Abstract
The present study tested the hypothesis that repetitive scratching provoked by either of two known pruritogens, compound 48/80 and 5′-guanidinonaltrindole (GNTI), is accompanied by activation of microglia cells in the mouse spinal cord. Immunohistochemical studies revealed that CD11b, a cell surface marker of microglia cells, was up-regulated in the spinal cord 10–30 min post subcutaneous (s.c.) injection of compound 48/80 (50 μg/100 μl) or GNTI (0.3 mg/kg) to the back of the mouse neck. Numerous intensely labeled CD11b immunoreactive (irCD11b) cells, with the appearance of hypertrophic reactive microglia, were distributed throughout the gray and white matter. In contrast, weakly labeled irCD11b cells were distributed in the spinal cord from mice injected with saline. Western blots showed that CD11b expression levels were significantly increased in spinal cords of mice injected s.c. with either pruritogen, reached a peak response in about 30 min, and declined toward the basal level in the ensuing 60 min. In addition, phospho-p38 (p-p38), but not p38, levels were up-regulated in spinal cords from mice injected with compound 48/80 or GNTI, with a time course parallel to that of CD11b expression. Pretreatment of the mice with nalfurafine (20 μg/kg; s.c.), a κ opioid receptor agonist that has been shown to suppress scratching, reduced CD11b and p-p38 expression induced by either pruritogen. The result demonstrates, for the first time, that scratch behavior induced by pruritogens GNTI and compound 48/80 is accompanied by a parallel activation of microglia cells in the spinal cord.
Keywords: compound 48/80, GNTI, glia, itch, nalfurafine, p38, phospho-p38
INTRODUCTION
Microglia, which are resident monocyte-lineaged cells in the central nervous system (CNS), together with oligodendrocytes and astrocytes, constitute a large population of glia cells that outnumber neurons in the CNS. There has been a surge of interest regarding the role of glia in various pathophysiological states of the CNS. More recent studies show that microglia activation is correlated with the development and/or severity of a number of neurological disorders, including Parkinson’s disease, Alzheimer’s disease and chronic pain (Milligan and Watkins, 2009; Tsuda et al., 2013). Microglia, upon encountering noxious/pathological agents, transform from resting or ramified cell morphology to reactive or amoeboid-like appearance (Ling and Wang, 1993; Kreutzberg, 1996; Watkins et al., 2001; Tsuda et al., 2005). Accordingly, reactive microglia cells exhibit amoeboid-like morphology, increase proliferation, and up-regulate cell surface markers such as the complement receptor 3 (CR3); also known as cluster determinant 11b (CD11b), which recognizes the antibody OX42 (Robinson et al., 1986; Eriksson et al., 1993). More importantly, reactive microglia liberate a panel of bioactive molecules including cytotoxic and/or neurotrophic molecules such as interleukin-1β (IL-1 β); interleukin-6 (IL-6); tumor necrosis factor-α (TNF-α), proteases, brain-derived neurotrophic factor (BDNF) and reactive oxygen species (Milligan and Watkins, 2009; Kim et al., 2010; Tsuda et al., 2013). Viewed in this context, microglia may play a neuroprotective or neurotoxic role, contingent upon the type(s) and quantity of cytokines/chemokines being liberated under different disease states.
In the case of chronic pain and/or inflammation associated with the peripheral nerve injury (PNI) animal model, a pro-inflammatory role of cytokines liberated from activated microglia cells has been postulated (Milligan and Watkins, 2009; Watkins et al., 2009; Tsuda et al., 2013). On the other hand, a dissociation of microglia activation from neuropathic pain following PNI has also been reported in rats (Colburn et al., 1997). As pain and itch are both innate behaviors and share certain similarities insofar as signal processing is concerned (Ikoma et al., 2006; Mishra and Hoon, 2013; Bautista et al., 2014), we hypothesized that microglia cells are activated following exposure to the chemically diverse pruritogens 5′-guanidinonaltrindole (GNTI) and compound 48/80, in a mouse model in which scratch behavior is used as a quantitative index for the measurement of itch described previously (Inan et al., 2009).
MATERIALS AND METHODS
Animals
Male Swiss-Webster mice (Ace Laboratories, Boyertown, PA), weighing 25–30 g, were used. Animals were housed under a 12 h light/dark cycle with food and water available ad libitum. Experimental procedures were approved by Temple University Institutional Animal Care and Use Committee, in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals. Experiments were designed to keep the number of mice to a minimum and care was taken to minimize suffering.
Scratch behaviors
The experimental protocol in eliciting scratch in mice was similar to that described in our earlier studies (Inan et al., 2009; 2011). Mice were acclimated individually in rectangular observation boxes for at least 2 h prior to experiments, which were conducted between 10:00 and 16:00 h. A fixed dose of two pruritogens, GNTI (0.3 mg/kg) and compound 48/80 (50 μg in 100 μl), or saline was injected subcutaneously (s.c.) to the midline behind the mouse neck to induce scratching (Inan et al., 2009). In the series of experiments where the effect of anti-scratch compound nalfurafine (Inan et al., 2009) was evaluated against pruritogen-induced scratching, saline or nalfurafine (20 μg/kg) was injected s.c. to the flank. Twenty minutes later, either GNTI (0.3 mg/kg) or compound 48/80 (50 μg in 100 μl) was administered s.c. along the midline behind the neck of each mouse. The incidence of hindpaw scratching directed to the back of the neck was monitored for 30 min (Inan et al., 2009). Behavioral experiments were not conducted blind because the endpoint was so objective; whereas, immunohistochemistry and Western blot analyses were blind to the experimenters.
Immunohistochemistry
Mice having undergone pruritogen or saline challenge, as described above, were anesthetized with 4% isofurane and perfused intracardially with chilled 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Cervical spinal segments were removed, post-fixed in the same fixative for 2 h and stored in 30% sucrose/PBS overnight. Transverse spinal sections of 40 μm were prepared using a vibratome. Spinal sections were processed for CD11b immunoreactivity (irCD11b) by the avidin-biotin complex procedure (Dun et al., 2013). Spinal sections were first treated with 3% H2O2 to quench endogenous peroxidase, washed several times, blocked with 10% normal goat serum, and incubated in CD11b antibody (1:500 dilution; a mouse anti-rat monoclonal, EMD Millipore, Temecula, CA). After thorough rinsing, sections were incubated in biotinylated anti-mouse IgG (1:100 dilution; Vector Laboratories, Burlingame, CA) for 2 h, rinsed with PBS, and incubated in avidin-biotin complex solution (1:100 dilution; Vector Laboratories) for 1.5 h. After several washes in Tris-buffered saline (TBS), sections were developed in 0.05% diaminobenzidine/0.001% H2O2 solution and washed for at least 2 h with TBS. Sections were mounted on slides with 0.25% gel alcohol, air dried, dehydrated with absolute alcohol followed by xylene, and coverslipped with Cytoseal XYZ (Richard-Allan Scientific, Kalamazoo, MI).
Western blotting
Mice were anesthetized with 4% isofurane and decapitated. Cervical spinal cords were rapidly excised. Tissues were homogenized in ice cold lysate buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.1 mmol phenylmethylsulfonyl fluoride, and 1% protease inhibitor cocktails (Roche Diagnostics GmbH Mannheim, Germany) using ultra sonication. Whole tissue lysate homogenates were spun at 12,000 g for 20 min, and the supernatant was removed. The protein concentration was determined by Bio-Rad Protein Assay. For Western blot analysis, an equal amount of protein (20 μg) was loaded in each well and subjected to 4–7.5% or 4–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred onto nitrocellulose membrane and blocked in 5% non-fat dry milk prepared in TBS. Membranes were incubated with the primary antibody overnight at 4°C. The following primary antibodies were used: CD11b (dilution 1: 400), phospho-p38 MAPK (Thr180/Tyr182, 1:1,000 dilution, a rabbit monoclonal, Cell Signaling, Denvers, MA), p38 MAPK (1:1,000, a rabbit polyclonal, Cell Signaling), and β-actin (1:10,000, a mouse monoclonal, Sigma, St. Louis, MO). After washing, membranes were incubated with appropriate secondary antibodies (1:15,000, LI-COR Inc., Lincoln, NE) for 1 h at room temperature. Blots were developed by LI-COR Odyssey System (ODY-1780). The relative band intensity was detected by Image J software.
Chemicals and reagents
GNTI (Tocris, Ellisville, MO), compound 48/80 (Sigma Aldrich, St. Louis, MO) and nalfurafine (a generous gift from Adolor Company, Exton, PA), were dissolved in saline. Unless stated otherwise, other chemicals and reagents were from Sigma Aldrich (St. Louis, MO).
Statistics and data analysis
Data analysis and statistics were performed with GraphPad Prism 5.0 software. The statistical significance of differences between experimental groups was calculated by one-way analysis of variance followed by Tukey’s multiple comparison test. Differences were considered to be statistically significant with *P < 0.05, **P < 0.01 and ***P < 0.001. Results are given as mean ± SEM.
RESULTS
Pruritogens and Scratch
Similar to that reported in our earlier studies (Inan et al., 2009), GNTI (0.3 mg/kg) or compound 48/80 (50 μg in 100 μl), by s.c. injection to the midline of the mouse neck, provoked robust, repetitive scratching by the hindpaws directed to the back of the neck, that peaked across 30 min post injection and declined over the ensuing hour. Compound 48/80 (50 μg in 100 μl) and GNTI (0.3 mg/kg) elicited an average scratches of 463 ± 48 (n=6) and 416 ± 53 (n=8), respectively, in 30 min post injection. In contrast, saline injected to the back of mouse neck evoked an average of 42 ± 25 (n=6) scratching bouts in 30 min post injection. Pretreatment (at −20 min) with our standard dose of nalfurafine (20 μg/kg) provided, as in the past (Wang et al., 2005; Inan et al., 2009), impressive antagonism of our standard dose of both GNTI and Compound 48/80. The mean number of scratching bouts evoked by GNTI and compound 48/80 in mice pretreated with nalfurafine (20 μg/kg) was 56 ± 18 (n=6) and 49 ± 13 (n=6), respectively, in 30 min post injection.
At 0, 10, 30, 60 and 90 min after s.c. injection of saline or pruritogen, mice were anesthetized with 4% isofurane and perfused intracardically with a fixative or anesthetized with isofurane and decapitated; spinal cords were excised and processed for immunohistochemistry or Western blot.
Immunohistochemical Assessment of CD11b Expression in Spinal Cords
One of every five spinal sections of cervical segments was collected from perfused mice injected with either saline (n=3), Compound 48/80 (n=6) or GNTI (n=5).
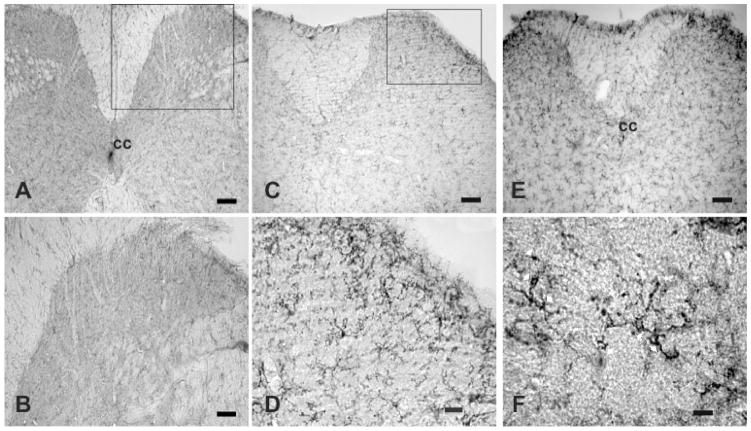
A representative section of cervical spinal cord from a mouse injected s.c. with saline, reveals that irCD11b cells had a small, weakly stained cell body with several thin processes, and are scattered throughout the gray and white matter (Fig. 1A and B). This type of irCD11b cells had the appearance of a “resting” or “ramified” microglia cell (Ling and Wang, 1993). In contrast, cervical spinal sections from mice treated with Compound 48/80 (Fig. 1C and D) or GNTI (Fig. 1E and F) expressed numerous, densely situated and strongly labeled irCD11b cells in both the grey and white matter; with the dorsal horn expressing the highest density of irCD11b cells (Fig. 1C, D and E). irCD11b cells from cervical cords of mice treated with either pruritogen appeared hypertrophic, with stout cell processes emanating from the enlarged cell bodies (Fig. 1F). This type of irCD11b cells is referred to herein as reactive microglia.
Fig. 1.
Distribution of CD11b immunoreactive cells in cervical segments from mice injected with saline, compound 48/80 or GNTI A and B, a lower and higher magnification of a cervical spinal segment from a mouse injected with saline; lightly labeled, small CD11b cells with fine cell processes are seen in the gray and white matter. C and D, a lower and higher magnification of a cervical spinal segment from a mouse injected with compound 48/80 (50 μg/100 μl) where strongly labeled, hypertrophic cells with stout cell processes densely distributed to the dorsal horn. E, a lower magnification of a cervical segment from a mouse injected with GNTI (0.3 mg/kg); numerous strongly labeled, hypertrophic cells with stout cell processes are distributed to the dorsal horn. F, a high magnification illustrating several strongly labeled irCD11 cells with stout cell processes, as indicated by white arrows. cc, central canal. Calibration bar: A, C and E, 100 μm; B and D, 50 μm; F, 10 μm.
Changes in Spinal Cord CD11b, p38 and p-p38 by compound 48/80
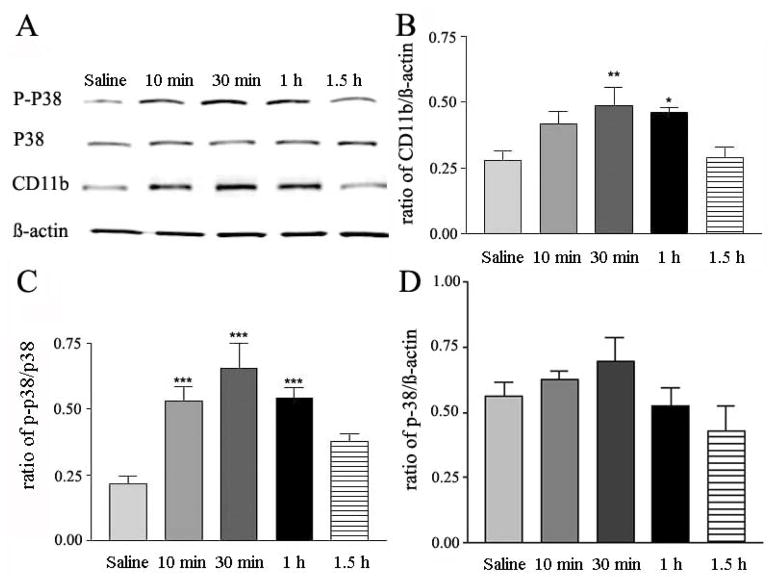
Fig. 2A shows changes in expression level of CD11b, p-p38, p38 and β-actin at various time intervals after saline or compound 48/80 injection; β-actin level serves as an internal standard in all the measurements. The mean ratio of expression of p-p38 and CD11b from five independent measurements is plotted against 30 min post injection of saline (Fig. 2B, first column) and 10, 30, 60 and 90 min post injection of Compound 48/80 (50 μg in 100 μl). The latter increased CD11b levels in spinal cords 10 min post injection, and reached a peak response in about 30 min (Fig. 2B).
Fig. 2.
Time course of compound 48/80-induced changes in CD11b, p38 and p-p38 expression in mouse spinal cords. A, representative Western blot of p-p38, p38, CD11b and β-actin induced by saline or compound 48/80 (50 μg in 100 μl) at 10, 30, 60 and 90 min post injection. B and C, histograms of ratios of CD11b/β-actin and p-p38/p38 induced by injection of saline or compound 48/80 (50 μg in 100 μl); mean ± SEM values are derived from 5 independent assays; *p <0.05, **p < 0.01 and ***p < 0.001.
The increase returned toward basal level in about an hour (Fig. 2B). The expression of p-p38 in spinal cords from mice treated with compound 48/80 was 2.5 fold higher than that from mice injected with saline (Fig. 2C). Further, the time course of rise and fall of spinal p-p38 in pruritogen-treated mice was similar to that of CD11b (Fig. 2B and C). The total p38 was not significantly changed (Fig. 2A and D).
Changes in CD11b, p38 and p-p38 Expression in Spinal Cords by GNTI
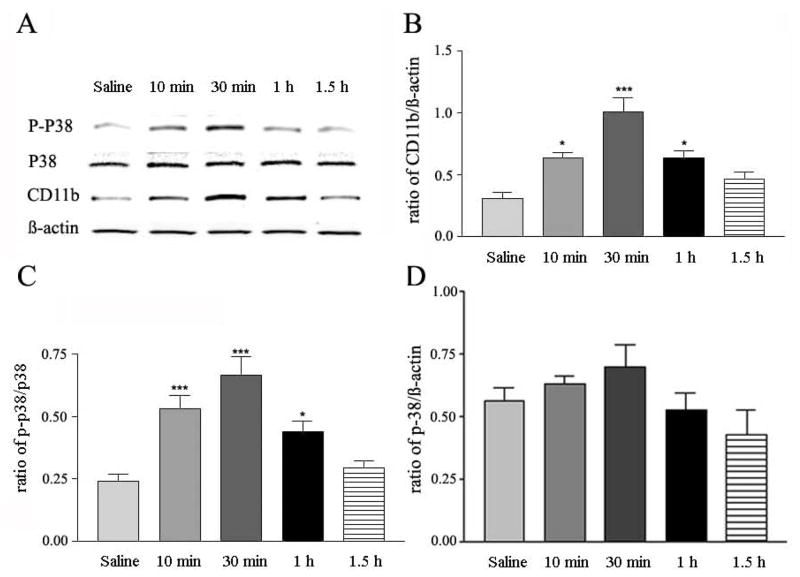
Similar to the situation with compound 48/80, Western blot showed that GNTI (0.3 mg/kg) raised the expression levels of CD11b and p-p38, but not p38 in spinal cords (Fig. 3A); the mean peak increase in CD11b and p-p38 expression amounted to about 2–2.5 fold over the saline control (Fig. 3B and C). The time course of GNTI-induced elevation of CD11b or p-p38 was parallel to that evoked by compound 48/80, reached a peak in about 30 min, and returned toward the basal level in about 1 h (Fig. 3B and C). With respect to p38, GNTI did not change the expression level of p38 at any time points measured (Fig. 3A and D).
Fig. 3.
Time course of GNTI-induced changes in CD11b, p-p38 and p38 expression in mouse spinal cords. A, representative Western blot of CD-11b, p-p38, p38 and β-actin, induced by GNTI (0.3 mg/kg) or saline at 10, 30, 60 and 90 min post injection. B, C and D, histograms of ratios of CD11b/β-actin, p-p38/p38, p-38/ β-actin induced by saline or GNTI (0.3 mg/kg). Mean ± SEM values are derived from 5 independent assays; *p <0.05, and ***p < 0.001.
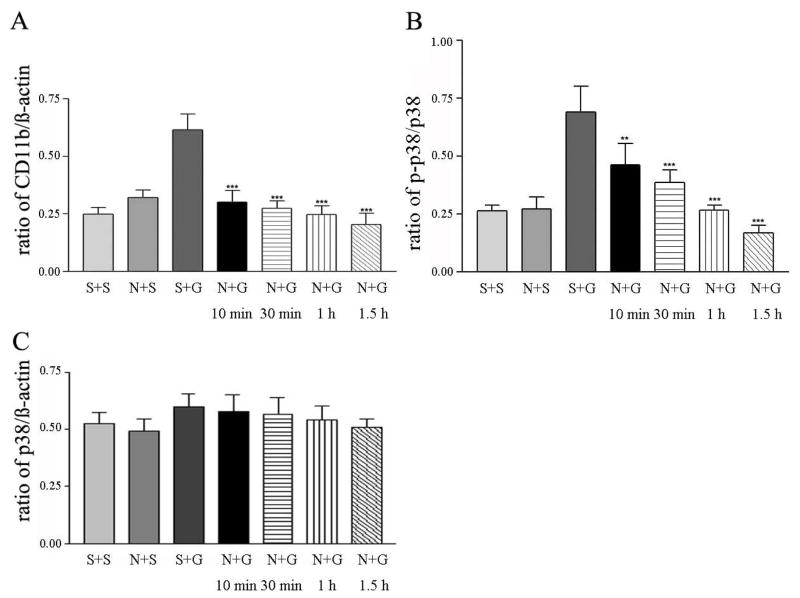
Attenuation of Pruritogen-induced CD11b and p-p38 expression by nalfurafine
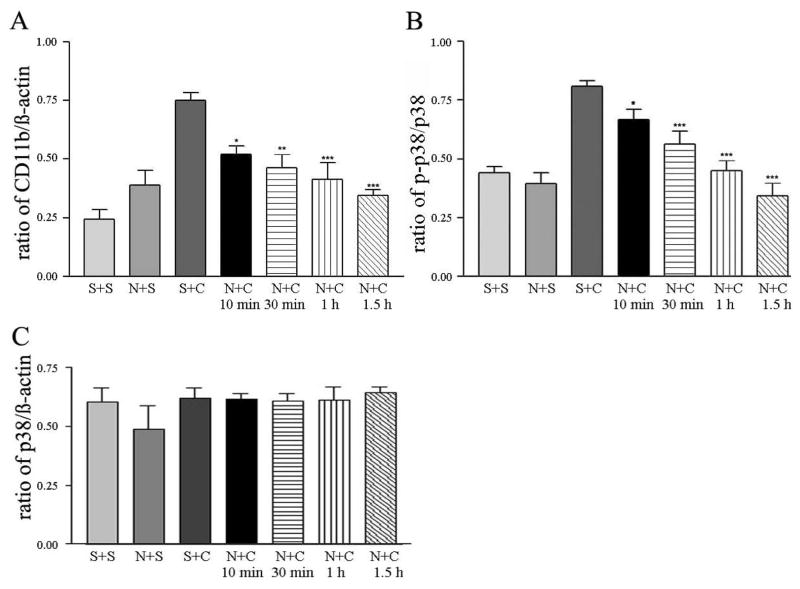
Results from our earlier studies demonstrated that nalfurafine, the κ-opioid agonist, significantly reduces scratching evoked by compound 48/80 or GNTI, a κ-opioid receptor antagonist (Wang et al., 2005; Inan et al., 2009). Accordingly, pretreatment of mice with nalfurafine (20 μg/kg) prior to compound 48/80 administration significantly reduced the number of scratches recorded 30 min post injection as compared to the number of scratches measured in mice injected with saline and compound 48/80 (49 ± 13 vs 416 ± 53; n=6). Under these conditions, expression levels of CD11b and p-p38 in the spinal cords removed from mice pretreated with nalfurafine (20 μg/kg) followed by compound 48/80 (50 μg in 100 μl) were lower as compared to levels obtained from mice injected with saline followed by compound 48/80 (Fig. 4A and B). The level of p-38 was not changed with or without pretreatment with nalfurafine (Fig. 4C).
Fig. 4.
Suppression of compound 48/80-induced changes in CD11b, p-p38 and p38 in mouse spinal cords by nalfurafine pretreatment. Histograms of ratios of CD11b to β-actin (A), p-p38 to p38 (B) and p-38/β-actin (C) at 10 min after subcutaneous injection of saline or compound 48/80 (50 μg in 100 μl) in the presence or absence of nalfurafine (20 μg/kg) pretreatment. Statistical comparison is made between responses evoked by compound 48/80, and responses of nalfurafine and compound 48/80 at 10, 30, 60 and 90 min after the last injection. Mean ± SEM values are derived from 5 independent assays; *p <0.05, **p < 0.01 and ***p < 0.001. Abbreviations: saline + saline (S+S); nalfurafine + saline (N+S); saline + compound 48/80 (S+C); nalfurafine + compound 48/80 (N+C).
Similarly, pretreatment of mice with nalfurafine (20 μg/kg) significantly and consistently reduced the number of scratches (56 ± 48, n=6) in mice recorded in a period of 30 min post GNTI administration as compared to the number of scratches (463 ± 48, n=6) recorded from mice injected with saline and GNTI. In this case, the level of expression of CD11b and p-p38 induced by GNTI in mice pretreated with nalfurafine was significantly lower as compared to that measured from spinal cords removed from mice pretreated with saline followed by GNTI (Fig. 5A and B). The level of p-38 was not altered in mice with or without pretreatment of nalfurafine (Fig. 5C).
Fig. 5.
Attenuation of CD11b and p-p38 expression induced by GNTI in mouse spinal cords by nalfurafine pretreatment. Histograms of ratios of CD11b to β-actin (A), p-p38 to p38 (B) and p-38/β-actin (C) at 10 min after subcutaneous injection of saline or GNTI (0.3 mg/kg) in the presence or absence of nalfurafine (20 μg/kg) pretreatment. Statistical comparison is made between responses evoked by GNTI, and responses of nalfurafine plus GNTI at 10, 30, 60 and 90 min after the last injection. Mean ± SEM values are derived from 5 independent assays; *p <0.05, **p < 0.01 and ***p < 0.001. Abbreviations: saline + saline (S+S); nalfurafine + saline (N+S), saline + GNTI (S+G); nalfurafine + GNTI (N+G).
DISCUSSION
Our immunohistochemical and Western blot studies show that the pruritogens GNTI and compound 48/80 injected s.c. to the back of mice evoked a robust expression of CD11b, a cell surface marker for microglia, and p-p38, also a marker of microglia, in spinal cords, reaching a peak response in about 30 min, Further, pretreatment of mice with nalfurafine, a clinically tested κ-opioid receptor agonist (Wikström et al., 2005), effectively mitigated the up-regulation of CD11b and p-p38 in spinal cords evoked by either pruritogen. Moreover, as with previous findings (Inan et al., 2009), compound 48/80 or GNTI when injected s.c. to the neck of mice provoked robust, repetitive scratching, which started 2–3 min post injection, peaked across 30 min, and lasted about 90 min; these features of scratch behaviors were similar to that observed here. In addition, scratch behaviors induced by these two pruritogens were effectively suppressed by nalfurafine (Wang et al., 2005; Inan et al., 2009). Thus, the onset, duration and pharmacology of CD11b up-regulation by compound 48/80 or GNTI were similar to those of scratch behaviors provoked by the same pruritogens. As a corollary, scratching behavior and CD11b up-regulation observed in these two studies may be causally correlated.
Microglia cells are one of the three major constituents of glia cells in the CNS; the other two being oligodendroglia and astrocytes. These three types of glia cell differ with respect to their cell morphology, molecular, cellular and functional characteristics (Watkins et al., 2001). Among the glia cells, microglia have received the most attention in recent years, as they were postulated to engage in several pathophysiological events by elaborating a panel of pro-inflammatory and/or anti-inflammatory cytokines and chemokines (Kreutzberg, 1996; Tsudo et al., 2005; Ubogu et al., 2006; Milligan and Watkins, 2009). For example, activated microglia cells liberate a number of signal molecules, including IL-1β, IL-6, and TNF-α, which contribute to chronic pain/inflammation in a rodent model of neuropathic pain (Tsudo et al., 2005; Milligan and Watkins, 2009). With respect to the other two types of glia cell, Wang et al. (2009) have shown that ligation-induced neuropathic pain is attenuated by inhibitors selective for astrocytes. As fewer reports have implicated the involvement of astrocytes or oligodendroglia in pathophysiological states of the CNS, microglia were the main target of our investigation. An involvement of oligodendroglia and/or astrocytes in scratch behavior directly or indirectly remains to be explored.
Microglia, in response to noxious stimuli, rapidly increase the expression of several surface antigens; among which CD11b, a member of the b2 integrin family, is detectable in resting (or ramified) microglia, and is up-regulated at the mRNA and protein level in activated (or amoeboid) microglia. In addition, resting microglia undergo a stereotypic transformation from “ramified” cell morphology to “amoeboid” shape, which is indicative of “reactive” state (Ling and Wang, 1993). In spinal cords from mice injected with saline, irCD11b cells were weakly labeled and less densely distributed; and assumed “ramified” cell morphology with several short, fine cell processes emanating from the cell body. In contrast, irCD11b cells in spinal cords from mice injected with pruritogens exhibited an enlarged cell body with several stout cell processes, and were densely spaced throughout the grey and white matter. Thus, the cell morphology and CD11b intensity suggest that CD11b cells in question are “reactive” microglia cells. In addition, Western blot showed that CD11b and p-p38 levels, but not p38, are elevated in spinal cords from pruritogen-treated mice as compared to those from saline-treated animals. Elevated expression of p-p38 followed a similar time course to that of CD11b, and pretreatment with nalfurafine attenuated the increase of CD11b as well as p-p38 expression. In a neuropathic pain model, up-regulation of p38 occurred in microglia, and not in neurons or astrocytes (Jin et al., 2003; Svensson et al., 2003; Ji and Suter, 2007). Viewed in this context, the activated microglia cells observed in the spinal cord following pruritogen challenge may more appropriately be labeled “reactive” microglia cells. It is pertinent to mention that, in addition to CD11b, ionized calcium binding adaptor molecule, abbreviated Iba1, has also been proposed to be a cell marker specific for microglia cells, and not neurons, astroglia or oligodendroglia (Ito et al., 1998).
In our previous study, compound 48/80 or GNTI injected s.c. to the back of the mouse neck, provoked fos-expression in neurons that are located mainly in the lateral side of the superficial layers of the cervical segment (Inan et al., 2009). Here, irCD11b cells are found densely distributed to the grey and white matter of the cervical cord. It is of interest to note that in the peripheral nerve injury model, a marked activation of microglia at the spinal cord level was detected in one to two days, reached a peak response in one to two weeks, and returned to the basal level by day 84 (Eriksson et al., 1993; Coyle, 1998). Further, activated microglia cells associated with chronic pain are confined mostly to the dorsal horn (Eriksson et al., 1993; Coyle, 1998). A rapid onset, as early as 3 h, and offset in 7 days, of spinal microglia activation was noted in a model of neuropathic pain induced by intrathecal injection of lysophosphatidic acid (Ma et al., 2010). In our scratch model, pruritogens cause a rapid up-regulation of microglia cells throughout the cervical spinal cord that is not limited to the dorsal horn. The reason for different spatial activation of spinal microglia cells under different pathophysiological conditions is not known.
Intrathecal administration of substance P or intradermal injection of formalin induced a rapid (< 5 min) expression of phosphorylated-p38 in spinal microglia (Svensson et al., 2003). Consistent with an early onset of CD11b up-regulation, our result showed that p-p38 is significantly up-regulated in 10 min, the earliest time point examined here. Further, the time course of increased CD11b was parallel to that of p-p38, implying a causal relationship between expression of p-p38 on the one hand and CD11b on the other. P38 mitogen-activated protein kinases are a class of mitogen-activated protein kinases that are responsive to stress stimuli, including cytokines, and are involved in Ca2+ sensitive intracellular signaling cascades, leading to the production of pro-inflammatory cytokines from microglia cells (Milligan and Watkins, 2009; Tsuda et al., 2004; 2013). There is evidence that chronic neuropathic pain subsequent to peripheral nerve injury may be caused by activation of microglia and/or astrocytes with subsequent release of pro-inflammatory cytokines (Coyle, 1998; Watkins et al., 2001; Cao and Zhang, 2008; Milligan and Watkins, 2009; Tsuda et al., 2013). Whether or not cytokines/ chemokines are being liberated from reactive microglia cells in response to pruritogen challenge is not known at present; neither is the nature of these signaling molecules. There is virtually no information relative to the involvement of microglia in scratch signaling in the spinal cord. In the case of pain signaling, microglia cells may contribute to chronic pain or inflammation by elaborating a panel of cytokines and/or chemokines (Milligan and Watkins, 2009; Watkins et al., 2009; Gao and Ji. 2010; Tsuda et al., 2013). On the basis of information derived from the chronic pain model, several possibilities regarding the activation of spinal microglia may be considered in our scratch model. First, pruritogens may activate peripheral primary afferents, which release glutamate and/or ATP from central primary afferents, which in turn, activate microglia. Second, pruritogens acting on central primary afferents and/or dorsal horn neurons may liberate one or more itch-producing mediators such as histamine, gastrin-releasing peptide (Sun and Chen, 2007), and natriuretic polypeptide b (Mishra and Hoon, 2013), which in turn activate microglia. Third, pruritogens may diffuse into the spinal cord and activate microglia directly. Lastly, toll-like receptors, in particular toll-like receptor 7 and toll-like receptor 3, may be involved in the pathogenesis of itch (Liu et al., 2012). Hence, pruritogens may directly or indirectly activate toll-like receptors, leading to the expression of itch sensation. It should, however, be stressed that the nature of upstream signaling molecules or downstream signaling cascade within the microglia remain to be systematically investigated.
Despite the long-term use of compound 48/80 as a standard pruritogen in both preclinical and clinical research, it is not yet known which of the many mediators this polymer releases from mast cells is/are responsible for the scratching behavior it elicits in mice since histamine itself, a likely candidate, was only weakly active in this species (Kuraishi et al., 1995). Similarly, just how prototype kappa opioid receptor antagonists such as the bimorphinan, norbinaltorphimine (Kamei and Nagase, 2001), and the indolomorphinan, 5′-GNTI, provoke repetitive scratching in mice has yet to be established. Previous work has shown that histamine H-1 and H-4 antagonists (Cowan and Inan, 2010), as well as antagonists of the gastrin-releasing peptide receptor (Inan et al., 2011), have no marked influence on 5′-GNTI-precipitated compulsive scratching. Non-histaminergic scratch-induced signaling, mediated by, for example, oxidative stress, Mas-related G protein-coupled receptors, protease-activated receptors, and toll-like receptors represent future areas of research with kappa opioid receptor antagonists such as 5′-GNTI.
Acknowledgments
Grant information: This work was supported in part by Grants R37NS18710 and P30DA13429 from the National Institutes of Health. Ying Zhang was supported in part by YunNan Provincial United Fund (No. 2012FB013).
Nalfurafine was a generous gift from Adolor Company, Exton, PA.
References
- Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. 2014;17:175–182. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang Y-Q. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmuno. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Cowan A, Inan S. Pharmacological analysis of GNTI-induced compulsive scratching in mice. Proc Brit Pharmacol Soc. 2010 http://www.pA2online.org/abstracts/Vol7Issue2abst054P.pdf.
- Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin immunoreactivity in neural and non-neural tissue of the rodent. Neuroscience. 2013;246:155–162. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson NP, Persson JK, Svensson M, Arvidsson J, Molander C, Aldskogius H. A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp Brain Res. 1993;96:19–27. doi: 10.1007/BF00230435. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nature Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A. Nalfurafine prevents 5′-guanidinonaltrindole- and compound 48/80-induced spinal c-fos expression and attenuates 5′-guanidinonaltrindole-elicited scratching behavior in mice. Neurosci. 2009;163:23–33. doi: 10.1016/j.neuroscience.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A. Investigation of gastrin-releasing peptide as a mediator for 5′-guanidinonaltrindole-induced compulsive scratching in mice. Peptides. 2011;32:286–292. doi: 10.1016/j.peptides.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007:3–33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Nagase H. Norbinaltorphimine, a selective κ-opioid receptor antagonist, induces an itch-associated response in mice. Eur J Pharmacol. 2001;418:141–145. doi: 10.1016/s0014-2999(01)00941-4. [DOI] [PubMed] [Google Scholar]
- Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci USA. 2010;107:14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wang WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Liu T1, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Nagai J, Ueda JH. Microglial activation mediates de novo lysophosphatidic acid production in a model of neuropathic pain. J Neurochem. 2010;115:643–653. doi: 10.1111/j.1471-4159.2010.06955.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- Sun Y-G, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Beggs S, Salter MW, Inoue K. Microglia and intractable chronic pain. Glia. 2013;61:55–61. doi: 10.1002/glia.22379. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neuroscience. 2005;28:1–16. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang W, Mei XP, Huang J, Wei YY, Wang YY, Wu SX, Li YG. Crosstalk between spinal astrocytes and neurons in nerve injury-induced neuropathic pain. PLoS One. 2009;4(9):e6973. doi: 10.1371/journal.pone.0006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DYW, Huang P, Li J-G, Cowan A, Liu-Chen L-Y. Comparison of pharmacological activities of three distinct κ ligands (Salvinorin A, TRK-820 and 3FLB) on κ opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Mater SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neuroscience. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y. κ-Opioid system in uremic pruritus; multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16:3742–3747. doi: 10.1681/ASN.2005020152. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;19:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]