Abstract
The majority of CXCR5+PD-1+CD4+ T follicular helper (Tfh) cells (>90%) are CD25−Bcl6hi, while a small subpopulation (<10%) are CD25+Bcl6low but do not express FoxP3 and are not T regulatory cells. We purified T:B-cell conjugates from tonsil and found they were enriched for the CD25+Bcl6low Tfh-cell subpopulation. In response to IL-2, these CD25+Tfh cells increased expression of costimulatory molecules ICOS or OX40, upregulated transcription factor c-Maf, produced cytokines IL-21, IL-17 and IL-10, and raised the levels of anti-apoptotic protein Bcl2. Conjugates formed with CD25+BCl6low Tfh cells included B cells expressing higher levels of activation-induced cytosine deaminase (AID), memory marker CD45RO, surface IgG or IgA, and MHC Class II compared to B-cell conjugates including CD25− Bcl6hi Tfh cells. While IL-2 suppresses early Tfh-cell differentiation, Tfh-cell recognition of antigen-presenting B cells and signaling through the T-cell receptor, likely triggers expression of the high affinity IL-2 receptor and responses to IL-2 including downregulation of Bcl6. CD25 expression on Tfh cells and local production of IL-2 in tonsil or lymph node, may support B helper T-cell function during later stages of B-cell maturation and the development of immune memory.
Keywords: T follicular helper cell, IL-2, CD25, B cells, germinal centers
Introduction
Germinal center (GC) development requires T-cell receptor recognition of peptide antigens on MHC Class II+ antigen-presenting B cells. This mechanism shapes the B-cell repertoire within individual GC and promotes affinity maturation of antibody through induction of activation-induced cytosine deaminase (AID), isotype switching and positive selection of B cells expressing high affinity antibodies capable of competing for antigen [1]. A specialized CD4+T follicular helper (Tfh) cell in lymphoid tissue GC [2–4] provides potent B helper T-cell function.
An early step in the differentiation of Tfh is upregulation of CXCR5 expression prior to Bcl6 appearance, under control of the achaetescute (Ascl2) transcription factor [5]. Bcl6 expression is regulated byIL-6/IL-21 cytokines; when it accumulates in excess of the available T-bet [6–8], Blimp-1 is inhibited and transcription of several important genesis derepressed [6–8]. The presence of cell surface CXCR5 guides Tfh cells along a gradient of CXCL13 chemokine into lymphoid follicles and nascent GC. There, Tfh cells recognize MHC Class II-restricted antigens presented by activated B cells and engage in multiple costimulatory receptor: ligand interactions or cytokine signaling events [9, 10]. Differentiated Tfh cells are distinguished from other CD4+T-cell subsets by loss of CCR7, expression of surface molecules CXCR5, ICOS, PD1, CD40L, OX40, secretion of IL-21 and IL-4 cytokines [2, 4, 6, 7, 10–14]. Tfh cells downregulate Bcl-6, CXCR5, and PD-1 in the memory phase [15]. A direct interaction of antigen-specific Tfh cells with cognate B cells promotes GC development, isotype switching, B-cell affinity maturation and memory B-cell formation [6, 7, 16–19].
In GC, cellular conjugates are formed when antigen-specific Tfh cells bind B cells capable of capturing antigen through surface immunoglobulins [1, 19]. During antibody affinity maturation, B cells compete for antigen that is processed and presented by MHC Class II to stimulate Tfh cells and maintain B-cell help. At the same time, Tfh cells are selected for T-cell receptor affinity above a threshold for antigen recognition [1]. Potent Tfh-cell function is associated with higher specific antibody-secreting cells, isotype-switched B cells and somatic mutations [19]. Imaging studies focusing on lymph node germinal centers showed an abundance of CXCR5+PD1+CD4 T cells [4] and even documented the capacity for some Tfh cells to emigrate from one germinal center to another within the same lymph node [20].
Several groups reported that high affinity IL-2 receptor alphachain (CD25) is not expressed on Tfh cells [21, 22] despite the fact that functional Tfh cells use TCR to recognize MHC Class II+ antigen-presenting B cells and the TCR signal often triggers CD25 expression with formation of the high affinity IL-2 receptor [23]. It was reported that IL-2 is a negative regulator of Tfh-cell differentiation [21] and antagonist of GC formation [22] because the IL-2-CD25-STAT5 axis inhibits Bcl6 expression by activating Blimp-1, negatively regulating Tfh-cell differentiation and suppressing primary antibody responses in mice [8, 21, 22, 24]. Chimerized mice showed preferential accumulation of CD25−/− T cells in germinal centers compared to wild-type cells [21], and high dose IL-2 at the time of primary immunization reduced antibody responses [22]. These studies depictIL-2 as an inhibitor of Tfh cells and antagonist of GC function despite older reports that B-cell proliferation and some IgG responses were positively regulated by IL-2 [25–27]. We wondered whether a small subset of IL-2-responsive Tfh cells might have specific roles in B-cell help and antibody responses. Using human tonsil tissues, we found a population of germinal center CD25+Bcl6low CD4+ Tfh cells present mainly in T:B-cell conjugates, that responded to IL-2 in vitro. Here, we report on the properties and functions of these CD25+Tfh cells and the B cells found conjugated to them in human tonsils.
Results
A subset of human tonsil Tfh cells express IL-2 receptor α (CD25)
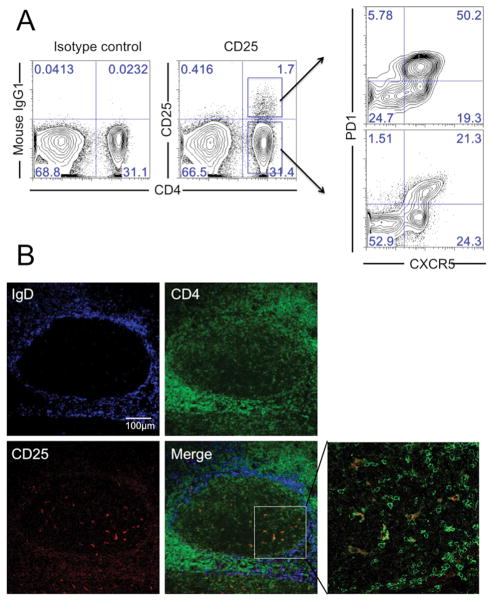
Antibody staining and flow cytometry of mononuclear cells from human tonsil revealed a small subset (approximately 5–10% of total CD4 T cells) that was positive for CD25 (Fig. 1A). Both CD25+and CD25− CD4 cells expressed the Tfh cells markers CXCR5 andPD1 with the CD25+ subset trending to higher expression of PD1. Most of our donors had higher frequencies of CD25+ cells in the PD-1 high subset of tonsil CD4 T cells. Some donors may also had higher frequency of CD25+ cells in the PD-1 low subset [28] but this was unusual and might due to donor variation. Imaging of human tonsil sections (Fig. 1B) localized CD25+CD4+ cells to germinal centers and, consistent with flow cytometry results, showed they were a small subset of total CD4 T cells. Among human tonsil cells, PD1 was mainly expressed on CD4 T cells (Supporting Information Fig. 1A) and PD1+CD4 T cells were almost exclusively localized to the GC (Supporting Information Fig. 1B).
Figure 1. A subset of human tonsil Tfh cells express IL-2 receptor (CD25).
(A) CD25+CXCR5+PD1+CD4 T cells were identified by flow cytometry in tonsil mononuclear cell preparations. Data are representative of seven experiments with similar results, each using a different donor. (B) Tonsil sections were stained with anti-IgD (blue), anti-CD4 (green) and anti-CD25 (red) antibodies, and analyzed by confocal immunofluorescence microscopy. The germinal center was within the ring of cells expressing IgD (blue) and contained CD25+CD4+T cells (Merge). Images are representative of two independent experiments.
CD25+ PD1+ CXCR5+CD4 T cells are not Treg
Because CD25 (together with FoxP3) is a marker for Treg cells and a subpopulation of follicular Treg cells (Tfr) also expresses Tfh cells markers [29, 30], we looked for FoxP3+Tfr cells in the population ofCD25+PD1+CD4 T cells. Among all tonsil CD4 T cells, FoxP3 was expressed mainly byCD25+PD1− cells (Fig. 2A). On average, about 70% of CD25+PD1− CD4 cells but only 10% ofCD25+PD1+CD4 cells expressed FoxP3 (Fig. 2B).
Figure 2. CD25+PD1+CXCR5+CD4+ T cells are not Treg cells.
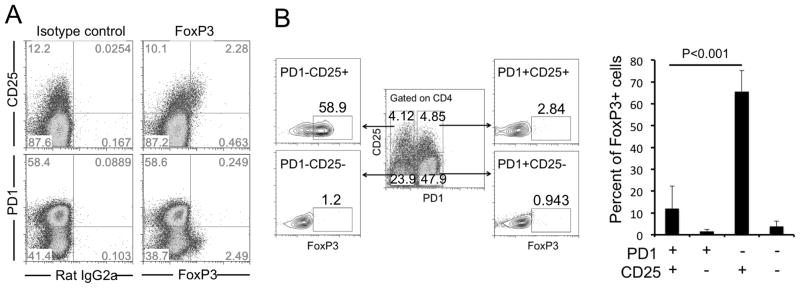
(A) Tonsil CD4+ T cells were purified, stained and analyzed by flow cytometry. (B) Tonsil cells were gated on CD4 expression to determine the percentage of FoxP3+ cells in PD1−CD25+ (58.9%), PD1−CD25− (1.2%), PD1+CD25+ (2.8%) or PD1+CD25− (0.9%). Data are summarized in a bar graph showing mean (+SEM) percentages of FoxP3+cells (n=7 donors) and are representative of 3 independent experiments. P < 0.05 was considered to be significant; Student’s t test.
CD25+CD4+GC T cells from tonsil have a Tfh cell phenotype despite only low levels of Bcl6
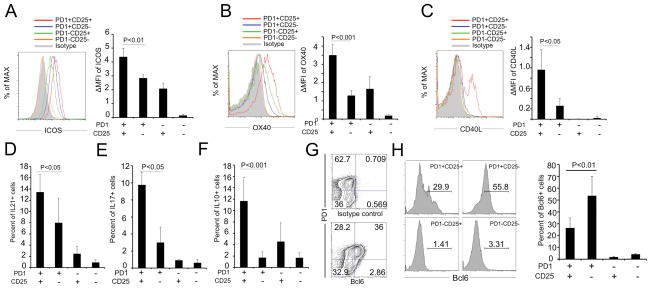
If the CD25+ population is a subset of Tfh cells, they should express costimulatory ligands and receptors plus cytokines required for cognate interactions with B cells. We tested several cell markers expected for Tfh cells including surface ICOS, OX40, CD40L (Fig. 3A–C) and intracellular IL-21 (Fig. 3D). These receptor/ligands and cytokines are critical for the reciprocal interactions of Tfh and B cells in GC. Surprisingly, CD25+PD1+Tfh cells expressed significantly higher levels of these molecules than did CD25−PD1+Tfh cells, and also had higher levels of IL-17 (Fig. 3E) and IL-10 (Fig. 3F) that can be important for B-cell help [31–36]. We next showed that Bcl6 was expressed only inPD1+Tfh cells (Fig. 3G) but was significantly lower among CD25+PD1+ Tfh cells compared to the CD25−PD1+ subset (Fig. 3H). The CD25+ Tfh cells comprise a cell subpopulation with many characteristics of Tfh cells but also express the high affinity IL-2 receptor and have significantly lower levels of Bcl6.
Figure 3. CD25+CD4+GC T cells from tonsil have a Tfh-cell phenotypewith low levels of Bcl6.
Fresh tonsil cells were stained and analyzed by flow cytometry using gating strategies shown in Figure 2B. Expression levels for (A) ICOS, (B) OX40 or (C) CD40L subsets were analyzed for specific cell subsets. Increases in the mean fluorescence intensity (ΔMFI) were calculated as: (MFI (specific mAb) − MFI (isotype control))/MFI (isotype control). The frequencies of (D) IL-21, (E) IL-17 or (F) IL-10positive cells in different subsets were analyzed. (G and H) Bcl6 expression on different subsets was detected by flow cytometry and the percentage of Bcl6+ cells was compared among CD4+ T-cell subsets defined by PD1 and CD25 expression.(A–H) Data are shown as mean+SEM (n=7 donors) and are representative of 3 independent experiments. P < 0.05 was considered to be significant (Student’s t test).
CD25+Tfh cells respond to IL-2 and provide enhanced B-cell help
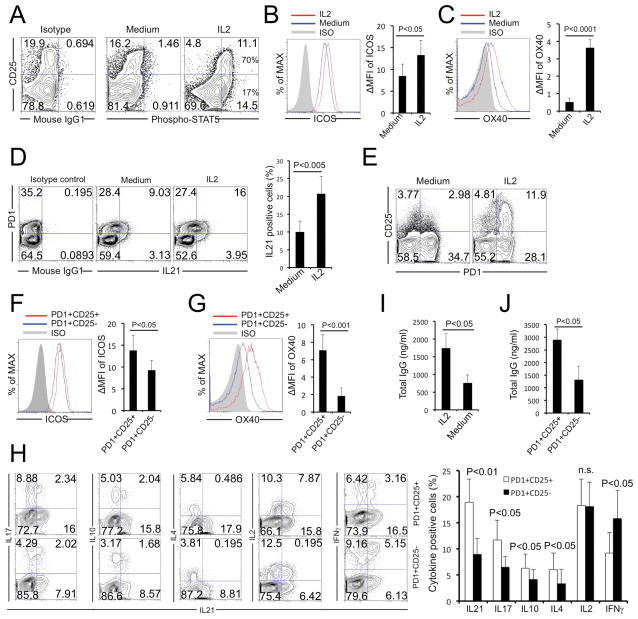
CD25+PD1+Bcl6low Tfh cells from tonsil expressed the high-affinity IL-2 receptor (Kd~10−11M) [23, 37], composed of IL-2Rα, IL-2Rβ and IL-2Rγ chains. Consistent with appearance of this cytokine receptor, CD25+Tfh cells phosphorylated STAT5 after exposure to low doses ofIL-2 (Fig. 4A). We wondered whether the enhanced Tfh cell phenotype was due to the IL-2-induced signaling. Tonsil CD4 T cells were purified by negative selection and treated with IL-2 for 24 h. IL-2 significantly increased expression levels for ICOS (Fig. 4B) or OX40 (Fig. 4C), and these cells produced greater amounts ofIL-21 after PMA plus Ionomycin stimulation (Fig. 4D). Interestingly, IL-2 preferentially induced CD25 expression onPD1+Tfh cells compared toPD1- non-Tfh cells (Fig. 4E). IL-2-treatment of CD25+PD1+Tfh cells also increased surface expression of ICOS (Fig. 4F) or OX40 (Fig. 4G). Further, CD25+Tfh cells produced more IL-21, IL-17, IL-10 and IL-4 (B-cell helper cytokines), similar amounts of IL-2 and lower amounts of IFN-γ compared to CD25− Tfh cells (Fig. 4H). We also tested whether Tfh cells provide help for B cells. IL-2 treatment significantly increased B helper T cell-dependent IgG secretion (Fig. 4I) and CD25+PD1+Bcl6low Tfh cells induced significantly higher IgG production by B cells than did CD25−PD1+Tfh cells (Fig. 4J). The CD25+ Tfh cells were highly responsive to IL-2 and cytokine treatment increased their ability to provide B-cell help.
Figure 4. CD25+ Tfh cells respond to IL-2 and provide B-cell help.
(A) Purified tonsil CD4+ T cells were treated with IL-2 (10U/mL) for 5 min and phosphorylated STAT5 levels were detected by flow cytometry. (B–H) Tonsil CD4+T cells were purified and treated with or without IL-2 (100U/mL) for 24 h. ICOS (B) andOX40 (C) expression and IL-21 (D) production among the CXCR5+PD1+cells were examined. For cytokine detection, cells were stimulated with PMA plus Ionomycin for 4 h, then stained with different antibody combinations against CXCR5, PD1 and CD25. (E) Purified tonsil cells were treated with IL-2 treatment for 24h and the percentage of CD25+PD1+CXCR5+ Tfh cells determined by flow cytometry.(F, G) In IL-2 treated tonsil CD4+ T cells, the PD1+CD25+ Tfh cells had higher expression of both ICOS and OX40 compared to the CD25− subset. MFI was calculated as: [MFI (specific mAb) – MFI (isotype control)]/MFI (isotype control). (H) Similarly, IL-2-treated Tfh cells were analyzed for IL-21, IL-17, IL-10, IL-4, IL-2 and IFN-γ production by intracellular staining and gating on PD1+CD25+ orPD1+CD25− cells.(I) B cells cocultured with Tfh cells in the presence or absence of IL-2 (100U/mL) were analyzed for levels of IgG by ELISA. (J) B cells cocultured with CD25+or CD25− Tfh cells were analyzed for levels of IgG by ELISA. Flow cytometry plots are representative of 4 experiments. (B–D, F–H) Data are expressed as mean +SEM (n=7 samples). P < 0.05 was considered to be significant; Student’s t test.
CD25+Tfh cells express transcription factor cMaf to promote cytokine responses
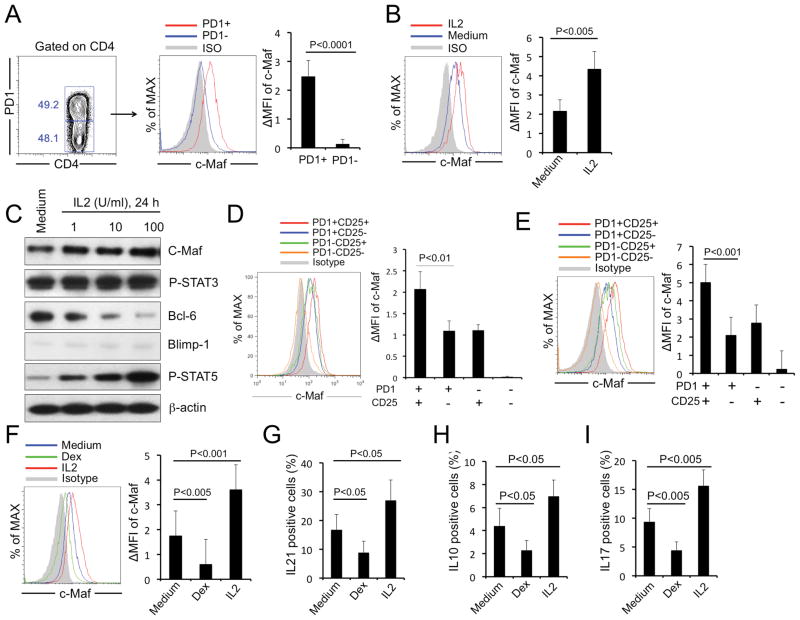
We know that IL-2 upregulates cMaf expression [38] and cMaf is important forIL-21 [14, 39], IL-10 [40, 41] or IL-17 [42] gene expression. Among all tonsil CD4 T cells, cMaf was mostly detected in PD1+Tfh cells (Fig. 5A) and expression levels were increased after IL-2 treatment (Fig. 5B and C). CD25+Tfh cells expressed higher levels of cMaf than did CD25− Tfh cells using either fresh (Fig. 5D) orIL-2-treated tonsil CD4 T cells (Fig. 5E). Dexamethasone (Dex) is an inhibitor of cMaf expression [43]. Dex treatment reduced cMaf levels (Fig. 5F) and inhibited IL-21 (Fig. 5G), IL-10 (Fig. 5H) or IL-17 (Fig. 5I) production by tonsil Tfh cells. STAT3 might also be involved in IL-21 regulation [7]. However, STAT3 is constitutively activated in tonsil Tfh cells and IL-2 had no effect on STAT3. As expected, IL-2 increased STAT5 phosphorylation and decreased Bcl6 levels (Fig. 5C). Our results showed that IL-2-dependent upregulation of cMaf is a mechanism for increasingIL-21, IL-10 orIL-17 production inCD25+Tfh cells, and that IL-2/STAT5-mediated modulation of Bcl6 was observed as expected.
Figure 5. CD25+ Tfh cells express high levels of transcription factor cMaf to promote cytokine responses and low levels of Blimp-1 to maintain a Tfh-cell phenotype in the absence of Bcl6.
(A) cMaf expression in PD1+ (Tfh) andPD1- (non-Tfh) tonsil CD4+T cells was determined by flow cytometry. (B) Purified tonsil Tfh CD4 T cells (CXCR5+PD1+) were treated with or without IL-2 (100U/mL) for 24 h, then cMaf expression was determined by flow cytometry. (C) Purified tonsil Tfh CD4+ T cells (CXCR5+PD1+) were treated without or with IL-2 at different concentrations for 24 h and protein expression was analyzed by western blot for cMaf, P-STAT3, Bcl6, Blimp-1, P-STAT5. Data are representative of 3 independent experiments. β-actin was used as a loading control. (D, E) cMaf expression was measured on T-cell subsets using (D) fresh or (E) IL-2-treated tonsil CD4+T cells. (F) cMaf expression was determined in tonsil Tfh cells treated with medium, IL-2 or Dexamethasone (Dex) for 24 h. (G–I) Purified tonsil Tfh cells were treated with medium, IL-2 or Dexamethasone (Dex) for 24 h and stimulated with PMA plus Ionomycin for 4 h;(G) IL-21, (H) IL-10 or (I) IL-17 production was detected by intracellular staining. (A, B, D–F) MFI was calculated as: (MFI (specific mAb) – MFI (isotype control))/MFI (isotype control).(A, B, D–I) Data are expressed as mean+SEM (n=7) and are representative of 3 independent experiments. P < 0.05 was considered to be significant; Student’s t test.
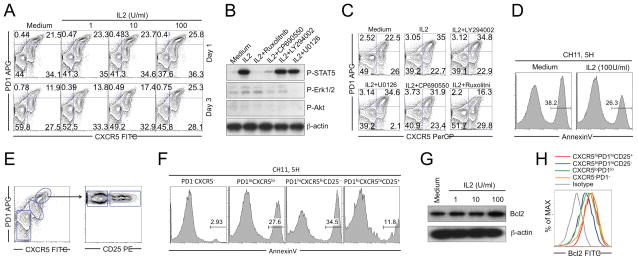
IL-2 increases Tfh-cell resistance to Fas-mediated apoptosis
As a terminally differentiated effector subset, Tfh cells express high levels of Fas and are prone to apoptotic death [44, 45]. Purified CD4 T cells in culture showed a gradual loss of GC Tfh cells due to apoptotic cell death [44]. Treating withIL-2 reduced the loss of GC Tfh cells in a dose dependent manner (Fig. 6A). Without IL-2, GC Tfh cells declined from ~25% of CXCR5+PD1+ cells at day 0, to ~11% by day 3 in this typical example (Fig. 6A). At 100 U/mL, IL-2 prevented the loss of GC Tfh cells (Fig. 6A). In mouse studies, GC Tfh cells eventually modulated their Bcl6 levels, ceased to proliferate and expressed theIL-7 receptor [46]. In human Tfh, IL-2 may be important for preserving the Tfh-cell population during late steps in the pathway for B-cell help.
Figure 6. An important function of IL-2 is to increase Tfh-cell resistance against Fas-mediated apoptosis.
(A) Purified tonsil CD4+T cells were cultured with or withoutIL-2 at different concentrations for 3 days. Frequencies of GC Tfh cells were detected. (B) Purified tonsil Tfh cells were pretreated without or with specific inhibitors and stimulated with IL-2 for 10 min. Protein levels of P-STAT5, P-Erk1/2, P-Akt were analyzed by western blot. (C) Purified tonsil CD4+T cells were cultured without or with IL-2 and different inhibitors for 3 days. Frequencies of GC Tfh cells were detected. (D) Purified tonsil Tfh cells cultured without or with IL-2 for 24 h and treated with Fas active antibody CH11 for 5 h; (E) Tonsil CD4+T cells were purified and treated with Fas agonist antibody CH11 for 5 h. (F) Cell apoptosis was analyzed by Annexin V staining. (G) Purified tonsil CD4 T cells were treated without or with IL-2 at different concentrations for 24 h. Bcl2 expression was detected by western blot. (H) Bcl2 expression by different subsets in tonsil CD4+T cells was detected by flow cytometry. (A–H) Data are representative of 3 independent experiments, each with different donors (n=5). (B, G) βactin was used as a loading control.
We next identified signaling pathways responsible for the IL-2 effect. Depending on the cell type, IL-2 can activate STAT5, PI3K/Aktor MEK/Erk [23, 47]. In tonsil Tfh cells, IL-2 activated STAT5 with little effect on Erk activation (Fig. 6B) and no significant activation of Akt (Fig. 6B). Further, Akt inhibitor LY294002 or Erk inhibitor U0126 did not block the effects ofIL-2 on STAT5 phosphorylation and Tfh-cell survival (Fig. 6B and C). A JAK1 inhibitor Ruxolitinib, completely inhibited STAT5 activation and blocked IL-2-induced maintenance of GC Tfh cells (Fig. 6B and C). The JAK3 inhibitor CP690550 partially blocked STAT5 activation but did not prevent theIL-2 effect (Fig. 6B and C), indicating that lower levels of STAT5 activation might be sufficient for GC Tfh-cell maintenance. These results demonstrated that IL-2-induced STAT5 activation is important for GC Tfh-cell survival.
IL-2 treatment also decreased Tfh-cell susceptibility to Fas-mediated apoptosis (Fig. 6D) and the CD25+GC Tfh cells were especially resistant to Fas-mediated apoptosis (Fig. 6E and F). Consistent with a previous study [48], IL-2 increased expression of the antiapoptotic protein Bcl2 in Tfh cells (Fig. 6G) and especially in CD25+ GC Tfh cells (Fig. 6H). We did not see significant changes in other antiapoptotic proteins including Mcl1 and BclXL. TheIL-2-induced Bcl2 expression depended on STAT5 activation and may be part of the mechanism for GC Tfh cells to resist F as-mediated killing.
CD25+Tfh cells are incorporated preferentially into T:B-cell conjugates
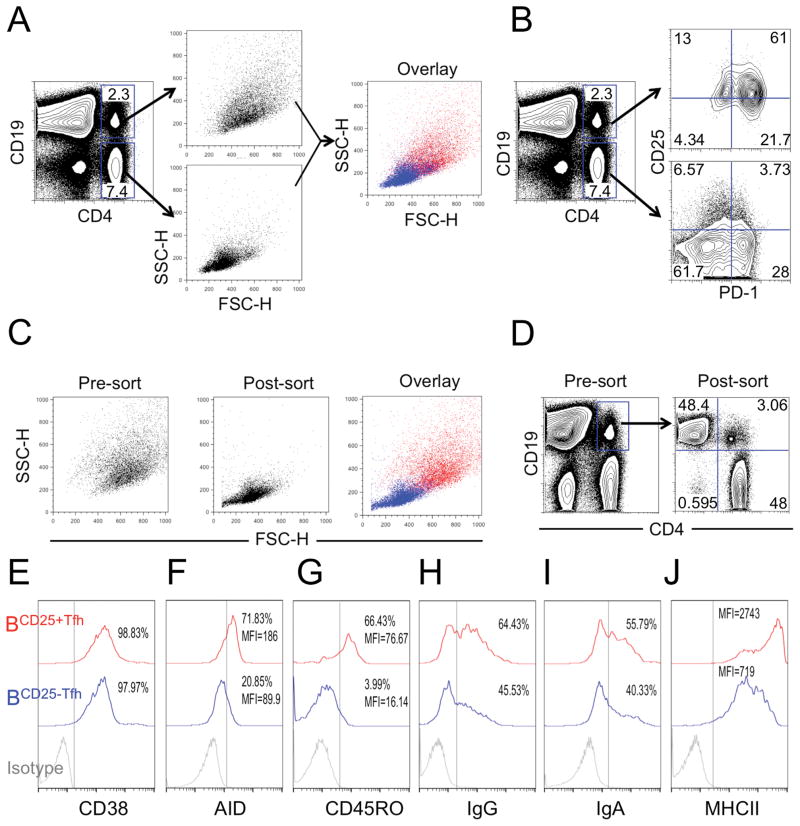
If CD25+CXCR5+PD1+Bcl6low CD4 T cells are authentic B helper T cells, they should be physically associated with germinal center B cells. We noticed that CD25+ Tfh cells from tonsil scattered light as if they were larger than single cells. To investigate this property, we stained tonsil cells with antibodies against CD4 and the B cell marker CD19. We observed objects larger than single cells that were positive for both of these markers (Fig. 7A). More than 60% of the CD4+/CD19+ objects were also PD1+ and CD25+(Fig. 7B). Since PD1 expression was not expected for B cells, we concluded that objects larger than single cells were conjugates, possibly Tfh: B-cell pairs. To rule out the possibility of finding CD4+CD25+CD19+B cells, we sorted double positive cells after staining for CD4+ and CD19+. We gated all live cells to minimize the loss of cell blasts. After sorting, the purified subsets scattered light as expected for single cells (Fig. 7C) and were either CD19+ or CD4+ with very few double positive cells remaining (Fig. 7D). We believe that shear forces during sorting disaggregated T:B-cell conjugates and released individual cells in the sorted material. This result is reinforced by the fact that single cells recovered from sorting were in a 1:1 ratio of T:B cells (Fig. 7D). We analyzed CD25 expression in both the CD19 and CD4 cells obtained after sorting for doublets. CD25 was expressed on sorted CD4 T cells, but not seen on CD19 B cells. Further, the relationship between CD25 expression and other parameters (including PD1, ICOS, OX40, CD40L, Bcl6 and FoxP3) for CD4 T cells that were separated from T:B conjugates were similar to what was shown in Fig. 2 and 3. Thus, the population of Tfh: B-cell conjugates from tonsil are dominated by the CD25+Tfh-cell subset, even though it is a minor subpopulation of total PD1+CD4 Tfh.
Figure 7. CD25+ Tfh cells are incorporated preferentially into T:B-cell conjugates.
(A and B) Total tonsil cells were stained with CD4, CD19, PD1 and CD25. Relative cell size based on (A) scattered light and (B) CD25/PD1 expression of different subsets was analyzed by flow cytometry. CD4 and CD19 double positive cells were purified by cell sorting. (C) Cell size is evaluated based on scattered light. (D) CD19+ (B cells) or CD4+ (Tfh cells) expression before and after sorting.(E–J) Expression of (E) CD38, (F) AID, (G) CD45RO, (H) IgG, (I) IgA and (J) MHCII on B cells conjugated with CD25+and CD25− Tfh cells were analyzed by flow cytometry. (A–J) Data are representative of three independent experiments each with different donors (n=5).
Next, we analyzed B cells conjugated with CD25+ (BCD25+Tfh) or CD25− (BCD25−Tfh) Tfh cells. As expected, both BCD25+Tfh and BCD25−Tfh expressed the human GC B-cell marker CD38 (Fig. 7E) that is known to be upregulated in human B cells but down regulated in mouse B cells [49, 50]. We found that BCD25+Tfh expressed higher levels of activation-induced cytidine deaminase (AID) (Fig. 7F) and memory marker CD45RO (Fig. 7G) compared to BCD25−Tfh, suggesting that BCD25+Tfh express antibodies with higher affinity for antigen than the antibodies found on BCD25−Tfh. Consistently, higher frequencies of BCD25+Tfh cells expressed IgG (Fig. 7H) and IgA (Fig. 7I) indicating they had already progressed beyond the class-switch recombination step. In cognate GC B:T interactions, B-cell receptors (BCR) capture and process antigen roughly in proportion to antibody affinity [49, 51, 52]. This allows GC B cells with higher affinity antibodies to process and present more peptide-MHC (pMHC) to cognate CD4 T cells. Here we found that BCD25+Tfh expressed higher levels of MHCII than did BCD25−Tfh cells (Fig. 7J), indicating that BCD25+Tfh cells have greater potential to form strong interactions with cognate Tfh cells.
Discussion
Tfh-cell differentiation is controlled initially by the achaetescute transcription factor that activates CXCR5 expression and suppresses CCR7 or PSGL1 expression to promote T-cell migration to toward B-cell follicles [5]. Subsequently, cytokine induction of transcriptional regulator Bcl6 is required for functional Tfh cells. In mouse models, IL-2 inhibited Tfh-cell differentiation and blocked germinal center formation [21, 22, 24]. This mechanism accounted for the inhibitory effects of IL-2 on the primary antibody responses against influenza in mice [22]. Thus, IL-2 has been viewed as an inhibitor of both Tfh-cell differentiation and antibody responses in mice. Our studies suggest that IL-2 may have a multiple effects on germinal centers and B-cell maturation, functioning early to inhibit germinal center formation [21, 22, 24] and later to support CD25+Tfh-cells that are conjugated to class-switched, memory B cells.
Dual effects of cytokines have been observed in other systems. For example, IL-2 activation of STAT5 inhibited Th17 polarization and lowered IL-17 production in mice [53]. However, therapeutic antibody against IL-2R was palliative for uveitis, an inflammatory disease associated with IL-17 production, and PBMC from scleritis or uveitis patients expressed IL-17 upon IL-2 stimulation [54].
The CD25+CXCR5+PD1+Tfh cells were a small subpopulation of Tfh cells in human oral tonsil. Low expression of Bcl6 compared to CD25− Tfh, was balanced by low expression of transcriptional repressor Blimp-1 that may allow maintenance of the Tfh-cell markers phenotype including CXCR5 expression. Higher levels of Bcl2 after IL-2 treatment seems to help CD25+PD1+CD4 Tfh cells resist apoptotic cell death. Our finding that T:B-cell conjugates mostly contain CD25+Tfh cells argues they are an important component of B-cell help and are present normally within germinal centers. If conjugates were formed during cell isolation or in vitro manipulation, the large excess of CD25− Tfh cells would have competed for available B cells and the CD25+Tfh: B-cell conjugates would have been much less abundant.
High-affinity B cells (producing high-affinity antibodies) are selected through clonal expansion, B-cell receptor somatic hypermutation (SHM), class-switch recombination (CSR), antigen affinity selection and contact with cognate Tfh cells [1, 49]. Activation-induced cytidine deaminase (AID) is required for SHM and CSR, and its levels are correlated with GC B-cell affinity [1, 49, 55]. Previous studies also showed that CD45RO is a marker for SHM and B-cell affinity [50, 56, 57]. We found that B cells conjugated to CD25+Tfh cells had higher AID, MHC Class II, CD45RO and IgG or IgA expression.
A similar result was seen in IL-4-knockout mice infected with Leishmania major. If the mice were reconstituted with eGFP+IL-4+T cells, the subset of B cells conjugated to eGFP+ T cells expressed higher levels of AID mRNA consistent with increased isotype switching and somatic mutation, and also contained higher levels of IgG mRNA [19]. In human tonsil tissue, CD25+ Tfh cells are associated preferentially with class-switched, CD45RO+, MHC Class IIhiB cells that are likely to express high affinity antibodies. Higher affinity surface antibodies compete for antigen and consequently, present more pMHC to Tfh cells to form higher avidity cell: cell interactions [49, 51, 52], that would increase TCR signaling, CD25 expression andIL-2 production, which further enhances CD25 expression [23]. Such a mechanism depends on affinity maturation to compete for antigen and MHC expression to compete for T-cell help; IL-2 may be one of the cytokines involved in this process of B-cell maturation and affinity selection, even though IL-2 inhibits early steps of germinal center formation. This mechanism is similar to the effects of IL-4 on T:B-cell conjugates and B-cell maturation in mice [19].
The dual effects of IL-2 may help to limit the supply of new Tfh cells during the germinal center reaction even while promoting mature Tfh-cell function. This dual control may help to constrict the B-cell repertoire responding to one antigen and drive the affinity maturation process.
Materials and Methods
Tonsil cell isolation and culture
All of the studies described here were approved by the Institutional Review Board at the University of Maryland, Baltimore. Tissue samples were obtained from young patients or adults undergoing tonsillectomy. Single cells were collected after mechanical disruption of dissected tonsil and density gradient purification of mononuclear cells (Ficoll-Paque; Amersham Biosciences). CD4 T cells and B cells were isolated by negative selection (Miltenyi Biotech). The Tfh cells were stained with anti-CXCR5, anti-PD1 or anti-CD25 antibodies, then sorted with a FACSAria cytometer (BD Bioscience). Purified tonsillar cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; GIBCO), 2 mMol/L L-glutamine, and penicillin–streptomycin (100 U/mL and 100 mg/mL, respectively). For coculture experiments, sorted Tfh-cell populations were cocultured with purified B cells (2×104 cells/well each) in the presence of SEB (1μg/mL) in U-bottomed 96-well plates for 8 days. The concentrations of IgG were determined by ELISA (IMMUNO-TEK, NY). In some experiments, IL-2 was added to the cocultures.
Immunofluorescence
Fresh tonsils were washed with PBS and fixed with paraformaldehyde for one hour at room temperature. Tonsils were placed in Tissue-Tek OCT (Sakura), frozen in liquid nitrogen, and stored at −80 °C. The following antibodies were used: IgD (allophycocyanin) clone IA6-2, CD4 (Alexa Fluor 488) clone RPA-T4, CD25 (PE) clone M-A251, PD1 (Alexa Fluor 488 or PE) clone Eh12.2h7, and FoxP3 (Alexa Fluor 647) clone 259d. Sections were blocked with the blocking solution (5% Goat Serum, 0.1% BSA, 1% DMSO in PBS) and incubated for 1 h in the antibodies diluted in Cyto Qimmunodiluent. Mount cover slip with Prolong Gold antifade reagent (Invitrogen) and sealed with nail polish. Slides were observed under Zeiss LSM 510 Meta Confocal Microscope.
Immunoblot analysis
Cell lysates were boiled for 10 min; proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes and probed with various primary antibodies. Secondary antibodies including HRP-conjugated, anti-rabbit, anti-rat, or anti-mouse (Cell Signaling Technology, Inc.), were visualized with enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK).
Flow cytometry
Unless noted, cells were stained with fluorophore-conjugated monoclonal antibodies from BioLegend, San Diego, CA. Generally, cells were washed and resuspended in 50–100 μL of RPMI 1640, then stained with mouse anti-human CD4 (PE or FITC) clone OKT4, mouse anti-human CD3 (FITC or PerCP or allophycocyanin) clone UCHT1, Mouse anti-human PD1 (FITC or PE or allophycocyanin) clone Eh12.2h7, mouse anti human CXCR5 (PerCP or FITC) clone J252D4, mouse anti-human ICOS (FITC) clone C398.4A, mouse anti-human OX40 (allophycocyanin) clone Ber-act35, mouse anti-human CD40L (PE) clone 24–31, mouse anti-human CD25 (PE or PerCP or allophycocyanin, BD Bioscience) clone M-a251, and isotype controls, including mouse IgG1-FITC clone X40, IgG1-PE clone X40, IgG1-PerCP clone X40, IgG1-allophycocyanin clone X40, and Mouse IgG2b PerCP clone Mpc-11. For intracellular Bcl-6, Bcl-2, c-Maf and FoxP3 staining, cells were stained with FoxP3 Fix/Perm Buffer Set (BioLegend, San Diego, CA). For intracellular cytokine staining, including IL-21, IL-17, IL-10, IL-4, IL-2 and IFN-γ, cells were stained with BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Bioscience). Data for at least 1×104 lymphocytes (gated on the basis of forward- and side-scatter profiles) were acquired from each sample on a FACSCalibur flow cytometer (BD Biosciences). All samples were analyzed using FlowJo software (FlowJo 8.8.2, Tree Star, San Carlos, CA). The increase in mean fluorescence intensity (ΔMFI) was calculated as: [MFI (specific mAb) – MFI (isotype control)]/MFI (isotype control).
Statistical analysis
Differences among groups were analyzed by Student’s t test. P< 0.05 was considered to be significant.
Supplementary Material
Acknowledgments
Haishan Li and C. David Pauza conceived the project, discussed all data, wrote and revised the manuscript together. Haishan Li performed the experimental studies. We are grateful to Dr. Scott Strome and Fleece Hubbard for help with obtaining human tonsil specimens and Dr. Felisa Diaz-Mendez for processing these materials. Special thanks go to Dr. Huiqing Li for help with confocal microscopy of human tonsil tissues. We also want to thank Dr. Marco Goicochea for assistance with cell sorting and to Dr. Yutaka Tagaya for helpful discussions and criticisms of the project. The work was funded by PHS grants AI096949 (C.D.P.) and AI102680 (C.D.P.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. This research was also supported by the Creative and Novel Ideas in HIV Research Program (CNIHR) (H.L.) through a supplement to the University of Alabama at Birmingham (UAB) Center For AIDS Research funding (P30 AI027767-24). This funding was made possible by collaborative efforts of the Office of AIDS Research, the National Institutes of Allergies and Infectious Diseases, and the International AIDS Society.
Abbreviations
- GC
germinal center
- Tfh
T follicular helper cell
- AID
activation-induced cytosine deaminase
- SHM
somatic hypermutation
- CSR
class-switch recombination
Footnotes
Conflict of Interest Disclosure:
The authors declare no commercial or financial conflict of interest.
References
- 1.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nature reviews Immunology. 2012;12:24–34. doi: 10.1038/nri3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. The Journal of experimental medicine. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, Nakatsukasa H, Neelapu SS, Chen W, Clevers H, Tian Q, Qi H, Wei L, Dong C. Transcription factor achaetescute homologue 2 initiates follicular T-helper-cell development. Nature. 2014 doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 7.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. The Journal of experimental medicine. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nature immunology. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- 10.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. Journal of immunology. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 11.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. Journal of immunology. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 12.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of immunology. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber JP, Fuhrmann F, Hutloff A. T-follicular helper cells survive as long-term memory cells. Eur J Immunol. 2012;42:1981–1988. doi: 10.1002/eji.201242540. [DOI] [PubMed] [Google Scholar]
- 16.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. Journal of immunology. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 17.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. Journal of immunology. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 18.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, Watowich SS, Qi H, Dong C, Wang D. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. The Journal of biological chemistry. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson-Leger C, Christenson JR, Holman M, Klaus GG. Evidence for a critical role for IL-2 in CD40-mediated activation of naive B cells by primary CD4 T cells. Journal of immunology. 1998;161:4618–4626. [PubMed] [Google Scholar]
- 26.Kishi H, Inui S, Muraguchi A, Hirano T, Yamamura Y, Kishimoto T. Induction of IgG secretion in a human B cell clone with recombinant IL 2. Journal of immunology. 1985;134:3104–3107. [PubMed] [Google Scholar]
- 27.Splawski JB, McAnally LM, Lipsky PE. IL-2 dependence of the promotion of human B cell differentiation by IL-6 (BSF-2) Journal of immunology. 1990;144:562–569. [PubMed] [Google Scholar]
- 28.Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC Immunol. 2011;12:53. doi: 10.1186/1471-2172-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature medicine. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nature medicine. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go NF, Castle BE, Barrett R, Kastelein R, Dang W, Mosmann TR, Moore KW, Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. The Journal of experimental medicine. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. The Journal of clinical investigation. 1994;93:424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh K, Hirohata S. The role of IL-10 in human B cell activation, proliferation, and differentiation. Journal of immunology. 1995;154:4341–4350. [PubMed] [Google Scholar]
- 34.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature immunology. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 35.Tarlinton D. IL-17 drives germinal center B cells? Nature immunology. 2008;9:124–126. doi: 10.1038/ni0208-124. [DOI] [PubMed] [Google Scholar]
- 36.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Nakamura M, Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 38.Rani A, Afzali B, Kelly A, Tewolde-Berhan L, Hackett M, Kanhere AS, Pedroza-Pacheco I, Bowen H, Jurcevic S, Jenner RG, Cousins DJ, Ragheb JA, Lavender P, John S. IL-2 regulates expression of C-MAF in human CD4 T cells. Journal of immunology. 2011;187:3721–3729. doi: 10.4049/jimmunol.1002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S, Ikeda K, Hirose K, Watanabe N, Grusby MJ, Iwamoto I, Nakajima H. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. Journal of leukocyte biology. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 40.Cao S, Liu J, Song L, Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. Journal of immunology. 2005;174:3484–3492. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. Journal of immunology. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato K, Miyoshi F, Yokota K, Araki Y, Asanuma Y, Akiyama Y, Yoh K, Takahashi S, Aburatani H, Mimura T. Marked induction of c-Maf protein during Th17 cell differentiation and its implication in memory Th cell development. The Journal of biological chemistry. 2011;286:14963–14971. doi: 10.1074/jbc.M111.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao X, Stewart AK, Hurren R, Datti A, Zhu X, Zhu Y, Shi C, Lee K, Tiedemann R, Eberhard Y, Trudel S, Liang S, Corey SJ, Gillis LC, Barber DL, Wrana JL, Ezzat S, Schimmer AD. A chemical biology screen identifies glucocorticoids that regulate c-maf expression by increasing its proteasomal degradation through up-regulation of ubiquitin. Blood. 2007;110:4047–4054. doi: 10.1182/blood-2007-05-088666. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Pauza CD. Critical roles for Akt kinase in controlling HIV envelope-mediated depletion of CD4 T cells. Retrovirology. 2013;10:60. doi: 10.1186/1742-4690-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E488–497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Li H, David Pauza C. Interplay of T-cell receptor and interleukin-2 signalling in Vgamma2Vdelta2 T-cell cytotoxicity. Immunology. 2011;132:96–103. doi: 10.1111/j.1365-2567.2010.03343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindemann MJ, Benczik M, Gaffen SL. Anti-apoptotic signaling by the interleukin-2 receptor reveals a function for cytoplasmic tyrosine residues within the common gamma (gamma c) receptor subunit. The Journal of biological chemistry. 2003;278:10239–10249. doi: 10.1074/jbc.M209471200. [DOI] [PubMed] [Google Scholar]
- 49.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 50.Jackson SM, Wilson PC, James JA, Capra JD. Human B cell subsets. Adv Immunol. 2008;98:151–224. doi: 10.1016/S0065-2776(08)00405-7. [DOI] [PubMed] [Google Scholar]
- 51.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, Utzny C, Muller S, Valitutti S. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 53.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 55.Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog. 2012;8:e1002920. doi: 10.1371/journal.ppat.1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson SM, Harp N, Patel D, Henderson M, Roy NM, Courtney MA, Johnson A, Capra JD. CD45RO: a marker for BCR-mediated selection. Scand J Immunol. 2007;66:249–260. doi: 10.1111/j.1365-3083.2007.01985.x. [DOI] [PubMed] [Google Scholar]
- 57.Jackson SM, Harp N, Patel D, Zhang J, Willson S, Kim YJ, Clanton C, Capra JD. CD45RO enriches for activated, highly mutated human germinal center B cells. Blood. 2007;110:3917–3925. doi: 10.1182/blood-2007-05-087767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.