Abstract
BACKGROUND
Intracellular Zn2+ levels decrease during prostate cancer progression and agents that modulate intracellular Zn2+ are cytotoxic to prostate cancer cells by an incompletely described mechanism. F10 is a new polymeric fluoropyrimidine drug-candidate that displays strong activity with minimal systemic toxicity in pre-clinical models of prostate cancer and other malignancies. The effects of exogenous Zn2+ or Zn2+ chelation for enhancing F10 cytotoxicity are investigated as is the role of Omi/HtrA2, a serine protease that promotes apoptosis in response to cellular stress.
METHODS
To test the hypothesis that the pro-apoptotic effects of F10 could be enhanced by modulating intracellular Zn2+ we investigated cell-permeable and cell-impermeable Zn2+ chelators and exogenous Zn2+ and evaluated cell viability and apoptosis in cellular models of castration-resistant prostate cancer (CRPC; PC3, C4-2). The role of Omi/HtrA2 for modulating apoptosis was evaluated by pharmacological inhibition and Western blotting.
RESULTS
Exogenous Zn2+ initially reduced prostate cancer cell viability but these effects were transitory and were ineffective at enhancing F10 cytotoxicity. The cell-permeable Zn2+-chelator tetrakis-(2-pyridylmethl)ethylenediamine (TPEN) induced apoptosis in prostate cancer cells and enhanced the pro-apoptotic effects of F10. The pro-apoptotic effects of Zn2+-chelation in combination with F10 treatment were enhanced by inhibiting Omi/HtrA2 implicating this serine protease as a novel target for prostate cancer treatment.
CONCLUSIONS
Zn2+-chelation enhances the pro-apoptotic effects of F10 and may be useful for enhancing the effectiveness of F10 for treatment of advanced prostate cancer. The serine protease Omi/HtrA2 modulates Zn2+-dependent apoptosis in prostate cancer cells and represents a new target for treatment of CRPC.
Keywords: Zn2+, castration-resistant prostate cancer, F10, fluoropyrimidine, Omi/HtrA2
INTRODUCTION
The relationship between Zn2+ and prostate cancer incidence, response to chemotherapy, and recurrence is complex. In general, prostate cancer cells have low intracellular Zn2+ levels [1]. Further, increased dietary Zn2+ is associated with prostate cancer survival [2] although chronic zinc oversupply may enhance risk of prostate cancer [3]. Either low or high dietary zinc increased tumor burden in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model of prostate cancer [4]. Low levels of Zn2+ and Fe2+ correlated with disease recurrence in prostate cancer patients [5]. The importance of Zn2+ levels for prostate cancer progression has resulted in development of imaging modalities to sense Zn2+ levels [6] as well as therapeutic approaches to modulate Zn2+ to enhance chemotherapy. The sensitivity of prostate cancer cells to both exogenous Zn2+ [7, 8] and Zn2+ chelation [9] suggests Zn2+ levels are tightly regulated and not simply minimized in prostate cancer cells.
The apparently complex relationship between Zn2+ levels and prostate cancer incidence and recurrence has resulted in studies to modulate intracellular levels of Zn2+ for therapeutic benefit. Zn2+ is transported into cells by the SLC39 (Zip-family) members and is transported out of cells by SLC30 (ZnT) zinc transporters [10]. Decreased expression of Zip-family members is characteristic of prostate cancer and as a consequence treatment of PCa cells with Zn2+ salts (e.g. ZnSO4) may have minimal effect on intracellular Zn2+ necessitating Zn2+-delivery with a cell-permeable chelate, such as pyrithione (ZnPy). Several studies have, however, reported that Zn2+ salts can be cytotoxic to prostate cancer cells. For example, zinc acetate was cytotoxic to PC3, LNCaP, and DU145 cells and direct intratumoral injection decreased tumor growth [7]. Other studies however, report that while zinc salts are readily taken up and retained by myeloid progenitor cells and protect these cells from the pro-apoptotic effects of docetaxel chemotherapy similar treatment does not result in increased Zn2+ levels in prostate cancer cells or protect these cells from docetaxel-induced apoptosis [11]. In contrast to Zn2+ salts, treatment with ZnPy increased Zn2+ levels in prostate cancer cells and was cytotoxic to DU145, PC3, and LNCaP cells [8] and enhanced paclitaxel- and TNFα-mediated apoptosis in PC3 cells [12]. Thus, the effects of Zn2+ on prostate cancer cells depends on the cell permeability of administered Zn2+ as well as on the chemotherapy agent co-administered.
The present studies focus on modulating intracellular Zn2+ in prostate cancer cells to enhance the effectiveness of chemotherapy with F10, a novel polymeric fluoropyrimidine that has shown promising activity in pre-clinical models of highly lethal malignancies including acute myeloid leukemia (AML) [13, 14], glioblastoma (GBM) [15], and advanced prostate cancer [16]. Our laboratory became interested in the potential of F10 for improved treatment of prostate cancer based upon results from the NCI 60 cell line screen indicating that cellular models of castration-resistant prostate cancer (CRPC; e.g. PC3, DU145) were highly sensitive to F10 with GI50 values in the nanomolar range [17]. F10 displays large advantages relative to current chemotherapy and PC3 cells were more than 600-fold more sensitive to F10 relative to the conventional fluoropyrimidine drug 5-fluorouracil (5FU). These results suggest that F10 might be useful for treating CRPC despite clinical studies with 5FU and capecitabine demonstrating these drugs lack efficacy for prostate cancer treatment [18–20]. We report herein investigations of treatment effects in cellular models of CRPC (C4-2 and PC3) with exogenous Zn2+ as well as with Zn2+-chelators both as single agents and in combination with F10. The cell permeable Zn2+ chelator tetrakis-(2-pyridylmethl)ethylenediamine (TPEN) displays strong single agent activity and enhanced the pro-apoptotic effects of F10 towards PC3 and C4-2 cells, demonstrating the potential for Zn2+-chelation to be used to enhance F10 efficacy for CRPC treatment.
The molecular targets affected by Zn2+-treatment in prostate cancer and other cells have been partly elucidated and include mediators of apoptosis such as caspase 3 and the caspase 3 substrate X-linked inhibitor of apoptosis (XIAP). Zn2+ inhibits the activity of caspase 3 [21] and cell-permeable Zn2+ chelators including TPEN [22] and PAC-1 [23] induce apoptosis through increased caspase 3 activation. Zinc depletion also was reported to induce XIAP cleavage prior to initiation of apoptosis in prostate cancer cells in a caspase-independent as well as proteasome-independent manner [24]. The Zn2+-dependent cleavage of XIAP was selective and neither cIAP1 nor cIAP2 were similarly affected by Zn2+ chelation. Zn2+-dependent XIAP cleavage was inhibited by Pefabloc implicating a serine protease for mediating XIAP-specific cleavage [11]. A potential candidate Ser protease for Zn2+-dependent XIAP degradation is Omi/HtrA2. Omi/HtrA2 undergoes auto-catalysis to release a form that has homology to Smac/DIABLO [25]. This cleaved product has pro-apoptotic properties resulting from sequestering XIAP, and thus indirectly activates caspase 3 [26]. Omi/HtrA2 is located in the mitochondria, however upon pro-apoptotic stimulation processed Omi/HtrA2 translocates into the cytosol and binds XIAP. Although Omi/HtrA2 is known to be important for promoting apoptosis in response to cellular stress [27], it has not been previously reported to contribute to apoptosis in PCa cells. We report that Omi/HtrA2 inhibition has single agent activity and induces apoptosis in prostate cancer cells. Our studies also show that inhibiting Omi/HtrA2 enhances F10-induced cytotoxicity and apoptosis towards CRPC cells. Both of these properties of Omi/HtrA2 are Zn2+-dependent. These studies demonstrate Omi/HtrA2 is a novel target for prostate cancer treatment.
Materials and Methods
Cell Maintenance
C4-2 cells were a gift from Dr. Elizabeth M. Wilson (UNC, Chapel Hill, NC). PC3 cells were purchased from cell and viral vector core laboratory at Wake Forest School of Medicine. All cells were maintained with RPMI 1640 (Gibco, Grand island, NY) with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA). All cells were incubated at 5% CO2 at 37 °C.
Cell-Viability and Apoptosis Assays
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise. F10 was synthesized as described previously [28]. PC3 and C4-2 cells were seeded at a density of 3,000 cells/well in 96-well plates and were treated the following day with the indicated agents for 24–72 h. Live cell counts were measured indirectly by measuring the ATP amounts using CellTiter-Glo® luminescent cell viability assay (Promega, Madison, WI) according to the manufacturer’s protocol and expressed as percent of control. Caspase 3/7, 8, and 9 activity and cell viability assays were performed using Promega Caspase-Glo Assay reagents for caspase activity.
Intracellular Zinc Measurements
PC3 and C4-2 cells were cultured as described for cell viability and apoptosis assays and four days later the media was removed and cells were incubated under minimal light for 30 min at 37 °C with 1 μM of FluoZin™-3 AM (Molecular Probes, Eugene, OR) or Zinquin in phosphate-free, HEPES-buffered Hanks’ balanced salt solution (HHBSS). After 30 min, the buffer with FluoZin-3 AM was discarded and the cells were incubated with ZnPy in HHBSS for 1 h at 37 °C followed by two washes with HHBSS. Fluorescence emitted by the Fluozin-3 AM that bound Zn2+ and trapped inside the cells was measured using a Tecan Genius luminescence plate reader with excitation/emission wavelengths of 485/520 nm. Fluorescence was visualized using a Zeiss LSM 510 Confocal Microscope (Carl Zeiss, Oberkochen, Germany).
Statistical Analysis
Assay values were expressed as mean +/− standard error and comparisons between mean values were made using Student’s t-test for paired samples with p ≤ 0.05 (two-tailed) considered significant.
RESULTS
Cytotoxicity of Exogenous Zn2+ and Zn2+-Chelation
The effects of exogenous Zn2+ or of Zn2+-chelation on the viability of prostate cancer cells were investigated using PC3 and C4-2 cells treated with either zinc sulfate or with the cell-permeable Zn2+ chelator pyrithione as the sodium complex in the presence or absence of ZnSO4. Alternatively, prostate cancer cells were treated with the cell-impermeable Zn2+-chelator diethylenetriaminepentaacetate (DTPA) to decrease Zn2+ concentrations in culture media and to indirectly decrease intracellular Zn2+. The results revealed a marked time-dependence to the effects of both exogenous Zn2+ and its chelation on prostate cancer cell viability (Figure 1). Treatment with zinc sulfate initially decreased cell viability, particularly for C4-2 cells, however 72 h of continuous treatment with elevated exogenous Zn2+ resulted in no significant change to the viability of PC3 cells and only a moderate (~20%) decrease in the viability of C4-2 cells consistent with cellular adaptation to increased Zn2+ in culture medium. The effects of zinc salts on cell viability were independent of the counterion with zinc chloride as effective as zinc sulfate consistent with a Zn2+-specific effect.
Figure 1.
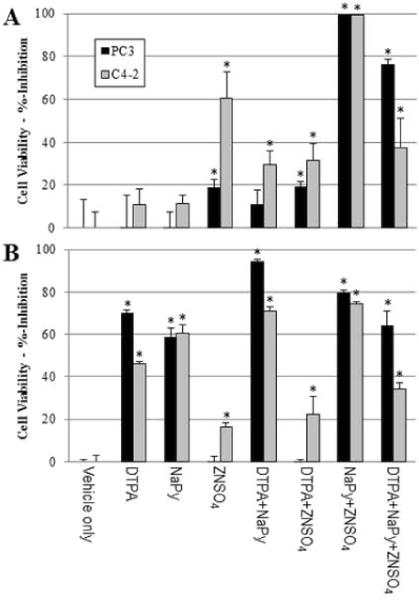
Time-dependent effects of Zn2+ chelation or treatment with exogenous Zn2+ to PC3 or C4-2 cell viability (expressed as %-inhibition). Cells were treated with either the cell-impermeable Zn2+-chelator DTPA (50 μM), ZnS04 (50 μM), NaPy (1.2 μM), or NaPy (1.2 μM) + ZnS04 (50 μM) for (A) 24 h or (B) 72 h. Statistical significance, Student’s two-tailed test: *: p<0.05.
Pyrithione is a pyridine N-oxide that forms a 2:1 complex with Zn2+ that is cell-permeable and widely used for studies evaluating the effects of increased intracellular Zn2+ on cell function independent of cell transport. Our studies showed that sodium pyrithione (NaPy) is cytotoxic to both C4-2 and PC3 cells in a time-dependent manner consistent with pyrithione facilitating uptake of Zn2+ from the culture media. Sodium pyrithione cytotoxicity is enhanced when combined with an excess of exogenous Zn2+ – conditions that favor ZnPy formation and enhance cell-uptake of Zn2+ independent of Zn2+-transporter function (Figure 1). The combination of pyrithione and Zn2+ showed only slightly reduced effects at later timepoints compared to Zn2+-only treatment, consistent with prostate cancer cells adapting less readily to increased intracellular Zn2+ that is internalized independent of facilitated uptake and likely by decreasing Zn2+-influx rather than increasing Zn2+-efflux.
While exogenous Zn2+ decreased prostate cancer cell viability, Zn2+-chelation with the cell-impermeable Zn2+-chelator DTPA also decreased viability consistent with cellular homeostasis requiring tight regulation of intracellular Zn2+ dependent on the concentration of free Zn2+ in culture medium. The reduction in prostate cancer cell viability due to chelating Zn2+ in culture medium is slower than effects resulting from exogenous Zn2+ treatment with significant reduction in viability at 72 h but not at 24 h of treatment (Figure 1). The cytotoxic effects of DTPA were completely reversed by co-treatment with zinc sulfate consistent with DTPA cytotoxicity solely resulting from Zn2+ chelation, although DTPA may chelate other metal ions. The combination of sodium pyrithione and DTPA was less effective than either single agent and this was partly reversed by addition of exogenous Zn2+ consistent with pyrithione cytotoxicity being mediated by Zn2+ transport.
Cell Imaging of Intracellular Zn2+
The cytotoxic effects of exogenous Zn2+ were investigated using the cell-permeable Zn2+-sensitive fluorescent dyes Zinquin and FluoZin-3 (Figure 2). Treatment of both PC3 and C4-2 cells with ZnPy resulted in concentration-dependent increases in fluorescence in cells pre-treated with FluoZin consistent with intracellular transport of Zn2+ being responsible for the cytotoxicity of ZnPy. The sub-cellular distribution of fluorescence resulting from FluoZin and Zinquin treatment was distinct although both fluorophores displayed strong concentration-dependent fluorescence that was responsive to ZnPy treatment consistent with the observed fluorescence being due to Zn2+ uptake into prostate cancer cells. Zinquin fluorescence was punctate consistent with sequestration into endosomes, lysososmes, or other organelles [29] while FluoZin fluorescence was diffuse consistent with predominantly cytoplasmic distribution. While the origin of Zn2+-dependent fluorescence by Zinquin and FluoZin remains under investigation and apparently involves interaction of the fluorphores with Zn2+-binding proteins rather than with an aqueous free-Zn2+ pool in cells [30], the results are consistent with ZnPy cytotoxicity being mediated through increased intra-cellular Zn2+ levels.
Figure 2.
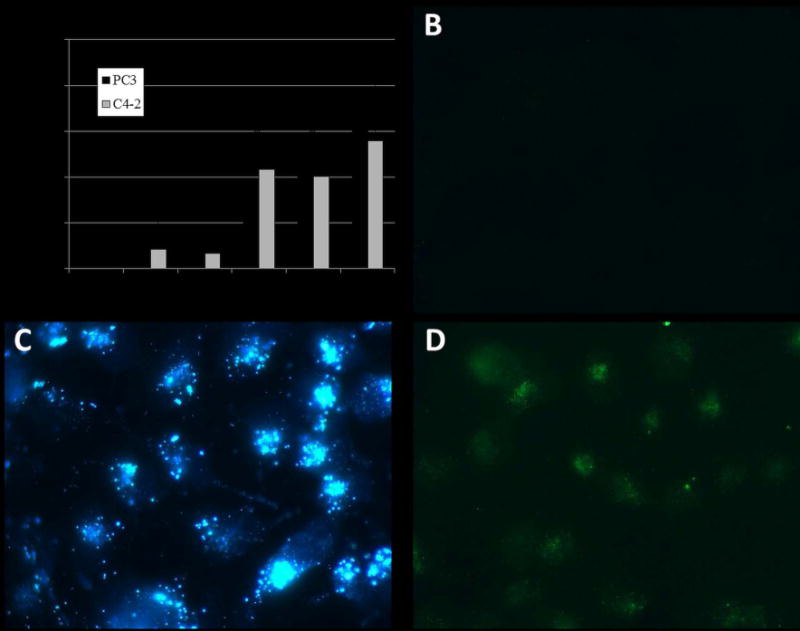
Exogenous Zn2+ causes sensor-dependent changes in fluorescence for intracellular Zn2+ sensors. (A) %-Increase in FluoZin fluorescence (relative to vehicle-treatment) in PC3 and C4-2 cells following treatment with ZnPy (“*” indicates p < 0.05 for both cell lines). B – D are confocal images of C4-2 cells: B – no treatment; C – 6 μM ZnPy with Zinquin detection; D – 6 μM ZnPy with FluoZin detection.
ZnPy Affects Chemotherapy Response
To determine to what extent ZnPy treatment affected the cytotoxic effects of chemotherapy drugs we investigated co-treatment of ZnPy with F10, a novel polymer of 5-fluoro-2′-deoxyuridine-5′-O-monophosphate, the active metabolite of fluoropyrimidine chemotherapy that displays strong anti-cancer activity and minimal systemic toxicity in pre-clinical models of AML [13, 14], GBM [15], and advanced prostate cancer [16]. F10 reduced the viability of both PC3 and C4-2 cells with IC50 values in the nanomolar range (Figure 3 – see data at 0 ZnPy). PC3 cells were markedly more sensitive to the effects of ZnPy relative to C4-2 cells although the IC50 for ZnPy in both cell lines exceeded 100 nM. Further, at concentrations of ZnPy that induced at least 50% reduction in viability for either cell line the effects of ZnPy treatment were independent of F10 concentration consistent with higher concentrations of ZnPy interfering with F10 cytotoxicity. Interestingly, while either F10 or ZnPy reduced the viability of prostate cancer cells, only F10 induced apoptosis. In fact, at higher concentrations ZnPy inhibited F10-induced apoptosis, but not viability. The results demonstrate that therapies to increase Zn2+ in PCa cells do not enhance and may actually diminish the cytotoxic effects of chemotherapy and modulate apoptosis.
Figure 3.
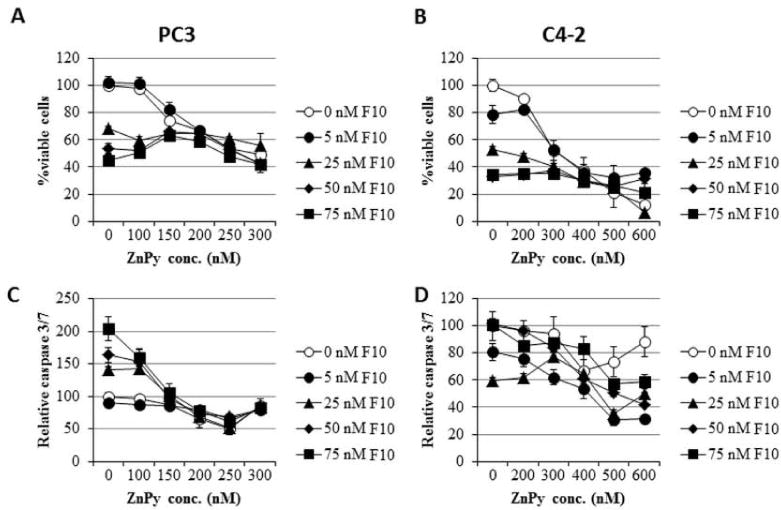
Continuous co-treatment with ZnPy enhances F10 cytotoxicity only under limited conditions. (A,B) Plots of cell viability for (A) PC3 and (B) C4-2 cells co-treated with F10 and ZnPy under the conditions indicated. (C,D) Plots of caspase 3/7 activity for (C) PC3 and (D) C4-2 cells under the conditions indicated. Cytotoxicity is independent of F10 concentration for ZnPy concentrations >150 nM for PC3 and > 300 nM for C4-2 cells.
Intracellular Zinc Chelation is Cytotoxic and Enhances F10-induced Apoptosis
In contrast to DTPA that is cell-impermeable and modulates Zn2+ levels in media, the Zn2+ chelator TPEN is readily internalized by prostate cancer cells. Our studies demonstrated that 6 h treatment with TPEN reduced the viability of prostate cancer cells (Figure 4) The effects of TPEN were more rapid than occurred with DTPA which had minimal effect with 6 h treatment, while cell-permeable pyrithione also displayed rapid effects. TPEN treatment also significantly reduced FluoZin fluorescence in C4-2 cells, consistent with TPEN-mediated effects on cell viability being Zn2+-dependent (Figure 4). Further, the effects of TPEN treatment on cell viability were completely reversed by co-treatment with zinc sulfate (Figure 4). Co-treatment of PC3 and C4-2 cells with TPEN during the final six hours of a 48 h treatment with 10 nM F10, conditions that result in ~15% decreased viability in the absence of TPEN, resulted in a significant further reduction in cell viability and increased apoptosis (Figure 5) and the effects of TPEN were negated by co-treatment with Zn2+. Treatment of C4-2 cells with F10 + TPEN did not, however, result in increased levels of either p21 or Bax relative to treatment with F10-only (Figure 6) suggesting that the apoptosis-enhancing properties of TPEN are mediated via pathways distinct from transcriptional upregulation of pro-apoptotic proteins that are induced by chemotherapy alone.
Figure 4.
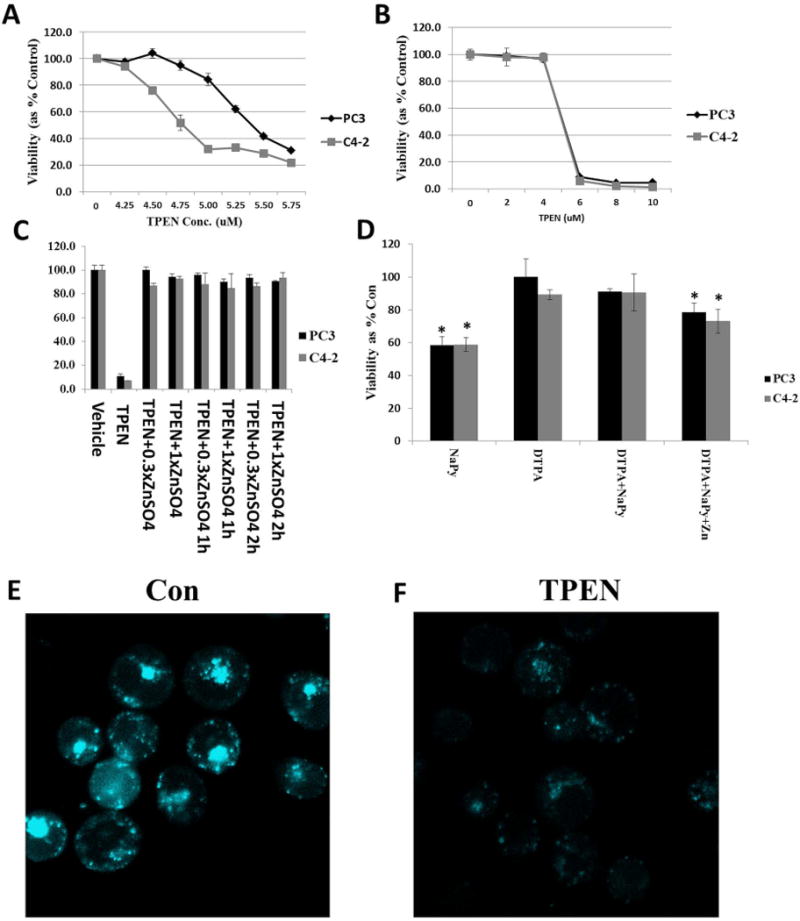
Treatment with the Zn2+ chelator TPEN reduces viability for PC3 and C4-2 cells and TPEN cytotoxicity is completely reversed by exogenous Zn2+. (A) Reduced viability upon treatment with the indicated TPEN concentration. (B) Expanded scale differentiates sensitivity of PC3 and C4-2 cells to TPEN cytotoxic effects. (C) Rescue of TPEN cytotoxicity with exogenous ZnSO4 at the indicated concentration relative to TPEN (6 μM) and with the delay indicated. (D) NaPy but not DTPA displays rapid effects. Treatments were as indicated in Figure 1 except for 6 h followed by 72 h in drug-free media. Statistical significance, Student’s two-tailed test: *: p<0.05. (E,F) FluoZin fluorescence for PC3 cells: (E) Control (non-treated cells); (F) Treatment with TPEN (5 μM for 4 h) with identical settings as in (E).
Figure 5.
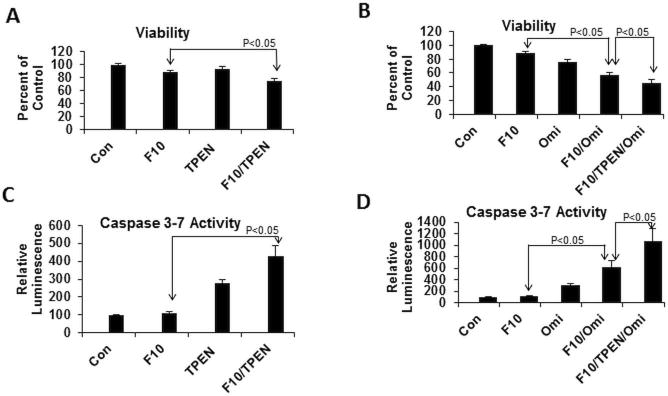
TPEN enhances F10 cytotoxicity and inhibiting the serine protease Omi/HtrA2 (Omi) further enhances F10 + TPEN. (A) Viability and (C) apoptosis in C4-2 cells upon treatment with F10 (10 nM; 48 h) + TPEN (6 μM). TPEN was administered during the final six hours of treatment. (B) Viability and (D) apoptosis upon treatment with F10 and TPEN as in (A,C) except additional co-treatment with the Omi/HtrA2 inhibitor Ucf-101 (10 μM).
Figure 6.
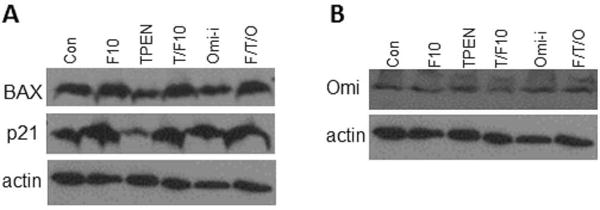
F10 enhances expression of p21 and Bax in C4-2 cells while neither TPEN nor UCF-101 (Omi) enhance expression as single agents or further enhance F10-induced increased expression. (A) Western blot for Bax and p21 for C4-2 cells treated as in Figure 5. (B) Western blot for Omi/HtrA2 that does not undergo changed expression under these treatment conditions.
We then sought to determine if the pro-apoptotic effects of Zn2+-chelation with TPEN might be mediated by the serine protease Omi/HtrA2. Omi/HtrA2 undergoes autocatalysis to release a product that has homology to Smac/DIABLO and which has pro-apoptotic properties resulting from sequestering the anti-apoptotic protein XIAP [25, 31]. As the pro-apoptotic effects of Zn2+-chelation treatment have been reported to be mediated via caspase 3 activation and inhibited by XIAP [23] Omi/HtrA2 activation may be expected to enhance the apoptotic effects of Zn2+-chelation by sequestering XIAP. Changes in Omi/HtrA2 expression were evaluated by Western blot and the effects of Omi/HtrA2 inhibition for mitigating the apoptosis-stimulating effects of Zn2+-chelation in F10-treated cells were evaluated using the inhibitor UCF-101 (Santa Cruz Biotechnology). Western blot revealed no change in the expression of Omi/HtrA2 under any of the conditions examined (Figure 6). Surprisingly, however, inhibiting Omi/HtrA2 enhanced the cytotoxic and pro-apoptotic effects of F10 and further enhanced the pro-apoptotic effects of Zn2+-chelation (Figure 5). These results reveal an anti-apoptotic role for Omi/HtrA2 in F10-treated cells and identify Omi/HtrA2 as a putative therapeutic target for treatment of prostate cancer.
DISCUSSION
Over the last decade six novel agents have been approved four use in patients in with CRPC based on improvement in overall survival from large randomized studies. However, the improvement in survival has been modest at about 4 months in all the trials. Therefore, there remains a clear and imminent need for drugs that produce more effective and prolonged response in this incurable disease state. Pre-clinical studies with the fluoropyrimidine polymer F10 have demonstrated this novel drug-candidate displays excellent anti-cancer activity in pre-clinical animal models of acute myeloid leukemia (AML) [13, 14], glioblastoma [15], and advanced prostate cancer [16], highly lethal malignancies for which new treatment options are urgently needed. Data from the NCI 60 cell line screen as well as from our laboratory have demonstrated that F10 is highly cytotoxic to prostate cancer cells [17] with the present studies demonstrating F10 is cytotoxic to C4-2 and PC3 cells at nanomolar levels. The present studies have demonstrated that the cytotoxic effects of F10 towards prostate cancer cells can be enhanced by modulating intracellular Zn2+ levels providing the basis for developing new therapeutic modalities that simultaneously deliver F10 and modulate Zn2+ for therapeutic benefit.
Zn2+ chelation presents a unique opportunity in patients with prostatic diseases as Zn2+ concentrations are higher in prostate than in other organs. Moreover, there is substantial data in the published lieterature that dietary, serum, or tissue Zn2+ concentrations affect prostate cancer incidence and outcomes. The progression of prostate cancer is paralleled by sequentially decreased levels of intracellular Zn2+ consistent with low levels of Zn2+ in prostate cancer cells presenting a survival advantage [32]. Consistent with this interpretation, prostate cancer cells in culture as well as in human malignant tissue express decreased levels of zinc transporters and increased levels of Zn2+ efflux pumps [10]. Further, administering exogenous Zn2+ has been found to decrease prostate cancer cell viability [7, 8] and studies from our laboratory demonstrate that strategies to deliver Zn2+ enhance the cytotoxicity of chemotherapy under some conditions [33, 34]. The present studies demonstrate that treatment of prostate cancer cells with Zn2+-salts initially decreases cell viability, however these effects are transitory and cells recover full viability within 72 h. The cell-permeable ionophore pyrithione reduces prostate cancer cell viability as the sodium complex and these effects are enhanced for the Zn2+ complex (ZnPy). As with cell-impermeable Zn2+-salts, the effects of Zn2+-Py are decreased at longer treatment times consistent with cellular adaptation to increased Zn2+. Further, ZnPy is effective at enhancing the cytotoxicity of F10 only at low concentrations with higher concentration of ZnPy that have substantial single-agent effects inhibiting F10 cytotoxicity. The mechanism by which Zn2+ levels modulate chemotherapy effectiveness require further investigation.
Although prostate cancer cells maintain Zn2+ levels at very low levels relative to normal prostate, further reduction in intracellular Zn2+ through Zn2+ chelation results in apoptosis. These observations are consistent with low levels of Zn2+ being important for maintaining a pro-survival balance in prostate cancer cells. Our studies show that both cell-impermeable (e.g. DTPA) and cell-permeable (e.g. TPEN) Zn2+-chelators each reduce the viability of prostate cancer cells. The effects of DTPA are considerably slower than TPEN demonstrating that cell-permeable Zn2+-chelation has potential for rapid induction of apoptosis in prostate cancer cells. Further, co-treatment with TPEN significantly enhances F10 cytotoxicity to prostate cancer cells demonstrating the potential for cell-permeable Zn2+ chelation to enhance chemotherapy. The mechanism by which Zn2+-chelation enhances F10-induced apoptosis is not known, however Zn2+ has been shown to form a specific complex with Cys-285 in caspase 3 that inhibits apoptosis [21]. F10-induced apoptosis may be attenuated through Zn2+-complexation of caspase 3 with Zn2+-chelation overcoming this attenuation.
The mechanism by which Zn2+-chelation tips the survival/apoptosis balance to apoptosis in prostate cancer cells is not known. Previous studies demonstrated TPEN treatment resulted in selective depletion of the X-linked inhibitor of apoptosis (XIAP) and this degradation was prevented by the serine protease inhibitor Pefabloc [9]. The present studies investigated a potential role for the serine protease Omi/HtrA2 in the Zn2+-dependent degradation of XIAP. Omi/HtrA2 undergoes auto-catalysis to release a form that has homology to Smac/DIABLO and that has pro-apoptotic properties resulting from sequestering XIAP [31]. Omi/HtrA2 has also been shown to promote apoptosis in response to cellular stress [27]. Surprisingly, our results implicate an anti-apoptotic role for Omi/HtrA2 with pharmacological inhibition markedly enhancing F10- and F10/TPEN-induced apoptosis (Figure 5). These results identify Omi/HtrA2 as a novel target for prostate cancer treatment with inhibitors of Omi/HtrA2 may have single agent activity as well as enhance the pro-apoptotic activities of chemotherapy towards prostate cancer cells.
We have previously shown that induction of cation dependent proteins such as Metallothioneins, MT2A isoform in particular, with ZnSO4 increases resistance to radiation and cisplatin chemotherapy [35]. As a corollary, Zn2+ chelation may increase sensitivity to chemotherapeutic agents. For the first time in the history of managing metastatic prostate cancer, chemotherapeutic agents demonstrated to improve overall survival in metastatic prostate cancer (e.g. docetaxel and cabazitaxel) are approved for clinical use. Novel clinical strategies to improve response to these chemotherapeutics are need and targeting Zn2+ through chelation may be an attractive option.
CONCLUSION
Zn2+ chelation with cell-permeable moieties represents a promising strategy for prostate cancer treatment that may also enhance the efficacy of chemotherapy. TPEN treatment enhances F10-induced apoptosis in PC3 and C4-2 cells demonstrating the potential for this combination therapy approach to be further evaluated in pre-clinical models of castration-resistant prostate cancer. In light of the low toxicity and strong anti-cancer activity of F10 in animal models of AML and GBM this combination is likely to be effective and well-tolerated for prostate cancer treatment. The serine protease Omi/HtrA2 contributes to prostate cancer cell survival and pharmacological inhibition of Omi/HtrA2 enhances the pro-apoptotic properties of F10 and F10/TPEN. These studies identify Omi/HtrA2 as a potential new target for prostate cancer treatment.
Acknowledgments
The authors are grateful for support from army: W81XWH1010132. Dr. Elizabeth Wilson (UNC-CH) supplied C4-2 cells.
Grant sponsor: DOD CDMRP PCRP W81XWH-10-1-0132 and W81XWH-12-1-0252; NIH-NCI CA 102532, NIH P30 CA12197.
Footnotes
Disclosure Statement: WG is President of Salzburg Therapeutics which holds licenses for F10 and its use for cancer treatment. Other authors have no disclosures.
References
- 1.Costello LC, Franklin RB. Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J Biol Inorg Chem. 2011;16(1):3–8. doi: 10.1007/s00775-010-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein MM, Kasperzyk JL, Andren O, Giovannucci EL, Wolk A, Hakansson N, Andersson SO, Johansson JE, Fall K, Mucci LA. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr. 2011;93(3):586–593. doi: 10.3945/ajcn.110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 4.Prasad AS, Mukhtar H, Beck FW, Adhami VM, Siddiqui IA, Din M, Hafeez BB, Kucuk O. Dietary zinc and prostate cancer in the TRAMP mouse model. J Med Food. 2010;13:70–76. doi: 10.1089/jmf.2009.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarafanov AG, Todorov TI, Centeno JA, Macias V, Gao W, Liang WM, Beam C, Gray MA, Kajdacsy-Balla AA. Prostate cancer outcome and tissue levels of metal ions. Prostate. 2011;71:1231–1238. doi: 10.1002/pros.21339. [DOI] [PubMed] [Google Scholar]
- 6.De Leon-Rodriguez L, Lubag AJ, Jr, Sherry AD. Imaging free zinc levels in vivo – what can be learned? Inorganica Chim Acta. 2012;393:12–23. doi: 10.1016/j.ica.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah MR, Kriedt CL, Lents NH, Hoyer MK, Jamaluddin N, Klein C, Baldassare J. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J Exp Clin Cancer Res. 2009;28:84. doi: 10.1186/1756-9966-28-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kriedt CL, Baldassare J, Shah M, Klein C. Zinc functions as a cytotoxic agent for prostate cancer cells independent of culture and growth conditions. J Exp Ther Oncol. 2010;8:287–295. [PubMed] [Google Scholar]
- 9.Makhov P, Golovine K, Uzzo RG, Rothman J, Crispen PL, Shaw T, Scoll BJ, Kolenko VM. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ. 2008;15:1745–1751. doi: 10.1038/cdd.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franz MC, Anderle P, Burzle M, Suzuki Y, Freeman MR, Hediger MA, Kovacs G. Zinc transporters in prostate cancer. Mol Aspects Med. 2013;34:735–741. doi: 10.1016/j.mam.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makhov P, Kutikov A, Golovine K, Uzzo RG, Canter DJ, Kolenko VM. Docetaxel-mediated apoptosis in myeloid progenitor TF-1 cells is mitigated by zinc: potential implication for prostate cancer therapy. Prostate. 2011;71:1413–1419. doi: 10.1002/pros.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006;27:1980–1990. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 13.Pardee TS, Gomes E, Jennings-Gee J, Caudell D, Gmeiner WH. Unique dual targeting of thymidylate synthase and topoisomerase 1 by FdUMP[10] results in high efficacy against AML and low toxicity. Blood. 2012;119:3561–3570. doi: 10.1182/blood-2011-06-362442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings-Gee J, Pardee TS, Gmeiner WH. Replication-dependent irreversible topoisomerase 1 poisoning is responsible for FdUMP[10] anti-leukemic activity. Exp Hematol. 2013;41:180–188. doi: 10.1016/j.exphem.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gmeiner WH, Lema-Tome C, Gibo D, Jennings-Gee J, Milligan C, Debinski W. Selective anti-tumor activity of the novel fluoropyrimidine polymer F10 towards G48a orthotopic GBM tumors. J Neurooncol. 2014;116:447–454. doi: 10.1007/s11060-013-1321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gmeiner WH, Bourland JD, Hatcher HC, Smith TL, D’Agostino RB, Jr, Blackstock W. F10 Inhibits Growth of PC3 Xenografts and Enhances the Effects of Radiation Therapy. JSM Clin Oncol Res. 2014;2:1028. [PMC free article] [PubMed] [Google Scholar]
- 17.Gmeiner WH, Reinhold WC, Pommier Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol Cancer Ther. 2010;9:3105–3114. doi: 10.1158/1535-7163.MCT-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berlin JD, Propert KJ, Trump D, Wilding G, Hudes G, Glick J, Burch P, Keller A, Loehrer P. 5-Fluorouracil and leucovorin therapy in patients with hormone refractory prostate cancer: an Eastern Cooperative Oncology Group phase II study (E1889) Am J Clin Oncol. 1998;21:171–176. doi: 10.1097/00000421-199804000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Huan SD, Aitken SE, Stewart DJ. A phase II study of 5-fluorouracil and high dose folinic acid in cisplatin-refractory metastatic bladder cancer. Ann Oncol. 1995;6:836–837. doi: 10.1093/oxfordjournals.annonc.a059325. [DOI] [PubMed] [Google Scholar]
- 20.Morant R, Bernhard, Dietrich D, Gillessen S, Bonomo M, Borner M, Bauer J, Cerny T, Rochlitz C, Wernli M, Gschwend A, Hanselmann S, Hering F, Schmid HP. Capecitabine in hormone-resistant metastatic prostatic carcinoma – a phase II trial. Br J Cancer. 2004;90:1312–1317. doi: 10.1038/sj.bjc.6601673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 22.Chimienti F, Seve M, Richard S, Mathieu J, Favier A. Role of cellular zinc in programmed cell death: temporal relationship between zinc depletion, activation of caspases, and cleavage of Sp family transcription factors. Biochem Pharmacol. 2001;62:51–62. doi: 10.1016/s0006-2952(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 23.Peterson QP, Goode DR, West DC, Ramsey KN, Lee JJ, Hergenrother PJ. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J Mol Biol. 2009;388:144–158. doi: 10.1016/j.jmb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo J, Schmitt SM, Zhang Z, Prakash J, Fan Y, Bi C, Kodanko JJ, Dou QP. Novel Polypyridyl chelators deplete cellular zinc and destabilize the X-linked inhibitor of apoptosis protein (XIAP) prior to induction of apoptosis in human prostate and breast cancer cells. J Cell Biochem. 2012;113:2567–2575. doi: 10.1002/jcb.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins LM, Iaccarino I, Tenev T, Gschmeissner S, Totty NF, Lemoine NR, Savopoulos J, Gray CW, Creasy CL, Dingwall C, Downward J. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J Biol Chem. 2002;277:439–444. doi: 10.1074/jbc.M109784200. [DOI] [PubMed] [Google Scholar]
- 26.Vande Walle L, Lamkanfi M, Vandenabeele P. The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ. 2008;15:453–460. doi: 10.1038/sj.cdd.4402291. [DOI] [PubMed] [Google Scholar]
- 27.Liu HR, Gao E, Hu A, Tao L, Qu Y, Most P, Koch WJ, Christopher TA, Lopez BL, Alnemri ES, Zervos AS, Ma XL. Role of Omi/HtrA2 in apoptotic cell death after myocardial ischemia and reperfusion. Circulation. 2005;111:90–96. doi: 10.1161/01.CIR.0000151613.90994.17. [DOI] [PubMed] [Google Scholar]
- 28.Gmeiner WH, Trump E, Wei C. Enhanced DNA-directed effects of FdUMP[10] compared to 5FU. Nucleosides Nucleotides Nucleic Acids. 2004;23:401–410. doi: 10.1081/ncn-120028336. [DOI] [PubMed] [Google Scholar]
- 29.Colvin RA, Laskowski M, Fontaine CP. Zinquin identifies subcellular compartmentalization of zinc in cortical neurons. Relation to the trafficking of zinc and the mitochondrial compartment. Brain Res. 2006;1085:1–10. doi: 10.1016/j.brainres.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 30.Meeusen JW, Nowakowski A, Petering DH. Reaction of metal-binding ligands with the zinc proteome: zinc sensors and N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine. Inorg Chem. 2012;51:3625–3632. doi: 10.1021/ic2025236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Takahashi-Niki K, Akagi T, Hashikawa T, Takahashi R. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ. 2004;11:208–216. doi: 10.1038/sj.cdd.4401343. [DOI] [PubMed] [Google Scholar]
- 32.Costello LC, Levy BA, Desouki MM, Zou J, Bagasra O, Johnson LA, Hanna N, Franklin RB. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol Ther. 2011;12:297–303. doi: 10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, Salsbury FR, Jr, Horita DA, Gmeiner WH. Zn2+ selectively stabilizes FdU-substituted DNA through a unique major groove binding motif. Nucleic Acids Res. 2011;39:4490–4498. doi: 10.1093/nar/gkr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh S, Salsbury FR, Jr, Horita DA, Gmeiner WH. Cooperative stabilization of Zn(2+):DNA complexes through netropsin binding in the minor groove of FdU-substituted DNA. J Biomol Struct Dyn. 2013;31:1301–1310. doi: 10.1080/07391102.2012.732343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DJ, Jaggi M, Zhang W, Galich A, Du C, Sterrett SP, Smith LM, Balaji KC. Metallothioneins and resistance to cisplatin and radiation in prostate cancer. Urology. 2006;67:1341–1347. doi: 10.1016/j.urology.2005.12.032. [DOI] [PubMed] [Google Scholar]


