Abstract
Problem
Gestational genitourinary infections are associated with life-long disabilities, but it is unknown if neonatal inflammation is involved.
Method
Mothers of 914 infants born before 28th gestation week reported cervical/vaginal infection (CVI), and/or urine/bladder/kidney infection (UTI), or neither. Inflammation proteins measured in baby’s blood on postnatal days 1, 7 and 14 were considered elevated if in the top quartile for gestational age. Logistic regression models adjusting for potential confounders assessed odds ratios.
Results
Compared to neither UTI/CVI, mothers with CVI were more likely to have infants with elevated CRP, SAA, MPO, IL-1β, IL-6, IL-6R, TNF-α, RANTES, ICAM-3, E-selectin and VEGF-R2 on day 1; those with UTI were more likely to have infants with elevated MPO, IL-6R, TNF-R1, TNF-R2, and RANTES on day 7. Placental anaerobes and genital micoplasma were more common in pregnancies with CVI.
Conclusion
Gestational UTI/CVI should be targeted for preventing systemic inflammation in the very preterm newborn.
Keywords: cervicitis, vaginitis, cytokines, acute phase proteins, preterm birth, placental microbiome
INTRODUCTION
Newborns whose mother had a genito-urinary infection during pregnancy appear to be at increased risk of a wide variety of life-long disabilities, including cerebral palsy [1], asthma [2], and low IQ [3]. Such adversities following gestational infections have been attributed to maternal immune activation [4–6], which promotes fetal epigenetic changes in genes involved in organ development [7] or influences fetal immune capabilities [8].
Infants born before 28 weeks of gestation are at especially high risk of inflammatory complications partially due to delayed ability to synthesize proteins with anti-inflammatory properties [9–11] and immaturity of the cytokine network related to gestational age [12–14]. Very preterm newborns who have had elevated concentrations of inflammation-related proteins measured in blood spots obtained during the first two postnatal weeks appear to be at increased risk of bronchopulmonary dysplasia [15] and enlarged lateral ventricles of the brain [16] when the infant is in the intensive care nursery, and at age two years – at increased risk of developmental delay [17], attention problems [18], and cerebral palsy [16, 19].
Early systemic inflammation in the very preterm newborn has been associated with maternal pre-pregnancy obesity [20], placenta inflammation and its correlates[21–23], necrotizing enterocolitis and isolated intestinal perforation [24] postnatal bacteremia [25], and prolonged ventilation [26]. In search of an intervention that might reduce the risk of developmental limitations in future generations of very preterm newborns, we wanted to evaluate to what extent maternal urinary tract infection and cervical/vaginal infection were also associated with early postnatal systemic inflammation in the very preterm offspring.
METHODS
The ELGAN Study
The ELGAN study was designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in ELGANs [27]. During the years 2002–2004, women delivering before 28 weeks gestation at one of 14 participating USA institutions were asked to enroll in the study. The enrollment and informed consent processes were approved by the individual institutional review boards (IRB). The protein analysis performed at the Fichorova laboratory was approved by the Brigham and Women’s Hospital IRB. A full description of the methods [27] and details of placental histology [21] and microbiology assessments in the ELGAN study [23, 28] have been published elsewhere. The sample for this report consists of all 914 newborns who had proteins measured on one or more days and whose mother provided information about urinary tract infection (UTI) and cervico-vaginal infections (CVI) during her pregnancy.
Maternal Variables
After delivery, a trained research nurse interviewed each mother in her native language using a structured data collection form and following procedures defined in a manual. Among the questions asked was “During this pregnancy, did you have any of these conditions or disorders?” Included among the options were “vaginal or cervical infection (specify)” and “urine, bladder or kidney infection.” The entities specified by the mothers reporting cervical/vaginal infection in this study were as follows: bacterial, bacterial vaginosis, mixed, yeast, chlamydia, trichomonas, herpes or unknown specific entity.
Newborn Variables
Estimation of gestational age at birth was based on a hierarchy ordered by the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), last menstrual period (7%), and gestational age recorded in the log of the neonatal intensive care unit (1%).
Blood Spot Collection
Drops of blood were collected on filter paper (Schleicher & Schuell 903, Whatman International Ltd, Florham Park, NJ) on postnatal day 1 (range: 1–3 days), postnatal day 7 (range: 5–8 days), and postnatal day 14 (range: 12–15 days), All blood was from the remainder after specimens were obtained for clinical indications. Dried blood spots were stored at −70°C in sealed bags with desiccant until processed.
Protein Measurement
Details about elution of blood spots are provided elsewhere [21, 23]. Proteins were measured in the Laboratory of Genital Tract Biology of the Department of Obstetrics, Gynecology and Reproductive Biology at Brigham and Women’s Hospital, Boston, with an electrochemiluminescence multiplex detection system (Sector Imager 2400 and Discovery Workbench Software, both from Meso Scale Discovery (MSD), Gaithersburg, MD, USA) that has been validated by comparisons with traditional ELISA [29]. The blood spot elution technique combined with the MSD technology for protein quantitation has shown high clinical content validity and less than 20% inter-assay variation based on quality control specimens repeatedly measured on each assay plate [21–23, 30]. Concentrations (pg/ml or U/ml) of each protein, measured in duplicate, were normalized to mg of total protein, determined by the BCA assay (Thermo Scientific, Rockford, IL, USA) using a multi-label Victor 2 counter (Perkin Elmer, Boston, MA, USA). The mean of normalized values served as the basis for all tables and analyses.
The following 25 proteins were chosen to represent the major types of inflammation mediators in our multiplex assays: 1) proinflammatory cytokines and cytokine receptors with known association with systemic immune responses to infection: IL-1β (Interleukin-1beta), IL-6 (Interleukin-6), IL-6R (interleukin-6 receptor), TNF-α (tumor necrosis factor-alpha), TNF-R1 (tumor necrosis factor-alpha-receptor1), TNF-R2 (tumor necrosis factor-alpha-receptor2); 2) chemokines for neutrophils, monocytes and T cells: IL-8 (interleukin-8, CXCL8), MCP-1 (monocyte chemotactic protein-1, CCL2), MCP-4 (monocyte chemoattractant protein-4, CCL13), MIP-1β (Macrophage Inflammatory Protein-1beta, CCL4), RANTES (regulated upon activation, normal T-cell expressed, and [presumably] secreted, CCL5), I-TAC (Interferon-inducible T cell alpha-chemoattractant, CXCL11); 3) adhesion molecules involved in leukocyte traffic across the vasculature: ICAM-1 (intercellular adhesion molecule-1, CD54), ICAM-3 (intercellular adhesion molecule-3, CD50), VCAM-1 (vascular cell adhesion molecule-1, CD106), E-SEL (E-selectin, CD62E); 4) metalloproteases aiding leukocyte traffic and tissue remodeling: MMP-1 (matrix metalloproteinase-1), MMP-9 (matrix metalloproteinase-9); 5) liver-derived acute phase reactants: CRP (C-Reactive Protein), SAA (serum amyloid A); 6) indicators of neutrophil activation: MPO (myeloperoxidase); 7) growth factors involved in inflammation and tissue damage: VEGF (vascular endothelial growth factor), VEGF-R1 (vascular endothelial growth factor-receptor 1, FLT-1), VEGF-R2 (vascular endothelial growth factor-receptor 2, KDR, CD309), and IGFBP-1 (insulin growth factor binding protein-1, PP12). Systemic inflammation was defined as having a concentration of a specific inflammation-related protein in the highest quartile for gestational age on the day the blood spot was collected.
Data Analysis
First, we sought potential confounders of the relationship between maternal UTI and/or CVI and systemic inflammation in the offspring. We did this by examining the relationships between UTI and/or CVI and demographic, pregnancy, delivery and placenta characteristics (Table 1) and characteristics of the newborn (Table 2). Information from this assessment prompted us to adjust all analyses for mother’s identification as Black and her eligibility for government-provided (public) insurance, as well as for gestational age category. Then we created two sets of logistic regression models. Each set contained one model for each protein on each of the three days.
Table 1A.
Percent of women who reported UTI and CVI, UTI only, CVI only, or neither infection during pregnancy with the demographic, pregnancy, and delivery characteristics listed on the left. All values are column percents except when N is noted. Percents that are higher than those in the “neither” category are bolded, and those that are lower are bolded and italicized.
| Characteristics | UTI and CVI | UTI Only | CVI only | Neither | Row N | |
|---|---|---|---|---|---|---|
| Racial identity | White | 55 | 53 | 52 | 64 | 553 |
| Black | 32 | 36 | 38 | 24 | 247 | |
| Other | 13 | 10 | 11 | 11 | 102 | |
|
| ||||||
| Maternal age (yrs) | < 21 | 13 | 18 | 18 | 11 | 118 |
| 21–35 | 68 | 66 | 70 | 68 | 623 | |
| > 35 | 19 | 16 | 12 | 20 | 173 | |
|
| ||||||
| Maternal education (yrs) | ≤ 12 | 19 | 20 | 23 | 14 | 114 |
| 13–15 | 71 | 62 | 53 | 48 | 462 | |
| ≥ 16 | 10 | 18 | 23 | 39 | 307 | |
|
| ||||||
| Single mother | Yes | 61 | 54 | 52 | 36 | 371 |
|
| ||||||
| Public insurance | Yes | 65 | 48 | 51 | 34 | 349 |
|
| ||||||
| Smoking in pregnancy | Yes | 26 | 21 | 23 | 20 | 126 |
|
| ||||||
| Pre-pregnancy BMI | < 25 | 48 | 57 | 51 | 60 | 529 |
| 25, < 30 | 29 | 20 | 26 | 20 | 188 | |
| ≥ 30 | 23 | 23 | 23 | 20 | 186 | |
|
| ||||||
| NSAID consumption Aspirin | Yes | 26 | 4 | 10 | 7 | 67 |
| Yes | 6 | 7 | 3 | 6 | 50 | |
|
| ||||||
| Antibiotic | Yes | 94 | 85 | 53 | 16 | 278 |
|
| ||||||
| Antenatal steroid course | Complete | 61 | 70 | 69 | 63 | 587 |
| Partial | 29 | 24 | 22 | 25 | 227 | |
| None | 10 | 7 | 9 | 12 | 99 | |
|
| ||||||
| Pregnancy complication | Preterm Labor | 61 | 46 | 45 | 44 | 412 |
| pPROM | 16 | 19 | 24 | 23 | 203 | |
| Preeclampsia | 10 | 19 | 10 | 13 | 118 | |
| Abruption | 3 | 8 | 12 | 12 | 101 | |
| Cervical insufficiency | 6 | 6 | 9 | 4 | 44 | |
| Fetal indication | 3 | 2 | 1 | 5 | 36 | |
|
| ||||||
| Magnesium | None | 32 | 20 | 30 | 35 | 294 |
| Tocolysis | 58 | 63 | 59 | 53 | 496 | |
| Seizure prophylaxis | 10 | 17 | 12 | 12 | 116 | |
|
| ||||||
| Cesarean delivery | Yes | 71 | 73 | 60 | 67 | 615 |
|
| ||||||
| CVI entitya | Bacterial vaginosis | 6 | - | 16 | - | 15 |
| Yeast | 48 | - | 45 | - | 57 | |
| Trichomonas | 10 | - | 0 | - | 3 | |
| Chlamydia | 3 | - | 3 | - | 4 | |
| Gonorrhoea | 0 | - | 0 | - | 0 | |
| Herpes | 0 | - | 2 | - | 2 | |
| Bacterial | 3 | - | 5 | - | 6 | |
| Viral | 0 | - | 0 | - | 0 | |
| Mixed | 0 | - | 1 | - | 1 | |
| Not specified | 29 | - | 28 | - | 35 | |
|
| ||||||
| Maximum column N | 31 | 106 | 94 | 683 | 914 | |
information obtained from interview of mother at the time of delivery; mothers were asked to specify entity only if the answered ‘yes’ to CVI
Table 2.
Percent of children whose mothers reported UTI and CVI, UTI one, CVI only, or neither infection during pregnancy with the characteristics listed on the left. All values are column percents except when N is noted. Percents that are higher than those in the “neither” category are bolded and those that are lower are bolded and italicized.
| Characteristics of the infant | UTI and CVI | UTI Only | CVI Only | Neither | Row N | |
|---|---|---|---|---|---|---|
| Sex | Male | 32 | 45 | 52 | 55 | 483 |
|
| ||||||
| Type of gestation | Multiple | 29 | 31 | 32 | 36 | 315 |
|
| ||||||
| Gestational age (weeks) | 23–24 | 23 | 22 | 31 | 18 | 183 |
| 25–26 | 48 | 38 | 36 | 48 | 420 | |
| 27 | 29 | 41 | 33 | 33 | 311 | |
|
| ||||||
| Birth weight (grams) | ≤ 750 | 29 | 38 | 36 | 37 | 333 |
| 751–1000 | 65 | 43 | 49 | 42 | 400 | |
| > 1000 | 6 | 19 | 15 | 21 | 181 | |
|
| ||||||
| Birth weight Z-score | < −2 | 0 | 5 | 3 | 6 | 50 |
| ≥ −2. < −1 | 10 | 21 | 10 | 12 | 119 | |
| ≥ −1 | 90 | 75 | 87 | 81 | 745 | |
|
| ||||||
| Days ventilation | 14+ | 45 | 44 | 43 | 44 | 399 |
|
| ||||||
| Necrotizing enterocolitis | Bell stage IIIb | 0 | 6 | 2 | 4 | 35 |
|
| ||||||
| Isolated intestinal perforation | Yes | 3 | 1 | 4 | 3 | 27 |
|
| ||||||
| Documented early bacteremia | Yes | 6 | 7 | 7 | 6 | 57 |
|
| ||||||
| Documented late sepsis | Yes | 29 | 32 | 25 | 25 | 235 |
|
| ||||||
| Maximum column N | 31 | 106 | 94 | 683 | 914 | |
includes Corynebacterium sp., Staphylococcus sp. and Propionibacterium sp.
includes Prevotella bivia, Lactobacillus sp, Peptostreptococcus magnus, Gardnerella vaginalis
BV=bacterial vaginosis; includes Prevotella bivia, Gardnerella vaginalis, Anaerobic Streptococcus, Peptostreptococcus magnus
includes any Mycoplasma sp and U. urealyticum
The first set of models classified each newborn by his/her mother’s acknowledgement of any UTI, and separately by acknowledgement of any CVI (Figure 1 and Supplemental Table 1). The referent group consisted of all infants whose mother denied both UTI and CVI. In the second set of models, we compared three levels of exposure, UTI and CVI, UTI alone, and CVI alone, to neither UTI nor CVI, using multinomial logistic regression models (Table 3). In both sets of models, odds ratios (OR) and their 95% confidence intervals were calculated for each protein elevation at each of the three time points. ORs with 95% confidence intervals that did not include 1.0 indicated statistically significant associations, at the 0.05 level.
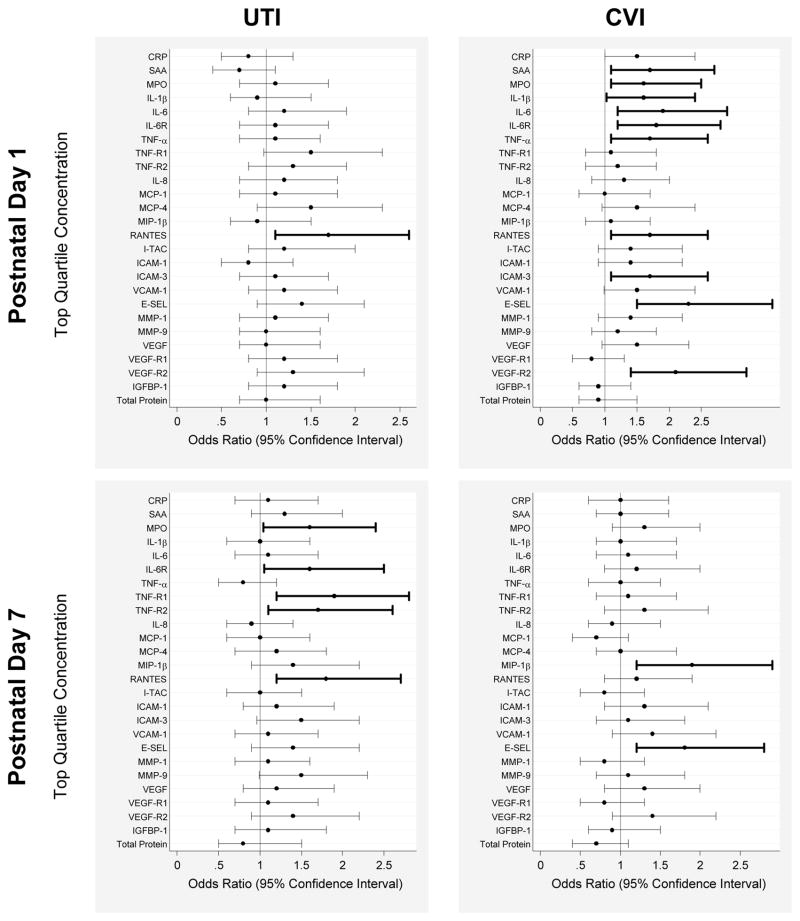
Figure 1.
Elevations of inflammation-associated proteins in the peripheral blood of newborns whose mothers acknowledged any UTI (uterine tract infection) or CVI (cervicovaginal infection) assessed as odds ratios (OR) and 95% confidence intervals of having top quatile protein concentrations for gestation age and day of blood collection. The reference group of each infection consisted of newborns whose mother denied both UTI and CVI (day 1, n=621, day 7, n=631). All ORs are adjusted for gestational age category, Black race, and public insurance. Odds ratios with 95% confidence intervals that do not include 1.0 are bolded.
Table 3.
The odds ratio (OR) (95% confidence interval) of a top-quartile concentration of the protein listed on the left on days 1, 7 and 14 with the newborn’s mother having acknowledged both a UTI (urinary tract infection) and CVI (cervicovaginal infection), A UTI without CVI, or a CVI without UTI. The referent group consists of all newborns whose mothers did not have either infection (day 1, N=621, day 7, N=631, day 14, N=574). All ORs are adjusted for gestational age category, Black race, and public insurance. Significantly elevated OR are bolded and shaded while significantly reduced OR are bolded, italicized, and shaded.
| Protein in top quartile | Both UTI and CVI | UTI no CVI | CVI no UTI | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Day 1 | Day 7 | Day 14 | Day 1 | Day 7 | Day 14 | Day 1 | Day 7 | Day 14 | |
| CRP | 1.1 (0.4, 2.6) | 0.9 (0.4, 2.2) | 0.7 (0.2, 3.0) | 0.7 (0.4, 1.2) | 1.2 (0.7, 1.9) | 1.2 (0.7, 2.0) | 1.7 (1.1, 2.8) | 1.1 (0.6, 1.8) | 0.8 (0.5, 1.5) |
| SAA | 1.0 (0.4, 2.5) | 1.7 (0.8, 3.7) | 0.9 (0.3, 2.2) | 0.6 (0.3,1.02) | 1.2 (0.7, 1.9) | 1.2 (0.7, 1.9) | 2.0 (1.3, 3.3) | 0.8 (0.5, 1.4) | 0.8 (0.4, 1.4) |
| MPO | 2.1 (0.95,4.6) | 2.2 (1.04,4.8) | 0.9 (0.3, 2.3) | 0.9 (0.5, 1.5) | 1.4 (0.9, 2.3) | 1.0 (0.7, 1.9) | 1.5 (0.9, 2.4) | 1.0 (0.6, 1.8) | 1.0 (0.6, 1.8) |
| IL-1β | 1.2 (0.5, 2.9) | 1.0 (0.4, 2.4) | 1.8 (0.8, 4.1) | 0.9 (0.5, 1.5) | 1.0 (0.6, 1.6) | 1.1 (0.7, 1.9) | 1.7 (1.1, 2.7) | 1.1 (0.6, 1.8) | 1.2 (0.7, 2.1) |
| IL-6 | 1.1 (0.4, 2.6) | 0.9 (0.4, 2.2) | 0.9 (0.4, 2.4) | 1.3 (0.8, 2.1) | 1.2 (0.7, 1.9) | 0.9 (0.6, 1.6) | 2.1 (1.3, 3.4) | 1.1 (0.7, 1.9) | 1.1 (0.7, 1.9) |
| IL-6R | 1.3 (0.6, 2.0) | 1.5 (0.7, 3.4) | 1.3 (0.5, 3.2) | 1.1 (0.6, 1.7) | 1.6 (1.02,2.6) | 1.1 (0.7, 1.9) | 2.0 (1.2, 3.2) | 1.2 (0.7, 2.0) | 1.0 (0.6, 1.8) |
| TNF-α | 1.6 (0.7, 3.8) | 1.1 (0.5, 2.4) | 2.0 (0.9, 4.7) | 1.0 (0.6, 1.6) | 0.7 (0.4, 1.2) | 1.1 (0.7, 1.9) | 1.8 (1.1, 2.9) | 0.9 (0.6, 1.6) | 1.3 (0.8, 2.2) |
| TNF-R1 | 1.1 (0.5, 2.7) | 2.1 (0.97,4.7) | 1.0 (0.4, 2.6) | 1.6 (1.01,2.5) | 1.8 (1.1, 2.8) | 0.9 (0.6, 1.6) | 1.1 (0.7, 1.9) | 0.8 (0.5, 1.4) | 1.0 (0.5, 1.7) |
| TNF-R2 | 1.4 (0.6, 3.2) | 2.1 (0.99,4.6) | 1.5 (0.7, 3.6) | 1.2 (0.8, 2.0) | 1.6 (1.02,2.5) | 0.9 (0.5, 1.5) | 1.1 (0.6, 1.8) | 1.1 (0.7, 1.9) | 0.6 (0.3, 1.2) |
| IL-8 (CXCL8) | 1.7 (0.8, 3.9) | 1.5 (0.7, 3.2) | 1.4 (0.6, 3.4) | 1.0 (0.6, 1.7) | 0.8 (0.5, 1.3) | 1.1 (0.7, 1.8) | 1.2 (0.7, 2.0) | 0.8 (0.5, 1.4) | 1.4 (0.8, 2.3) |
| MCP-1 (CCL2) | 0.8 (0.3, 2.1) | 1.7 (0.8, 3.9) | 1.0 (0.4, 2.6) | 1.2 (0.7, 2.0) | 0.9 (0.5, 1.4) | 0.9 (0.5, 1.5) | 1.1 (0.7, 1.9) | 0.4 (0.2, 0,8) | 1.2 (0.7, 2.0) |
| MCP-4 (CCL13) | 2.6 (1.2, 5.9) | 2.5 (1.2, 5.4) | 1.7 (0.7, 4.0) | 1.2 (0.7, 1.9) | 0.9 (0.5, 1.5) | 1.2 (0.7, 1.9) | 1.3 (0.7, 2.1) | 0.7 (0.4, 1.3) | 1.0 (0.6, 1.8) |
| MIP-1β (CCL4) | 1.2 (0.5, 2.7) | 2.3 (1.1, 4.9) | 1.1 (0.5, 2.8) | 0.9 (0.5, 1.5) | 1.2 (0.8, 2.0) | 1.1 (0.7, 1.9) | 1.0 (0.6, 1.7) | 1.7 (1.03,2.7) | 0.8 (0.4, 1.4) |
| RANTES (CCL5) | 1.8 (0.8, 4.0) | 1.8 (0.8, 3.9) | 1.4 (0.5, 3.4) | 1.6 (1.02,2.6) | 1.8 (1.1, 2.8) | 1.2 (0.7, 2.0) | 1.7 (1.01,2.7) | 1.1 (0.6, 1.8) | 1.1 (0.7, 2.0) |
| I-TAC (CXCL11) | 2.5 (1.2, 5.5) | 1.2 (0.6, 2.8) | 1.7 (0.7, 4.0) | 1.1 (0.6, 1.7) | 0.9 (0.6, 1.5) | 1.0 (0.6, 1.8) | 1.2 (0.7, 1.9) | 0.7 (0.4, 1.2) | 1.1 (0.6, 1.9) |
| ICAM-1 (CD54) | 1.5 (0.7, 3.4) | 1.3 (0.6, 3.0) | 1.6 (0.7, 3.8) | 0.7 (0.4, 1.2) | 1.2 (0.7, 1.9) | 0.6 (0.3, 1.1) | 1.4 (0.8, 2.2) | 1.4 (0.8, 2.2) | 1.2 (0.7, 2.1) |
| ICAM-3 (CD50) | 1.7 (0.7, 3.9) | 1.3 (0.6, 3.1) | 1.4 (0.6, 3.5) | 1.0 (0.6, 1.6) | 1.5 (0.97,2.5) | 1.1 (0.7, 1.9) | 1.6 (1.00,2.6) | 1.0 (0.6, 1.8) | 0.7 (0.4, 1.2) |
| VCAM-1 (CD106) | 1.4 (0.6, 3.2) | 1.2 (0.5, 2.9) | 0.9 (0.4, 2.4) | 1.1 (0.7, 1.8) | 1.1 (0.7, 1.8) | 0.6 (0.3, 1.1) | 1.6 (0.98,2.6) | 1.5 (0.9, 2.4) | 1.1 (0.6, 1.8) |
| E-SEL (CD62E) | 1.8 (0.8, 4.1) | 1.7 (0.9, 3.8) | 0.8 (0.3, 2.1) | 1.3 (0.8, 2.0) | 1.4 (0.8, 2.2) | 1.2 (0.8, 2.0) | 2.4 (1.5, 3.9) | 1.8 (1.1, 3.0) | 1.4 (0.8, 2.3) |
| MMP-1 | 1.1 (0.4, 2.6) | 0.6 (0.2, 1.6) | 0.6 (0.2, 1.8) | 1.1 (0.7, 1.8) | 1.2 (0.7, 1.9) | 0.7 (0.4, 1.2) | 1.4 (0.9, 2.3) | 0.9 (0.5, 1.5) | 1.4 (0.8, 2.3) |
| MMP-9 | 1.4 (0.6, 3.2) | 1.4 (0.6, 3.2) | 1.1 (0.5, 2.8) | 0.9 (0.6, 1.6) | 1.6 (0.99,2.5) | 0.9 (0.6, 2.6) | 1.1 (0.7, 1.8) | 1.0 (0.6, 1.7) | 1. 1 (0.6, 1.8) |
| VEGF | 1.5 (0.7, 3.5) | 2.1 (0.98,4.6) | 2.0 (0.9, 4.7) | 0.9 (0.6, 1.6) | 1.1 (0.6, 1.7) | 09 (0.6, 1.6) | 1.4 (0.9, 2.4) | 1.0 (0.6, 1.7) | 0.8 (0.4, 1.4) |
| VEGF-R1 | 0.7 (0.3, 1.9) | 1.0 (0.4, 2.5) | 0.5 (0.2, 1.6) | 1.3 (0.8, 2.1) | 1.1 (0.7, 1.8) | 0.6 (0.3,1.02) | 0.9 (0.5, 1.5) | 0.7 (0.4, 1.3) | 0.6 (0.4, 1.2) |
| VEGF-R2 | 1.8 (0.8, 3.9) | 1.2 (0.5, 2.7) | 1.0 (0.4, 2.4) | 1.2 (0.8, 2.0) | 1.5 (0.96,2.4) | 0.9 (0.5, 1.5) | 2.2 (1.4, 3.5) | 1.5 (0.9, 2.5) | 1.0 (0.6, 1.7) |
| IGFBP-1 | 0.8 (0.3, 2.1) | 0.4 (0.1, 1.3) | 0.8 (0.3, 2.2) | 1.3 (0.8, 2.0) | 1.4 (0.9, 2.3) | 0.9 (0.5, 1.5) | 0.9 (0.5, 1.5) | 1.2 (0.7, 2.0) | 0.8 (0.5, 1.5) |
|
| |||||||||
| Total Protein | 1.0 (0.4, 2.4) | 0.7 (0.3, 1.8) | 0.4 (0.1, 1.3) | 1.1 (0.7, 1.8) | 0.8 (0.5, 1.3) | 0.8 (0.5, 1.4) | 0.9 (0.5, 1.5) | 0.6 (0.4, 1.1) | 1.1 (0.6, 1.8) |
|
| |||||||||
| N specimens | 28 | 29 | 25 | 100 | 100 | 91 | 91 | 87 | 78 |
RESULTS
Demographic, Pregnancy, Maternal and Placental Characteristics (Table 1A and 1B)
Table 1B.
Percent of women who reported UTI and CVI, UTI only, CVI only, or neither infection during pregnancy with placenta characteristics listed on the left. All values are column percents except when N is noted. Percents that are higher than those in the “neither” category are bolded, and those that are lower are bolded and italicized.
| Characteristics | UTI and CVI | UTI Only | CVI only | Neither | Row N | |
|---|---|---|---|---|---|---|
| Placental microbes: all deliveries | Any microorganism | 46 | 41 | 53 | 49 | 404 |
| Skin organisms a | 8 | 14 | 24 | 19 | 158 | |
| Any aerobe | 21 | 28 | 34 | 32 | 265 | |
| Any anaerobe | 33 | 19 | 34 | 27 | 231 | |
| E. coli | 8 | 5 | 8 | 6 | 49 | |
| Alpha streptococcus | 0 | 4 | 6 | 7 | 52 | |
| Vaginal organisms b | 0 | 5 | 20 | 17 | 129 | |
| Lactobacillus sp | 0 | 4 | 3 | 7 | 48 | |
| BV-associated c | 4 | 8 | 17 | 15 | 117 | |
| Genital mycoplasma d | 8 | 9 | 21 | 9 | 83 | |
|
| ||||||
| Placental microbes: Cesarean delivery | Any microorganism | 39 | 32 | 36 | 42 | 226 |
| Skin organisms a | 6 | 12 | 10 | 12 | 66 | |
| Any aerobe | 17 | 23 | 18 | 24 | 129 | |
| Any anaerobe | 28 | 12 | 24 | 22 | 118 | |
| E. coli | 6 | 4 | 4 | 5 | 27 | |
| Alpha streptococcus | 0 | 3 | 8 | 5 | 28 | |
| Vaginal organisms b | 0 | 0 | 8 | 12 | 53 | |
| Lactobacillus sp | 0 | 0 | 2 | 5 | 24 | |
| BV-associated c | 6 | 4 | 8 | 10 | 48 | |
| Genital mycoplasma d | 11 | 5 | 16 | 6 | 39 | |
|
| ||||||
| Umbilical cord vasculitis | Grade 3–5 | 37 | 10 | 25 | 15 | 135 |
|
| ||||||
| Neutrophilic infiltration of fetal stem vessels of chorionic plate | Present | 45 | 14 | 32 | 24 | 205 |
|
| ||||||
| Inflammation of the chorion/decidua | Grade 3–4 | 47 | 28 | 45 | 35 | 295 |
|
| ||||||
| Infarct | Present | 13 | 18 | 15 | 17 | 142 |
|
| ||||||
| Syncytial knots | Increased | 20 | 25 | 18 | 20 | 173 |
|
| ||||||
| Trrombosis fetal stem vessels | Present | 3 | 3 | 4 | 5 | 40 |
|
| ||||||
| Maximum column N | 31 | 106 | 94 | 683 | 914 | |
Of the 914 babies, 31 had a mother with both UTI and CVI, 94 had a mother with UTI only, 106 had a mother who had a CVI only, and the mother of 683 denied both forms of infection. Compared to women who did not have either type of infection, those who had either or both were more likely to identify as Black, to have lower educational attainment, and to be single and eligible for government-provided (public) health care insurance. Women who had both UTI and CVI were more likely than others to present with preterm labor and to have used non-steroidal anti-inflammatory drugs; they were less likely than others to have a pre-pregnancy body mass index < 25, pPROM and placental abruption. Among those who reported any CVI, yeast and bacterial vaginosis were the most prevalent entities specified by the mothers, and bacterial vaginosis was more commonly associated with ‘CVI only’ as compared to ‘both CVI and UTI’. Antibiotic consumption was higher in women who reported either or both CVI and UTI; among those, it was highest in women who reported both types of infection and lowest in women who reported CVI only.
Women either or both UTI and CVI were less likely to have Lactobacilli isolated from the placenta. In comparison to all others, women who had both UTI and CVI were more likely to have inflammation of the umbilical cord, the chorion and/or decidua and the fetal stem vessels of the chorionic plate. Women with any UTI were less likely to have vaginal bacteria isolated from the placenta. Women who had only CVI were more likely to have genital mycoplasma isolated from the placenta. Women who had CVI, with or without UTI, were more likely than others to have umbilical cord vasculitis, inflammation of the chorion and/or decidua and neutrophilic infiltration of the fetal stem vessels of the chorionic plate. In addition, compared to women with UTI only, women with any CVI were more likely to have anaerobes isolated from the placenta.
Newborn Characteristics (Table 2)
Children born to women who had CVI only were more likely than others to be born before the 25th week of gestation. Children born to women with UTI, with or without CVI, were somewhat more likely than others to have late bacteremia.
Odds Ratios of Top Quartile Concentrations: Any UTI and Any CVI Compared to Neither Infection (Figure 1 and Supplemental Table 1)
On postnatal day 1, infants born to mothers with UTI (alone or combined with CVI) were more likely than others to have an elevated concentration of RANTES. By day 7 they were more likely to have significantly elevated concentrations of RANTES and four other proteins (MPO, IL-6R, TNF-R1, and TNF-R2). By day 14, newborns whose mother had a UTI during this pregnancy were significantly less likely than others to have an elevated concentration of VEGF-R1.
On postnatal day 1, infants whose mother reported CVI (alone or combined with UTI) were more likely than others to have significantly elevated concentrations of 10 proteins (SAA, MPO, IL-1β, IL-6, IL-6R, TNF-α, RANTES, ICAM-3, E-SEL, and VEGF-R2). By day 7, these children were more likely to have elevated concentrations of only MIP-1β and E-SEL. There were no associations between protein levels and CVI on day 14.
Odds Ratios of Top Quartile Concentrations: Both UTI and CVI, UTI Only, and CVI Only Compared to Neither Infection (Table 3)
Newborns whose mother had both UTI and CVI were more likely than women with neither to have elevated day 1 concentrations of MCP-4 and I-TAC, and elevated day 7 concentrations of MPO, MCP-4, and MIP-1β. No associations were significant on postnatal day 14.
Newborns whose mother had a UTI but no CVI were more likely than women who reported no genitourinary infection to have elevated day 1 concentrations of TNF-R1 and RANTES, and elevated day 7 concentrations of IL-6R, TNF-R1, TNF-R2, and RANTES. No significant OR was observed on day 14.
Compared to newborns whose mother had neither UTI nor CVI, those whose mother had a CVI but no UTI were more likely to have elevated day 1 concentrations of 9 proteins (CRP, SAA, IL-1β, IL-6, IL-6R, TNF-α, RANTES, E-SEL, and VEGF-R2). On day 7 they had significantly elevated concentrations of MIP-1β and E-SEL and a significantly reduced concentration of MCP-1. Similar to children of mothers with both UTI and CVI, or UTI only, children of mothers who had CVI only were not at increased risk of an elevated concentration of any inflammation-related protein on day 14.
DISCUSSION
Our main findings are that infants born to women who acknowledged a gestational UTI and/or CVI were more likely than others to have systemic inflammation during the first week after very preterm birth, and this inflammation is no longer evident by the end of the second postnatal week. CVI was associated with a diffuse inflammatory response on postnatal day 1, which gradually subsided by day 14. In contrast, the neonatal systemic inflammation that followed maternal UTI was barely evident at birth, and manifested with a more limited inflammatory protein repertoire on day 7. Like the infants of mothers who had a CVI, these infants of mothers who had a UTI had no evidence of systemic inflammation on day 14.
The obvious inference is that the gestational genitourinary infection contributed to the increased risk of early postnatal systemic inflammation in the very preterm newborn. Such an inference, however, might not be appropriate. Social inequality and low economic status place women at increased risk of genital infections [31–33]. Indeed, in our study, self-reported genitourinary infections were associated with low educational achievement, eligibility for government-provided medical care insurance, underweight, and self-identification as Black. Some unmeasured or unidentified correlate of low socioeconomic status could have also accounted for what we found. Given the possibility that the genitourinary tract infection is not in the causal pathway and is merely a marker for other correlates of low socioeconomic status, we adjusted for eligibility for government-provided medical care insurance and Black race. This adjustment, however, did not appreciably reduce the associations between genitourinary infections and fetal systemic inflammation, suggesting that these associations are likely to transcend correlates of socioeconomic status.
The predominant role of CVI in the pathogenesis of newborns’ inflammation shortly after birth, evident from the greater number of proteins elevated in the CVI-only group, might be attributable to vaginal bacteria capable of ascending to the uterus and perhaps colonizing the placenta. We did not find that the placentas of women who reported CVI were more likely than the placentas of women without CVI to harbor an organism. In our sample, however, every other women had bacteria detectable in the placenta parenchyma, and as we have previously shown, the magnitude of inflammatory responses associated with placental bacterial colonization depends on the type organism, with bacterial vaginosis bacteria placing the newborn at increased risk of systemic inflammation as compared to no bacteria or to Lactobacilli, which actually decreased the risk of systemic inflammation and promoted a non-inflammatory state [23]. Lactobacilli were less commonly found in the placentas of women with any CVI or UTI as compared to women who reported neither CVI nor UTI. This is in keeping with our previous findings that infants whose placenta harbored Lactobacilli were at reduced risk of systemic inflammation [23].
We cannot exclude the possibility that the infant systemic inflammation is a response to the mother’s systemic inflammation, which might in turn be a systemic response to the localized inflammation in the vaginal and cervical mucosa, or to the ascendance of bacteria from the vagina to the uterus. While our study had no information on the levels of inflammatory proteins in the maternal systemic circulation, association of CVI with elevated inflammation proteins in the maternal blood has been reported in human [34] and mouse studies [35].
For UTI to promote intrauterine and fetal inflammation probably requires systemic dissemination (urinary source bacteremia), which is certainly possible for E. coli [36] and is supported by the somewhat higher prevalence of late sepsis in infants born to mothers with UTI in our ELGAN sample. While human data are still to be gathered, cystitis in mice caused by uropathogenic E. coli leads to robust cellular inflammatory infiltration in uteroplacental tissue and to significantly increased levels of inflammation-related proteins (including IL-6 and TNF-α) in the maternal serum shortly after infection and during delivery [37].
Pregnant women with bacterial vaginosis (a condition where the vaginal microbiome is dominated by potentially pathogenic vaginal bacteria) are more likely than others to also have a UTI [38]. No information on the vaginal microbiota and BV diagnostics was routinely collected for our study subjects. Since bacterial vaginosis is often asymptomatic, yet associated with local and systemic proinflammatory cytokine upregulation [34, 39], and shows some tendency to co-occur with UTI [40] we cannot exclude the possibility that pathogenic vaginal bacteria might ascend to the uterus and have partially contributed to some inflammatory responses in the UTI only group.
In our ELGAN sample, women who reported any infection, including UTI or CVI, were much more likely to receive an antibiotic than women who did not report an infection. Among women with an infection, those who received an antibiotic might have had more severe symptoms than those who did not. Antibiotic treatment has the capacity to alter the vaginal microbiota composition [41], to exacerbate maternal inflammatory responses in some sexually transmitted infections [42, 43], and to alter the newborns colonization by maternal bacteria [44]. Consequently, mother’s receipt of antibiotics during this pregnancy might have contributed to what we found. Confounding by indication, which some feel can never be entirely eliminated [45], prevents us from assessing contributions of antibiotic receipt to what we found.
We found that women with CVI and UTI were more likely to deliver before the 25th week of gestation and those who reported both forms of infection were more likely to experience preterm labor. Although both UTI and CVI have been implicated in contributing to preterm birth, this is still controversial because of the way the data were collected for this association [46, 47].
Our study has several strengths. First, our large sample size makes it unlikely that we have missed important associations due to lack of statistical power, or claimed associations that might reflect the instability of small numbers. Second, we selected infants based on gestational age, not birth weight, in order to minimize confounding due to factors related to fetal growth restriction [48]. Third, we collected all of our data prospectively. Fourth, our protein data are of high analytic quality [29] and have clinical content validity [16, 17, 21–23, 25, 30].
The weaknesses of our study are those of all observational studies. We are unable to distinguish between causation and association as explanations for what we found. In addition, although our sample is large, the number of women who had both CVI and UTI was small (N=31), limiting the power to perceive statistical significance and thereby limiting the inferences we can make about this group of women and their offspring. Our study is limited to genitourinary infections reported by the mothers. The evidence presented here that these maternal infections predispose the newborn to systemic inflammation warrants further studies applying sensitive molecular techniques to clarify the role of specific genitourinary and sexually transmitted pathogens in the process. We also did not collect information on onset and duration of infection. Consequently, we might have misclassified a newborn as recently exposed when the exposure was remote in time. We do not know how much of what we found is limited to infants born before 28th gestation week. Future studies will need to address this.
Here we offer the first documentation that very preterm infants born to women who have a genitourinary infection during their pregnancy are at increased risk of systemic inflammation, particularly during the first postnatal week. Because systemic inflammation appears to place very preterm newborns at increased risk of life-long disabilities such as cerebral palsy and mental retardation, our findings have the potential to target an intervention that might reduce the risk of developmental limitations in future generations of very preterm newborns.
In conclusion, maternal UTI and/or CVI during the pregnancy appear to place extremely low gestational age newborns at increased risk of systemic inflammation during the first postnatal week and should be a subject to a more rigorous management and prevention strategies.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05 and 2R01NS040069 - 06A2), the National Eye Institute (1R01EY021820-01A1), and the National Institute of Child Health and Human Development (5P30HD018655-28).
We thank the ELGAN subjects and families, the research assistants who processed the blood spots and analysed proteins in Fichorova’s Laboratory (Yoshika Yamamoto, Olimpia Sociu, Bi Yu Li) as well as the following ELGAN study colleagues:
Children’s Hospital, Boston, MA
Kathleen Lee, Anne McGovern, Jill Gambardella, Susan Ursprung, Ruth Blomquist
Baystate Medical Center, Springfield, MA
Bhavesh Shah, Karen Christianson
Beth Israel Deaconess Medical Center, Boston, MA
Camilia R. Martin
Brigham & Women’s Hospital, Boston, MA
Linda J. Van Marter
Massachusetts General Hospital, Boston, MA
Robert M. Insoft, Jennifer G. Wilson, Maureen Pimental
Floating Hospital for Children at Tufts Medical Center, Boston, MA
Cynthia Cole, John M. Fiascone, Janet Madden, Ellen Nylen, Anne Furey
U Mass Memorial Health Care, Worcester, MA
Francis Bednarek (deceased), Mary Naples, Beth Powers
Yale University School of Medicine, New Haven, CT
Richard Ehrenkranz, Joanne Williams, Elaine Romano
Wake Forest University, Baptist and Forsyth Medical Centers, Winston-Salem, NC
T. Michael O’Shea, Debbie Gordon, Teresa Harold
University Health Systems of Eastern Carolina, Greenville, NC
Stephen C. Engelke, Sherry Moseley, Donna Pare
North Carolina Children’s Hospital, Chapel Hill, NC
Carl Bose, Gennie Bose
Helen DeVos Children’s Hospital, Grand Rapids, MI
Mariel Poortenga, Dinah Sutton
Sparrow Hospital, Lansing, MI
Carolyn Solomon
Michigan State University, East Lansing, MI
Nigel Paneth, Padmani Karna, Madeleine Lenski
University of Chicago Medical Center, Chicago, IL
Michael D. Schreiber, Grace Yoon
William Beaumont Hospital, Royal Oak, MI
Daniel Batton, Beth Kring
Frontier Science and Technology Research Foundation, Amherst, NY
Ken Wood
National Institute of Neurological Disorders and Stroke
Deborah Hirtz
Footnotes
Conflicts of Interest
None of the authors have commercial or other associations that may pose a conflict of interest with the content of this article.
References
- 1.Miller JE, Pedersen LH, Streja E, Bech BH, Yeargin-Allsopp M, Van Naarden Braun K, Schendel DE, Christensen D, Uldall P, Olsen J. Maternal infections during pregnancy and cerebral palsy: a population-based cohort study. Paediatric and perinatal epidemiology. 2013;27(6):542–552. doi: 10.1111/ppe.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collier CH, Risnes K, Norwitz ER, Bracken MB, Illuzzi JL. Maternal infection in pregnancy and risk of asthma in offspring. Maternal and child health journal. 2013;17(10):1940–1950. doi: 10.1007/s10995-013-1220-2. [DOI] [PubMed] [Google Scholar]
- 3.Camp BW, Broman SH, Nichols PL, Leff M. Maternal and neonatal risk factors for mental retardation: defining the ‘at-risk’ child. Early Hum Dev. 1998;50(2):159–173. doi: 10.1016/s0378-3732(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 4.Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biological psychiatry. 2014;75(4):332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canetta SE, Brown AS. Prenatal Infection, Maternal Immune Activation, and Risk for Schizophrenia. Translational neuroscience. 2012;3(4):320–327. doi: 10.2478/s13380-012-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin P, Liu J, Li Z, Wang YY, Qiao NN, Huang SY, Li BM, Sun RP. Prenatal immune challenge in rats increases susceptibility to seizure-induced brain injury in adulthood. Brain research. 2013;1519:78–86. doi: 10.1016/j.brainres.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Tang B, Jia H, Kast RJ, Thomas EA. Epigenetic changes at gene promoters in response to immune activation in utero. Brain, behavior, and immunity. 2013;30:168–175. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 8.Mandal M, Donnelly R, Elkabes S, Zhang P, Davini D, David BT, Ponzio NM. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain, behavior, and immunity. 2013;33:33–45. doi: 10.1016/j.bbi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Yoon BH, Romero R, Moon J, Chaiworapongsa T, Espinoza J, Kim YM, Edwin S, Kim JC, Camacho N, Bujold E, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med. 2003;13(1):32–38. doi: 10.1080/jmf.13.1.32.38. [DOI] [PubMed] [Google Scholar]
- 10.Jones CA, Cayabyab RG, Kwong KY, Stotts C, Wong B, Hamdan H, Minoo P, deLemos RA. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39(6):966–975. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 12.Matoba N, Yu Y, Mestan K, Pearson C, Ortiz K, Porta N, Thorsen P, Skogstrand K, Hougaard DM, Zuckerman B, et al. Differential patterns of 27 cord blood immune biomarkers across gestational age. Pediatrics. 2009;123(5):1320–1328. doi: 10.1542/peds.2008-1222. [DOI] [PubMed] [Google Scholar]
- 13.Narendran V, Visscher MO, Abril I, Hendrix SW, Hoath SB. Biomarkers of epidermal innate immunity in premature and full-term infants. Pediatr Res. 2010;67(4):382–386. doi: 10.1203/PDR.0b013e3181d00b73. [DOI] [PubMed] [Google Scholar]
- 14.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatric research. 2014;75(3):376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose C, Laughon M, Allred EN, Van Marter LJ, O’Shea TM, Ehrenkranz RA, Fichorova R, Leviton A Elgan Study I. Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr Res. 2011;69(4):347–353. doi: 10.1203/PDR.0b013e31820a58f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leviton A, Kuban K, O’Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. The Journal of pediatrics. 2011;158(6):897–903. e891–895. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 17.O’Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, Hirtz D, Leviton A Extremely Low Gestational Age Newborn Study I. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. The Journal of pediatrics. 2012;160(3):395–401. e394. doi: 10.1016/j.jpeds.2011.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Shea TM, Joseph RM, Kuban KC, Allred EN, Ware J, Coster T, Fichorova RN, Dammann O, Leviton A. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr Res. 2014;75(6):781–787. doi: 10.1038/pr.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuban KC, O’Shea TM, Allred EN, Paneth N, Hirtz D, Fichorova RN, Leviton A for the ESI. Systemic Inflammation and Cerebral Palsy Risk in Extremely Preterm Infants. J Child Neurol. 2014 doi: 10.1177/0883073813513335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Burg JW, Allred EN, McElrath TF, Fichorova RN, Kuban K, O’Shea TM, Dammann O, Leviton A. Is maternal obesity associated with sustained inflammation in extremely low gestational age newborns? Early Hum Dev. 2013;89(12):945–955. doi: 10.1016/j.earlhumdev.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A. The relationship between neonatal blood protein profiles and placenta histologic characteristics in extremely low gestation age newborns. Pediatr Res. 2011;69:68–73. doi: 10.1203/PDR.0b013e3181fed334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElrath TF, Fichorova RN, Allred EN, Hecht JL, Ismail MA, Yuan H, Leviton A, Investigators ES. Blood protein profiles of infants born before 28 weeks differ by pregnancy complication. American journal of obstetrics and gynecology. 2011;204(5):418 e411–418 e412. doi: 10.1016/j.ajog.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Fichorova RN, Onderdonk AB, Yamamoto H, Delaney ML, DuBois AM, Allred E, Leviton A Extremely Low Gestation Age Newborns Study I. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio. 2011;2(1):e00280–00210. doi: 10.1128/mBio.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin CR, Bellomy M, Allred EN, Fichorova RN, Leviton A. Systemic inflammation associated with severe intestinal injury in extremely low gestational age newborns. Fetal and pediatric pathology. 2013;32(3):222–234. doi: 10.3109/15513815.2012.721477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leviton A, O’Shea TM, Bednarek FJ, Allred EN, Fichorova RN, Dammann O, Investigators ES. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta paediatrica. 2012;101(4):355–359. doi: 10.1111/j.1651-2227.2011.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bose CL, Laughon MM, Allred EN, O’Shea TM, Van Marter LJ, Ehrenkranz RA, Fichorova RN, Leviton A, Investigators ES. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine. 2013;61(1):315–322. doi: 10.1016/j.cyto.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht JL, Onderdonk A, Delaney M, Allred EN, Kliman HJ, Zambrano E, Pflueger SM, Livasy CA, Bhan I, Leviton A. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr Dev Pathol. 2008;11(1):15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 29.Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. 2008;80(12):4741–4751. doi: 10.1021/ac702628q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leviton A, Fichorova R, Yamamoto Y, Allred EN, Dammann O, Hecht J, Kuban K, McElrath T, O’Shea TM, Paneth N. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine. 2011;53(1):66–73. doi: 10.1016/j.cyto.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harling G, Subramanian SV, Barnighausen T, Kawachi I. Income inequality and sexually transmitted in the United States: who bears the burden? Social science & medicine. 2014;102:174–182. doi: 10.1016/j.socscimed.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Patel V, Weiss HA, Mabey D, West B, D’Souza S, Patil V, Nevrekar P, Gupte S, Kirkwood BR. The burden and determinants of reproductive tract infections in India: a population based study of women in Goa, India. Sexually transmitted infections. 2006;82(3):243–249. doi: 10.1136/sti.2005.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss HA, Patel V, West B, Peeling RW, Kirkwood BR, Mabey D. Spousal sexual violence and poverty are risk factors for sexually transmitted infections in women: a longitudinal study of women in Goa, India. Sexually transmitted infections. 2008;84(2):133–139. doi: 10.1136/sti.2007.026039. [DOI] [PubMed] [Google Scholar]
- 34.Bogavac M, Brkic S, Simin N, Grujic Z, Bozin B. Do bacterial vaginosis and chlamydial infection affect serum cytokine level? Srpski arhiv za celokupno lekarstvo. 2010;138(7–8):444–448. doi: 10.2298/sarh1008444b. [DOI] [PubMed] [Google Scholar]
- 35.Fidel PL, Lynch ME, Sobel JD. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infection and Immunity. 1993;61(10):4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marschall J, Zhang L, Foxman B, Warren DK, Henderson JP Program CDCPE. Both host and pathogen factors predispose to Escherichia coli urinary-source bacteremia in hospitalized patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(12):1692–1698. doi: 10.1093/cid/cis252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolton M, Horvath DJ, Jr, Li B, Cortado H, Newsom D, White P, Partida-Sanchez S, Justice SS. Intrauterine growth restriction is a direct consequence of localized maternal uropathogenic Escherichia coli cystitis. PloS one. 2012;7(3):e33897. doi: 10.1371/journal.pone.0033897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharami SH, Afrakhteh M, Shakiba M. Urinary tract infections in pregnant women with bacterial vaginosis. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2007;27(3):252–254. doi: 10.1080/01443610701194846. [DOI] [PubMed] [Google Scholar]
- 39.Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193(4):556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 40.Harmanli OH, Cheng GY, Nyirjesy P, Chatwani A, Gaughan JP. Urinary tract infections in women with bacterial vaginosis. Obstetrics and gynecology. 2000;95(5):710–712. doi: 10.1016/s0029-7844(99)00632-8. [DOI] [PubMed] [Google Scholar]
- 41.Stokholm J, Schjorring S, Eskildsen CE, Pedersen L, Bischoff AL, Folsgaard N, Carson CG, Chawes BL, Bonnelykke K, Molgaard A, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013 doi: 10.1111/1469-0691.12411. [DOI] [PubMed] [Google Scholar]
- 42.Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, et al. The Villain Team-Up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sexually transmitted infections. 2013;89(6):460–466. doi: 10.1136/sextrans-2013-051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, Goodman RP, Chepa-Lotrea X, Buck OR, Murray R, Kula T, et al. Endobiont viruses sensed by the human host - beyond conventional antiparasitic therapy. PloS one. 2012;7(11):e48418. doi: 10.1371/journal.pone.0048418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, Di Gioia D. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Applied microbiology and biotechnology. 2014 doi: 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- 45.Bosco JL, Silliman RA, Thwin SS, Geiger AM, Buist DS, Prout MN, Yood MU, Haque R, Wei F, Lash TL. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giraldo PC, Araujo ED, Junior JE, do Amaral RL, Passos MR, Goncalves AK. The prevalence of urogenital infections in pregnant women experiencing preterm and full-term labor. Infectious diseases in obstetrics and gynecology. 2012;2012:878241. doi: 10.1155/2012/878241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunnington M, Kortsalioudaki C, Heath P. Genitourinary pathogens and preterm birth. Curr Opin Infect Dis. 2013;26(3):219–230. doi: 10.1097/QCO.0b013e328360dc31. [DOI] [PubMed] [Google Scholar]
- 48.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.