Abstract
Purpose
To assess the impact of a multimodal antiemetic protocol on postoperative nausea and vomiting (PONV) after LeFort I osteotomy.
Methods
Consecutive subjects undergoing LeFort I osteotomy with or without additional procedures at a single academic institution were recruited as the intervention cohort for an IRB-approved prospective clinical trial with a retrospective comparison group. The intervention cohort was managed with a multimodal antiemetic protocol including total intravenous anesthesia; prophylactic ondansetron, steroids, scopolamine, and droperidol; gastric decompression at surgery end; opioid-sparing analgesia; avoidance of morphine and codeine; prokinetic erythromycin; and minimum 25 mL/kg fluids. The comparison group consisted of consecutive subjects from a larger study who underwent similar surgical procedures prior to protocol implementation. Data including occurrence of PONV were extracted from medical records. Data were analyzed bivariately with Fisher’s Exact and Wilcoxon Rank Sum Tests. Logistic regression was used to compare the likelihood of nausea and vomiting in the two cohorts controlling for demographic and surgery characteristics. P<0.05 was considered significant.
Results
The intervention (n=93) and comparison (n=137) groups were similar in terms of gender (58% and 65% female, P=0.29), race (72% and 71% Caucasian, P=0.85), age (median 19 and 20 years old, P=0.75), proportion of subjects with known risk factors for PONV (P=0.34), percentage undergoing bimaxillary surgery (60% for both groups), and percentage for whom surgery time was over 180 minutes (63% versus 59%, P=0.51).
Prevalence of PON was significantly lower in the intervention group than the comparison group (24% versus 70%, P<0.0001). Prevalence of POV was likewise significantly lower in the intervention group (11% versus 28%, P=0.0013). The likelihood that subjects in the comparison group would experience nausea was 8.9 and vomiting 3.7 times higher than in the intervention group.
Conclusion
This multimodal protocol was associated with substantially decreased prevalence of PONV in subjects undergoing LeFort I osteotomy.
INTRODUCTION
Postoperative nausea and vomiting (PONV) complicate post-anesthesia and post-surgical care. This remains especially true for orthognathic surgery patients undergoing procedures involving the maxilla. Current estimates of the prevalence of PONV in the 24 hours after surgery for patients undergoing LeFort I osteotomy is between 44 and 68%, as compared to 8 to 30% in general surgical populations, despite application of conventional antiemetic therapies.1–7 Patients undergoing LeFort I osteotomy also seem to have a higher prevalence of PONV than those undergoing isolated mandibular osteotomy; swallowed blood, altered diet and deliberate hypotension may contribute.1,2,8
The implications of PONV for both individuals and the health care system are considerable. For individual patients, PONV can result in hematoma, wound dehiscence, intra-oral bleeding with continued swallowing of blood, anxiety and agitation (particularly for orthognathic surgery patients who often have maxillomandibular elastics in place after surgery), dehydration, electrolyte imbalances, and, in extreme cases, esophageal damage or aspiration.8,9 Evidence shows fear of PONV overshadows concerns about pain in surgical patients4,11–13 and that patients are willing to pay out of pocket to avoid PONV.13 PONV additionally leads to decreased patient satisfaction. In two large reviews of patient complaints after anesthesia and associated patient satisfaction, PONV was a leading complaint;15 patient dissatisfaction was significantly associated with the occurrence of PONV.15,16 PONV can also be very distressing to non-professional caregivers responsible for the patient.
At the health care system level, evidence suggests PONV can significantly increase health care costs through prolonged stay in the post-anesthesia care unit (PACU), delay of discharge, and unplanned hospital admissions or readmissions following outpatient procedures.5,6,10,14 In one seminal study the odds ratio for unplanned admission due to vomiting after outpatient surgery was an alarmingly high 3.4 (95% CI 1.8–6.4); patients were readmitted only slightly more frequently for pain or bleeding than for emesis.10
PONV prevention for orthognathic surgery patients remains an under-investigated domain of clinical care. As more orthognathic surgical procedures are performed on an ambulatory basis (often outside of the hospital setting), tactics to reduce the high prevalence of PONV in this surgical population become important to maximize patient safety and satisfaction, and, potentially, to contain costs. Patients undergoing LeFort I osteotomy are often underappreciated as a high-risk group and, though well-established protocols for prevention of PONV exist, these modalities are rarely maximized in this population.
The aim of this study was to compare the experience of PONV in subjects managed with a multimodal antiemetic protocol and those managed conventionally at the discretion of the anesthesia care team with the hypothesis that implementation of the protocol would reduce the prevalence of this postoperative complication. A secondary aim was to compare those managed with and without the protocol in terms of time to wake-up as well as length of PACU stay and length of hospital stay.
MATERIALS AND METHODS
Multimodal Protocol
A multimodal antiemetic protocol synthesizing several recommendations to reduce risk of PONV was developed primarily based on Society of Ambulatory Anesthesia (SAMBA) consensus guidelines (Box 1).17 Volatile anesthetics and nitrous oxide were avoided in favor of a total intravenous anesthetic (TIVA) based around propofol and remifentanil infusions titrated by the anesthesia care team.17–19 Neostigmine use was limited to 2.5 mg unless safety mandated otherwise.17,20 Hydration goals were set to a minimum of 25 mL/kg combined fluid in the operating room (OR) and PACU.17,21 Thirty mg of IV ketorolac were administered at the conclusion of the procedure and every 6 hours postoperatively as an opioid-sparing strategy. Once ketorolac was discontinued, ibuprofen 600 mg po every 6 hours was initiated.17 Morphine and codeine were avoided both intraoperatively and postoperatively.2,22–25 Fentanyl was preferred over hydromorphone as an IV agent due to its shorter duration of action.
Box 1. Protocol Summary.
-
Preoperative
Scopolamine patch
250 mg oral erythromycin
Midazolam 1–2 mg IV per anesthesia care team
Fluids: Crystalloid will be administered during the case – minimum 10 mL/kg.
-
Induction/maintenance/anti-emetics
No nitrous oxide. No volatile agents.
-
Propofol/remifentanil total intravenous anesthetic (TIVA)
Esmolol first line to achieve target SBP < 90 at key parts of procedure
-
Additional anti-hypertensive agents as needed
Avoid excessive opioids
Avoid volatile agents as above
Neostigmine: Avoid if possible (paralysis not surgically necessary); if used, limit to maximum 2.5 mg unless patient safety dictates otherwise
Steroids per surgeon preference. Typically 125 mg Solu-Medrol IV at beginning of case
0.625 mg IV droperidol near end of case
4 mg IV ondansetron near end of case
Surgeons to evacuate gastric contents with a nasogastric tube at end of case
-
Analgesia in the OR
Ketorolac 30 mg IV at end of case
No morphine. Fentanyl preferred, hydromorphone if necessary
-
PACU/Floor
-
Rescue medications for nausea/emesis
Promethazine 6.25–25 mg IV, po or PR
Dexamethasone 4mg IV (PACU only)
Diphenhydramine 25–50 mg IV or po
Ondansetron 4 mg IV or 4–8 mg ODT (if 6+ hours after first dose)
Fluids: Continue crystalloid in PACU until goal of at least 25 mL/kg reached
-
Analgesia
No morphine. Fentanyl preferred, hydromorphone if necessary
No codeine. Hydrocodone/acetaminophen preferred. Oxycodone if necessary
Ketorolac 30 mg IV q6h until conversion to po analgesics, then ibuprofen 600 mg q6h
Pro-kinetics: Repeat 250 mg oral erythromycin 8 hours after first dose in OR
Steroids: Redosed per surgeon preference
-
Combination therapy with prophylactic agents from multiple classes is recommended for patients at high risk for PONV.17 Agents known to reduce PONV include 5-HT3 receptor antagonists, droperidol, dexamethasone, and transdermal scopolamine.17,18,26,27 The protocol therefore included application of a transdermal scopolamine patch in the pre-operative holding area, intended to prolong duration of prevention of PONV. Droperidol (0.625 mg IV) and ondansetron (4 mg IV) were administered near the end of the case to time peak action with emergence from anesthesia. Administration of high-dose methylprednisolone was part of the surgical protocol, thus no additional steroids were given.
The constant swallowing of small amounts of blood and its retention in the stomach following LeFort I osteotomy likely contribute to the high prevalence of PONV in this population. Therefore, sub-bacteriostatic doses of erythromycin (250 mg) were administered orally pre-operatively and again after 8 hours to capitalize on erythromycin’s established properties as a motilin agonist. Erythromycin was chosen over metoclopramide due to its safety profile and its well-characterized impact on gastric motility and even PONV.28–31 Additional measures to minimize ingestion of blood that are common practice at this institution include placement of a throat pack and evacuation of gastric contents at the end of surgery. These practices were in place for the comparison cohort as well as the intervention cohort.
Rescue anti-nausea and anti-emetic therapy options that work through several mechanisms were selected based on the SAMBA guidelines and on data from a randomized, double-blind, placebo-controlled study of over 2000 subjects.17,32 Providers could administer 25–50 mg diphenhydramine po or IV; 4 mg IV or 4–8 mg ODT ondansetron; 6.25–25 mg of promethazine po, IV, or PR; or 4 mg dexamethasone IV.
Study Design
The authors designed and implemented a prospective, IRB-approved clinical trial with a retrospective comparison group to test the hypothesis that protocol implementation would reduce prevalence of PON and POV. The trial was registered with ClinicalTrials.gov (NCT01592708). Helsinki Declaration guidelines were followed.
Consecutive subjects at least 15 years old undergoing LeFort I osteotomy with or without additional procedures at a single academic institution between 7/2012 and 2/2013 were recruited as the intervention cohort receiving the above described protocol. Only patients undergoing a LeFort I osteotomy were recruited due to prior evidence that these subjects experience more PONV than those undergoing isolated mandibular osteotomy.1,2 Exclusion criteria included known or incidentally discovered prolonged QT interval (QTc>460), uncontrolled GERD or hiatal hernia, pre-existing hypertension per AHA guidelines, glaucoma, seizure disorder, COPD, chronic kidney disease stage III or greater, history of severe constipation, pre-existing chronic nausea or vomiting, allergies or contraindications to protocol medications or patient insistence on inhalational induction of anesthesia. A trained research associate described the project to each subject and obtained written consent with verbiage consistent with ClinicalTrials.gov requirements.
The comparison group consisted of consecutively enrolled ASA I or II subjects from a larger IRB-approved study who underwent LeFort I osteotomy with or without additional procedures at the same institution from 6/2008 to 6/2012, prior to implementation of the PONV protocol.2 Several exclusion criteria were added for the intervention cohort based on contraindications to protocol medications.
All orthognathic surgical procedures were performed by oral and maxillofacial surgery faculty and residents at The University of North Carolina. Rigid fixation was used to stabilize osteotomy sites. Intermaxillary elastic traction was used postoperatively.
Variables and Data Collection
The primary predictor variable in this study was treatment modality – protocol implementation in the intervention group versus anesthetic technique at providers’ discretion in the comparison group.
The primary outcome variables, experience of PON or POV, were extracted from medical records. A subject was considered to have experienced PON if there were nursing or provider notes about nausea or if rescue medications were administered for nausea. A subject was considered to have experienced POV if there were nursing or provider notes about emesis or if emesis was recorded in the subject’s intake and output record.
Secondary outcome variables were time to wake-up, length of PACU stay and length of hospital stay; all were collected from time stamps in the medical record. Time to wake-up was defined as the interval from surgery end to leaving the OR. Length of PACU stay was defined as time from arrival in the PACU till the patient was ready to leave the PACU to be transported to the floor, a time point noted in the record by PACU nursing staff when standard criteria were met. This interval was used in lieu of total time in the PACU as subjects may remain in the PACU once suitable for transport secondary to a lack of staff or bed availability rather than a patient-based need for prolonged PACU care. Length of hospital stay was defined as time from admission order to discharge order.
Additional patient-related, intraoperative, and postoperative variables were collected from medical records for both the intervention and comparison groups. Patient-related variables included demographic characteristics, as well as established patient-related risk factors for PONV including female gender, non-smoking status, and history of PONV or motion sickness.3,33 Other implicated factors were also assessed, such as migraine headaches, low ASA status, and younger age.1,3,34 Potential surgery-related intraoperative risk factors for PONV included duration of surgery (from incision to surgery end), subsets of surgery type (LeFort I alone or LeFort I and mandibular surgery), and additional procedures performed.6,17,34 Protocol violations in the intervention cohort and anesthesia medications in the retrospective cohort, as well as total IV fluids given in the OR and PACU in mL/kg were recorded. Postoperative variables included analgesics given in the PACU and on the floor and postoperative steroid dosing regimen.
All data were independently reviewed by two individuals; if differences were noted the record was jointly reviewed to arrive at a consensus and assure accuracy.
Data Analysis
Bivariate comparisons between the intervention and control groups were performed using Fisher’s Exact Test for nominal variables and Exact Wilcoxon Rank Sum Test for continuous variables. Logistic regression was used to compare the likelihood of nausea and vomiting in the two cohorts controlling for age, risk category, type of surgery, segmental maxillary osteotomy, genioplasty, third molar removal, additional procedures, and surgery time (SAS V9.1). Level of significance was set at 0.05.
RESULTS
Ninety-six consecutively enrolled subjects operated between 7/2012 and 1/2014 were recruited for the intervention cohort. Three intervention subjects for whom the multimodal protocol was grossly violated (ie a volatile agent was used for maintenance and no adjunctive medications were administered) were excluded. Data for the remaining 93 subjects were analyzed based on intention to treat.
The comparison cohort included 137 subjects operated between 6/2008 and 6/2012.
The intervention and comparison groups did not differ statistically and were similar overall in terms of gender, age, race, and proportion of subjects with known risk factors for PONV (Table 1).
Table 1.
Comparison of demographic and surgery-related traits between comparison and intervention cohorts
| Trait | Comparison Cohort n (%) |
Intervention Cohort n (%) |
P value |
|---|---|---|---|
| Female | 89 (65%) | 54 (58%) | 0.29 |
| Caucasian | 95 (71%)* | 67 (72%) | 0.85 |
| Bimaxillary surgery | 82 (60%) | 56 (60%) | 0.96 |
| Segmental LeFort | 46 (34%) | 29 (31%) | 0.70 |
| 3rd molars removed | 60 (44%) | 46 (49%) | 0.40 |
| Genioplasty | 52 (38%) | 33 (35%) | 0.70 |
| Surgery time > 180 minutes | 81 (59%) | 59 (63%) | 0.51 |
| Median (IQR) | Median (IQR) | ||
| Age | 20 (17–24) | 19 (17–23) | 0.75 |
| Risk factorsǂ | Comparison Cohort n (%) |
Intervention Cohort n (%) |
P value |
|---|---|---|---|
| 0 or 1 | 43 (31%) | 37 (40%) | 0.34 |
| 2 | 73 (53%) | 42 (45%) | |
| 3+ | 21 (15%) | 14 (15%) |
Risk factors include female gender, non-smoking status, history of migraines, history of PONV, and history of motion sickness.
Data missing for 3 subjects
Surgical factors
A similar percentage of intervention and comparison subjects underwent bimaxillary surgery, were treated with segmental LeFort I osteotomy, had third molars removed, and had genioplasty performed. Surgery time was greater than 180 minutes for a similar percentage of subjects in each cohort (Table 1).
Thirty three percent of subjects in each group underwent mandibular bone graft harvest. Additional procedures such as cranial bone graft harvest, anterior iliac crest bone graft harvest, condylectomy, and submental liposuction were performed in 31% of intervention subjects and in 28% of comparison subjects.
Anesthesia factors
The intervention protocol was followed precisely in 26 of the 93 subjects. Minor violations of the protocol, such as missing a dose of erythromycin or droperidol (most common), hydration to <25 mL/kg or very brief administration of nitrous oxide or a volatile agent were seen in the remaining subjects.
In the comparison group, all but 3 subjects (98%) received volatile anesthetic and 54% (n=74) received nitrous oxide. Most (97%, n=133) received ondansetron in the OR and 50% (n=68) received droperidol. Thirty six percent (n=49) received ketorolac. Eighty percent (n=109) received at least 25 mL/kg of intravenous fluids.
Postoperative factors
Sixty nine percent (n=94) of comparison subjects received morphine either during or after surgery. Sixty one percent (n=84) received acetaminophen with codeine.
Most subjects, 72% in each cohort, received more than one dose of postoperative methylprednisolone.
PACU and Hospital Length of Stay, Time to Wake Up
Median time to wake up was 12 minutes (IQR 9–20) for the intervention cohort and 12 minutes (IQR 9–18) for the comparison cohort.
Overall there was little difference between the intervention cohort and the comparison cohort in terms of PACU stay or total hospital stay: Median PACU stay was 87.5 minutes (IQR 72–107.5) for the intervention cohort and 87 minutes (IQR 64–112) for the comparison cohort (P=0.67). Median length of hospital stay was 26.4 hours (IQR 23.5–32.6) for the intervention cohort and 28.2 hours (IQR 23.5–41.4) for the comparison cohort (P=0.07). When evaluating LeFort I surgery alone versus bimaxillary surgery, though, median length of hospital stay for those who underwent bimaxillary surgery tended to be shorter in the intervention cohort – 27.3 hours (IQR 24–40.7) – than in the comparison cohort – 37.7 hours (IQR 25.5–42.6). This difference approached statistical significance (P=0.07).
Postoperative nausea and vomiting
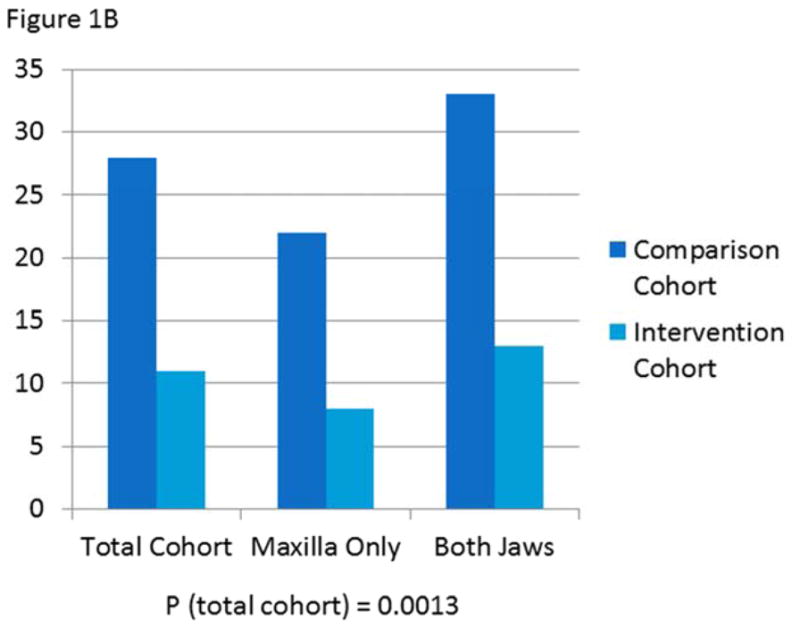
When evaluated by surgery type (LeFort I only or bimaxillary), those who underwent bimaxillary surgery experienced a non-statistically significant trend towards slightly more nausea and vomiting than those who had LeFort I osteotomies. For those who had LeFort I osteotomies, 22% of the intervention cohort and 64% of the comparison cohort experienced postoperative nausea (PON). Of those who had bimaxillary osteotomies, 25% of the intervention cohort and 74% of the comparison cohort experienced PON. For those who had LeFort I osteotomies, 8% of the intervention cohort and 22% of the comparison cohort experienced postoperative vomiting (POV). Of those who had bimaxillary osteotomies, 13% of the intervention cohort and 33% of the comparison cohort experienced POV. (Figure 1)
Figure 1.
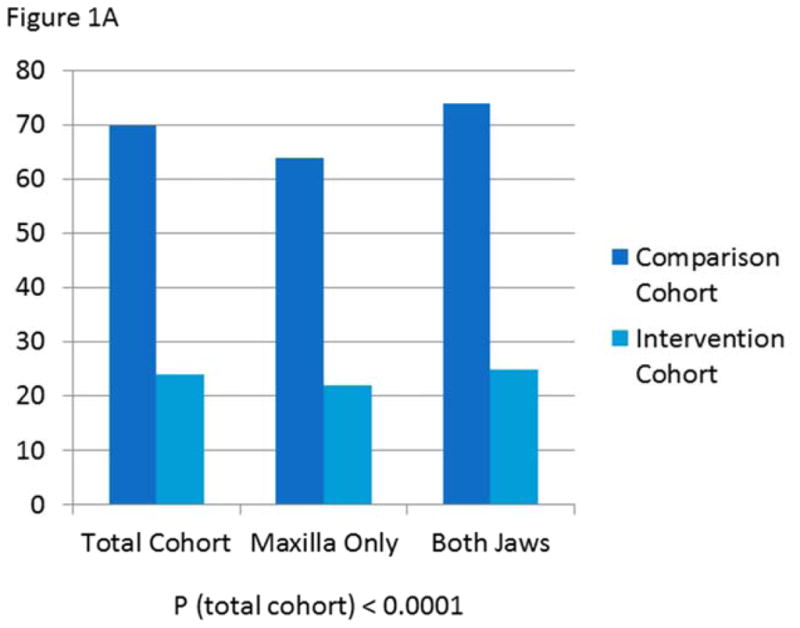
A) Prevalence of postoperative nausea in comparison versus intervention cohorts overall and by surgery type
B) Prevalence of postoperative vomiting in comparison versus intervention cohorts overall and by surgery type
Prevalence of postoperative nausea was significantly lower in the intervention group than the comparison group (24 versus 70%, P<0.0001). After controlling for demographic and surgical characteristics, patients in the comparison cohort were 8.9 times more likely to experience nausea than those in the intervention cohort (Table 2). Prevalence of postoperative vomiting was likewise significantly lower in the intervention group than the comparison group (11 versus 28%, P=0.0013). After controlling for demographic and surgical characteristics, patients in the comparison cohort were 3.7 times more likely to experience vomiting than those in the intervention cohort (Table 3).
Table 2.
Likelihood of the Occurrence of Nausea: Odds Ratios and 95% Confidence Intervals
| Trait | Odds Ratio | Conf Int Lower Bound |
Conf Int Upper Bound |
|---|---|---|---|
| Group | 8.9 | 4.6 | 16.9 |
| Age | 1.3 | 0.6 | 2.6 |
| Risk Category | 1.0 | 0.5 | 2.1 |
| Bimaxillary surgery | 1.1 | 0.5 | 2.6 |
| Segmental LeFort | 1.2 | 0.8 | 1.80 |
| 3rd molars removed | 1.9 | 1.0 | 3.9 |
| Genioplasty | 1.6 | 0.8 | 3.2 |
| Additional procedures | 1.2 | 0.6 | 2.4 |
| Surgery time | 0.3 | 0.1 | 0.8 |
Table 3.
Likelihood of the Occurrence of Vomiting: Odds Ratios and 95% Confidence Intervals
| Trait | Odds Ratio | Conf Int Lower Bound |
Conf Int Upper Bound |
|---|---|---|---|
| Group | 3.7 | 1.7 | 8.1 |
| Age | 0.9 | 0.4 | 2.0 |
| Risk Category | 1.3 | 0.6 | 3.0 |
| Bimaxillary surgery | 0.5 | 0.2 | 1.4 |
| Segmental LeFort | 0.4 | 0.2 | 1.00 |
| 3rd molars removed | 1.3 | 0.6 | 2.6 |
| Genioplasty | 2.1 | 0.9 | 4.6 |
| Additional procedures | 0.4 | 0.2 | 1.0 |
| Surgery time | 1.4 | 0.5 | 3.7 |
Time to First Recorded Nausea or Vomiting
Median time to first recorded nausea or vomiting for the intervention cohort was 5.4 hours (IQR 3.2–7.7). Median time to first recorded nausea or vomiting for the comparison cohort was 4.4 hours (IQR 2.7–6.3).
In the first hour after surgery, only 4% (4 of 93) of the intervention cohort and 6% (8 of 137) of the comparison cohort experienced PONV. Including those who experienced nausea in the first hour, during the first four hours, 9% (8 of 93) of the intervention cohort and 28% (39 of 137) of the comparison cohort experienced PONV. The remainder experienced PONV after 4 hours. The timing of PONV was not statistically significantly different between the groups (P=0.31).
DISCUSSION
This study’s primary objective was to compare experience of PONV in subjects undergoing LeFort I osteotomy managed with a multimodal antiemetic protocol as compared to subjects managed conventionally. The protocol synthesizes multiple modalities shown to be effective both in orthognathic surgery patients22,35 and in other surgical populations.17,21 As hypothesized, the protocol resulted in a clinically and statistically significant reduction in experience of both PON and POV after LeFort I osteotomy with or without additional procedures.
The study’s secondary aim was to assess time to wake-up, length of PACU stay, and length of hospital stay in the comparison versus intervention cohorts. There was no significant difference in time to wake-up or length of PACU stay between cohorts, nor was there a difference in time to discharge for those undergoing isolated LeFort I osteotomy. There was a trend towards decreased length of hospital stay in intervention subjects undergoing bimaxillary osteotomies; the impact of reduction in PONV on length of hospital stay merits continued attention.
Anesthesia factors and postoperative analgesic regimens were the primary targets of this multimodal protocol to prevent PONV. Most subjects in the comparison group did receive standard therapies directed at reduction of nausea and vomiting such as steroids and ondansetron with or without droperidol. Despite this the comparison subjects in this study fared significantly worse in terms of PONV than did intervention subjects, for whom multiple therapies were implemented to maximize control of PONV. These findings are consistent with reports that multimodal therapy is superior to single agent therapy in other high-risk surgical populations.17,21
The high prevalence of PONV in the comparison group of patients undergoing LeFort I osteotomy is consistent with that reported in the largest retrospective study examining PONV in 514 orthognathic surgery patients. Of the 126 subjects in that study who underwent maxillary osteotomy alone 44% experienced PONV. Of the 101 subjects who underwent bimaxillary surgery, 56% experienced PONV. The statistically significant risk factors for PONV cited as most important by the authors included younger age, a history of predisposing factors especially a prior history of PONV, use of a volatile agent, longer surgery duration, procedures involving the maxilla, and use of postoperative opioids.1
These findings highlight strengths of the current study: The intervention and comparison groups are similar to one another in terms of many acknowledged risk factors for PONV, including age; gender; predisposing factors for PONV such as non-smoking status, history of motion sickness, and prior history of PONV; and surgery duration. Increased prevalence of PONV in the current comparison group compared with those reported by Silva et al can be attributed to increased surgery time;6 one group reported a 60% increase in risk of PONV in ambulatory surgery patients with each 30 minute increase in surgery time up to 180 minutes.34 Most (61%) of the comparison subjects in this study had a surgery time of > 180 minutes while few (less than 10%) of those described by Silva et al had a surgery time exceeding 120 minutes. This is further supported by another group’s assertion that 89% of subjects experienced PONV when orthognathic surgery time was longer than 165 minutes.7
There have been conflicting reports of the impact of TIVA on PONV in the orthognathic surgery population.7,36,37 Farah et al examined two regimens to achieve deliberate hypotension in just 20 orthognathic surgery patients and concluded that, in contrast to prior studies, those subjects anesthetized without the use of a volatile agent experienced more PON than those managed with a volatile anesthetic. However, though they report a 40% prevalence of PON in those managed without a volatile agent, they also describe a more alarming 40% prevalence of POV in those who received volatile anesthetic.36 Tabrizi et al reported a trend towards more PONV while in the PACU in 30 subjects managed with a volatile agent (17%) than in 32 subjects managed with TIVA (3%).7 Chegini et al also examined the impact of volatile anesthetic with longer-acting opioids (n=30) versus a TIVA with remifentanil and propofol (n=21). There was a nonsignificant trend towards more PONV in the first four hours after surgery in the group receiving a TIVA (14%) than in the group receiving a volatile agent (10%).37
Though both of the latter groups report lower prevalence of PONV than presented in this study, this is likely attributable to the short duration of evaluation: Tabrizi et al only examined PONV while subjects were in the PACU (mean 66 and 72 minutes for each group). Their findings are congruent with those of the current study, in which only 4% of the intervention group and 6% of the comparison group experienced PONV in the first hour after surgery. Chegini et al limited their evaluation of PONV to the first four hours after surgery, during which time our rates of PONV were 9% for the intervention arm and 28% for the comparison arm. Perhaps differences in response to the regimens would have emerged with longer follow up duration.
A handful of studies have examined the impact of other individual variables on PONV in orthognathic surgery patients. Piper et al suggested that monotherapy with a 5-HT3 receptor antagonist can reduce PONV in orthognathic surgery patients.35 Another group demonstrated that subjects whose postoperative pain was managed primarily with codeine experienced statistically significantly more nausea after orthognathic surgery (60%) than did those managed by naproxen with codeine for breakthrough or with morphine sulfate (16%); there was no difference in POV (19%).22 Ichinohe et al reported no difference in PONV after bimaxillary surgery when adding nitrous oxide to a propofol-based TIVA; their report focused on ASA I, female, nonsmoking patients with no history of PONV or motion sickness. Subjects also received prophylactic dexamethasone, had no neostigmine, and had a nasogastric tube left in place overnight. The authors reported similarly low rates of PON (21%) and POV (7%) in this comparatively low risk group to those found in the current study.38
The multiple modalities employed in this study achieved clinically and statistically significant reduction in both PON and POV after LeFort I osteotomies. Though some investigation has been carried out to determine the impact of individual strategies employed in the multimodal regimen presented in this study, the use of a multimodal protocol impedes the ability to discern the impact of individual protocol components in this particular study. Many separate studies would be required to fully characterize the effect of each component. The SAMBA guidelines were updated in 2014 and no longer recommend minimization of neostigmine as a strategy to reduce risk of PONV due to conflicting evidence about its efficacy, suggesting this portion of the protocol may have limited benefit.39 Additional investigation will be especially important as newer agents, such as the neurokinin–1 (NK-1) receptor antagonists and longer acting 5HT-3 receptor antagonists as in palonosetron, become more widely available and affordable.40–43
Even though the comparison subjects were prospectively enrolled, the medical record assessment was retrospective. A randomized clinical trial was not warranted at the initiation of this study given the lack of evidence to support the effectiveness of the intervention protocol. If a randomized clinical trial is pursued in the future, the difficulty and expense of masking the medical staff who are responsible for reporting perioperative outcomes would need to be addressed. Another reason for the non-concurrent comparison group was to allow comparison to a group treated at the same institution with “as is” practice without the potential introduction of bias by alerting anesthesia providers to the extent of the PONV problem in patients undergoing LeFort I osteotomy. The under-recognition of this group as high risk likely contributes to the high rate of PONV these patients experience; education of the anesthesia community is critical. Limitations related to the use of a retrospective comparison cohort include slight differences in exclusion criteria that could be confounding factors. Evaluation of patient satisfaction with control of PONV was not feasible as this question was not part of the larger study containing the comparison subjects. However, prior studies suggest that patient satisfaction is strongly impacted by experience of PONV;15,16 this is an important issue in an elective surgical population.
CONCLUSION
This multimodal protocol significantly reduced both PON and POV after LeFort I osteotomy with or without additional procedures. The investigation alerted the authors to the need to communicate about the high prevalence of PONV after LeFort I osteotomies with anesthesia providers. Additional investigation into the role of individual protocol components in reduction of PONV, impact of improved control of PONV on duration of hospital stay, experience of nausea and vomiting following discharge from the hospital, and impact of PONV on patient satisfaction are warranted.
Acknowledgments
Supported in part by NIH/NIDCR R01 DE 005215.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silva AC, O’Ryan F, Poor DB. Postoperative nausea and vomiting (PONV) after orthognathic surgery: A retrospective study and literature review. J Oral Maxillofac Surg. 2006;64:1385. doi: 10.1016/j.joms.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Dicus CDB. Evaluation of post-operative and post-discharge nausea and vomiting in orthognathic surgery patients. Oral Abstract; 2011; American Association of Oral and Maxillofacial Surgeons 93rd Annual Meeting. [Google Scholar]
- 3.Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. 1994;78:7. doi: 10.1213/00000539-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Chung F, Un V, Su J. Postoperative symptoms 24h after ambulatory anesthesia. Can J Anaesth. 1996;43:1121. doi: 10.1007/BF03011838. [DOI] [PubMed] [Google Scholar]
- 5.Phillip BK. Patients’ assessment of ambulatory anesthesia and surgery. J Clin Anesth. 1992;4:355. doi: 10.1016/0952-8180(92)90155-t. [DOI] [PubMed] [Google Scholar]
- 6.Watcha MF, White PF. Postoperative nausea and vomiting: Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Tabrizi R, Eftekharian HR, Langner NJ, Ozkan BT. Comparison of the effect of 2 hypotensive anesthetic techniques on early recovery complications after orthognathic surgery. J Craniofac Surg. 2012;23:e203. doi: 10.1097/SCS.0b013e31824de3d3. [DOI] [PubMed] [Google Scholar]
- 8.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Scuderi PE, Conlay LA. Postoperative nausea and vomiting and outcome. Int Anesthesiol Clin. 2003;41:165. doi: 10.1097/00004311-200341040-00012. [DOI] [PubMed] [Google Scholar]
- 10.Gold BS, Kitz DS, Lecky JH, et al. Unanticipated admission to the hospital following ambulatory surgery. JAMA. 1989;262:3008. [PubMed] [Google Scholar]
- 11.Koivuranta M, Laara E, Snare L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- 12.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Gan TJ, Sloan F, Dear GdL, et al. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth Analg. 2001;92:393. doi: 10.1097/00000539-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Carroll NV, Miederhoff P, Cox FM, Hirsch JD. Postoperative nausea and vomiting after discharge from outpatient surgery centers. Anesth Analg. 1995;80:903. doi: 10.1097/00000539-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann M, Monte K, Barach P, Kindler CH. Postoperative patient complaints: a prospective interview study of 12,276 patients. J Clin Anesth. 2010;22:13. doi: 10.1016/j.jclinane.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 17.Gan TJ, Meyer TA, Apfel CC. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 18.Apfel CC, Korttila K, Abdalla M, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. NEJM. 2004;350:2441. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tramer M, Moore A, McQuay H. Propofol anaesthesia and postoperative nausea and vomiting: quantitative systematic review of randomized controlled studies. Br J Anesth. 1997;78:247. doi: 10.1093/bja/78.3.247. [DOI] [PubMed] [Google Scholar]
- 20.Tramer MR, Fuchs-Buder T. Omitting antagonism of neuromuscular blockade: effect on postoperative nausea and vomiting and risk of residual paralysis. A systematic review. Br J Anaesth. 1999;82:379. doi: 10.1093/bja/82.3.379. [DOI] [PubMed] [Google Scholar]
- 21.Scuderi PE, James RL, Harris L, et al. Multimodal antiemetic management prevents early postoperative vomiting after outpatient laparoscopy. Anesth Analg. 2000;91:1408. doi: 10.1097/00000539-200012000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Precious DS, Multari J, Finley GA, McGrath P. A comparison of patient-controlled and fixed schedule analgesia after orthognathic surgery. J Oral Maxillofac Surg. 1997;55:33. doi: 10.1016/s0278-2391(97)90442-0. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez RF, Castillo JM, Castillo MP, et al. Codeine/acetaminophen and hydrocodone/acetaminophen combination tablets for the management of chronic cancer pain in adults: A 23-day, prospective, double-blind, randomized, parallel-group study. Clin Ther. 2007;29:581. doi: 10.1016/j.clinthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee K, Esuvaranathan V, Streets C, Johnson A, Carr AS. Adenotonsillectomy in children: a comparison of morphine and fentanyl for peri-operative analgesia. Anaesthesia. 2001;56:1193. doi: 10.1046/j.1365-2044.2001.02084-4.x. [DOI] [PubMed] [Google Scholar]
- 25.Claxton AR, McGuire G, Chung F, Cruise C. Evaluation of morphine versus fentanyl for postoperative analgesia after ambulatory surgical procedures. Anesth Analg. 1997;84:509. doi: 10.1097/00000539-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Fortney JT, Gan TJ, Graczyk S. A comparison of the efficacy, safety and patient satisfaction of ondansetron versus droperidol as antiemetics for elective outpatient surgical procedures. Anesth Analg. 1998;86:731. doi: 10.1097/00000539-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Kranke P, Morin AM, Roewer N, Wulf H, Eberhart LH. The efficacy and safety of transdermal scopolamine for the prevention of postoperative nausea and vomiting: a quanititative systematic review. Anesth Analg. 2002;95:133. doi: 10.1097/00000539-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Kopp VJ, Mayer DC, Shaheen NJ. Intravenous erythromycin promotes gastric emptying prior to emergency anesthesia. Anesthesiology. 1997;87:703. doi: 10.1097/00000542-199709000-00037. [DOI] [PubMed] [Google Scholar]
- 29.Itoh Z, Nakaya M, Suzuki T, et al. Erythromycin mimics exogenous motilin in gastrointestinal contractile activity in the dog. Am J Physiol. 1984;247:G688. doi: 10.1152/ajpgi.1984.247.6.G688. [DOI] [PubMed] [Google Scholar]
- 30.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Int Med. 2010;152:101. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 31.Barkun AN, Bardou M, Martel M, et al. Prokinetics in acute upper GI bleeding: a meta-analysis. Gastrointest Endosc. 2010;72:1138. doi: 10.1016/j.gie.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Habib AS, Gan TJ. The effectiveness of rescue antiemetics after failure of prophylaxis with ondansetron or droperidol: a preliminary report. J Clin Anesth. 2005;17:62. doi: 10.1016/j.jclinane.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting. Anesthesiology. 1999;91:693. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91:109. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Piper SN, Rohm K, Boldt J, Kranke P, Maleck W, Seifert R, Suttner S. Postoperative nausea and vomiting after surgery for prognathism: not only a question of patients’ comfort. A placebo-controlled comparison of dolasetron and droperidol. J Craniomaxillofac Surg. 2008;36:173. doi: 10.1016/j.jcms.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Farah GJ, de Moraes M, Filho LI, et al. Induced hypotension in orthognathic surgery: a comparative study of 2 pharmacological protocols. J Oral Maxillofac Surg. 2008;66:2261. doi: 10.1016/j.joms.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 37.Chegini S, Johnston KD, Kalantzis A, Dhariwal DK. The effect of anesthetic technique on recovery after orthognathic surgery: A retrospective audit. Anesth Prog. 2012;59:69. doi: 10.2344/11-10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichinohe T, Kaneko Y. Nitrous oxide does not aggravate postoperative emesis after orthognathic surgery in female and nonsmoking patients. J Oral Maxillofac Surg. 2007;65:936. doi: 10.1016/j.joms.2006.06.283. [DOI] [PubMed] [Google Scholar]
- 39.Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 40.Kloth DD. New pharmacologic findings for the treatment of PONV and PDNV. Am J Health-Syst Pharm. 2009;66:S11. doi: 10.2146/ashp080462. [DOI] [PubMed] [Google Scholar]
- 41.Muchatuta N, Paech M. Management of postoperative nausea and vomiting: focus on palonsetron. Therapeutics Clin Risk Manag. 2009;5:21. [PMC free article] [PubMed] [Google Scholar]
- 42.George E, Hornuss C, Apfel CC. Neurokinin-1 and novel serotonin antagonists for postoperative and postdischarge nausea and vomiting. Curr Op Anesth. 2010;23:714. doi: 10.1097/ACO.0b013e32833f9f7b. [DOI] [PubMed] [Google Scholar]
- 43.Kovac AL, Eberhart L, Kotarski J, et al. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107:439. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]


