Abstract
Objective
Common neurological diseases or injuries that can affect the right hemisphere, including stroke, traumatic brain injury, and frontotemporal dementia, disrupt emotional empathy – the ability to share in and make inferences about how other people feel. This impairment negatively impacts social interactions and relationships. Accumulating evidence indicates that emotional empathy depends on coordinated functions of orbitofrontal cortex, anterior insula, anterior cingulate, temporal pole, and amygdala, but few studies have investigated effects of lesions to white matter tracts that connect these structures. We tested the hypothesis that percent damage to specific white matter tracts connecting these gray matter structures predicts error rate in an emotional empathy task after acute right hemisphere ischemic stroke.
Methods
We used multivariable linear variable linear regression with percent damage to 8 white matter tracts, age, and education as independent variables and error rate on emotional empathy as the dependent variable to test a predictive model of emotional empathy in 30 patients with acute ischemic right hemisphere stroke.
Results
Percent damage to 8 white matter tracts along with age and education predicted the error rate in emotional empathy; but only percent damage to the uncinate fasciculus was independently associated with error rate. Participants with right uncinate fasciculus lesions were significantly more impaired than right hemisphere stroke patients without uncinate fasciculus lesions in the emotional empathy task.
Interpretation
The right uncinate fasciculus plays an important role in the emotional empathy network. Patients with lesions in this network should be evaluated for empathy, so deficits can be addressed.
A coherent hypothesis about the neural network underlying emotional empathy has emerged from various sources: functional MRI of healthy individuals experiencing empathy1–7, resting state functional connectivity studies of individuals with frontotemporal dementia (who have impaired empathy)8, focal lesion studies9–11 and voxel-based morphometry studies12, 13 of individuals with impaired empathy. Together, these studies have identified the important roles of several cortical and limbic areas, including prefrontal cortex, orbitofrontal cortex, amygdala, and temporal pole, particularly in the right hemisphere. Some of components of this network may be especially critical for specific processes underlying emotional empathy14–24. These areas are strongly interconnected with the anterior insula and anterior cingulate cortex1, 25, 26, areas which themselves are clearly engaged when healthy people empathize with others1, 2, 4–7. Seeley and colleagues8 have raised the possibility that Von Economo neurons, found in anterior cingulate and anterior insula, are selectively targeted in behavioral variant frontotemporal dementia (bvFTD), a neurodegenerative disease in which impaired empathy is prominent feature. Loss of Von Economo neurons and fork cells in right anterior anterior insular cortex correlates with severity of clinical disease in bvFTD27.
If areas found to be critical for emotional empathy comprise a functional network, then focal lesions to white matter connections between them should disrupt emotional empathy. There is some evidence favoring this hypothesis from patients with FTD. One of the most important white matter connections between orbitofrontal cortex, temporal pole, insula, and amygdala is the uncinate fasciculus. In a diffusion tensor imaging study of FTD compared to controls, patients with FTD had reduced fractional anisotropy (FA) in uncinate fasciculus, anterior corpus callosum, and bilateral anterior descending cingulum tracts, compared to controls28. Likewise, even carriers of progranulin mutations (one gene mutation underlying FTD) had reduced FA in the uncinate fasciculus29; and patients with advanced FTD had reduced FA only in the uncinate fasciculus in another study30. However, reduced FA in the uncinate fasciculus in FTD could be a result of degeneration of any of the cortical areas to which the uncinate fasciculus is connected rather than direct evidence that the “lesion” itself is associated with clinical symptom of impaired empathy in FTD. In the present study, we tested the hypothesis that impaired emotional empathy immediately after acute right hemisphere ischemic stroke is associated with lesions in the (right) uncinate fasciculus.
Methods
Participants
Stroke patients were a consecutive series of 30 individuals who had met the following inclusion criteria: (1) acute ischemic right hemisphere stroke; (2) premorbid proficiency in English; (3) provided informed consent to participate in the study and were able to complete the testing; and none of the exclusion criteria: (1) reduced level of consciousness or on-going sedation; (2) neurological disease other than stroke; and (3) inability to have MRI due to implanted ferrous metal, claustrophobia, or weight >300 pounds. The study protocol was approved by the Johns Hopkins Medicine Institutional Review Board. Patients were enrolled from March 17, 2009–November 27, 2012. An additional 19 patients met all criteria and were enrolled in the study but could not complete the testing (either the empathy testing or the MRI); another 220 patients were screened, but excluded because they met one of the above exclusion criteria. Performance on empathy testing was compared to previous data from hospitalized controls with normal MRI and normal neurological examination at the time of testing with the same demographic characteristics and exclusion criteria as the stroke patients.
Imaging
Stroke protocol MRI, included the following sequences: diffusion weighted imaging (DWI), Apparent Diffusion Coefficient (ADC), fluid-attenuated inversion recovery (FLAIR), Susceptibility Weighted Imaging (SWI), T2 weighted imaging, and 3D time-of-flight angiography of the intracranial vessels. Sequences were acquired using single-shot spin-echo echo-planar imaging, in the transverse plane parallel to the AC-PC line, with whole brain coverage. DWI was obtained as an average of diffusion weighted echo planar images acquired in three orthogonal gradient directions with a b-value of 1000 (s/mm2), and ADC was calculated from the diffusion weighted echo planar images with a least diffusion weighting (b0). While edema surrounding acute stroke can affect white matter tracts, edema is greatest at 3–10 days after stroke. Edema is present in the first 24 hours only in very large infarcts that impair level of consciousness (not included in this study). We evaluated the T2 sequences for edema, and ruled out significant edema in these cases.
Image processing
To define boundary(s) of acute stroke lesion(s) (hereafter, stroke map) of each participant, a threshold of > 30% intensity increase from the unaffected area in the diffusion-weighted image was applied, and a neurologist (KO), masked to the clinical information, manually modified the boundary to avoid false-positive and false-negative areas on RoiEditor (www.MRIstudio.org)31. We then transformed the least diffusion weighted image (b0) with T2-weighted contrast to the JHU-MNI-b0 atlas using affine transformation followed by the large deformation diffeomorphic metric mapping31. We applied the resultant matrices to the stroke map for the normalization. We overlaid the customized version of the JHU-MNI Brain Parcellation Map (cmrm.med.jhmi.edu) on the normalized stroke map to investigate percentage volume of each of the following white matter tracts (fornix; stria terminalis, inferior frontooccipital fasciculus; posterior thalamic radiation; sagittal stratum, superior frontooccipital fasciculus; superior longitudinal fasciculus; uncinate fasciculus) that might be affected by acute stroke (Figure 1) on DiffeoMap (www.MRIstudio.org). Ten randomly selected images were used to test intra and inter-operator reproducibility of the stroke map. The Dice overlap coefficient was used to evaluate overlap of the stroke maps, and intraclass correlation coefficient (ICC) was used to evaluate consistency of the stroke volumes measured by the stroke maps. Intra- and inter-observer reliability of the stroke map were excellent; the intra-operator Dice coefficient was: 0.90 (+/− 0.044) with more than 6 months interval; the inter-operator Dice coefficient by 2 different neurologists (KO and AVF) was: 0.86 (+/− 0.085). The ICC was 0.98 both within and across observers.
Figure 1.
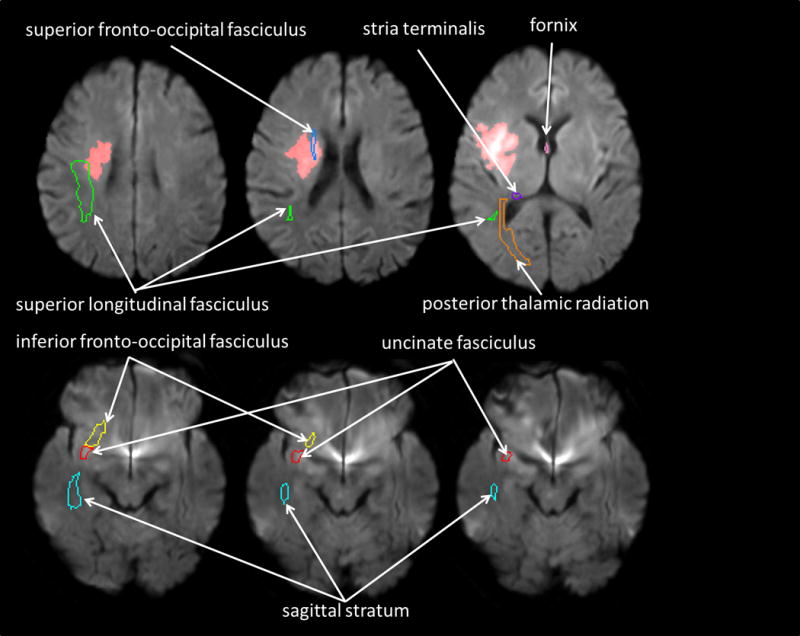
A pre-defined set of three-dimensional ROIs [right fornix (pink contour), right stria terminalis (purple contour), right inferior fronto-occipital fasciculus (yellow contour), right posterior thalamic radiation (orange contour), sagittal stratum (cyan contour), superior fronto-occipital fasciculus (blue contour), superior longitudinal fasciculus (green contour), and uncinate fasciculus (red contour)] on the atlas space was overlaid on the normalized stroke map to report % volume of each ROI affected by the infarction. In this figure, the normalized stroke map (red area) was overlaid on the normalized DWI.
Emotional Empathy Testing
Patients underwent testing of emotional empathy within 24 hours of admission to the hospital. Testing was restricted to one aspect of emotional empathy – affective perspective-taking, as described in more detail elsewhere10. In brief, participants were asked yes/no and multiple choice questions requiring inferences about emotions of individuals in short videotapes or stories that were read to them. To control for deficits in sustained attention and recent memory, they were also asked factual questions about the stories. A cut-off score for impaired emotional empathy of >20% errors was determined by the score that had the highest specificity for acute stroke (i.e., no normal control made >20% errors).
A questionnaire was given to caregivers of participants on the first follow up visit after hospitalization, regarding sequelae of stroke, including items regarding change in: personality or behavior, strength, coordination, motor speech, word retrieval, reading, writing, sensation, mood, walking, swallowing, sleep, empathy (understanding emotions of others and expressing emotion through tone of voice and facial expression), and sexual function. A subset of 14 caregivers provided responses at follow-up; 50% of the caregivers, including all caregivers of participants with impaired empathy on our testing, reported that the stroke survivor had impaired understanding of the emotions of others.
Statistical Analysis
We used multivariable linear regression analysis to identify the independent predictors of severity of emotional empathy impairment (error rate on the emotional empathy task). The following independent variables were entered as potential predicators: percentage of damage to fornix; stria terminalis, inferior frontooccipital fasciculus; posterior thalamic radiation; sagittal stratum, superior frontooccipital fasciculus; superior longitudinal fasciculus; uncinate fasciculus; age; and education. Education was not recorded at the time of testing in 10 patients. We were not able to contact 6 patients to determine the education. In 4 patients who were contacted, education was 12–14 (mean 12.5) years, not significantly different from the entire group.
After finding that the uncinate fasciculus was the main white matter tract where the degree of damage was associated with the severity of empathy impairment, we then evaluated differences between stroke patients with lesions in the uncinate fasciculus and patients without lesions in the uncinate fasciculus, with regard to score on our emotional empathy test, age, education, and volume of infarct, using unpaired t-tests.
Finally, to rule out the possibility that damage to the uncinate fasciculus was not simply a reflection of damage to nearby gray matter structures, we evaluated the association between any damage to the uncinate fasciculus and damage to any of the components of the cortical network associated with empathy in our previous study: right prefrontal cortex, orbitofrontal cortex, anterior insula, amygdala, temporal pole, or anterior cingulate cortex.
Results
The prediction model contained all of the nine predictors with no variables removed. The model was statistically significant, F(10, 10) = 5.7, p = 0.005, and accounted for approximately 70% of the variance of empathy error rate (r2 = 0.851, adjusted r2 = 0.70). Empathy error rate was primarily predicated by degree of damage to the uncinate fasciculus. The raw and standardized regression coefficients of the predictors and with their correlations with empathy error rate, are shown in Table 1. The degree of damage to the uncinate fasciculus received the strongest positive weight in the model followed by the degree of damage to the superior frontooccipital fasciculus. No other predictors, other than uncinate fasciculus, were independently and significantly associated with empathy error rate.
Table 1.
The Multivariable Model Associated with Percent Error Rate on Affective Perspective-Taking
| Variable | Unstandardized Coefficients | Standardized Coefficients | t | Significance | |
|---|---|---|---|---|---|
| Beta | Standard Error | Beta | |||
| Constant | −0.263 | 0.337 | Value? | −0.779 | 0.454 |
| Age | 0.006 | 0.003 | 0.294 | 1.937 | 0.081 |
| Education | 0.014 | 0.017 | 0.126 | 0.871 | 0.404 |
| Stria Terminalis | −104.932 | 131.963 | −8.37 | −0.799 | 0.443 |
| Fornix | −396.969 | 503.616 | −5.931 | −0.788 | 0.449 |
| Inferior Frontoocciptal Fasciculus | −1.025 | 0.603 | −0.934 | −1.69 | 0.120 |
| Posterior Thalamic Radiation | −10.108 | 19.605 | −2.306 | −0.516 | 0.617 |
| Sagittal Stratum | −3.686 | 2.354 | −0.523 | −1.57 | 0.148 |
| Superior Frontoocciptal Fasciculus | 0.209 | 0.291 | 0.231 | 0.718 | 0.489 |
| Superior Longitudinal Fasciculus | −0.458 | 0.258 | −0.278 | −1.78 | 0.106 |
| Uncinate Fasciculus | 0.729 | 0.175 | 0.660 | 4.169 | 0.002 |
Patients with uncinate fasciculus lesions had significantly higher error rates than patients without uncinate fasciculus lesions (64% vs. 19% errors; t=4.12; p<0.0001), but the two groups were not significantly different in terms of age (56.8 vs. 53.7 years; t=0.42;p=0.68), education (13.0 vs. 14.47 years; t=−1.15; p=0.26), or total error rate on a test of prosody comprehension (40% vs. 53%; t=1.44; p=0.17).
Finally, to evaluate the possibility that damage to the right uncinate fasciculus reflected damage to the nearby cortical structures already found to be associated with impaired empathy, we directly evaluated the association between the presence of damage the right uncinate fasciculus and presence of damage to any of the gray matter structures found to be associated with impaired emotional empathy in our previous study10. We found no association between a lesion in the uncinate fasciculus and a lesion in this gray matter network (chi squared = 0.73; p=0.39). In fact, 60% of the patients with uncinate fasciculus lesions in this study did not have lesions in any component of the gray matter network (prefrontal cortex, orbitofrontal cortex, anterior insula, anterior cingulate cortex, temporal pole, or amygdala). Thus, even “pure” uncinate fasciculus lesions can cause deficits (plausibly by disrupting input to cortical areas or connections between cortical areas). At least in those 60% of patients, the empathy deficit cannot be explained by damage to cortical areas alone (i.e. cannot be an artifact of the anatomical proximity to important cortical areas). However, other patients did have damage to adjacent areas of cortex that may have contributed to their impairment (see examples of cases of patients with empathy deficits in Figure 2).
Figure 2.
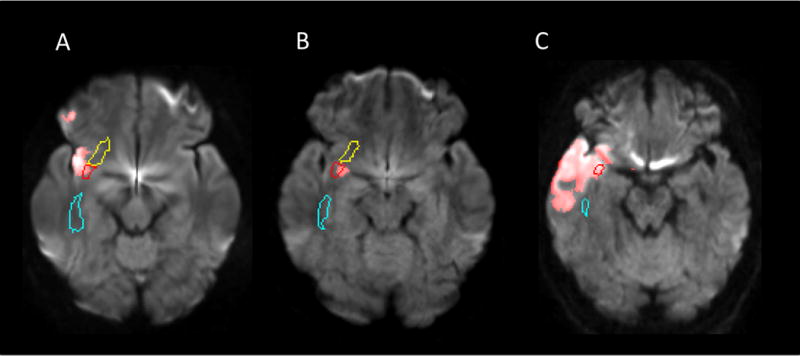
Representative three individuals (A, B and C) with acute infarction in the uncinate fasciculus (red contour). The normalized stroke maps (red area) were overlaid on the DWIs normalized to the JHU-MNI atlas space and pre-defined ROIs were overlaid on the normalized images. The right inferior fronto-occipital fasciculus (yellow contour) and the sagittal stratum (cyan contour) were also visualized in these slices.
Discussion
Results confirm that even acute damage to the right uncinate fasciculus can disrupt performance on a task of emotional empathy. While clearly the right uncinate fasciculus is not the only important neural structure underlying empathy, it does seem to be among the most important white matter tracts in this network. The critical role of the uncinate fasciculus is not surprising, as it serves as a critical link between structures that have been implicated in components of emotional empathy – particularly between orbitofrontal cortex, anterior insula, temporal pole, and amygdala. The majority of participants did not have damage to the gray matter structures themselves, indicating that disruption of the white matter tracts that connect them can also disrupt emotional empathy, as we would expect if these structures operate as a network underlying this critical aspect of social cognition.
Studies of neurodegenerative disease have also shown a relationship between reduced volume in the uncinate fasciculus and errors on empathy tasks, primarily in FTD28–30. Our data are complementary, as they show that individuals who were neurologically normal just days before show acute disruption of empathy that correlates with the degree of damage to the uncinate fasciculus. Several cases of herpes encephalitis32, limbic encephalitis due to Potassium channel antibody associated encephalopathy33, or Juvenile Neuronal Ceroid Lipofuscinosis34 have been reported to have impaired empathy, in some cases associated with Klüver–Bucy syndrome32,34. While the damage to bilateral mesial temporal lobe, especial temporal pole, is usually emphasized, these individuals likely have damage to the uncinate fasciculus as well. Children with neuropsychiatric disorders due to brain injury have significantly lower fractional anisotropy in bilateral uncinate fasciculus35.
Our study provides a unique contribution by showing that acute lesions of the uncinate fasciculus can also cause impaired empathy. By evaluating patients within the first 24 hours of acute ischemic stroke with both MRI and empathy testing, we showed that the empathy impairment was associated with damage to the white matter tract itself, rather than secondary degeneration of the white matter tract due to a cortical lesion. While either could cause empathy deficits, an association between a deficit and secondary degeneration of the white matter tract could be due to either the damaged cortical lesion alone (not the degeneration of the tract itself), or the disconnection between the cortical area and other areas caused by degeneration of the white matter tract. In contrast, if a lesion is associated with acute disruption in the white matter tract, it must due to the disconnection between the areas connected by that tract (e.g. impaired input to one or more of the cortical areas).
One weakness of our study is that we did not attempt to determine which cognitive process underlying empathy depends on the uncinate fasciculus. That is, emotional empathy requires a number of cognitive and emotional regulation functions, often broadly divided into stages or levels of emotional contagion (sharing in the emotions of another) and perspective-taking (making inferences about the emotions of another) (Table 2). There is evidence from functional imaging and lesion studies that certain structures within the neural network supporting emotional empathy may have differential roles for discrete cognitive components or processes. For example, orbitofrontal cortex may have a critical role in emotional contagion5, perhaps through recognition of other’s emotions through vocal prosody14 and facial expression. Or this area may be important for modulating empathy, depending on potential consequences or the relationship between the empathizer and the target of empathy15. Right anterior temporal cortex seems to have a role in integrating distinct components of emotional empathy, as indicated by case studies of patients with temporal pole atrophy who are impaired in several aspects of emotional empathy16, 17 or a more general process, such as representing social concepts18, 19. The amygdala also plays an important role in emotional empathy, as shown by functional imaging of healthy controls20–22 and individuals with amygdala lesions10, 23. The role of the amygdalae may be in identifying emotional valence of stimuli24. Understanding and sharing in another’s emotions requires all of these components of emotional empathy, as well as integration of these components. The uncinate fasciculus, which connects many of the key structures, may be critical for their integration.
Table 2.
Hypothesized cognitive and emotional processes underlying emotional empathy
| EMOTIONAL CONTAGION |
| Suppression of one’s own (earlier) affective state |
| Arousal and awareness (conscious or unconscious) of the affective state of the other person (through observation or imagination) |
| Adoption of a new affective state that is isomorphic to that of another person |
| AFFECTIVE PERSPECTIVE-TAKING |
| Suppression of one’s own perspective |
| Recognition of the affective state of another person by adopting the other’s perspective (through observation or imagination) e.g., recognizing another’s anger or joy by adopting that person’s perspective |
| INTEGRATION OF AFFECTIVE PERSPECTIVE-TAKING & EMOTIONAL CONTAGION |
| Attribution of the source of one’s newly adopted affective state and perspective to the other person Emotional regulation |
Another weakness is that several white matter tracks were not well evaluated because there were too few patients who had any damage to the tract to determine its contribution. In fact, in the cases where only a few patients had damage (e.g., the fornix), it appeared that the greater the damage, the lower the error rate (so the predictive weight was negative, although non-significant; see Table 1). Although there was little power to evaluate these tracts, it does not seem that damage caused acute disruptions in empathy (as the trend was in the opposite direction). We also did not evaluate the effects of damage to the left uncinate fasciculus. Finally, we have not reported stability or recovery of empathy over time; we are evaluating the course of recovery of empathy in an ongoing study. The current study does not yield evidence regarding the importance of the uncinate fasciculus for recovery of empathy.
Despite the study’s limitations, our results add to the accumulating evidence for a network of structures involving at least right orbitofrontal cortex, anterior insula, temporal pole, anterior cingulate, insula, amygdala, and uncinate fasciculus in supporting the ability to share in, and make inferences about, the emotions of others. The clinical implications of the findings of the current study are that patients with deep right frontal and temporal lesions, particularly involving the uncinate fasciculus, may have difficulty inferring how other people feel. This important social disability should be recognized, and appropriate counseling provided to caregivers. Additionally, innovative treatments to improve empathy, such as intranasal oxytocin23,36, should be evaluated in individuals with uncinate fasciculus lesions.
Acknowledgments
This work was supported by the National Institutes of Health (National Institute of Neurological Disorders and Stroke) through award RO1NS47691 (to AEH), and through NICHD award R01 HD065955 and The Yousem Family Research Fund (to KO). The content is solely the responsibility of the authors and does not necessarily represent the views the National Institutes of Health.
All of the authors have been supported by grants from NIH during the conduct of the study; Dr. Faria was also supported by a grant from the American Heart Association during the conduct of the study. In addition, Dr. Mori has patents US 12/743,169, US 12/747,816, and 61/357,361 licensed to AnatomyWorks and Dr. Mori owns and is the CEO of “AnatomyWorks.”
Footnotes
Conflict of Interest
This arrangement is being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Soc Neurosci. 2006;1:364–384. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- 3.Decety J, Porges EC. Imagining being the agent of actions that carry different moral consequences: an fMRI study. Neuropsychologia. 2011;49:2994–3001. doi: 10.1016/j.neuropsychologia.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Gu X, Gao Z, Wang X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Singer T, Seymour B, O’Doherty J, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 8.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- 10.Leigh R, Oishi K, Hsu J, et al. Acute lesions that impair affective empathy. Brain. 2013;136:2539–2549. doi: 10.1093/brain/awt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain. 2014;137:981–997. doi: 10.1093/brain/awt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- 13.Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross ED, Monnot M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang. 2008;104:51–74. doi: 10.1016/j.bandl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Perry RJ, Rosen HR, Kramer JH, et al. Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–160. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- 17.Narvid J, Gorno-Tempini ML, Slavotinek A, et al. Of brain and bone: the unusual case of Dr. A. Neurocase. 2009;15:190–205. doi: 10.1080/13554790802632967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zahn R, Moll J, Iyengar V, et al. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132:604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahn R, Moll J, Krueger F, et al. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bzdok D, Schilbach L, Vogeley K, et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu X, Liu X, Guise KG, et al. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30:3739–3744. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunnlieb C, Munte TF, Tempelmann C, Heldmann M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013;1499:29–42. doi: 10.1016/j.brainres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 26.Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–545. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]
- 27.Kim EJ, Sidhu M, Gaus SE, Huang EJ. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb Cortex. 2012;22:251–9. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Schuff N, Du AT, Rosen HJ, et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132:2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borroni B, Alberici A, Premi E, Archetti S, et al. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res. 2008;11:585–595. doi: 10.1089/rej.2007.0623. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo K, Mizuno T, Yamada K, Akazawa K, et al. Cerebral white matter damage in frontotemporal dementia assessed by diffusion tensor tractography. Neuroradiology. 2008;50:605–611. doi: 10.1007/s00234-008-0379-5. [DOI] [PubMed] [Google Scholar]
- 31.Oishi K, Faria A, Jiang H, Akhter K, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito Y, Yokoyama A, Nishio S, Asai K. Effects of selective serotonin re-uptake inhibitors on behavior in Klüver-Bucy syndrome during childhood. Pediatr Int. 2009;51:736–739. doi: 10.1111/j.1442-200X.2009.02896.x. [DOI] [PubMed] [Google Scholar]
- 33.Vincent A, Buckley C, Schott JM, Baker I, Dewar B-K, Detert N, Clover L, Parkinson A, Bien6 CG, Omer S, Lang B, Rossor MN, Palace J. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 34.Lanska DJ, Lanska MJ. Klüver-Bucy Syndrome in Juvenile Neuronal Ceroid Lipofuscinosis. J Child Neurol January. 1994;9:67–69. doi: 10.1177/088307389400900117. [DOI] [PubMed] [Google Scholar]
- 35.Max JE, Wilde EA, Bigler ED, Thompson WK, MacLeod M, Vasquez AC, Merkley TL, Hunter JV, Chu ZD, Yallampalli R, Hotz G, Chapman SB, Yang TT, Levin HS. Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2012;51:1208–201736. doi: 10.1016/j.jaac.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hétu S, Taschereau-Dumouchel V, Jackson PL. Stimulating the brain to study social interactions and empathy. Brain Stimulation: Basic, translational, and clinical research in neuromodulation. 2012;5:95–102. doi: 10.1016/j.brs.2012.03.005. [DOI] [PubMed] [Google Scholar]


