scholar]
- 19.Lombardi F, Mäkikallio TH, Myerburg RJ, Huikuri HV. Sudden cardiac death: Role of heart rate variability to identify patients at risk. Cardiovasc Res. 2001;50:210–7. doi: 10.1016/s0008-6363(01)00221-8. [DOI] [PubMed] [Google Scholar]
- 20.Braune HJ, Geisendörfer U. Measurement of heart rate variations: Influencing factors, normal values and diagnostic impact on diabetic autonomic neuropathy. Diabetes Res ClinPract. 1995;29:179–87. doi: 10.1016/0168-8227(95)01133-1. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability. Hypertension. 2003;42:1106–11. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 22*.Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability: The ARIC study. Am J Cardiol. 1995;76:906–12. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 23*.Sloan RP, Huang MH, McCreath H, Sidney S, Liu K, Williams OD, Seeman T. Cardiac autonomic control and the effects of age, race, and sex: The CARDIA study. AutonNeurosci-Basic. 2008;139:78–85. doi: 10.1016/j.autneu.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzzetti S, Mayet J, Shahi M, Mezzetti S, Foale RA, Sever PS, Poulter NR, Porta A, Malliani A, Thom SA. Absence of sympathetic overactivity in Afro-Caribbean hypertensive subjects studied by heart rate variability. J Hum Hypertens. 2000;14:337–42. doi: 10.1038/sj.jhh.1001009. [DOI] [PubMed] [Google Scholar]
- 25*.Dorr N, Brosschot JF, Sollers JJ, III, Thayer JF. Damned if you do, damned if you don't: The differential effect of expression and inhibition of anger on cardiovascular recovery in black and white males. Int J Psychophysiol. 2007;66:125–34. doi: 10.1016/j.ijpsycho.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 26*.Heffernan KS, Jae SY, Vieira VJ, Iwamoto GA, Wilund KR, Woods JA, Fernhall B. C-reactive protein and cardiac vagal activity following resistance exercise training in young African-American and white men. Am J Physiol-Reg I. 2009;296:R1098–1105. doi: 10.1152/ajpregu.90936.2008. [DOI] [PubMed] [Google Scholar]
- 27*.Hinnant JB, Elmore-Staton L, El-Sheikh M. Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. DevPsychobiol. 2011;53:59–68. doi: 10.1002/dev.20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Ding X, Su S, Li Z, Riese H, Thayer JF, Treiber F, Sneider H. Genetic influences on heart rate variability at rest and during stress. Psychophysiology. 2009;46:458–65. doi: 10.1111/j.1469-8986.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Li Z, Snieder H, Su S, Ding X, Thayer JF, Treiber FA, Wang X. A longitudinal study in youth of heart rate variability at rest and in response to stress. Int J Psychophysiol. 2009;73:212–17. doi: 10.1016/j.ijpsycho.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Ohira T, Roux AVD, Prineas RJ, Kizilbash MA, Carnethon MR, Folsom AR. Associations of psychosocial factors with heart rate and its short-term variability: Multi-ethnic study of atherosclerosis. Psychosom Med. 2008;70:141–6. doi: 10.1097/PSY.0b013e318160686a. [DOI] [PubMed] [Google Scholar]
- 31*.Earnest CP, Lavie CJ, Blair SN, Church TS. Heart rate variability characteristics in sedentary postmenopausal women following six months of exercise training: The DREW study. PLoS One. 2008;3:e2288. doi: 10.1371/journal.pone.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Esco M, Olson M, Williford H, Blessing D, Shannon D, Grandjean P. The relationship between resting heart rate variability and heart rate recovery. ClinAutonRes. 2010;20:33–8. doi: 10.1007/s10286-009-0033-2. [DOI] [PubMed] [Google Scholar]
- 33.Martin LA, Doster JA, Critelli JW, Lambert PL, Purdum M, Powers C, Prazak M. Ethnicity and type D personality as predictors of heart rate variability. Int J Psychophysiol. 2010;76:118–21. doi: 10.1016/j.ijpsycho.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 34*.Wang X, Thayer JF, Treiber F, Snieder H. Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol. 2005;96(8):66–72. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 35.Murali R, Chen E. Exposure to violence and cardiovascular and neuroendocrine measures in adolescents. Ann Behav Med. 2005;30:155–63. doi: 10.1207/s15324796abm3002_8. [DOI] [PubMed] [Google Scholar]
- 36*.Gutin B, Howe C, Johnson MH, Humphries MC, Snieder H, Barbeau P. Heart rate variability in adolescents: Relations to physical activity, fitness, and adiposity. Med SciSports Exerc. 2005;37:1856–63. doi: 10.1249/01.mss.0000175867.98628.27. [DOI] [PubMed] [Google Scholar]
- 37.Urbina EM, Bao W, Pickoff AS, Berenson GS. Ethnic (black-white) contrasts in heart rate variability during cardiovascular reactivity testing in male adolescents with high and low blood pressure: The Bogalusa Heart Study. Am J Hypertens. 1998;11:196–202. doi: 10.1016/s0895-7061(97)00314-2. [DOI] [PubMed] [Google Scholar]
- 38*.Salomon K. respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychol. 2005;24:68–76. doi: 10.1037/0278-6133.24.1.68. [DOI] [PubMed] [Google Scholar]
- 39*.Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, Carbone MA, Cox M. Gene-environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Dev. 2008;79:1377–94. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 40*.Zion AS, Bond V, Adams RG, Williams D, Fullilove RE, Sloan RP, Bartels MN, Downey JA, De Meersman RE. Low arterial compliance in young African-American males. Am J Physiol-Heart C. 2003;285:H457–62. doi: 10.1152/ajpheart.00497.2002. [DOI] [PubMed] [Google Scholar]
- 41.Faulkner MS, Hathaway D, Tolley B. Cardiovascular autonomic function in healthy adolescents. Heart Lung. 2003;32:10–22. doi: 10.1067/mhl.2003.6. [DOI] [PubMed] [Google Scholar]
- 42.Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: A potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150:153–60. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 43*.Choi JB, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, Dimsdale JE. Age and ethnicity differences in short-term heart-rate variability. Psychosom Med. 2006;68:421–6. doi: 10.1097/01.psy.0000221378.09239.6a. [DOI] [PubMed] [Google Scholar]
- 44.Stein PK, Freedland KE, Skala JA, Carney RM, Davila-Roman V, Rich MW, Kleiger RE. Heart rate variability is independent of age, gender, and race in congestive heart failure with a recent acute exacerbation. Am J Cardiol. 1997;79:511–12. doi: 10.1016/s0002-9149(96)00798-9. [DOI] [PubMed] [Google Scholar]
- 45.Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 46.Guolo A, Varin C. The R Package and metaLik for likelihood inference in meta-analysis. J Stat Software. 2012;50:1–14. [Google Scholar]
- 47.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 48.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 49.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36:1–48. [Google Scholar]
- 50.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 51.Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med. 2001;20:825–840. doi: 10.1002/sim.650. [DOI] [PubMed] [Google Scholar]
- 52.Viechtbauer W. Bias and efficiency in meta-analytic variance estimators in the random-effects model. J EducBehav Stat. 2005;30:261–293. [Google Scholar]
- 53.Raudenbush SW. Analyzing effect sizes: Random-effects models. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd. New York: Russell Sage Foundation; 2009. pp. 295–315. [Google Scholar]
- 54.Lotufo PA, Valiengo L, Benseñor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53:272–82. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 55.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 56.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Software. 2012;49:1–15. [Google Scholar]
- 58*.Fuller-Rowell TE, Williams DR, Love GD, McKinley PS, Sloan RP, Ryff CD. Race differences in age-trends of autonomic nervous system functioning. J Aging Health. 2013;25:839. doi: 10.1177/0898264313491427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Hall MH, Middleton K, Thayer JF, Lewis TT, Kline CE, Matthews KA, Kravitz HM, Krafty RT, Buysse DJ. Racial differences in heart rate variability during sleep in women: The Study of Women Across the Nation sleep study. Psychosom Med. 2013;75:783–90. doi: 10.1097/PSY.0b013e3182a7ec5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kemp A, Brunoni A, Nunes M, Dantas E, Carvalho de Figueiredo R, Pereira A, Ribeiro A, Mill J, Andreão R, Thayer JF, Bensenor I, Lotufo P. Effects of depression, anxiety, comorbidity and antidepressants on resting-state, heart rate and its variability: An ELSA-Brasil cohort baseline study. The American Journal of Psychiatry. doi: 10.1176/appi.ajp.2014.13121605. In Press. [DOI] [PubMed] [Google Scholar]
- 61.Allen B, Jennings JR, Gianaros PJ, Thayer J, Manuck SB. Resting High-Frequency Heart Rate Variability is related to Resting Brain Perfusion. Psychophysiology. doi: 10.1111/psyp.12321. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 63.Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/S0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- 64.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarczok MN, Li J, Mauss D, Fischer JE, Thayer JF. Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome. Int J Cardiol. 2013;167(3):855–61. doi: 10.1016/j.ijcard.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Jarczok MN, Koenig J, Schuster AK, Thayer JF, Fischer JE. Nighttime heart rate variability, overnight urinary norepinephrine, and glycemic status in apparently healthy human adults. Int J Cardiol. 2013;168(3):3025–6. doi: 10.1016/j.ijcard.2013.04.147. [DOI] [PubMed] [Google Scholar]
- 67.vonKänel R, Thayer JF, Fischer JE. Nighttime vagal cardiac control and plasma fibrinogen levels in a population of working men and women. Ann Noninvasive Electrocardiol. 2009;14(2):176–84. doi: 10.1111/j.1542-474X.2009.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Offord DR, Kraemer HC. Risk factors and prevention. Evid Based Mental Health. 2000;3:70–71. [Google Scholar]
- 69.Schuster DP, Gaillard T, Osei K. The cardiometabolic syndrome in persons of the African diaspora: challenges and opportunities. J CardiometabSyndr. 2007;2(4):260–266. doi: 10.1111/j.1559-4564.2007.07484.x. [DOI] [PubMed] [Google Scholar]
- 70.Gaillard T, Schuster D, Osei K. Metabolic syndrome in Black people of the African diaspora: the paradox of current classification, definition and criteria. Ethn Dis. 2009;19(2):S2-1–7. [PubMed] [Google Scholar]
- 71.Stein CM, Lang CC, Singh I, He HB, Wood AJJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks: Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–51. doi: 10.1161/01.hyp.36.6.945. 01. [DOI] [PubMed] [Google Scholar]
- 72.Kahn DF, Duffy SJ, Tomasian D, Holbrook M, Rescorl L, Russell J, Gocke N, Loscalzo J, Vita JA. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40:195–201. doi: 10.1161/01.hyp.0000024571.69634.ed. [DOI] [PubMed] [Google Scholar]
- 73.Frohlich ED. Hemodynamic differences between black patients and white patients with essential hypertension: State of the art lecture. Hypertension. 1990;15:675–80. doi: 10.1161/01.hyp.15.6.675. [DOI] [PubMed] [Google Scholar]
- 74.Taherzadeh Z, Brewster LM, Van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J ClinHypertens. 2010;12:431–8. doi: 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benarroch EE. The arterial baroreflex. Neurology. 2008;71:1733–8. doi: 10.1212/01.wnl.0000335246.93495.92. [DOI] [PubMed] [Google Scholar]
- 76.Rowlands DB, De Giovanni J, McLeay RA, Watson RD, Stallard TJ, Littler WA. Cardiovascular response in black and white hypertensives. Hypertension. 1982;4:817–20. doi: 10.1161/01.hyp.4.6.817. [DOI] [PubMed] [Google Scholar]
- 77.Sanderson JE, Billingham JD, Floras J. Baroreceptor function in the hypertensive black African. ClinExpHypertens A. 1983;5:339–51. doi: 10.3109/10641968309069493. [DOI] [PubMed] [Google Scholar]
- 78.Allen MT, Matthews KA, Kenyon KL. The relationships of resting baroreflex sensitivity, heart rate variability, and measures of impulse control in children and adolescents. Int J Psychophysiol. 2000;37:185–94. doi: 10.1016/s0167-8760(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 79.Hinds K, Stachenfeld NS. Greater orthostatic tolerance in young black compared with white women. Hypertension. 2010;56:75–81. doi: 10.1161/HYPERTENSIONAHA.110.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas KS, Nelesen RA, Ziegler MG, Bardwell WA, Dimsdale JE. Job strain, ethnicity, and sympathetic nervous system activity. Hypertension. 2004;44:891–6. doi: 10.1161/01.HYP.0000148499.54730.0d. [DOI] [PubMed] [Google Scholar]
- 81.Parmer R, Cervenka J, Stone R. Baroreflex sensitivity and heredity in essential hypertension. Circulation. 1992;85:497–503. doi: 10.1161/01.cir.85.2.497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.